Abstract
Background
Negative experiences of needle procedures in childhood can lead to medical avoidance and vaccine hesitancy into adulthood. We evaluated the feasibility of two new interventions provided by clinical nurses to reduce the negative impact of vaccinations: divided attention (DA) and positive memory reframing (PMR).
Methods
Children (8–12 years) were randomized into four groups: usual care (UC), DA, PMR or combined (DA + PMR). To evaluate feasibility, we undertook in‐depth analysis of video‐recorded interventions, nurse experiences (phone interviews) and child/parent memory recall of interventions (phone interviews at 2 weeks post‐vaccination). Key clinical outcomes included child and parent ratings of needle‐related pain intensity and fear assessed at baseline, immediately post‐vaccination and 2 weeks post‐vaccination (recalled).
Results
A total of 54 child–parent dyads were screened, with 41 included (10/group, except PMR [n = 11]). The interventions were not always completed as intended: 10%–22% of participants received complete interventions and two had adverse events related to protocol breach. Preliminary within‐group analyses showed no effects on child/parent pain ratings. However, children in DA + PMR had reduced recalled fear (p = 0.008), and PMR (p = 0.025) and DA + PMR (p = 0.003) had reduced fear of future needles. Parent ratings of child fear were also reduced immediately post‐vaccination for UC (p = 0.035) and PMR (p = 0.035).
Conclusions
The interventions were feasible, although enhanced nurse training is required to improve fidelity. Preliminary clinical results appear promising, particularly for reducing needle‐related fear. Protocol registration: Protocol number ACTRN12618000687291 at ANZCTR.org.au
Significance
Two new nurse‐led interventions to reduce negative impacts of vaccinations in children, divided attention and positive memory reframing, were feasible and may reduce needle‐related fear. Nurses were able to deliver the interventions in various environments including non‐clinical settings (schools). These interventions have potential to facilitate broader dissemination of vaccinations for children in a manner that minimizes distress.
1. INTRODUCTION
For many children, undergoing a needle procedure can be a painful and distressing experience. Such negative experiences can develop into fear of needles (McMurtry et al., 2015), which can have devastating consequences such as vaccine hesitancy and outbreaks of preventable diseases. Reducing fear/phobias of needles are a critical research priority given the current COVID‐19 pandemic and the need for mass global vaccination uptake (Love & Love, 2021).
Pain influences children long after the painful stimulus is removed (Noel, Chambers, Petter, et al., 2012). Children's memories of needle‐related pain are a powerful predictor of future pain experiences and may have greater influence on future pain than the initial experience of pain itself (Noel et al., 2017; Noel et al., 2012a; von Baeyer et al., 2004). Children's pain memory development is influenced by multiple factors (Noel, Chambers, Petter, et al., 2012; Noel, Palermo, et al., 2015), including anxiety (Noel et al., 2012b), pain‐related fear (Kain et al., 2006), sex (Hechler et al., 2009), and age (Noel, Rabbitts, et al., 2015). Recalling higher levels of childhood pain (compared with initial pain reports) is associated with higher subsequent pain, distress, medical non‐compliance and future pain expectancies (Arntz et al., 1990; Noel, Chambers, McGrath, et al., 2012; von Baeyer et al., 2004). Children's pain memories are malleable through post‐event information (Noel, 2016). For example, positive memory reframing (PMR) involves talking to children after a painful experience to emphasize its positive aspects, correct negative exaggerations in recall, and foster a sense of self‐efficacy in their pain coping (Noel et al., 2018).
While reframing the memory of a painful experience may reduce the negative impact of needle‐related pain, so might decreasing the intensity of the painful experience itself. Attention and expectation are important contributors to the pain experience (Johnston et al., 2012; Miron et al., 1989; Quevedo & Coghill, 2007). For example, expecting to receive noxious stimuli in two spatially discrete locations on the arm (divided attention [DA]) resulted in an analgesic effect for a noxious stimulus that was provided between those two locations (Stanton et al., 2016). That is, the stimulus was rated as significantly less painful when expectancy was spatially divided than when there was no expectancy concerning stimulus location. Analgesia during DA is thought to be mediated by changes in receptive field (RF) properties of dorsal horn neurons (Hayes et al., 1981; Quevedo & Coghill, 2007). When spatially dividing attention between two spatially discrete skin locations, psychophysical findings suggest that nociceptive RFs become more ‘focussed’ (smaller and shifted towards the two attended areas), creating a relative ‘silent zone’ between the two attended areas where sensitivity to nociceptive stimuli may be reduced (Quevedo & Coghill, 2007). Thus, the DA paradigm aims to take advantage of inherent properties of nociception involving attentional modulation of pain and may reduce pain intensity experienced from an acutely painful procedure. Given the spatially localized nature of needles and the resultant experience of pain, use of such a paradigm may provide analgesia in a clinical vaccination situation.
Applying PMR and DA together may also have synergistic or super‐additive effects. If the pain experienced during a needle procedure is decreased and post‐intervention information positively reframes the experience, the pain and fear that is remembered may be substantially reduced, as might expectancies of future pain. Few studies have examined memory‐reframing interventions targeting children's recall of (needle) pain (Bruck et al., 1995), and these interventions were delivered by researchers. Childhood vaccinations are typically provided by nurses in schools, particularly from Grade 1 (typically aged ≥6 years) onward. We targeted primary school children aged 8–12 years old to reflect this, and also, to ensure the validity of self‐report of pain, fear and the other constructs (Birnie et al., 2019). Thus, in the current study, we trained practicing clinical nurses to administer these interventions in a variety of environments, including local schools. Given the complexity of the interventions and the novelty of nurse‐led delivery, it is crucial to determine feasible procedures prior to undertaking a large‐scale trial that can formally analyse clinical efficacy. Therefore, we aimed to: (i) evaluate the feasibility of implementing the two new interventions (DA, PMR, both or neither) to reduce the negative impact of needle procedures in children undergoing flu vaccination; (ii) explore preliminary effects on needle‐related pain/fear.
2. METHODS
2.1. Study design
This was a randomized, outcome assessor blinded, 2 × 2 factorial feasibility trial. Clinical outcomes were assessed at baseline, immediately post‐vaccination and at 2‐week post‐vaccination. This study was conducted in accordance with the Declaration of Helsinki and was approved by the University of South Australia (UniSA) Human Research Ethics Committee on the 9th of March 2018 (protocol number 200775) and the Department of Child Education, Government of South Australia (reference number 2018‐0010) on 13 February 2018. The protocol was registered on 19 April 2018 with the Australian and New Zealand Clinical Trials Registry (ANZCTR.org.au; protocol number ACTRN12618000687291).
Participants were randomly allocated to groups (1:1:1:1) using a computer‐generated randomization schedule, involving random permuted blocks of four and eight, that was generated by an independent investigator using Excel. Allocation was concealed in sequentially numbered, sealed, opaque envelopes created by an investigator not involved in the study. The clinical nurse opened each envelope just prior to the commencement of each participant's vaccination appointment.
Participants (parents and children) were advised that they would be allocated to one of four groups, but that all children would receive at minimum, best‐practice usual care (UC). The additional interventions were briefly described, with participants advised that some children would be allocated to receive these additional interventions. Research staff, including the outcome assessor and the researcher performing analysis of clinical data, were blinded to group allocation. Clinical nurses were unavoidably aware of group allocation but were not involved in outcome assessment.
2.2. Protocol deviations
Initially, this study was intended to be a randomized clinical trial (n = 256 total), but due to limited funding available, this study was modified to be a feasibility trial (ANZCTR protocol registration formally updated on 2 February 2022). The feasibility design was also considered appropriate given the novelty of the interventions being nurse‐delivered. Consequently, no initial pre‐specified feasibility criteria were set in the official trial registration. However, prior to any data analysis, consensus amongst the research team was used to set feasibility priorities. These priorities included recruitment rate, retention rate, feasibility/acceptability of the interventions (based upon parent, child, nurse experiences), and fidelity of the intervention provision. We also retained all outcome measures and monitored missing data to evaluate the feasibility of including all potentially relevant measures, including predictors of pain and pain memory at baseline, in a large‐scale clinical trial. Thus, the target sample size and analytical procedures were adjusted to enable the evaluation of intervention feasibility, rather than efficacy. Accordingly, we aimed to recruit at least 10 participants per group to allow in‐depth video analysis of each intervention session and thorough evaluation of the feasibility of clinical nurses delivering each intervention.
2.3. Participants
Participants were recruited from the community in South Australia via social media, community flyers and emails to local schools. Eligible participants were child–parent dyads who consented for the child to receive a yearly influenza vaccination. Eligible children were aged 8–12 years who were currently attending school. Children were excluded if they had a history of severe allergic reaction after a vaccine (e.g., anaphylaxis), severe egg allergies (e.g., respiratory distress/required epinephrine), diagnosed anxiety disorder or post‐traumatic stress disorder, or moderate to severe illness (with or without fever) on the day of vaccination. While severe egg allergies are no longer considered a contraindication for the influenza vaccine (Australian Government Department of Health, 2019), at the time of the study the Government of South Australia website suggested caution in those with severe egg allergies, hence exclusion in this study.
2.4. Procedure
Figure 1 outlines the clinical trial procedures. Volunteers first underwent an initial screen (either online or paper‐based) for basic eligibility criteria to exclude children with severe allergies or who had been diagnosed with anxiety or post‐traumatic stress disorders. Eligible participants were then scheduled for an appointment at the relevant testing site, where parents completed a further pre‐vaccination eligibility screening (i.e., parent report of medical exclusions via a paper‐based needle safety checklist, reviewed by the clinical nurse prior to the vaccination) to ensure the child was safe to receive the vaccination.
FIGURE 1.
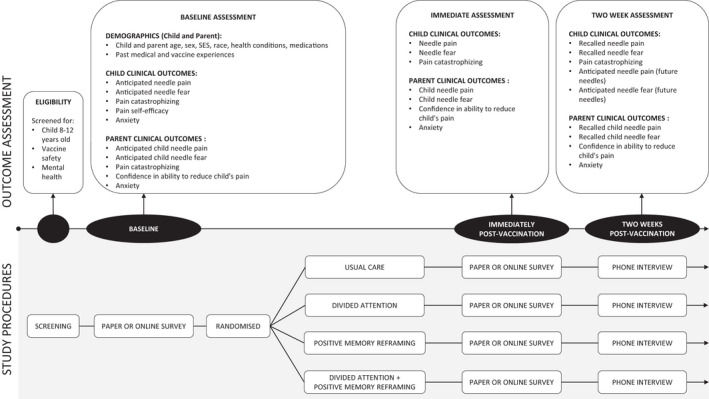
Clinical trial procedures
Data were collected at three sites: The UniSA Physiotherapy Clinic and at two local schools. The research team explained the study and obtained written informed consent from participants (child and parent) upon their arrival at the testing site. Permission to video record the intervention was also sought. The child and their parent then completed the baseline questionnaires either on paper or on iPads (using SurveyMonkey). A modified procedure was used for testing at schools, whereby verbal consent was attained and baseline questionnaires were sent out to the participant to complete prior to, but on the same day as, attending the vaccination. No appointments proceeded until written informed consent was attained. At baseline, demographic information (age, sex, race, school, socioeconomic status) and psychometrically‐sound outcome measures were collected from the child and their parent. For children, baseline outcomes included expectancies of needle‐related pain intensity (0–5) and fear (0–4), collected using face scales (Faces Pain Scale‐Revised [FPSR] and Children's Fear Scale [CFS], respectively) (Hicks et al., 2001; McMurtry et al., 2011), and the Pain Catastrophizing Scale (PCS‐State [3‐item]) (Durand et al., 2017). For parents, baseline outcomes were assessed using 11‐point Numeric Rating Scales (NRS), including expectancies of their child's needle‐related pain intensity and fear (Durand et al., 2017), state anxiety (Parent State Anxiety NRS) (Durand et al., 2017), and their confidence that they could decrease their child's pain if they tried (Parent Stop Tendency NRS) (Caes et al., 2011). At baseline only, children and parents completed further outcome measures that have been shown to be predictors of pain and pain memory, and parents completed medical history and vaccination preparation questionnaires. See Supplementary File 1 for full details regarding the clinical outcome measures. The child then entered the designated, private intervention room accompanied by their parent, and received the allocated intervention.
Follow‐up clinical outcome assessments occurred immediately post‐vaccination (in‐person), collected either electronically or in paper‐based format, and then verbally (via phone call) 2 weeks post‐vaccination. Immediately post‐vaccination, the child's needle‐related pain intensity and fear were assessed using the face scales (child) and NRS (parent). In addition, the child completed the PCS‐State and the parent completed the Parent State Anxiety NRS and the Parent Stop Tendency NRS (confidence). Two weeks post‐vaccination, children and parents performed an established memory interview with the blinded outcome assessor via phone call (Noel et al., 2009; Noel et al., 2012a; Noel, Palermo, et al., 2015) (Supplementary File 1). Telephone interviews were audio‐recorded and transcribed. Recall of pain and fear related to the needle procedure were assessed, as well as expectancies for pain/fear for future needles, using both free recall and the same pain and fear scales previously administered (i.e. face scales, which were emailed to the parent ahead of the interview). The PCS‐State (child) and the Parent State Anxiety NRS and Parent Stop Tendency NRS (parent) were also assessed during this interview at 2 weeks post‐vaccination.
2.5. Interventions
Interventions have been described in accordance with the TIDieR checklist (Hoffmann et al., 2014). Clinical nurses (n = 3; 30–41 years of nursing experience) who were also experienced in paediatric vaccination (3–17 years of experience) and trained in the study procedures provided all four interventions. The recruited clinical nurses each received ~4 h of study‐specific training, including written information on the intervention procedures for each group, guided practice of each of the intervention, a video recording of ‘best practice’ provision of PMR and DA, and assessment of intervention fidelity using pilot participants (n = 4). Training and assessment of intervention fidelity prior to trial commencement was completed by experts in the field and chief investigators of this trial (T.R.S. and M.N.). The nurses were also provided with intervention ‘information sheets’ to guide them during data collection (Supplementary File 2). All children received a single 0.5 ml intramuscular injection to the upper arm of Fluarix Tetra Inactivated influenza Vaccine (year‐relevant strains as recommended by the World Health Organization [WHO] for the Southern Hemisphere vaccine). In all interventions, the clinical nurse began by reviewing the vaccine safety checklist and providing practical information on what to expect after the vaccination (e.g., feeling a bit tired or sluggish, sore arm) and strategies to deal with potential side effects (e.g., drink plenty of water, ice pack on arm).
Participants were randomized into one of four groups:
Usual care (UC): This condition followed usual best‐practice standard care procedures as normally employed by the clinical nurses (Birnie et al., 2018). This condition typically included having the nurse engage with the child, including calming them down if anxious using their own usual strategies (e.g., verbal reassurance) and using a method of verbal and physical distraction when providing the needle (e.g., verbally advising the child to wiggle their toes). While topical anaesthesia can be used as part of normal clinical care during needle‐related procedures, this was not used in the present study because at the time of the study, topical anaesthesia was not recommended for routine use as part of standard care in Australia (ATAGI, 2016). Additionally, for our study purposes and particularly for interventions involving DA, we were interested in seeing if pain relief could occur without topical anaesthetics. Usual care was provided in all four groups; however, the other three groups also received DA, PMR or both.
Divided attention (DA): The DA intervention aimed to take advantage of spatially‐precise analgesic effects of expectation and attention by using a tactile localization game. Prior to receiving the vaccination, children in this group engaged in a sensory discrimination task that required them to divide their attention between two areas on their upper arm. The nurse identified two spots on the child's arm, one above and one below the location where the vaccine injection would be given. Using either the rubber end of a pencil or their index finger, the nurse touched the child's skin in spot one or spot two (random order, slightly varying exact location each time) and asked the child to identify which spot was touched. If this was too easy (i.e., child getting all correct), then the nurse first increased the speed of touching and then if necessary, provided the touch in triplets or quadruplets, asking the child to identify the pattern of touch (e.g., spot one, spot two, then spot two). The nurse also provided an effect statement (‘touching the skin like this makes it go a bit numb’). The game was played for 1–2 min, and then the nurse informed the child that an important question would be asked about those two spots later. Specifically, the nurse asked the child to remember the spatial location of the two spots from the game; this aimed to maximize potential for analgesia. Consistent with past work (Stanton et al., 2016), the nurse provided the needle at a skin location situated in between the two spots used in the game. The child was then asked at the end of the appointment to identify to which spot the needle was given (i.e., the ‘important question’). We anticipated that this intervention would take approximately 4 min to provide.
Positive memory reframing (PMR): Children in this group received an individualized intervention that aimed to focus on and emphasize positive aspects of a previous needle experience (or other previous painful experience if they could not recall a needle experience), correct negative exaggerations in recall, and to promote a feeling of self‐efficacy in their abilities to cope with needles. This intervention drew from existing memory reframing techniques used in the context of children's needle procedures (Bruck et al., 1995; Noel et al., 2018). PMR involved talking to the child and parent for a few minutes both before and immediately following the vaccine injection. To individualize the intervention, the clinical nurse first spoke with the parent about the child's previous needle experience with the aim of determining 1–2 positive things that occurred during the past experience. The parent was also able to identify whether the child was inaccurately recalling the past needle or if their memory was negatively exaggerated. The nurse then engaged with the child and had the child recall positive features of their past needle experience, even if minimal (e.g., what went well, a friendly nurse), supplemented by parent input. Any exaggerations of recall were addressed (e.g., the previous needle procedure actually only took 2 min not 10 min), supported by parent input. The nurse also praised specific strategies that the child used well (e.g., deep breaths, looking away) and affirmed that the child was brave, together promoting increased self‐efficacy for pain coping. If the child had not previously received a needle or could not recall receiving a needle, then discussion of another past medical procedure or painful experience was undertaken. Following this, the vaccination was provided. After the vaccine injection, with the clinical nurse praised how well the child handled the experience, pointing out specific coping strategies that the child used that were helpful and adaptive in managing pain. The nurse affirmed that their chosen strategy is actually known to help, praising their bravery (e.g., ‘you handled this really well’, ‘you were really brave’), minimizing the distressing aspects (de‐emphasizing distressing and painful aspects), and bolstering children's sense of self‐efficacy in their ability to cope with needles by providing positive statements about how they will handle future needles (e.g., ‘your next needle is going to go super well because you know how to make it go well by doing all these helpful strategies’). We anticipated that this intervention would take approximately 6 min to provide, but that time would vary depending on the time taken for parents and/or children to provide information about the previous vaccine experience.
Divided attention + positive memory reframing (DA + PMR): This group received both the PMR and DA interventions to test whether there is a synergistic or super‐additive effect on pain and fear when high levels of self‐efficacy for pain coping are promoted and when attention is divided to potentially decrease the pain of the needle itself. Identical procedures were used for each intervention (as described above), with PMR first, followed by the DA game prior to the needle, followed by PMR after the needle. We anticipated that the combined interventions would take approximately eight to 10 minutes to provide.
2.6. Feasibility outcomes
2.6.1. Study procedures
Recruitment rates and retention rates were monitored to evaluate the feasibility of study procedures.
2.6.2. Feasibility of intervention delivery
To evaluate the feasibility of intervention delivery by clinical nurses, the video‐recorded interventions were assessed by two independent researchers (H.G.J. and C.G.N.) who evaluated numerous aspects of the intervention delivery. Conflicts were resolved via discussion.
Intervention fidelity
A standardized coding scheme was used to characterize the exact procedures used by clinical nurses for each participant. Interventions were coded according to the number of intervention components that were delivered as intended for each participant (UC = 2 components, DA = 10 components, PMR = 7 components, DA + PMR = 17 components; see Supplementary File 3). Examples of intervention components include ‘Nurse introduces game to the child’ (DA) and ‘Nurse acknowledges a good coping strategy that the child used’ (PMR). This was completed using a protocol checklist with three response categories: yes, no and partially. The proportion of components delivered as intended for each intervention overall (for all participants combined), as well as the proportion of participants for which the intervention was delivered in full, were calculated. Additional strategies used by nurses in each intervention were described (e.g., wiggling the toes, sitting on the parent's lap). Last, the timing of side effect information delivery was coded as ‘before’, ‘between’ or ‘after’ the intervention/needle because this timing could influence how the child experiences the needle procedure and thus, could impact the therapeutic effects of the interventions. The intervention protocol included specific instructions for nurses to provide vaccination side effect information before beginning the intervention, which was particularly important for the interventions involving PMR. Thus, the fidelity criteria were that providing side effect information before commencing the intervention was considered acceptable, and providing information after or between the intervention/needle was unacceptable.
Child, parent and nurse distress and coping (promoting) behaviours during interventions
The short‐form Child‐Adult Medical Procedure Interaction Scale (CAMPIS‐SF) (Blount et al., 2001) was used to assess children's distress and coping, as well as the coping and distress promoting behaviours of their parent and the nurse, by the same two independent researchers (H.G.J. and C.G.N.). The CAMPIS‐SF uses a 5‐point Likert scale (1 = none, 5 = maximum or nearly continuous) to rate the frequency of verbal and non‐verbal coping and distress behaviours for the child and coping‐promoting and distress‐promoting behaviours of the parent and nurse separately (Blount et al., 2001). Ratings were made for the periods occurring before, during and after the vaccination. Conflicts where there was a one‐point difference on the scale were averaged, and conflicts where there was a two‐point or greater difference were resolved through discussion and re‐watching the videos to reach consensus. The nurses were also assessed for rapport (0–10 NRS: 0 = extremely poor quality, 10 = extremely high quality) and communication pattern (adapted for the nurses based upon prototypical parent communication patterns in painful paediatric contexts) (Cline et al., 2006). See Supplementary File 3 for these assessment tools.
2.6.3. Child/parent recall of interventions
The content of each memory recall interview with children and their parents (i.e., follow‐up phone calls at 2 weeks post‐vaccination) was also coded by the two independent researchers (H.G.J. and C.G.N.) according to recalled verbal and non‐verbal expressions that occurred during the vaccination. Notably, this did not involve coding verbal/non‐verbal communication occuring during the phone call between the researcher and the child/parent; coding was specific to their recall of the vaccination experience. Codes included explanations of pain/vaccination, coping, pain, anxiety/fear and references to body parts and medical procedures (Noel et al., 2019) (see Supplementary File 4 for the coding scheme: Table S4.1). Non‐verbal behaviours (e.g., crying, smile and frowning) were coded when the parent or child recalled verbally that the child displayed these behaviours at the time of the vaccination. Conflicts were resolved via discussion.
2.6.4. Nurse perception of interventions
After data collection was completed, each nurse was interviewed via phone (by F.A.B.) to assess their perceptions of how each intervention went (i.e., what went well and what was challenging). Nurses were also asked about their beliefs regarding the purpose of each intervention, their confidence with delivering each intervention, and for suggestions on how to improve both the training procedures and the interventions themselves. Phone interviews with the nurses were audio‐recorded and descriptively synthesized by an independent researcher (H.G.J.).
2.7. Analyses
2.7.1. Feasibility outcomes
Feasibility of study procedures and intervention fidelity were examined in terms of frequencies and percentages. Descriptive statistics (mean, standard deviation [SD], frequency and range) were used to summarize other intervention factors, including the rapport scores for the nurses, the CAMPIS‐SF scores and the coding of verbal and non‐verbal content from the 2‐week post‐vaccination memory interview phone calls with the children and parents. Descriptive syntheses were used to summarize nurse communication patterns during interventions and nurse perceptions of the interventions provided via the phone interviews.
2.7.2. Preliminary clinical outcomes
Given the feasibility nature of this study, we conducted exploratory analyses on available data for child and parent clinical outcomes, including child and parent ratings of child needle‐related pain intensity and fear, child catastrophizing and parent anxiety and confidence. Missing data were excluded from the analyses. Overall changes over time (all four groups combined) and within‐group changes over time were analysed using repeated measures analyses of variances with pairwise comparisons (if main effect significant) and paired t‐tests, respectively. Cohen's d was used to estimate effect sizes for within‐group changes. Sensitivity analyses excluding participants who had adverse events were completed. Given the exploratory nature of this analysis, correction for multiple comparisons was not undertaken. Within‐group change scores and their 95% confidence intervals were calculated, with Cohen's d (for dependent samples) reported.
3. RESULTS
Table 1 presents demographic characteristics of children and their parents, with Supplementary File 5 (Table S5) presenting further detail on participant vaccine and painful medical procedure history and vaccine preparation strategies. Of the 41 eligible child–parent dyads, one involved a non‐biological parent (foster carer), and this carer had known the child for 6 years (DA group). Eight parents had two eligible children, therefore, these parents completed study assessments twice, one with respect to each child. A third family had three eligible children (the mother of these three children completed the baseline survey three times and follow‐up assessments with respect to two children, however, the father attended the vaccination of one child, so he completed follow‐up assessments with respect to that child).
TABLE 1.
Participant demographics
| Characteristic | Usual care (n = 10) | Divided attention (n = 10) | Positive memory reframing (n = 11) | Divided attention + positive memory reframing (n = 10) | Overall (n = 41) |
|---|---|---|---|---|---|
| Sex (n, female:male) | |||||
| Child | 5F:5M | 7F:3M | 7F:4M | 7F:3M | 26F:15M |
| Parent | 8F:2M | 10F:0M a | 9F:2M | 5F:5M | 32F:9M |
| Age (child) | 9.90 (1.30) | 9.30 (1.42) | 9.64 (1.50) | 9.30 (0.95) | 9.54 (1.28) |
| Number of people in household | |||||
| Total | 4.00 (0.83) | 4.50 (1.43) | 4.27 (1.10) | 4.60 (1.07) | 4.34 (1.10) |
| Children | 2.10 (0.88) | 2.70 (1.57) | 2.36 (1.36) | 2.70 (1.34) | 2.46 (1.29) |
| Marital status (n, parent) | |||||
| Married | 8 | 6 | 9 | 9 | 32 |
| Common‐law | 1 | 0 | 1 | 1 | 3 |
| Separated/divorced | 1 | 3 | 1 | 0 | 5 |
| Single | 0 | 1 | 0 | 0 | 1 |
| Education level (n, parent) | |||||
| Graduate/professional school, Master's degree, PhD | 3 | 4 | 3 | 3 | 14 |
| College/Bachelor's degree | 3 | 4 | 4 | 3 | 13 |
| Vocational school/some college, no degree | 4 | 2 | 2 | 1 | 9 |
| High school or less | 0 | 0 | 2 | 3 | 5 |
| Household income (n) | |||||
| Less than $15,000 | 0 | 0 | 0 | 1 | 1 |
| $15,000–$29,999 | 0 | 0 | 1 | 1 | 2 |
| $30,000–49,999 | 1 | 1 | 1 | 1 | 4 |
| $50,000–$74,999 | 2 | 0 | 0 | 1 | 3 |
| $75,000–$99,999 | 2 | 1 | 1 | 1 | 5 |
| $100,000–$149,999 | 4 | 4 | 5 | 2 | 15 |
| Over $150,000 | 1 | 3 | 2 | 3 | 9 |
| NR | 0 | 1 | 1 | 0 | 2 |
| Race (n, child) | |||||
| Caucasian/white | 9 | 10 | 10 | 9 | 38 |
| Asian | 0 | 0 | 1 | 1 | 2 |
| Other | 1 | 0 | 0 | 0 | 1 |
| Race (n, parent) | |||||
| Caucasian/white | 9 | 9 | 10 | 9 | 37 |
| Asian | 1 | 0 | 1 | 1 | 3 |
| Other | 0 | 0 | 0 | 0 | 0 |
| NR | 0 | 1 | 0 | 0 | 1 |
| Pain catastrophizing | |||||
| Child (PCS‐C) | 19.10 (12.02) | 20.40 (10.72) | 15.36 (6.44) | 22.60 (12.06) | 19.27 (10.43) |
| Parent (PCS‐P) | 13.20 (5.75) | 12.11 (4.14) b | 12.60 (5.58) b | 21.20 (10.22) | 14.82 (7.59) c |
| Anxiety | |||||
| Child (0–10 CSA‐NRS) | 4.60 (2.41) | 4.10 (3.31) | 4.18 (3.71) | 6.20 (2.53) | 4.76 (3.06) |
| Child (STAI‐C) | 35.70 (5.27) | 33.50 (7.78) | 30.09 (6.46) | 36.60 (5.34) | 33.88 (6.58) |
| Child (CASI) | 29.50 (5.97) | 32.00 (7.77) | 26.73 (3.04) | 31.20 (7.15) | 29.78 (6.30) |
| Parent (STAI‐S) | 45.20 (5.71) | 43.22 (5.47) b | 45.60 (5.04) b | 46.20 (4.69) | 45.10 (5.08) c |
| Parent (STAI‐T) | 45.40 (2.59) | 43.44 (2.96) b | 45.40 (4.06) b | 47.20 (5.14) | 45.41 (3.92) c |
| Child pain self‐efficacy (0–10 NRS) | 5.20 (3.58) | 5.90 (2.85) | 5.82 (3.09) | 4.50 (2.59) | 5.37 (2.99) |
| Physical or mental health diagnoses (n, parent) |
1 Anxiety 1 ASD, ADHD 1 Primary sclerosing cholangitis, ulcerative colitis |
1 Behcet's disease 1 Depression 1 Rheumatoid Arthritis 2 NR |
1 Anxiety, post‐natal depression 1 Breast cancer 1 Dershen Axide 1 Rheumatoid Arthritis 1 NR |
1 ADHD 1 Brain injury, gout 1 Depression, anxiety 1 Depression |
5 Depression and/or anxiety 2 Rheumatoid Arthritis 2 ASD and/or ADHD 5 Other 3 NR |
|
Physical or mental health diagnoses (n, child, reported by parent) |
1 ASD, ADHD 1 Dyslexia 1 Swelling |
1 Finger fracture 1 NR |
1 NR |
1 ASD 1 Small wrist fracture |
2 ASD and/or ADHD 3 Injury 1 Dyslexia 2 NR |
Note: Values are mean (SD) uncles otherwise indicated. Child pain self‐efficacy was assessed using a vaccination‐specific 0–10 point Numeric Rating Scale (0 = Not well at all; 10 = very well).
Abbreviations: ADHD, attention deficit hyperactivity disorder; ASD, autism spectrum disorder; CASI, Childhood Anxiety Sensitivity Index (18 items, 1–3 Likert scale, maximum score 54, lower score represents lower anxiety); CSA‐NRS, Child State Anxiety Numeric Rating Scale (0–10, 0 = not at all nervous or anxious, 10 = most nervous or anxious, maximum score 10, lower score represents lower state anxiety/nervousness); NR, not reported; NRS, Numeric Rating Scale; PCS‐C, Pain Catastrophizing Scale for Children (13 items, 0–4 Likert scale, maximum score 52, lower score represents less catastrophizing); PCS‐P, Pain Catastrophizing Scale for Parents (13 items, 0–4 Likert scale, maximum score 52, lower score represents less catastrophizing); STAI‐C, State Trait Anxiety Inventory for Children (20 items, 1–3 Likert scale, maximum score 60, lower score represents lower anxiety); STAI‐S, State Trait Anxiety Inventory State subscale for parents (20 items, 1–4 Likert scale, maximum score 80, lower score represents lower state anxiety); STAI‐T, State Trait Anxiety Inventory Trait subscale for parents (20 items, 1–4 Likert scale, maximum score 80, lower score represents lower trait anxiety).
The mother of one child completed the baseline assessment but then the father attended the vaccination and completed follow‐up assessments.
n = 1 missing data.
n = 2 missing data.
3.1. Feasibility outcomes
3.1.1. Feasibility of study procedures (recruitment and retention)
Data collection occurred over two flu seasons (19th of April 2018 to 23rd of June 2018; 24th of July 2019). Figure 2 presents the flow of participants through the study using the Consolidated Standards of Reporting Trials (CONSORT) for feasibility studies (Eldridge et al., 2010). Over 3 months of the 2018 flu season, 50 participants were screened and 37 were recruited. In order to reach our recruitment target of 10 per group, we re‐started data collection in the subsequent flu season. In 2019, four participants were screened, recruited and immunized on a single day (via one school). This corresponds to a recruitment rate of approximately 3–4 participants per week. A total of 54 child–parent dyads were screened, with 51 eligible and 41 included. Ten dyads were randomized to each group except the PMR group (n = 11). Three exclusions related to the child being outside the 8–12‐year age‐range; the other 10 exclusions were eligible but never received the vaccination due to non‐response to follow‐up contact (n = 7) and scheduling issues (n = 3), including two whose appointments were cancelled due to vaccine shortage and could not be rescheduled. Four participant appointments in total had to be cancelled due to a vaccine shortage, but two were able to be rescheduled. Missing clinical data (7% across all timepoints) were excluded from the analyses. The main reason for missing data was loss to follow‐up at the 2‐week post‐vaccination timepoint (n = 6).
FIGURE 2.
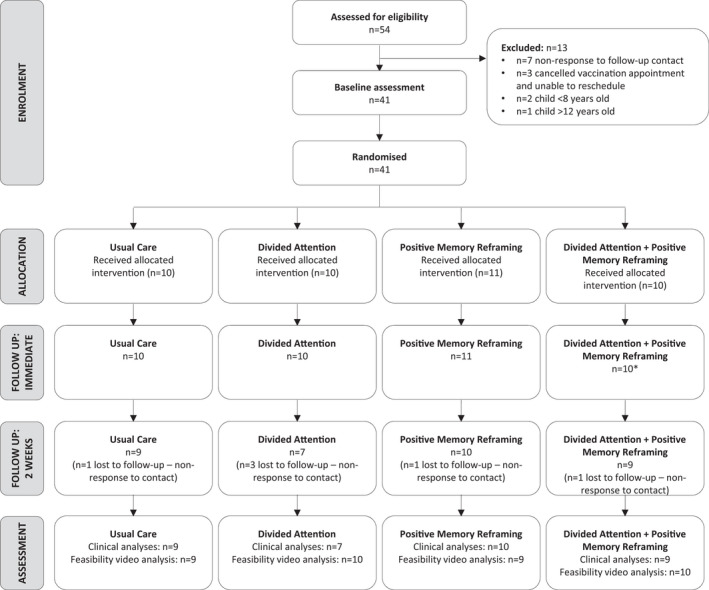
CONSORT flow diagram for feasibility studies. *n = 1 child in this group did not complete the immediately post‐vaccination assessment (reason unknown), but their parent did.
3.1.2. Feasibility of intervention delivery
Videos were available for analysis for 93% of participants (n = 38/41). The three missing videos were due to camera battery failure, researcher error (accidentally did not record), and one participant declined to be recorded. Durations of interventions were generally shorter than anticipated (except for DA): mean (SD) duration (minutes:seconds) were 3:46 (0.07) for UC, 5:47 (0.21) for DA, 5:00 (0.10) for PMR and 6:14 (0.09) for DA + PMR.
Intervention fidelity
Overall, 85% of intervention components were delivered as intended. For the UC group, 100% of intervention components were met for each participant; however, for the three intervention groups, only 10%–22% of participants received the intervention in full (Table 2). The most frequently omitted components of each intervention were as follows:
DA group: Nurse played the sensory discrimination game for <1 min (i.e., not the full 1–2 min; n = 5); prior to delivering the needle the nurse did not prime the child to remember the spatial location of the two spots from the game (aim: to maximize potential for analgesia; n = 4).
PMR group: Nurse did not tell the child “I see hundreds of kids for these needles and you handled this so well” (n = 5); nurse did not tell the child that they were brave (aim: to enhance self‐efficacy; n = 4).
DA + PMR group: Nurse played the sensory discrimination game for <1 min (i.e., not the full 1–2 min; n = 5); nurse did not tell the child that they were brave (n = 4).
Additional strategies were adopted by nurses in each group, with 92% of interventions involving ‘distraction’ techniques. The most frequent techniques included overt distraction such as instructions to wiggle toes and engaging with the parent (e.g., sitting on the parent's lap and stroking the child's leg) during needle provision as well as techniques related to breathing and relaxation (e.g., taking deep breaths).
TABLE 2.
Analyses of intervention videos
| Group a | Intervention fidelity | Rapport rating [mean (SD), range] | Communication pattern (n) | |
|---|---|---|---|---|
| Components delivered as intended | Intervention delivered in full (all components—% participants) | |||
| Usual care (n = 9) | 100% (2 components) | 100 | 7.67 (0.87), 6–9 |
8 Supportive 1 Invalidating |
| Divided attention (n = 10) | 84% (10 components) | 20 | 7.10 (0.99), 5–8 |
9 Supportive 1 Invalidating |
| Positive memory reframing (n = 9) | 76% (7 components) | 22 | 7.44 (0.53), 7–8 | 9 Supportive |
| Divided attention + positive memory reframing (n = 10) | 87% (17 components) | 10 | 8.10 (0.32), 8–9 | 10 Supportive |
n = 3 videos were not available for analysis. Intervention fidelity: Interventions were coded according to the number of intervention components that were delivered as intended for each participant via a protocol checklist with three response categories: yes, no and partially. The proportion of components delivered as intended for each intervention overall (for all participants combined), as well as the proportion of participants for which the intervention was delivered in full, were then calculated. Rapport: assessed using a 0–10 point scale (0 = extremely poor quality, 10 = extremely high quality). Communication pattern: assessed using the scale provided in Cline et al. (2006) (adapted for nurses).
Side effect information was delivered before the needle for 71% of interventions (n = 27), after the needle for 18% of interventions (n = 7), between the intervention and the needle for 5% of interventions (n = 2), and for the remainder, it was not evident in video (n = 2). For the PMR and DA + PMR interventions specifically, side effect information was delivered before the intervention for 67% and 70% of interventions, respectively.
Child, parent and nurse distress and coping (promoting) behaviours during interventions
Table 3 presents the CAMPIS‐SF scores for children and parents. For children, in all four groups, verbal coping was highest before the needle and lowest during the needle, and non‐verbal coping was highest during the needle and lowest after the needle. There were no clear patterns for verbal or non‐verbal distress. For nurses, verbal coping‐promoting was highest during the needle (except for DA + PMR where it was highest before the needle) and verbal distress‐promoting was highest before the needle in all four groups, but there was no clear pattern for non‐verbal behaviours. For parents, verbal and non‐verbal coping‐promoting was highest before the needle, except for the DA + PMR group, where it was highest during the needle. Parents' non‐verbal distress‐promoting was highest during the needle for all four groups, but there was no clear pattern for verbal distress‐promoting. The two independent raters had moderate to almost perfect agreement on all items on the CAMPIS‐SF (Cohen's kappa = 0.65–0.92) (McHugh, 2012).
TABLE 3.
The short‐form child‐adult medical procedure interaction scale (CAMPIS‐SF) results
| Intervention [mean (SD), range] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual care (n = 9) | Divided attention (n = 10) | Positive memory reframing (n = 9) | Divded attention + positive memory reframing (n = 10) | ||||||||||
| Before | During | After | Before | During | After | Before | During | After | Before | During | After | ||
| Child | Coping: Verbal |
2.44 (0.53) 2.00–3.00 |
1.00 (0.00) 1.00–1.00 |
1.78 (0.83) 1.00–3.00 |
2.10 (0.74) 1.00–3.00 |
1.40 (0.97) 1.00–4.00 |
1.60 (0.70) 1.00–3.00 |
2.33 (0.87) 1.00–4.00 |
1.00 (0.00) 1.00–1.00 |
1.44 (0.88) 1.00–3.00 |
2.50 (0.85) 2.00–4.00 |
1.50 (0.97) 1.00–4.00 |
1.70 (0.67) 1.00–3.00 |
| Coping: Non‐verbal |
2.11 (0.60) 1.00–3.00 |
4.11 (1.30) 1.00–5.00 |
1.67 (0.87) 1.003.00 |
2.10 (0.74) 1.00–3.00 |
2.40 (1.58) 1.00–5.00 |
1.30 (0.48) 1.00–2.00 |
1.89 (0.33) 1.00–2.00 |
3.44 (1.67) 1.00–5.00 |
1.67 (0.87) 1.00–3.00 |
2.30 (0.48) 2.00–3.00 |
4.10 (0.99) 2.00–5.00 |
1.70 (0.48) 1.00–2.00 | |
| Distress: Verbal |
1.89 (1.30) 1.00–5.00 |
1.56 (1.13) 1.00–4.00 |
1.56 (0.88) 1.00–3.00 |
1.90 (1.29) 1.00–4.00 |
1.90 (1.45) 1.00–5.00 |
1.60 (0.97) 1.00–4.00 |
1.44 (0.73) 1.00–3.00 |
1.33 (1.00) 1.00–4.00 |
1.44 (1.01) 1.00–4.00 |
1.30 (0.95) 1.00–4.00 |
1.10 (0.32) 1.00–2.00 |
1.50 (0.71) 1.00–3.00 | |
| Distress: Non‐verbal |
1.44 (1.33) 1.00–5.00 |
1.56 (1.13) 1.00–4.00 |
1.11 (1.13) 1.00–4.00 |
2.00 (1.63) 1.00–5.00 |
2.00 (1.70) 1.00–5.00 |
1.80 (1.23) 1.00–4.00 |
1.33 (0.71) 1.00–3.00 |
1.56 (1.33) 1.00–5.00 |
1.56 (1.13) 1.00–4.00 |
1.10 (0.32) 1.00–2.00 |
1.60 (0.70) 1.00–3.00 |
1.10 (0.32) 1.00–2.00 | |
| Nurse | Coping promoting: Verbal |
2.89 (0.60) 2.00–4.00 |
4.22 (0.97) 3.00–5.00 |
2.89 (0.33) 2.00–3.00 |
3.10 (0.88) 2.00–4.00 |
3.30 (1.42) 1.00–5.00 |
3.00 (1.05) 2.00–5.00 |
3.22 (0.67) 2.00–4.00 |
3.89 (1.27) 1.00–5.00 |
2.67 (0.87) 1.00–4.00 |
3.40 (0.52) 3.00–4.00 |
3.30 (1.06) 2.00–5.00 |
3.00 (0.47) 2.00–4.00 |
| Coping promoting: Non‐verbal |
3.11 (0.33) 3.00–4.00 |
3.22 (0.97) 2.00–5.00 |
2.67 (0.71) 2.00–4.00 |
3.20 (0.79) 2.00–5.00 |
2.60 (1.17) 1.00–4.00 |
2.50 (0.85) 1.00–4.00 |
3.22 (0.67) 2.00–4.00 |
3.44 (0.73) 2.00–4.00 |
2.89 (0.78) 1.00–4.00 |
3.70 (0.67) 3.00–5.00 |
2.80 (1.03) 1.00–5.00 |
3.00 (0.47) 2.00–4.00 | |
| Distress promoting: Verbal |
1.56 (1.01) 1.00–4.00 |
1.22 (0.44) 1.00–2.00 |
1.22 (0.44) 1.00–2.00 |
1.80 (1.03) 1.00–4.00 |
1.20 (0.42) 1.00–2.00 |
1.50 (0.53) 1.00–2.00 |
1.56 (0.53) 1.00–2.00 |
1.11 (0.33) 1.00–2.00 |
1.44 (0.53) 1.00–2.00 |
1.60 (0.84) 1.00–3.00 |
1.20 (0.42) 1.00–2.00 |
1.20 (0.42) 1.00–2.00 | |
| Distress promoting: Non‐verbal |
1.33 (1.00) 1.00–4.00 |
1.33 (1.00) 1.00–4.00 |
1.00 (0.00) 1.00–1.00 |
1.50 (1.08) 1.00–4.00 |
1.50 (1.27) 1.00–5.00 |
1.20 (0.42) 1.00–2.00 |
1.00 (0.00) 1.00–1.00 |
1.00 (0.00) 1.00–1.00 |
1.22 (0.44) 1.00–2.00 |
1.10 (0.32) 1.00–2.00 |
1.00 (0.00) 1.00–1.00 |
1.00 (0.00) 1.00–1.00 | |
| Parent | Coping promoting: Verbal |
1.78 (0.44) 1.00–2.00 |
1.33 (0.50) 1.00–2.00 |
1.67 (0.71) 1.00–3.00 |
2.00 (0.94) 1.00–4.00 |
1.30 (0.67) 1.00–3.00 |
1.40 (0.52) 1.00–2.00 |
2.11 (0.60) 1.00–3.00 |
1.67 (0.87) 1.00–3.00 |
1.89 (0.60) 1.00–3.00 |
2.00 (0.67) 1.00–3.00 |
2.40 (1.71) 1.00–5.00 |
1.70 (0.67) 1.00–3.00 |
| Coping promoting: Non‐verbal |
2.11 (1.05) 1.00–4.00 |
1.67 (1.12) 1.00–4.00 |
1.56 (0.53) 1.00–2.00 |
2.00 (0.82) 1.00–3.00 |
1.50 (0.97) 1.00–4.00 |
1.60 (0.84) 1.00–3.00 |
2.44 (0.88) 1.00–4.00 |
2.33 (1.50) 1.00–5.00 |
1.89 (0.78) 1.00–3.00 |
2.50 (0.97) 1.00–4.00 |
2.40 (1.84) 1.00–5.00 |
1.90 (0.74) 1.00–3.00 | |
| Distress promoting: Verbal |
1.22 (0.44) 1.00–2.00 |
1.00 (0.00) 1.00–1.00 |
1.33 (0.50) 1.00–2.00 |
1.40 (0.70) 1.00–3.00 |
1.30 (0.95) 1.00–4.00 |
1.20 (0.42) 1.00–2.00 |
1.44 (0.73) 1.00–3.00 |
1.67 (1.12) 1.00–4.00 |
1.67 (1.12) 1.00–4.00 |
1.30 (0.67) 1.00–3.00 |
1.00 (0.00) 1.00–1.00 |
1.20 (0.42) 1.00–2.00 | |
| Distress promoting: Non‐verbal |
1.56 (1.01) 1.00–4.00 |
1.78 (1.56) 1.00–5.00 |
1.56 (1.01) 1.00–4.00 |
1.70 (1.06) 1.00–4.00 |
2.20 (1.75) 1.00–5.00 |
1.70 (1.06) 1.00–4.00 |
1.67 (0.87) 1.00–3.00 |
2.22 (1.56) 1.00–5.00 |
1.67 (1.12) 1.00–4.00 |
1.70 (1.05) 1.00–4.00 |
2.20 (1.69) 1.00–5.00 |
1.30 (0.48) 1.00–2.00 | |
Note: Each domain of the CAMPIS‐SF is rated using a 5‐point Likert scale (1 = none, 5 = maximum or nearly constant) to rate the frequency of verbal and non‐verbal coping and distress promoting behaviours. The higher the mean, the more frequently the behaviour was displayed. Dark shading represents scores ≥3; Light shading represents scores ≥2.
Overall, the mean (SD) rating of rapport between the nurse and the child was 7.68 (0.79) (range 5–9). For 95% (n = 36/38) of interventions, the nurse's communication pattern was considered supportive, with the remaining 5% (n = 2/38) considered invalidating. Table 2 presents the nurse rapport scores and communication patterns for each intervention group.
3.1.3. Child/parent recall of interventions
Supplementary File 4 (Table S4.2) presents content coding in terms of verbal and non‐verbal expressions of the parent and child during the memory recall interviews (phone calls at 2 weeks). In general, parents in the new intervention groups more frequently expressed positive and negative emotions and pain (vs. UC). Children in the new intervention groups also more frequently expressed emotions but had fewer pain expressions (vs. UC).
3.1.4. Nurse perceptions of interventions
Interviews of the three nurses after data collection was complete revealed that overall, they understood the purpose of each intervention, and they felt confident in their ability to deliver the interventions and had sufficient training. However, it should be noted that one nurse stated that she did not have sufficient recollection of PMR and DA + PMR to respond to the interview questions regarding these interventions. Table 4 presents a summary of the three nurses' perceptions of each intervention. The nurses provided overall positive feedback about the PMR intervention, but the feedback about the DA intervention was less positive and they provided suggestions for improvements. The nurses agreed the PMR intervention was good because it was individualized and helped build rapport, however, a barrier was that some children were unable to recall a previous painful procedure, or something positive about a previous experience. The DA sensory game was perceived to be less suitable for older children and the nurses suggested that it should be adapted based on the child's age (e.g., use of alternative distraction methods). The nurses also suggested that the DA and DA + PMR interventions were less suitable for anxious children because the time taken to complete the interventions, particularly to play the sensory game, could contribute to heightened child anxiety.
TABLE 4.
Summary of nurse perceptions of interventions
| Domain | Divided attention (n = 3) | Positive memory reframing (n = 2) a | Divided attention + positive memory reframing (n = 2) a |
|---|---|---|---|
| Perceived purpose of intervention |
3 Distraction away from vaccination 1 Reduce sensory aspect of vaccination |
2 Recall a previously positive aspect and apply to current experience to make current experience better |
2 Mixture of DA and PMR 1 Harder to describe—more detailed |
| How it went overall |
2 Good, but may not be suitable for all children (more suited to younger children) 1 Not good, no better than usual practice |
2 Good (has merit, no hiccups) | 2 Intervention took too long |
| What went well |
2 Nothing specific (intervention works as a whole, nothing special) 2 No children were bad/pulled away 1 Well accepted by children |
2 Individualized to child (better than DA which is not individualized) 1 Helps develop rapport with parent and child 1 Uses something that works for them rather than something that works for me 1 Simplicity of it was good 1 Providing praise was good |
2 Participation/involving the child in the process 1 Involves the good aspects of both DA and PMR interventions |
| What did not go well/was challenging |
1 Children wary about why the nurse wanted to touch them 1 Need to be more confident 1 Difficult for anxious children, takes a long time which can build anxiety more |
2 Child not being able to recall previous experience or something positive | 2 Length of intervention, less suitable for anxious children |
| Confidence |
2 Confident, but less than usual because they were using a different procedure 1 Confident with young but not older children |
2 Confident |
2 Confident, but felt pressure to follow study procedures appropriately 2 Confidence increased with experience |
| Feedback on training |
3 Training was sufficient 1 Suggested video recordings of ‘ideal’ intervention delivery to provide clear expectations |
2 Training was sufficient 2 Simplicity of intervention meant that no further training was required |
2 Training was sufficient 1 Suggested video recordings of ‘ideal’ intervention delivery to provide clear expectations 1 Provide strategies to deal with anxious children |
| Feedback on intervention itself |
1 Adapt for the age of the child 1 Give the child power to decide where they are being touched 1 Ability to use alternative distraction methods (e.g., bribery with lollies, wiggle toes) |
2 No suggestions |
1 Environmental set‐up was not ideal 1 Ability to individualize based on age group 1 Ability to use alternative distraction methods |
Abbreviations: DA, divided attention; PMR, positive memory reframing.
n = 1 nurse reported that they did not have sufficient recollection of these interventions. The results presented in this table summarize absolute frequencies of statements provided by nurses during phone interviews after data collection was complete.
3.1.5. Preliminary clinical outcomes
There were no significant between‐group differences in clinical outcomes at baseline.
Overall effects (all four groups combined)
The overall analysis (Figure 3) showed main effects over time for children's ratings of fear (F 2,66 = 3.685, p = 0.039), children's needle‐related pain catastrophizing (F 2,66 = 11.178, p < 0.001), parent ratings of child fear (F 2,66 = 6.983, p = 0.005), and parent ratings of child pain (F 2,66 = 11.206, p = 004). There were no main effects over time for children's ratings of pain (F 2,66 = 1.889, p = 0.159), parent anxiety (Parent State Anxiety NRS; F 2,66 = 1.764, p = 0.186), or parent confidence that they could decrease their child's pain if they tried (Parent Stop Tendency Scale; F 2,68 = 0.665, p = 0.491).
FIGURE 3.
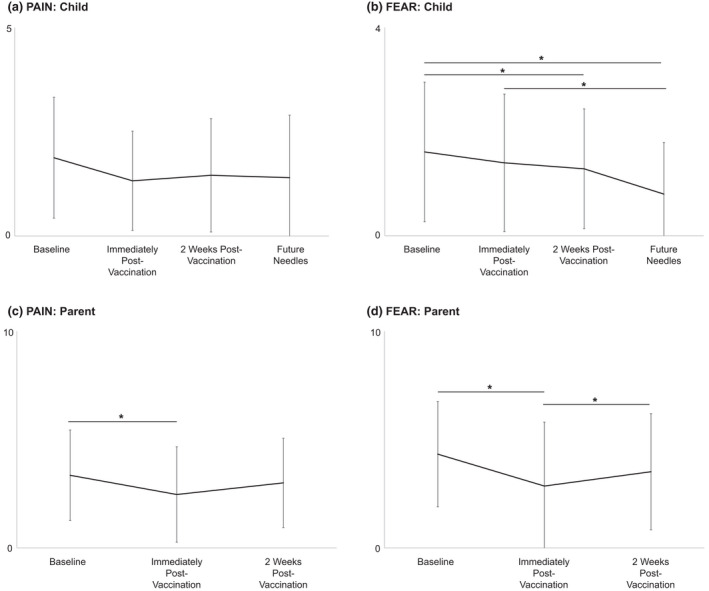
Overall effects (all four groups combined) for child and parent ratings of needle‐related pain intensity and fear (means and SDs). *p < 0.05. (a) Child Pain was assessed using a 0–5 point face scale (Faces Pain Scale‐Revised; 0 = no pain, 5 = very much pain). (b) Child Fear was assessed using a 0–4 point face scale (Children's Fear Scale; 0 = not scared at all, 5 = most scared possible). (c, d) Parent ratings of Child Pain and Fear were assessed using 0–10 point Numeric Rating Scales (0 = no pain, 10 = most pain; 0 = not at all scary, 10 = most scary, respectively).
Pairwise comparisons showed a reduction in children's recall of their needle‐related fear at 2 weeks post‐vaccination (vs. baseline, p = 0.027). Children's fear of future needles was also reduced (vs. immediately post‐vaccination, p = 005). Needle‐related pain catastrophizing also reduced immediately (vs. baseline, p = 0.005) and 2 weeks post‐vaccination (vs. baseline, p = 0.001). There was also an overall reduction in children's anticipated fear of future needles (vs. baseline; p < 0.001) but no effect on children's anticipated pain intensity during future needles. Overall, parents' ratings of child fear were lower immediately post‐vaccination than anticipated at baseline (p = 0.012), but this rating increased at 2 weeks post‐vaccination (vs. immediately post‐vaccination; p = 0.03). Parents' perceptions of their child's needle‐related pain intensity were also lower immediately post‐vaccination than anticipated at baseline (p = 0.008).
Within‐group effects
Within‐group analyses (Figure 4) showed reductions immediately post‐vaccination (vs. baseline) for parents' ratings of child fear in the UC group (p = 0.035, d = 0.784) and the PMR group (p = 0.035, d = 0.733), and child catastrophizing in the PMR group at 2 weeks post‐vaccination (p = 0.013, d = 0.980). The DA + PMR group showed reductions in child fear (p = 0.008, d = 1.167) and catastrophizing (p = 0.007, d = 1.213) at 2 weeks post‐vaccination (vs. baseline) and, at 2 weeks, child recalled fear of the needle was higher than their fear of future needles (p = 0.008, d = 1.167). Children in PMR (p = 0.025, d = 0.850) and DA + PMR (p = 0.003, d = 1.376) groups also showed reduced fear of future needles (vs. baseline). The DA group had no significant within‐group changes. There were also no significant changes over time in child or parent ratings of child pain for any group.
FIGURE 4.
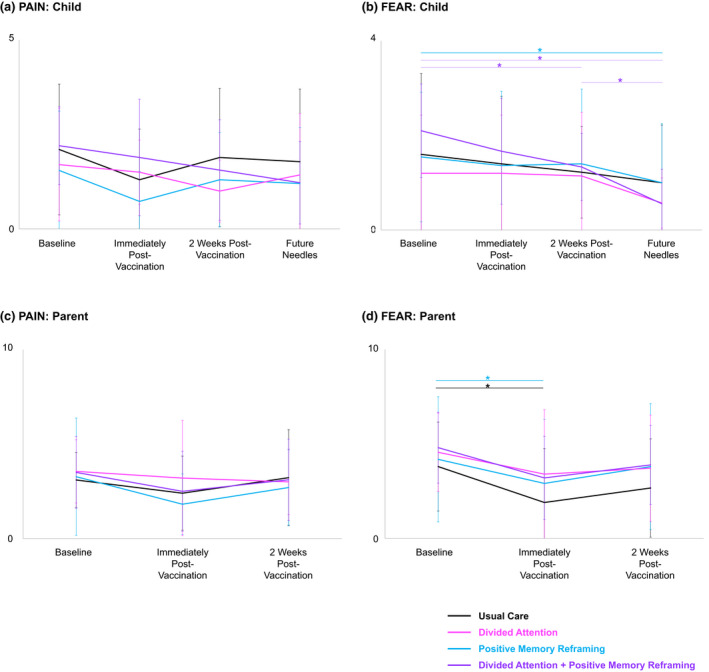
Within‐group effects for child and parent ratings of needle‐related pain intensity and fear (means and SDs). *p < 0.05. (a) Child Pain was assessed using a 0–5 point face scale (Faces Pain Scale‐Revised; 0 = no pain, 5 = very much pain). (b) Child Fear was assessed using a 0–4 point face scale (Children's Fear Scale; 0 = not scared at all, 5 = most scared possible). (c, d) Parent ratings of Child Pain and Fear were assessed using 0–10 point Numeric Rating Scales (0 = no pain, 10 = most pain; 0 = not at all scary, 10 = most scary, respectively).
In the DA group, parent anxiety increased from immediately post‐vaccination to 2 weeks post‐vaccination (p = 0.017). There were no within‐group changes in parent anxiety or parent confidence.
Supplementary File 6 (Table S6) provides within‐group change scores with 95% confidence intervals, and effect sizes (Cohen's d).
Adverse events and sensitivity analyses
Two participants (n = 1 UC; n = 1 DA) had adverse events related to study protocol breach, where the needle was delivered despite child distress. Sensitivity analyses with these participants' data removed showed no differences in the findings, barring a reduction in the overall parents' ratings of child fear at 2 weeks (vs. baseline; p = 0.029), and a reduction in child catastrophizing post‐vaccination in the UC group (vs. baseline; p = 0.010).
4. DISCUSSION
This study evaluated the feasibility of two new interventions delivered by clinical nurses to reduce the negative impact of needle procedures in children. The data collection methods appear feasible, however, recruitment rates for flu vaccinations in this age group were low. This study was the first to recruit and train clinical nurses to administer these interventions and the nurses felt confident delivering them. However, the interventions were rarely delivered fully as intended, with nurses frequently omitting intervention components. Improved nurse training will be required prior to a large‐scale randomized controlled trial (RCT) to ensure that these interventions are delivered accurately and consistently in any environment, including schools. Preliminary statistical analyses suggest that the interventions reduced needle‐related fear but did not reduce pain. However, the combined intervention (DA + PMR) showed medium‐large effects on child fear (Supplementary File 6 ‐ within group effect sizes) potentially providing support for hypothesized super‐additive effects. However, these effects may have been driven by high anticipated pain and fear at baseline in the DA + PMR group. That these simple interventions were applied by clinical nurses in various environments and had promising preliminary results supports continued inquiry towards improved vaccination protocols for children.
4.1. Intervention feasibility
Overall, nurses were able to deliver each intervention component as intended. However, adherence to delivering interventions in full for each child was poor. Video analysis of the clinical encounters, as well as interviews with the nurses, allow potential explanations for poor adherence. The most frequently omitted protocol items were providing explicit statements in PMR (e.g., ‘you were brave’), and for DA the sensory discrimination game was often not played for the full duration. Additional distraction strategies were also implemented for nearly every child, suggesting the nurses perceived distraction during the needle application itself remained important. Interestingly, interviews revealed the nurses felt confident and they had received sufficient training, but they perceived problems with the new interventions. Nurses suggested these interventions took too long, heightening child anxiety—a perception likely driven by conflict with usual practice of providing vaccinations as quickly as possible. This feedback also explains why mean intervention durations were generally shorter than anticipated. In addition, one nurse reported that the DA game was less suitable for older children, which may have also contributed to lack of adherence. Indeed, video analysis revealed that nurse distress promoting behaviours were highest and rapport scores were lowest for DA. It is possible that deviations from intervention protocols were purposeful, reflecting the nurses' clinical experience and wisdom, rather than accidental or due to lack of training. Each nurse had >30 years clinical experience, so these protocol omissions might also reflect difficulties in training highly experienced nurses to change their behaviour.
Strategies to improve intervention protocol adherence are warranted prior to progression to a clinical trial. Nurses may require more practice so they are comfortable relaxing into longer intervention delivery times. Expanded psychoeducation about the importance of pain management during needles could also be important. For DA specifically, highlighting the scientific rationale for playing the DA game in full might promote better adherence. Additionally, bolstering aspects of the interventions that the nurses perceived as positive might improve adherence. For example, nurses reported that the best aspects of PMR were individualization and child empowerment (e.g., using strategies that work for the child, not just the nurse), as well as facilitation of both child and parent rapport development. Last, given that the timing of side effect information delivery may impact intervention effects, timing should be included on future nurse intervention information sheets.
4.2. Recommendations and future directions
Additional pilot and feasibility testing following iterative changes to the nurse training and intervention implementation procedures is required prior to progression to a large‐scale RCT. Improved training of nurses will be necessary so interventions are delivered effectively and in full, regardless of the environment. Approximately one‐third of PMR components were frequently omitted, although little information exists regarding what components are key. Dismantling studies to identify PMR components critical to positively altering pain memories may provide insight into components that cannot be omitted. Similarly, mechanistic studies should seek to determine key components of DA, including whether the 1–2 min duration is required for analgesic effects. Given issues identified in providing DA, combined with a lack of pain reduction experienced during the needle (the hypothesized effect), such additional work is needed to determine if this intervention should be continued. Further, given low intervention fidelity despite extensive nurse training, getting experts to deliver the interventions (e.g., psychologists for PMR) or the child's parent [which has been shown to be an effective strategy with high intervention fidelity for PMR (Pavlova et al., 2021)] to deliver the interventions might be an important initial step to determine clinical efficacy when the interventions are delivered in full. Then, if clinical results are promising, future studies could investigate efficacy in conjunction with implementation into regular clinical practice (i.e., nurses providing all aspects of the intervention). Given our small sample size we were unable to determine if any clinical effects were driven by additional distraction techniques provided by the nurses or the absence/presence of certain intervention components. Future a priori analyses should seek to evaluate the component‐specific effects of the interventions, for example, a mechanism‐based RCT where each group gets differing combinations of intervention components. We allowed the nurses to use additional distraction techniques in the intervention protocols for ethical reasons—this is considered best‐practice care (Birnie et al., 2018) and thus, we recommend future studies do the same and seek to determine the effects of the new interventions in addition to best‐practice care. Secondly, recruitment rates were low, likely due to the absence of government‐funded influenza vaccines for children aged 8–12, a feature shown to influence vaccine uptake (Howard et al., 2021). Thus, future studies should consider another type of injection that is typically scheduled (e.g., measles/mumps/rubella) rather than an entirely voluntary vaccine. In addition, four appointments were cancelled due to flu vaccine shortage, and shortages may be less likely for a scheduled vaccine. Finally, strategies to improve retention are needed, given high loss to follow‐up at 2 weeks post‐vaccination (15%). Ensuring sufficient emphasis on the importance of continued follow‐up, and incentivizing full participation (e.g., honoraria), may be relevant.
Based on the preliminary clinical results and nurse feedback, it seems a focus on PMR going forward is warranted, but adaptation of DA may be needed. For example, recruitment of younger children to maximize the suitability of DA for all participants (i.e., younger children may be more amenable to playing the DA game), or providing specific protocol adaptation strategies for older and/or more anxious children. For PMR, a potential strategy to reduce intervention delivery time, while still ensuring memories are reframed and positive expectancies are instilled, is via parent engagement. Parents could easily be taught PMR strategies to use prior to the needle, with nurses then assisting with PMR during and after the needle. This ‘hybrid’ strategy has advantages beyond reducing overall delivery time. Parents have the advantage of being present with their child during previous painful procedures and thus are equipped with first‐hand knowledge of what is accurate and effective for their child. This strategy would also offer more time to reflect on previous painful experience, which could address our nurse feedback that some children were unable to recall/reflect on previous experiences during the intervention. Preliminary evidence from an RCT suggests that parent‐led PMR may lead to a reduction in negatively‐biased memories of pain in children after surgery (Pavlova et al., 2021), which suggests a super‐additive effect may be possible when both parents and nurses are engaged. Parent‐led PMR was also found to be acceptable and feasible (Pavlova et al., 2021). Translation of pain management to the family unit can be empowering and anxiety‐relieving, particularly for parents who may not be confident in how to manage their child's needle‐related fear and pain. Thus, the ability of this research to directly translate to clinical management of needle‐related pain and fear in children is high.
The preliminary clinical findings provide exciting potential for the application of these interventions to other needle contexts (IV cannulation, venipunctures), other painful medical procedures, interventionists (parents) and formats (online education, text messaging). These interventions may have particular relevance for children undergoing needle procedures frequently. One of the first memory‐reframing interventions developed was used in the context of children undergoing repeated needle procedures for cancer treatment, and it was found to be effective in reducing negative exaggerations in children's memories (Chen et al., 1999). Future research examining these interventions for children with other chronic illnesses is warranted. Future investigations could also compare efficacy in children and parents to determine developmental differences in intervention efficacy/delivery, as well as the possibility of treatment‐tailoring based on baseline risk factors. Further exploration of the combined intervention appears warranted—it had large effects on child fear (with the caveats of preliminary analyses and high baseline fear), and also had positive findings from our video analyses (nurse coping promoting behaviours were high and distress promoting behaviours were low, and rapport scores were high). Future work should explore the types of children who would be best suited for this more comprehensive intervention (e.g., highly fearful/anxious). A key future direction is to follow children long‐term and examine the impact of the interventions on subsequent pain and fear at future needle procedures. Last, we recommend that future studies use topical anaesthetic for all children, consistent with best practice care recommendations, and examine the added benefit of these interventions.
4.3. Strengths and limitations
Strengths of this study include protocol registration and rigorous reporting with adherence to CONSORT (Eldridge et al., 2010; Thabane et al., 2016) and TiDieR (Hoffmann et al., 2014) checklists. While we did not have pre‐registered feasibility criteria, these were determined by the team prior to data analysis. Further, a unique strength of our study was video‐recorded interventions, rigorously analysed by two independent reviewers using standardized coding schemes/rating scales, and supplemented by interview of the clinical nurses, allowing comprehensive evaluation of intervention fidelity. Future studies of similar interventions should use video recording to detect adherence issues. Together, these feasibility outcomes provide clear directions for protocol amendments and further pilot/feasibility testing.
A limitation of our study was that one of the three nurses had insufficient recollection of the intervention protocols to answer the interview questions. As such, the comprehensiveness and quality of the nurse perception data may be limited, and this speaks to feasibility challenges in training nurses to perform these interventions. Further limitations include those inherent to feasibility studies, namely, it was not powered for efficacy analyses. Therefore, caution in interpreting the clinical outcomes is needed. We only recruited three nurses, so challenges related to differing experience/skill levels may not have been captured. Further, that one nurse provided the majority of interventions (76%), suggests practitioner generalizability will be important to evaluate in future work.
4.4. Conclusions
Protocol amendments and further pilot and feasibility testing are required prior to progression to a large‐scale RCT. Recommended amendments include enhanced training of nurses, use of a vaccine that is scheduled rather than voluntary to improve recruitment rates, and strategies to improve participant retention. Promising preliminary clinical results, particularly with respect to reducing child fear, suggest further research into these new interventions is warranted. Mechanistic studies should seek to identify key intervention components to optimize efficacy and feasibility via omission of unnecessary components and, given the lack of effects on pain, analgesic potential of the interventions requires further exploration. That these interventions can be applied by clinical nurses in various environments, including non‐clinical environments such as schools, suggests potential for these interventions to improve clinical protocols and facilitate broader dissemination of vaccinations for children in a manner that minimizes distress.
AUTHOR CONTRIBUTIONS
All authors contributed substantially to this manuscript. All authors discussed the results and commented on the manuscript and approved the final version for publication. Felicity A. Braithwaite contributed to data collection, data analysis, interpretation of data, drafting the article. Melanie Noel contributed to conception and design, interpretation of data, critically revising the article for important intellectual content. Hannah G. Jones contributed to data analysis, interpretation of data, critically revising the article for important intellectual content. Michael D. Weise contributed to data collection, interpretation of data, critically revising the article for important intellectual content. Cara G. Nania contributed to data analysis, interpretation of data, critically revising the article for important intellectual content. Emily Watson contributed to data collection, interpretation of data, critically revising the article for important intellectual content. Tasha R. Stanton contributed to conception and design, data collection, data analysis, interpretation of data, critically revising the article for important intellectual content.
FUNDING INFORMATION
This work was supported via funding from the Australian Pain Society, Australian Pain Relief Association, Cops for Kids Clinical Research Grant (2018). T.R.S. is supported by a National Health & Medical Research Career Development Fellowship (ID1141735). F.A.B. is supported by the John Stuart Colville Fellowship (Arthritis Foundation of South Australia). Funding sources had no involvement in the conduct of the research or preparation of the article.
CONFLICT OF INTEREST
T.R.S. has received support from Eli Lilly Ltd for travel and accommodation cost (unrelated to the present topic) and receives payment for lectures on pain and rehabilitation. F.A.B. has received speaker fees for providing lectures related to pain and blinding in clinical trials. M.N., H.G.J., C.G.N., M.D.W. and E.W. have no conflicts of interest to declare.
Supporting information
Data S1
Data S2
Data S3
Data S4
Data S5
Data S6
ACKNOWLEDGEMENTS
We would like to thank the clinical nurses who delivered the interventions: Rebecca Haysman, Sandy Mallison and Lesley McCauley. Open access publishing facilitated by University of South Australia, as part of the Wiley ‐ University of South Australia agreement via the Council of Australian University Librarians.
Braithwaite, F. A. , Noel, M. , Jones, H. G. , Wiese, M. D. , Nania, C. G. , Watson, E. , & Stanton, T. R. (2022). Reframe the pain: Divided attention and positive memory reframing to reduce needle pain and distress in children—A feasibility randomized controlled trial. European Journal of Pain, 26, 1702–1722. 10.1002/ejp.1992
Institutional URL: https://people.unisa.edu.au/felicity.braithwaite
REFERENCES
- Arntz, A. , Van Eck, M. , & Heijmans, M. (1990). Predictions of dental pain: The fear of any expected evil, is worse than the evil itself. Behaviour Research and Therapy, 28, 29–41. [DOI] [PubMed] [Google Scholar]
- Australian Government Department of Health . (2019). Vaccination for people who have had an adverse event following immunisation . https://immunisationhandbook.health.gov.au/vaccination‐for‐special‐risk‐groups/vaccination‐for‐people‐who‐have‐had‐an‐adverse‐event‐following#vaccinating‐people‐with‐a‐known‐egg‐allergy
- Australian Technical Advisory Group on Immunisation (ATAGI) . (2016). Australian immunisation handbook 10th edition . immunisationhandbook.health.gov.au.
- Birnie, K. A. , Hundert, A. S. , Lalloo, C. , Nguyen, C. , & Stinson, J. N. (2019). Recommendations for selection of self‐report pain intensity measures in children and adolescents: A systematic review and quality assessment of measurement properties. Pain, 160, 5–18. [DOI] [PubMed] [Google Scholar]
- Birnie, K. A. , Noel, M. , Chambers, C. T. , Uman, L. S. , & Parker, J. A. (2018). Psychological interventions for needle‐related procedural pain and distress in children and adolescents. Cochrane Database of Systematic Reviews, 10, CD005179. 10.1002/14651858.CD005179.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount, R. L. , Bunke, V. , Cohen, L. L. , & Forbes, C. J. (2001). The Child–Adult Medical Procedure Interaction Scale‐Short Form (CAMPIS‐SF): Validation of a rating scale for children's and adults' behaviors during painful medical procedures. Journal of Pain and Symptom Management, 22, 591–599. [DOI] [PubMed] [Google Scholar]
- Bruck, M. , Ceci, S. J. , Francoeur, E. , & Barr, R. (1995). “I hardly cried when I got my shot!” influencing children's reports about a visit to their pediatrician. Child Development, 66, 193–208. [DOI] [PubMed] [Google Scholar]
- Caes, L. , Vervoort, T. , Eccleston, C. , Vandenhende, M. , & Goubert, L. (2011). Parental catastrophizing about child's pain and its relationship with activity restriction: The mediating role of parental distress. Pain, 152, 212–222. [DOI] [PubMed] [Google Scholar]
- Chen, E. , Zeltzer, L. K. , Craske, M. G. , & Katz, E. R. (1999). Alteration of memory in the reduction of children's distress during repeated aversive medical procedures. Journal of Consulting and Clinical Psychology, 67, 481–490. [DOI] [PubMed] [Google Scholar]
- Cline, R. J. , Harper, F. W. , Penner, L. A. , Peterson, A. M. , Taub, J. W. , & Albrecht, T. L. (2006). Parent communication and child pain and distress during painful pediatric cancer treatments. Social Science & Medicine, 63, 883–898. [DOI] [PubMed] [Google Scholar]
- Durand, H. , Birnie, K. A. , Noel, M. , Vervoort, T. , Goubert, L. , Boerner, K. E. , Chambers, C. T. , & Caes, L. (2017). State versus trait: Validating state assessment of child and parental catastrophic thinking about children's acute pain. The Journal of Pain, 18, 385–395. [DOI] [PubMed] [Google Scholar]
- Eldridge, S. M. , Chan, C. L. , Campbell, M. J. , Bond, C. M. , Hopewell, S. , Thabane, L. , & Lancaster, G. A. (2010). CONSORT statement: Extension to randomised pilot and feasibility trials. BMJ, 355, i5239. 10.1136/bmj.i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, R. , Dubner, R. , & Hoffman, D. (1981). Neuronal activity in medullary dorsal horn of awake monkeys trained in a thermal discrimination task. II. Behavioral modulation of responses to thermal and mechanical stimuli. Journal of Neurophysiology, 46, 428–443. [DOI] [PubMed] [Google Scholar]
- Hechler, T. , Chalkiadis, G. A. , Hasan, C. , Kosfelder, J. , Meyerhoff, U. , Vocks, S. , & Zernikow, B. (2009). Sex differences in pain intensity in adolescents suffering from cancer: Differences in pain memories? The Journal of Pain, 10, 586–593. [DOI] [PubMed] [Google Scholar]
- Hicks, C. L. , von Baeyer, C. L. , Spafford, P. A. , van Korlaar, I. , & Goodenough, B. (2001). The faces pain scale–revised: Toward a common metric in pediatric pain measurement. Pain, 93, 173–183. [DOI] [PubMed] [Google Scholar]
- Hoffmann, T. C. , Glasziou, P. P. , Boutron, I. , Milne, R. , Perera, R. , Moher, D. , Altman, D. G. , Barbour, V. , Macdonald, H. , & Johnston, M. (2014). Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ, 348, g1687. [DOI] [PubMed] [Google Scholar]
- Howard, Z. , Dalton, C. , Carlson, S. , Baldwin, Z. , & Durrheim, D. (2021). Impact of funding on influenza vaccine uptake in Australian children. Public Health Research and Practice, 31, e3112104. 10.17061/phrp3112104 [DOI] [PubMed] [Google Scholar]
- Johnston, N. E. , Atlas, L. Y. , & Wager, T. D. (2012). Opposing effects of expectancy and somatic focus on pain. PLoS One, 7, e38854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain, Z. N. , Mayes, L. C. , Caldwell‐Andrews, A. A. , Karas, D. E. , & McClain, B. C. (2006). Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics, 118, 651–658. [DOI] [PubMed] [Google Scholar]
- Love, A. S. , & Love, R. J. (2021). Considering needle phobia among adult patients during mass COVID‐19 vaccinations. Journal of Primary Care and Community Health, 12, 21501327211007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh, M. L. (2012). Interrater reliability: The kappa statistic. Biochemia Medica, 22, 276–282. [PMC free article] [PubMed] [Google Scholar]
- McMurtry, C. M. , Noel, M. , Chambers, C. T. , & McGrath, P. J. (2011). Children's fear during procedural pain: Preliminary investigation of the Children's fear scale. Health Psychology, 30, 780–788. [DOI] [PubMed] [Google Scholar]
- McMurtry, C. M. , Riddell, R. P. , Taddio, A. , Racine, N. , Asmundson, G. J. , Noel, M. , Chambers, C. T. , & Shah, V. (2015). Far from “just a poke”: Common painful needle procedures and the development of needle fear. The Clinical Journal of Pain, 31, S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron, D. , Duncan, G. H. , & Bushnell, M. C. (1989). Effects of attention on the intensity and unpleasantness of thermal pain. Pain, 39, 345–352. [DOI] [PubMed] [Google Scholar]
- Noel, M. (2016). Commentary: Harnessing the fragility of pain memories to help children forget: A new avenue for pediatric psychology interventions? Journal of Pediatric Psychology, 41, 232–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel, M. , Chambers, C. T. , McGrath, P. J. , Klein, R. M. , & Stewart, S. H. (2012a). The influence of children's pain memories on subsequent pain experience. Pain, 153, 1563–1572. [DOI] [PubMed] [Google Scholar]
- Noel, M. , Chambers, C. T. , McGrath, P. J. , Klein, R. M. , & Stewart, S. H. (2012b). The role of state anxiety in children's memories for pain. Journal of Pediatric Psychology, 37, 567–579. [DOI] [PubMed] [Google Scholar]
- Noel, M. , Chambers, C. T. , Petter, M. , McGrath, P. J. , Klein, R. M. , & Stewart, S. H. (2012). Pain is not over when the needle ends: A review and preliminary model of acute pain memory development in childhood. Pain Management, 2, 487–497. [DOI] [PubMed] [Google Scholar]
- Noel, M. , McMurtry, C. M. , Chambers, C. T. , & McGrath, P. J. (2009). Children's memory for painful procedures: The relationship of pain intensity, anxiety, and adult behaviors to subsequent recall. Journal of Pediatric Psychology, 35, 626–636. [DOI] [PubMed] [Google Scholar]
- Noel, M. , McMurtry, C. M. , Pavlova, M. , & Taddio, A. (2018). Brief clinical report: A systematic review and meta‐analysis of pain memory‐reframing interventions for Children's needle procedures. Pain Practice, 18, 123–129. [DOI] [PubMed] [Google Scholar]
- Noel, M. , Palermo, T. M. , Chambers, C. T. , Taddio, A. , & Hermann, C. (2015). Remembering the pain of childhood: Applying a developmental perspective to the study of pain memories. Pain, 156, 31–34. [DOI] [PubMed] [Google Scholar]
- Noel, M. , Pavlova, M. , Lund, T. , Jordan, A. , Chorney, J. , Rasic, N. , Brookes, J. , Hoy, M. , Yunker, W. K. , & Graham, S. (2019). The role of narrative in the development of children's pain memories: Influences of father–and mother–child reminiscing on children's recall of pain. Pain, 160, 1866–1875. [DOI] [PubMed] [Google Scholar]
- Noel, M. , Pavlova, M. , McCallum, L. , & Vinall, J. (2017). Remembering the hurt of childhood: A psychological review and call for future research. Canadian Psychologist, 58, 58–68. [Google Scholar]
- Noel, M. , Rabbitts, J. A. , Tai, G. G. , & Palermo, T. M. (2015). Remembering pain after surgery: A longitudinal examination of the role of pain catastrophizing in children's and parents' recall. Pain, 156, 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova, M. , Lund, T. , Nania, C. , Kennedy, M. , Graham, S. , & Noel, M. (2021). Reframe the pain: A randomized controlled trial of a parent‐led memory‐reframing intervention. The Journal of Pain, 23, 263–275. [DOI] [PubMed] [Google Scholar]
- Quevedo, A. S. , & Coghill, R. C. (2007). Attentional modulation of spatial integration of pain: Evidence for dynamic spatial tuning. The Journal of Neuroscience, 27, 11635–11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton, T. , Gilpin, H. , Reid, E. , Mancini, F. , Spence, C. , & Moseley, G. (2016). Modulation of pain via expectation of its location. European Journal of Pain, 20, 753–766. [DOI] [PubMed] [Google Scholar]
- Thabane, L. , Hopewell, S. , Lancaster, G. A. , Bond, C. M. , Coleman, C. L. , Campbell, M. J. , & Eldridge, S. M. (2016). Methods and processes for development of a CONSORT extension for reporting pilot randomized controlled trials. Pilot Feasibility Studies, 2, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Baeyer, C. L. , Marche, T. A. , Rocha, E. M. , & Salmon, K. (2004). Children's memory for pain: Overview and implications for practice. The Journal of Pain, 5, 241–249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data S2
Data S3
Data S4
Data S5
Data S6


