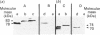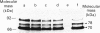Abstract
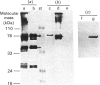
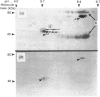
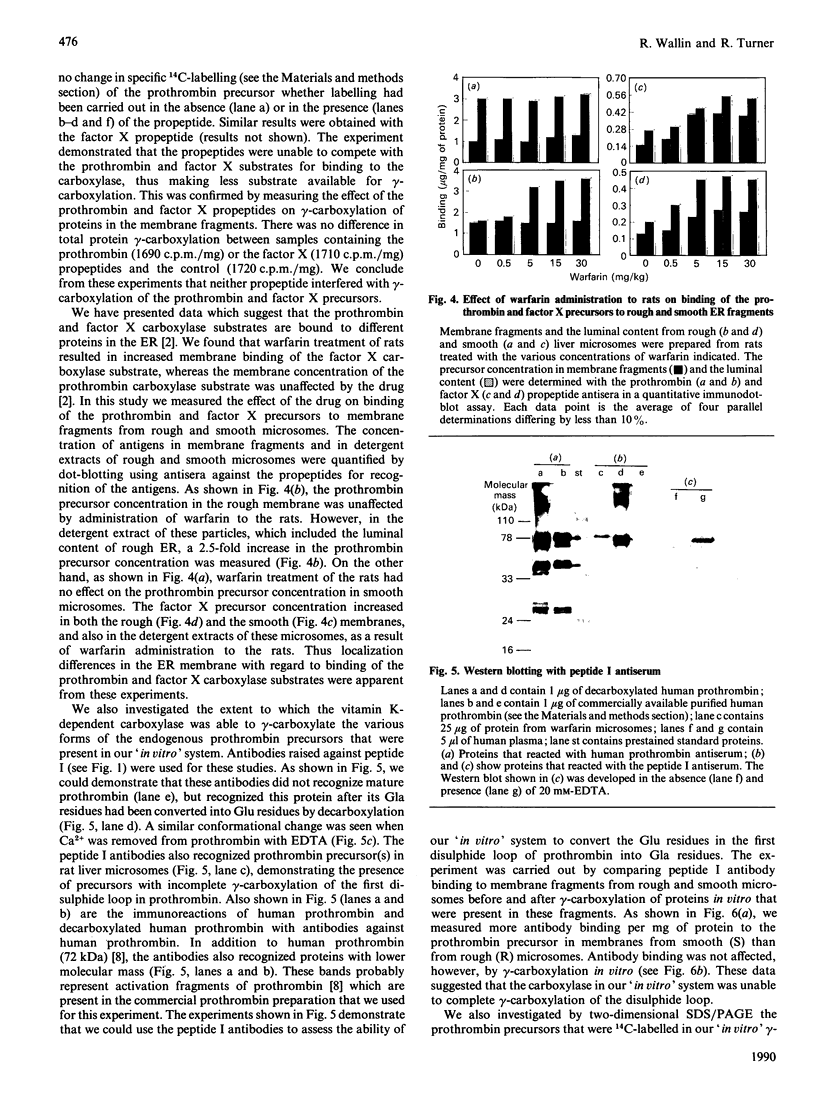
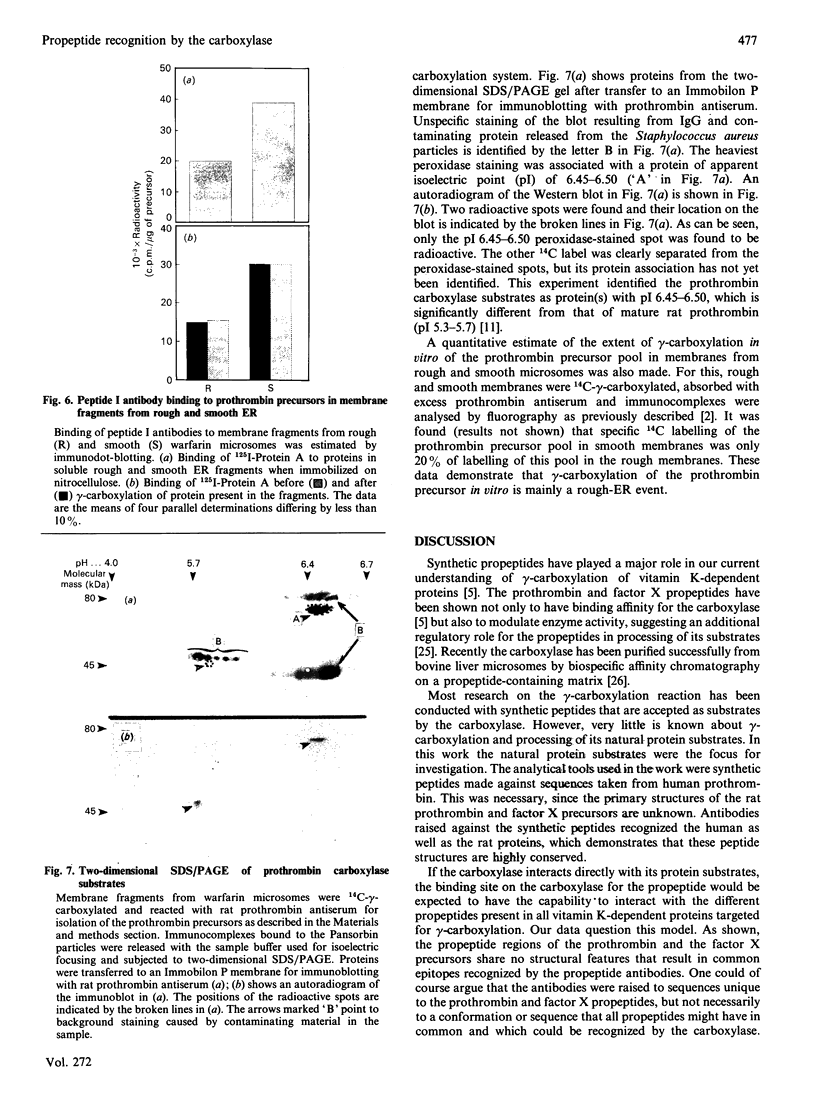
Precursors of vitamin K-dependent proteins are synthesized with a propeptide that is believed to target these proteins for gamma-carboxylation by the vitamin K-dependent carboxylase. In this study synthetic propeptides were used to investigate gamma-carboxylation of the prothrombin and factor X precursors in rat liver microsomes. The extent of prothrombin processing by the carboxylase was also investigated. Antisera raised against the human prothrombin and factor X propeptides only recognized precursors with the respective propeptide regions. The data demonstrate structural differences in the propeptide region of the prothrombin and the factor X carboxylase substrates which raises questions about the hypothesis of a common propeptide binding site on the carboxylase for all precursors of vitamin K-dependent proteins. The hypothesis of separate binding sites is supported by data which demonstrate differences in binding of the prothrombin and factor X precursors to membrane fragments from rough and smooth microsomes. gamma-Carboxylation of the prothrombin precursors in vitro was investigated with conformational specific antibodies raised against a portion of the Gla (gamma-carboxyglutamic acid) region extending from residue 15 to 24. The synthetic peptide used as antigen contains three of the ten potential Gla sites in prothrombin. It is shown that these antibodies do not recognize mature prothrombin but recognize the decarboxylated protein. It is also demonstrated that the epitope is Ca2(+)-dependent. The antibodies were used to assess gamma-carboxylation of the prothrombin precursor in membrane fragments from microsomal membranes. The results suggest that microsomal gamma-carboxylation does not involve Glu residues 16, 19 and 20 of the Gla region.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z. Preparation of monoclonal antibodies to preselected protein regions. Methods Enzymol. 1986;121:69–95. doi: 10.1016/0076-6879(86)21010-1. [DOI] [PubMed] [Google Scholar]
- Carlisle T. L., Shah D. V., Schlegel R., Suttie J. W. Plasma abnormal prothrombin and microsomal prothrombin precursor in various species (38492). Proc Soc Exp Biol Med. 1975 Jan;148(1):140–144. doi: 10.3181/00379727-148-38492. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A., Fair D. S. Molecular recognition in the activation of human blood coagulation factor X. J Biol Chem. 1989 Jul 5;264(19):11035–11043. [PubMed] [Google Scholar]
- Dallner G. Isolation of rough and smooth microsomes--general. Methods Enzymol. 1974;31:191–201. [PubMed] [Google Scholar]
- Degen S. J., Davie E. W. Nucleotide sequence of the gene for human prothrombin. Biochemistry. 1987 Sep 22;26(19):6165–6177. doi: 10.1021/bi00393a033. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Suttie J. W. Vitamin K-dependent carboxylase. Solubilization and properties. J Biol Chem. 1976 Oct 25;251(20):6238–6243. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hubbard B. R., Ulrich M. M., Jacobs M., Vermeer C., Walsh C., Furie B., Furie B. C. Vitamin K-dependent carboxylase: affinity purification from bovine liver by using a synthetic propeptide containing the gamma-carboxylation recognition site. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6893–6897. doi: 10.1073/pnas.86.18.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch J. E., Suttie J. W. Vitamin K-dependent carboxylase. Control of enzyme activity by the "propeptide" region of factor X. J Biol Chem. 1987 Nov 15;262(32):15334–15337. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Novoa E., Seegers W. H., Hassouna H. I. Improved procedures for the purification of selected vitamin K-dependent proteins. Prep Biochem. 1976;6(5):307–338. doi: 10.1080/00327487608061622. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Poser J. W., Price P. A. A method for decarboxylation of gamma-carboxyglutamic acid in proteins. Properties of the decarboxylated gamma-carboxyglutamic acid protein from calf bone. J Biol Chem. 1979 Jan 25;254(2):431–436. [PubMed] [Google Scholar]
- Price P. A., Fraser J. D., Metz-Virca G. Molecular cloning of matrix Gla protein: implications for substrate recognition by the vitamin K-dependent gamma-carboxylase. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8335–8339. doi: 10.1073/pnas.84.23.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiet M. J., Jorgensen M. J., Furie B., Furie B. C. Effect of propeptide mutations on post-translational processing of factor IX. Evidence that beta-hydroxylation and gamma-carboxylation are independent events. J Biol Chem. 1987 Nov 5;262(31):14895–14898. [PubMed] [Google Scholar]
- Riederer B. M., Zagon I. S., Goodman S. R. Brain spectrin(240/235) and brain spectrin(240/235E): differential expression during mouse brain development. J Neurosci. 1987 Mar;7(3):864–874. doi: 10.1523/JNEUROSCI.07-03-00864.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski J. A., Esmon C. T., Suttie J. W. Vitamin K-dependent carboxylase. Requirements of the rat liver microsomal enzyme system. J Biol Chem. 1976 May 10;251(9):2770–2776. [PubMed] [Google Scholar]
- Suttie J. W., Jackson C. M. Prothrombin structure, activation, and biosynthesis. Physiol Rev. 1977 Jan;57(1):1–70. doi: 10.1152/physrev.1977.57.1.1. [DOI] [PubMed] [Google Scholar]
- Suttie J. W. Vitamin K-dependent carboxylase. Annu Rev Biochem. 1985;54:459–477. doi: 10.1146/annurev.bi.54.070185.002331. [DOI] [PubMed] [Google Scholar]
- Swanson J. C., Suttie J. W. Prothrombin biosynthesis: characterization of processing events in rat liver microsomes. Biochemistry. 1985 Jul 16;24(15):3890–3897. doi: 10.1021/bi00336a012. [DOI] [PubMed] [Google Scholar]
- Swanson J. C., Suttie J. W. Vitamin K dependent in vitro production of prothrombin. Biochemistry. 1982 Nov 9;21(23):6011–6018. doi: 10.1021/bi00266a044. [DOI] [PubMed] [Google Scholar]
- Ulrich M. M., Furie B., Jacobs M. R., Vermeer C., Furie B. C. Vitamin K-dependent carboxylation. A synthetic peptide based upon the gamma-carboxylation recognition site sequence of the prothrombin propeptide is an active substrate for the carboxylase in vitro. J Biol Chem. 1988 Jul 15;263(20):9697–9702. [PubMed] [Google Scholar]
- Wallin R., Culp E. N., Coleman D. B., Goodman S. R. A structural model of human erythrocyte band 2.1: alignment of chemical and functional domains. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4095–4099. doi: 10.1073/pnas.81.13.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin R., Martin L. F. Early processing of prothrombin and factor X by the vitamin K-dependent carboxylase. J Biol Chem. 1988 Jul 15;263(20):9994–10001. [PubMed] [Google Scholar]
- Wallin R., Martin L. F. Warfarin poisoning and vitamin K antagonism in rat and human liver. Design of a system in vitro that mimics the situation in vivo. Biochem J. 1987 Jan 15;241(2):389–396. doi: 10.1042/bj2410389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin R., Prydz H. Studies on a subcellular system for vitamin K-dependent carboxylation. Thromb Haemost. 1979 May 25;41(3):529–536. [PubMed] [Google Scholar]
- de Metz M., Vermeer C., Soute B. A., van Scharrenburg G. J., Slotboom A. J., Hemker H. C. Partial purification of bovine liver vitamin K-dependent carboxylase by immunospecific adsorption onto antifactor X. FEBS Lett. 1981 Jan 26;123(2):215–218. doi: 10.1016/0014-5793(81)80290-6. [DOI] [PubMed] [Google Scholar]