Abstract
In high income countries, approximately 10% of pregnancies are complicated by pre‐eclampsia (PE), preterm birth (PTB), fetal growth restriction (FGR) and/or macrosomia resulting from gestational diabetes (GDM). Despite the burden of disease this places on pregnant people and their newborns, there are still few, if any, effective ways of preventing or treating these conditions. There are also gaps in our understanding of the underlying pathophysiologies and our ability to predict which mothers will be affected. The placenta plays a crucial role in pregnancy, and alterations in placental structure and function have been implicated in all of these conditions. As extracellular vesicles (EVs) have emerged as important molecules in cell‐to‐cell communication in health and disease, recent research involving maternal‐ and placental‐derived EV has demonstrated their potential as predictive and diagnostic biomarkers of obstetric disorders. This review will consider how placental and maternal EVs have been investigated in pregnancies complicated by PE, PTB, FGR and GDM and aims to highlight areas where further research is required to enhance the management and eventual treatment of these pathologies.

Keywords: extracellular vesicles, fetal growth restriction, gestational diabetes, placenta, pre‐eclampsia, pre‐term birth
Abstract figure legend Summary of the salient points covered within this review.

Introduction
In high income countries, approximately 10% of pregnancies are complicated by pre‐eclampsia (PE), preterm birth (PTB), fetal growth restriction (FGR) and/or large‐for‐gestational age (LGA) infants resulting from gestational diabetes (GDM) (Table 1). Despite the burden of disease placed on pregnant people and their newborns, there are still few, if any, effective ways of preventing or treating these conditions. Current management relies on clinical surveillance and optimising the time, place and route of delivery (Alberry & Soothill, 2007; Burton et al., 2019; Goldenberg et al., 2008; Quintanilla Rodriguez & Mahdy, 2022). These obstetric conditions are not only responsible for maternal, fetal and neonatal morbidity and mortality; they are also associated with long‐term increased risks of cardiometabolic disease in the mothers and children (Bendix et al., 2020; Colella et al., 2018; Graves et al., 2019; Lees et al., 2013).
Table 1.
Brief overview of the obstetric conditions discussed in this article
| Obstetric Condition | Definition | Diagnosis |
|---|---|---|
| Gestational diabetes | Glucose intolerance above an agreed threshold that develops during pregnancy and usually resolves after delivery | Elevated fasting plasma glucose and/or elevated 1 or 2 h plasma glucose following a glucose tolerance test (thresholds vary) (National Institute for Health & Care Excellence, 2015 (updated 2020); Kapur et al., 2020) |
| Pre‐eclampsia | A multisystem disorder characterised by new‐onset hypertension at 20+0 weeks of pregnancy or later with one or more additional features | Blood pressure ≥140/≥90 mmHg with proteinuria and/or evidence of maternal acute kidney injury, liver dysfunction, neurological features, haemolysis or thrombocytopenia, and/or fetal growth restriction (American College of Obstetricians & Gynecologists, 2020; Brown et al., 2018) |
| Preterm birth | Birth before 37 completed weeks of gestation | Birth before 37 completed weeks of gestation |
| Fetal growth restriction | A failure of the fetus to reach its growth potential (Malhotra et al., 2019) | Estimated fetal weight (EFW) or abdominal circumference (AC) <3rd centile or EFW or AC <10th centile with abnormal Doppler velocimetry and/or slowing of fetal growth (Gordijn et al., 2018) |
The definitions and diagnostic criteria provided are widely used but not universally agreed.
There is a growing body of evidence suggesting extracellular vesicles (EVs) play an important role in communication between the mother, placenta and fetus (Chiarello et al., 2018; Tong & Chamley, 2015). This makes them a promising means of investigating the mechanisms underlying obstetric diseases, as well as a potential source of predictive, diagnostic and prognostic biomarkers (Familari et al., 2017; Miranda et al., 2018a). It may also be possible to harness their capacity for inter‐organ communication for use in future therapeutics (Keshtkar et al., 2018; Merino‐Gonzalez et al., 2016). This review aims to highlight the recent advances in the study of extracellular vesicles in pregnancy with a particular focus on gestational diabetes, pre‐eclampsia, preterm birth and fetal growth restriction. It also aims to expose gaps in our current understanding and potential areas for future study in the pursuit of treatment for these pathologies.
The placenta
The placenta plays a central role in healthy pregnancy and is both a source and recipient of EVs. From 16 weeks of gestation the placenta comprises a supporting mesodermal core, containing an extensive network of fetal blood vessels (Fig. 1) (Kingdom et al., 2000). These form a branching, villous structure overlain by the syncytiotrophoblast, a continuously replenished syncytium that sheds fragments into the maternal circulation. Placental angiogenesis and vasculogenesis is heavily influenced by members of the vascular endothelial growth factor (VEGF) family, including placental growth factor (PlGF) and VEGF‐A and their receptors (Umapathy et al., 2019).
Figure 1. Anatomical cross‐section of the human placenta.
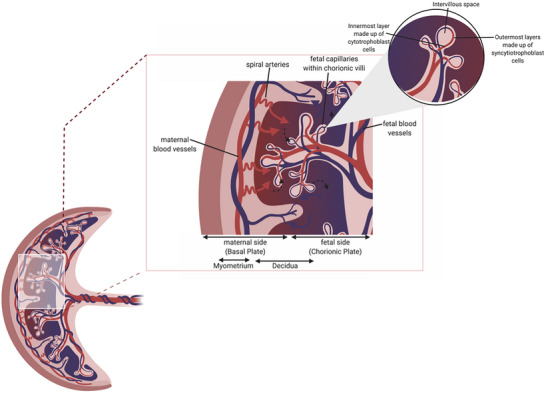
Adapted from Jansen et al. (2020). Created in Biorender.com.
Maternal blood enters the intervillous space via the spiral arteries to supply the fetus and placenta with oxygen and nutrients. During pregnancy the spiral arteries are remodelled following extravillous trophoblast invasion, under the influence of decidual immune cells, to reduce vascular resistance and increase flow velocity (Cartwright et al., 2010b; Williams et al., 2009). Incomplete spiral artery remodelling has been associated with early‐onset fetal growth restriction and pre‐eclampsia (Fig. 2) (Cartwright et al., 2010a) due to impaired placental function, but and the precise mechanisms responsible for this remain elusive.
Figure 2. Schematic diagram of the spiral artery in non‐pregnant state, healthy pregnancy and pregnancies with insufficiently remodelled spiral arteries.
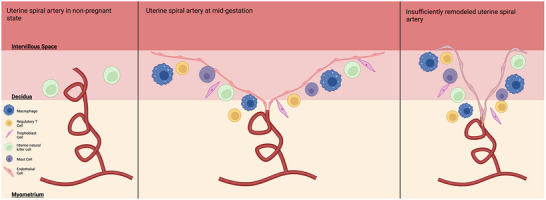
A number of other cells in addition to trophoblast cells are thought to be involved in the vascular remodelling of the spiral artery. These include macrophages, uterine natural killer cells (uNK) and uterine mast cells. Adapted from Schumacher et al. (2018). Created in Biorender.com.
One of the major challenges in studying normal and abnormal placental function is the inaccessibility of the placenta during pregnancy. Placental sampling during pregnancy carries a risk of miscarriage or preterm birth, so placentas from uncomplicated pregnancies can only be analysed following term deliveries. Therefore, placentas from spontaneous or iatrogenic preterm deliveries cannot be compared to ‘normal’ placentas of the same gestation. Analysis of placental EVs offers a possible solution to this challenge.
Extracellular vesicles
Extracellular vesicles are a non‐replicating, lipid bilayer‐delimited particles produced by all cells for cell‐to‐cell communication (Yáñez‐Mó et al., 2015). They are transported within extracellular spaces, in biofluids such as amniotic fluid (Balbi et al., 2017; Hell et al., 2017) and in plasma (Arraud et al., 2014). EVs are characterised by their pathway of release from cells into three subtypes of overlapping sizes: exosomes (∼40–150 nm), microvesicles (∼100–1000 nm) and apoptotic bodies (∼500–2000 nm) (Fig. 3; Skotland et al., 2017). Each of these subtypes is distinguished by its mechanism of biogenesis; exosomes, for example, are generated from the inward budding of the endosomal membrane to form a multivesicular body (MVB) (McVey & Kuebler, 2018; Skotland et al., 2017). The MVB then releases these intraluminal vesicles as exosomes upon fusion with the plasma membrane. Microvesicles, on the other hand, are derived from the outward budding of the plasma membrane and are typically released under conditions of cellular stress and activation (McVey & Kuebler, 2018; Skotland et al., 2017). Lastly, apoptotic bodies are formed during apoptosis, where cellular components are fragmented and packaged into EVs (Kalra et al., 2016).
Figure 3. Biogenesis of exosomes, microvesicles and apoptotic bodies (small, medium and large EVs; El Andaloussi et al., 2013).
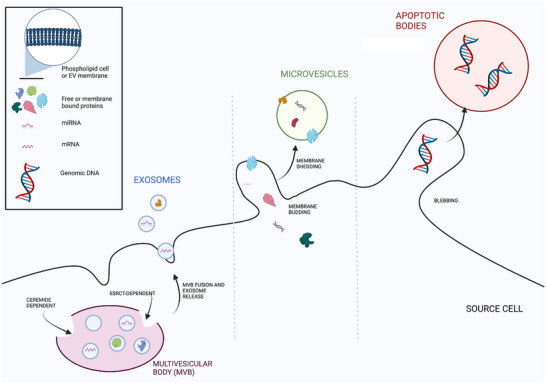
Exosomes range from 30 to 150 nm in diameter and are released from the multivesicular body through fusion with the cell membrane. They show the same membrane orientation as the source cells. Whilst this is also the case with microvesicles (100 nm to 1 μm in diameter), they are formed from a heterogeneous process involving the budding of a cell membrane around the intended contents and the shedding of this membrane. Apoptotic bodies range from 50 to 5000 nm in diameter (Doyle & Wang, 2019) and are formed by the separation of source cell plasma from the cytoskeleton as a reaction to increased hydrostatic pressure after cell contraction (Wickman et al., 2012). Created with Biorender.com.
Due to the overlapping size distributions of the subtypes, in combination with the lack of a definitive specific marker for each subtype, the field is now moving away from using these terms to describe EVs in publications (Lancaster & Febbraio, 2005; Théry et al., 2018). The ‘Minimal Information for Studies of Extracellular Vesicles’ set by the International Society for Extracellular Vesicles (ISEV) instead recommends that EVs are described by their physical characteristics, ergo, their size with defined ranges that do not overlap (Théry et al., 2018). For example, EVs <200 nm in diameter are now referred to as small EVs (sEV) and EVs >200 nm are large EVs (LEVs).
Extracellular vesicle cargo
All EVs transport a range of cellular cargo, including phospholipids, miRNA, mRNA, DNA and transmembrane as well as cytosolic proteins (Kalra et al., 2016; Sáez et al., 2018). In 2007, a role for EVs as mediators of cell–cell communication was first described when Valadi et al. demonstrated that EVs can transfer mRNA from one cell to another, leading to protein transcription and hence a potentially functional effect (Lotvall & Valadi, 2007; Valadi et al., 2007). It is now well established that in addition to mRNA, EVs transport their other cargo, including protein, lipid and non‐coding RNAs between cells from different areas of the body, and as such, they are key regulators of cellular communication (Boon & Vickers, 2013; McVey & Kuebler, 2018).
In addition to their key roles in cell–cell communication, the stability of EVs and their ability to protect their cargo, specifically miRNAs, from degradation (Ge et al., 2014) has sparked research interest into the use of EVs as diagnostic biomarkers (Simeone et al., 2020; Zhao & Yang, 2021), as indicators of disease progression (Lee et al., 2021; Simionescu et al., 2021) and, through preliminary exploration, as targeted therapies (D'Arrigo et al., 2019; Pezzana et al., 2021). The benefit of EVs over non‐vesicular methods of transportation is that the phospholipid bilayer protects cargo and could allow for tissue‐specific delivery (Hoshino et al., 2015; Jiao et al., 2017; Manier et al., 2017).
Whilst the role of EVs as biomarkers and functional mediators of cell–cell communication in different physiological and pathological conditions, including pregnancy, has only emerged in recent years, the production of EVs from the placenta has been long established. During normal pregnancy the syncytiotrophobast releases EVs of different sizes, including macro‐vesicles (such as syncytial nuclear aggregates; SNAs), micro‐vesicles (MVs) and exosomes, into the maternal circulation (Heazell et al., 2007; Out et al., 1991; Warrander et al., 2012). Much of the original work that examined EVs in pregnancy primarily focused on large EVs such as MVs and SNAs, establishing that levels increase across gestation and that they have key roles in the pathogenesis of pre‐eclampsia by influencing the maternal immune response (Holder et al., 2012; Holder et al., 2016). In more recent years, the focus has shifted to studying small EVs in obstetric complications.
The role of large EVs in pregnancy is well reviewed (Redman & Sargent, 2008; Redman et al., 2012) and small EVs continue to be the most commonly studied EV subtype across multiple conditions including cancer (Azmi et al., 2013; Xu et al., 2018; Hoshino et al., 2015; Lee et al., 2021), heart disease (Bei et al., 2017; Saheera et al., 2021; Akhmerov et al., 2022) and obstetric complications. Therefore, whilst some of the discussed work may include large EVs due to the overlap in size categorisation between sEVs and microvesicles, the main focus will be on sEVs.
Extracellular vesicles in healthy pregnancy
Endometrial, embryonic and placental EVs all contribute to early pregnancy development, influencing implantation, immunomodulation and spiral artery remodelling (Andronico et al., 2019; Chen et al., 2022; Morelli & Sadovsky, 2022; Zhang et al., 2020). Endometrial epithelial EVs are present in the uterine fluid of humans and other animals (Li et al., 2021; Mishra et al., 2021). EVs from cultured endometrial epithelium are able to enter trophectoderm cells (Evans et al., 2019) and have been shown to increase blastocyst adhesion and invasion and promote embryo development (Mishra et al., 2021). Conversely, EVs from cultured embryos can enter endometrial epithelial cells (Giacomini et al., 2017), and trophoblast EVs are readily taken up by monocytes, increasing migration and altering cytokine production (Atay et al., 2011). Placental EVs may also be responsible for presenting paternal minor histocompatility antigens to maternal T and B cells in such a way that they produce immunotolerance (Morelli & Sadovsky, 2022).
As discussed, spiral artery remodelling is important for meeting the metabolic demands of the fetus and placenta. Salomon, Yee et al. (2014) have demonstrated the capacity for extravillous trophoblast‐derived EVs to increase vascular smooth muscle cell migration, while Jia et al. (2018) found that both maternal and umbilical cord derived porcine EVs could significantly enhance the migration and proliferation of human umbilical vein endothelial cells. Placental EVs have also been found to contain and release VEGF‐A, a member of the VEGF family and one of the major contributors to angiogenesis and vasculogenesis (Fig. 2) (Condrat et al., 2021; Patton et al., 2015).
Bidirectional extracellular vesicle trafficking via the placenta
The placenta releases EVs into the maternal and fetal circulations, takes up maternal EVs and allows transit of maternal and exogenous EVs to the fetus (Buca et al., 2020; Chiarello et al., 2018). The capacity for placental EVs (pEVs) to enter maternal cells has been demonstrated by Tong et al. (2017) who found uptake of fluorescently labelled EVs from cultured placenta localised to maternal lungs, liver and kidneys after venous administration in pregnant mice. The concentration of pEVs in maternal plasma increases over the course of pregnancy, as indicated by exosomal placental alkaline phosphatase (PLAP) concentration (Salomon, Torres et al., 2014), with 12−25% of all maternal circulating EVs being of placental origin (Elfeky et al., 2017). Bidirectional trafficking of placenta‐specific chromosome 14 and chromosome 19 cluster miRNAs into maternal and fetal compartments has been demonstrated both in vivo in mice and in matched patient placental biopsies, maternal and fetal plasma (Chang et al., 2017 2018; Paquette et al., 2018).
Holder et al.’s visualisation of the internalisation of fluorescent‐labelled maternal macrophage‐derived EVs by the placenta shows that this EV‐ mediated communication between maternal tissues and the placenta is bidirectional. They also found maternal macrophage EVs that modulate placental cytokine production were actively transported into the placenta by clathrin‐ mediated endocytosis (Holder et al., 2016). This bidirectional communication is supported by the ability of maternal adipose tissue EVs to influence glucose metabolism in the placenta, by upregulating genes involved in glycolysis and gluconeogenesis (Jayabalan et al., 2018) EV cargo of dietary origin has also been shown to enter into and influence events in the placenta, highlighting the potential for dietary‐derived EVs/cargo to reach even distal organs (Timms et al., 2022).
Extracellular vesicles in obstetric diseases
Given the increasing evidence for EV function in healthy pregnancy, it is unsurprising that interest in the roles of EVs in obstetric diseases has also grown. This includes both their possible actions as mediators of pregnancy complications and their potential as a source of protein and RNA biomarkers to predict and diagnose disease.
Extracellular vesicles in gestational diabetes
GDM affects 6% of pregnancies worldwide and is defined as a glucose intolerance that develops during pregnancy and resolves post‐partum (Coustan, 2013; Mack & Tomich, 2017). Pregnancies complicated by GDM have been shown to have higher concentrations of circulating EVs than uncomplicated pregnancies, and a large percentage of these are PLAP‐positive, suggesting that there may be increased pEV biogenesis and release in GDM pregnancies (Salomon et al., 2016). pEVs have been shown to interact with maternal organs, influencing skeletal muscle biology (Kupper & Huppertz, 2022) and contributing to insulin resistance in the mother (Kandzija et al., 2019; Palma et al., 2022). Whilst the importance of pEVs in the pathogenesis of GDM is of obvious importance, there is also evidence that EVs in maternal circulation, and their miRNA cargo, could potentially influence placental development and dysfunction in GDM (Kennedy et al., 2019; Quilang et al., 2022). Gillet et al. (2019) detected 10 maternal serum EV miRNAs that were upregulated in GDM at early gestation (6–15 weeks). Whilst the functional role of these was not reported, eight of the altered miRNAs have predicted functions related to vascular development. Given that placental vascular dysfunction is a feature of GDM, it is possible that these circulating EV‐enclosed miRNAs may contribute to placental dysfunction in GDM. Indeed our recent observation that vascular regulatory miRNAs are also altered in EVs in both maternal circulation and placenta in GDM pregnancies that go on to deliver LGA babies (Kennedy et al., 2019) supports this hypothesis. Although further work is required to confirm this, a potential role for EV miRNAs in pathogenesis of GDM and associated fetal growth could provide both novel diagnostic and therapeutic opportunities to reduce clinical burden associated with GDM.
Extracellular vesicles in pre‐eclampsia
PE is a multisystem disease characterised by new‐onset hypertension after 20 weeks of gestation and one or more additional indicators of organ dysfunction, such as proteinuria (American College of Obstetricians & Gynecologists, 2020; Brown et al., 2018). Its incidence ranges from 2% to 5% and it can result in both fetal and maternal morbidity and mortality (Huppertz, 2008; Jin & Menon, 2018; Lisonkova & Joseph, 2013). To date, pre‐eclampsia is the obstetric complication in which EVs have been most extensively studied.
Several studies have suggested pEVs provide a link between placental damage and the maternal phenotype in PE. Dutta et al. (2020) demonstrated that the injection of EVs from trophoblast cultured in hypoxic (1% oxygen) conditions into the tail veins of pregnant rats increased the mean systolic blood pressure more than the administration of trophoblast‐derived EVs produced in normoxic (8% oxygen) conditions. Similarly, Han et al. (2020) found EVs from freeze–thaw‐injured placenta induced hypertension and proteinuria when administered to non‐pregnant mice, disrupted endothelial integrity and induced vasoconstriction. Powell et al. (2022) demonstrated the uptake of sEVs from human maternal plasma into the vessel wall of mouse mesenteric arteries. Exposure to sEVs isolated from the plasma of individuals with PE produced significantly more vasoconstriction and less endothelium‐induced relaxation in these vessels than sEVs from plasma of individuals without PE.
In addition to peripheral vascular changes, pre‐eclampsia is also characterised by neurological changes, manifesting as hyperreflexia and potentially seizures (eclampsia). Leon et al. (2021) found that human brain endothelial cells, used as an in vitro model of the blood–brain barrier, showed increased permeability and reduced transendothelial electrical resistance when exposed to plasma EVs from mothers with PE compared to plasma EVs from mothers without PE. A similar response was seen to EVs from placenta cultured in hypoxic conditions (1% oxygen) compared with EVs from placenta cultured in normoxic conditions (8% oxygen) (Fig. 4). These changes were mitigated by co‐administration of magnesium sulphate, a drug that is used clinically to prevent seizures in pre‐eclampsia.
Figure 4. Oxygen tension in pEV biogenesis and bioactivity.
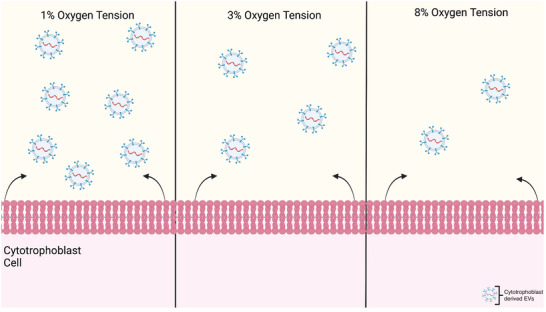
Saloman et al. carried out a study by which they treated cytotrophoblast (CT) cell cultures with different levels of oxygen tension. They found that in the higher oxygen tensions, fewer EVs were secreted by CT cells than in the lower oxygen tensions. They hypothesised that CT‐derived exosomes are formed under hypoxic conditions within early pregnancy in the placenta and that this may be an adaptive response to encourage trophoblast invasion (Salomon et al., 2015). This supports the further investigation into the role of early pregnancy pEVs in both healthy and abnormal pregnancies (Dutta et al., 2020; Truong et al., 2017). Created in Biorender.com.
While placental EVs may negatively impact maternal health in the later stages of pre‐eclampsia, there is also evidence to suggest maternal EVs may negatively impact the placenta earlier in pregnancy. Kohli et al. (2016) administered EVs from serum‐starved cultured mouse endothelial cells to pregnant mice at days 10.5 and 11.5 of gestation (E10.5, E11.5), the time at which the mature mouse placenta is established. This resulted in significant increases in maternal blood pressure, proteinuria and soluble fms‐like tyrosine kinase (sflt1) compared with control medium. Fetal and placental sizes were reduced, fetal survival was reduced and placental inflammasome activation was increased in pregnancies administered EVs versus control media. The placentas of EV‐administered mice also showed structural changes consistent with malperfusion.
Li et al. (2020) isolated EVs from maternal plasma to quantify their concentration and size, as well as determine whether components of their cargo were up‐ or downregulated across 60 healthy, PE and FGR pregnancies. Their findings displayed a twofold upregulation of the miRNA cargos miR‐153‐3p and miR‐325‐3p in pre‐eclamptic pregnancies compared with healthy pregnancies (Fig. 5). The overexpression of miR‐153 in humans is hypothesised to inhibit cell proliferation and invasion and encourage apoptosis (Zeng et al., 2017). Further to this, miR‐153 has also been shown to bind to the 3′ untranslated region of hypoxia inducible factor 1 (HIF1) and supresses expression. This is associated with reduced tube formation in the endothelial cells of the primary human umbilical vein, as well as reduced VEGFA expression and thus inhibited angiogenesis.
Figure 5. Summary of method and findings of Li et al. (2020) .
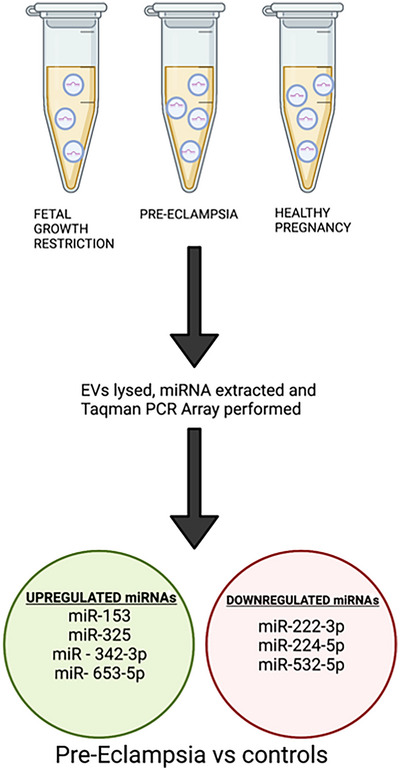
Li et al extracted maternal plasma from 60 mothers who had either a healthy pregnancy or suffered from FGR and pre‐eclampsia. They isolated EVs and lysed them to determine whether their miRNA cargo was altered between each control group and found a collection were up‐ and downregulated in pre‐eclampsia compared to the two control groups. Created in Biorender.com.
One notable finding of Li et al.’s work was that the seven miRNAs that were differentially expressed in pEVs from healthy and PE pregnancies did not show differences in miRNA sequencing of the whole plasma free miRNA. This was further shown in a study by Hromadnikova et al. (2019), in which the capacity of C19MC miRNAs to predict FGR was higher when expression was analysed from lysed circulating maternal exosomes rather than whole plasma miRNA. Li et al. also demonstrated an association between pEV cargo and the placental transcriptome, thus providing evidence that pEVs isolated from maternal blood can act as a non‐invasive placental biopsy (Tannetta et al., 2017).
As well as contributing to the development of pre‐eclampsia, EVs may have potential as therapies. One example of this is decidual mesenchymal stem/stromal cell (DMSC) EVs. DMSCs have been shown to reduce endothelial cell dysfunction in response to oxidative and inflammatory damage when co‐cultured with them (Alshabibi et al., 2018). Zheng et al. (2020) hypothesised that the beneficial effects of DMSCs may be mediated by EVs, which in turn could be used to mitigate the endothelial dysfunction seen in pre‐eclampsia. This was supported by their finding of increased cell attachment and proliferation and decreased IL‐6 expression and lipid peroxidation when human umbilical vein endothelial cells (HUVECs) were exposed to DMSC EVs in combination with serum from pre‐eclamptic mothers compared with pre‐eclamptic serum alone.
Extracellular vesicles in preterm birth
There are multiple mechanisms that lead to the same final common pathway of preterm birth, which in turn is the leading cause of perinatal mortality and morbidity in developed countries (Goldenberg et al., 2008). EVs appear to contribute to the normal initiation of labour at term (Palomares et al., 2021; Yadava et al., 2021), making it plausible that they could form part of the mechanistic pathway in some cases of preterm birth.
In an in vivo mouse study (Sheller‐Miller et al., 2019), maternal plasma EVs at gestational day 18 (E18, late pregnancy) were found to contain proinflammatory cargo, thought to contribute to labour and delivery. Administration of E18 EVs to pregnant mice at E15 resulted in preterm birth in four out of five dams, while none of the dams administered E9 EVs delivered prematurely.
Intraperitoneally administered EVs were delivered to the uterine tissues regardless of gestational age, while E18 EVs were found to both prepare the uterus and cervix for parturition and promote prepartum proinflammation in fetal membranes. Whilst this is a murine model with a small sample of mice (n = 15), the study highlights the importance of pEVs in paracrine signalling within pregnancies.
When studying human pregnancy, Menon et al. (2020) found fewer PLAP+ (placental) EVs in maternal plasma from the first and second trimesters of pregnancies ending in preterm birth compared with pregnancies ending in term birth. In contrast, the number and size distribution of PLAP− EVs was similar between the two groups. Using sequential windowed acquisition of all theoretical mass spectra (SWATH) mass spectrometry of pEV cargo, they identified 96 proteins which differed significantly across gestation between pregnancies ending in term and preterm birth. The highest scoring network for these proteins related to cell death and survival. Analysing total circulating EV miRNAs using next generation sequencing, they also identified 173 miRNAs that differed significantly across gestation between pregnancies ending in term and preterm birth (Menon, Debnath et al., 2019). Signalling pathways targeted by these miRNAs included p53, fitting with the proteomic finding of differences relating to cell death and survival, TGF‐β signalling and glucocorticoid receptor signalling.
An additional cross‐sectional study by Menon, Dixon et al. (2019) compared the protein cargo of total circulating EVs in maternal plasma between pregnancies at term not in labour (TNIL) and at term in labour (TL) with preterm premature rupture of membranes (pPROM) and ending in preterm birth (PTB). Canonical pathway analysis of the results of SWATH mass spectrometry showed similarities between TNIL and PTB EV cargo when compared with TL EVs. Differences were evident, however, between pPROM EV and PTB EV cargo, with increased concentrations of proteins related to inflammation, coagulation activation and response to oxidative stress in PTB EVs. Individual proteins related to cholesterol signalling, cell proliferation and classical immune activation showed significant differences between the four groups on pairwise comparison (Menon, Dixon et al., 2019) (Fig. 6). These findings suggest further investigation of different preterm birth phenotypes may provide greater insights than studying preterm birth as a single entity. Menon's research team makes up the majority of the recent literature in this field, highlighting the novelty of this area and the resulting literature gaps. Further weight will be added to their findings if they can be replicated by other researchers.
Figure 6. Summary of method and findings of Menon, Dixon et al. (2019).
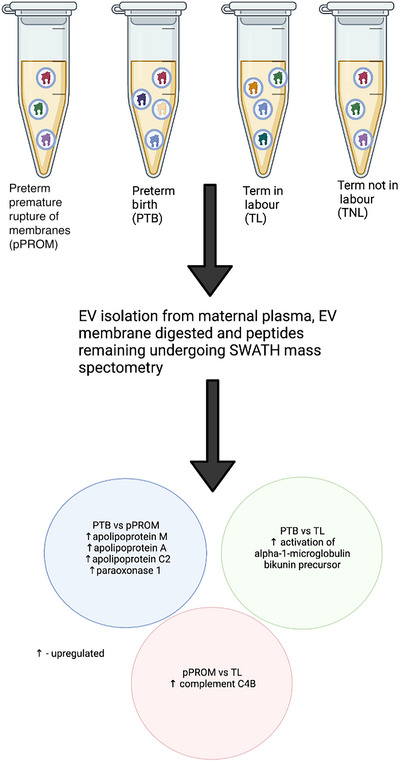
EVs isolated from maternal plasma were collected from four groups: term not in labour (TNIL, n = 13), term in labour (TL, n = 11), preterm premature rupture of membranes (pPROM, n = 8), and preterm birth (PTB, n = 13). SWATH mass spectrometry protieomics were performed displaying notable differences between the four groups. Created in Biorender.com.
McElrath et al. (2019) displayed the potential of using EVs isolated from maternal plasma in the first trimester of singleton pregnancies as a source of predictive biomarkers for PTB before 35 weeks of gestation. Using a 1:2 case‐controlled study, a panel of five EV proteins was selected based on analysis of samples from a training set of 135 pregnancies. When tested on a further 126 pregnancies, their marker panel had an area under the curve (AUC) of 0.74 (95% CI 0.63–0.81), with a positive likelihood ratio of 2.70 and a negative likelihood ratio of 0.27. Zhao et al. (2020) analysed EV lipids in second trimester maternal plasma and found higher levels of PS(34:0) in microvesicles from 27 pregnancies ending before 37 weeks of gestation compared with 66 full term pregnancies. When tested in a validation set of a further 83 pregnancies, microvesicle PS(34:0) had an AUC of 0.71 (95% CI 0.60–0.82) for predicting birth before 37 weeks. Although both of these findings have yet to be externally validated, they support the utility of EVs as a source of predictive markers in pregnancy.
Extracellular vesicles in FGR
There are fewer studies focussing specifically on FGR than the other obstetric conditions discussed in this review. However, there is considerable overlap, in terms of both pathophysiology and incidence, between early‐onset FGR (<32 weeks of gestation) and early‐onset pre‐eclampsia (<34 weeks of gestation). This may mean some of the findings about EVs in pre‐eclampsia may prove relevant to FGR in the future.
Miranda et al. (2018b) demonstrated an association between the ratio of pEVs (CD63+ and PLAP+) to total circulating EVs (CD63+) and fetal growth in utero. They found the ratio of pEVs to total EVs was significantly reduced in small‐for‐gestational‐age (SGA) fetuses (estimated fetal weight (EFW) <10th centile) compared with appropriate‐for‐gestational‐age (AGA) fetuses, with a further reduction in the ratio for fetuses with FGR (EFW <10th centile and abnormal Doppler studies or EFW <3rd centile). This raises the possibility of monitoring pEV quantity as a marker for placental insufficiency and thus fetal growth.
Ariyakumar et al. (2021) also found total EV concentration was significantly lower in maternal plasma from pregnancies with FGR (birth weight <10th centile with absent or reversed umbilical artery end‐diastolic flow) than in normal pregnancies, with FGR maternal plasma EV concentrations similar to that of non‐pregnant individuals. The concentration of Fas ligand (FasL), which promotes immune tolerance through suppression of the nuclear factor κB subunit p65, was lower also in EVs from FGR pregnancies than AGA pregnancies. This suggests a possible pathophysiological effect of EVs in FGR.
Conclusion
Extracellular vesicles are a key mediator of cell–cell communication, carrying miRNA, proteins and surface antigens. It is perhaps unsurprising, therefore, that they appear to be involved in so many key areas of cross‐talk between the mother, the placenta and the fetus. In healthy pregnancy this includes implantation, immunomodulation and the initiation of labour. In pregnancy complications it includes the production of maternal pre‐eclampsia manifestations in response to EVs from hypoxic or otherwise damaged placenta.
Studying maternal, placental and fetal EVs in healthy and complicated pregnancies is providing novel insights into the causes and pathophysiology of obstetric diseases. It is also allowing researchers to identify novel biomarkers to predict which pregnancies are at higher risk of complications. In time we may be able to harness this knowledge to use EVs as a therapy, either by utilising naturally produced EVs that have beneficial effects, as in the case of DMSC EVs, or by using artificially created EVs as a drug delivery system. Given the lack of current effective treatments for many obstetric complications, this would be of great benefit to the health of pregnant people and their future children.
Additional information
Competing interests
No competing interests declared
Author contributions
R.F., M.K., R.S. and K.F.: conception or design of the work; drafting the work or revising it critically for important intellectual content. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Academy of Medical Sciences (The Academy of Medical Sciences): R.S., SGL026∖1008; Mary and Alice Smith Memorial Scholarship from University of Leeds: M.G.K.; Medical Research Council (MRC) New Investigator Grant: K.F., MR/Ro23166/1.
Supporting information
Peer Review History
Biography
Rachel Farrelly is a medical student at the University of Leeds with a keen interest in academic obstetrics. She gained a distinction in her Master of Research degree studying extracellular vesicles in maternal plasma of pregnancies complicated by fetal growth restriction, won the Basil Lee Bursary award from the Royal Society of Medicine for innovation in communication, and is the recipient of a British Society of Haematology elective Scholarship and British Society of Immunology elective award. Specifically, her research interests surround early antenatal detection of pregnancy complications. She hopes to pursue a career which balances her clinical and academic interest in the field of maternal–fetal medicine. Her hopes for the future include completing her medical degree and pursuing a further research qualification in the form of a PhD or MD.

Handling Editors: Laura Bennet & Rebecca Simmons
The peer review history is available in the Supporting Information section of this article (https://doi.org/10.1113/JP282849#support‐information‐section).
References
- Alberry, M. , & Soothill, P. (2007). Management of fetal growth restriction. Archives of Disease in Childhood Fetal and Neonatal Edition, 92(1), F62–F67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmerov, A. , & Parimon, T. (2022). Extracellular vesicles, inflammation, and cardiovascular disease. Cells, 11(14), 2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshabibi, M. A. , Khatlani, T. , Abomaray, F. M. , AlAskar, A. S. , Kalionis, B. , Messaoudi, S. A. , Khanabdali, R. , Alawad, A. O. , & Abumaree, M. H. (2018). Human decidua basalis mesenchymal stem/stromal cells protect endothelial cell functions from oxidative stress induced by hydrogen peroxide and monocytes. Stem Cell Research and Therapy, 9(1), 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists (2020). Gestational hypertension and preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstetrics and Gynecology, 135(6), 1492–1495. [DOI] [PubMed] [Google Scholar]
- Andronico, F. , Battaglia, R. , Ragusa, M. , Barbagallo, D. , Purrello, M. , & Di Pietro, C. (2019). Extracellular vesicles in human oogenesis and implantation. International Journal of Molecular Sciences, 20(9), 2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyakumar, G. , Morris, J. M. , McKelvey, K. J. , Ashton, A. W. , & McCracken, S. A. (2021). NF‐κB regulation in maternal immunity during normal and IUGR pregnancies. Scientific Reports, 11(1), 20971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arraud, N. , Linares, R. , Tan, S. , Gounou, C. , Pasquet, J. M. , Mornet, S. , & Brisson, A. R. (2014). Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. Journal of Thrombosis and Haemostasis, 12(5), 614–627. [DOI] [PubMed] [Google Scholar]
- Atay, S. , Gercel‐Taylor, C. , Suttles, J. , Mor, G. , & Taylor, D. D. (2011). Trophoblast‐derived exosomes mediate monocyte recruitment and differentiation. American Journal of Reproductive Immunology, 65(1), 65–77. [DOI] [PubMed] [Google Scholar]
- Azmi, A. S. , Bao, B. , & Sarkar, F. H. (2013). Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer and Metastasis Reviews, 32(3–4), 623–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbi, C. , Piccoli, M. , Barile, L. , Papait, A. , Armirotti, A. , Principi, E. , Reverberi, D. , Pascucci, L. , Becherini, P. , Varesio, L. , Mogni, M. , Coviello, D. , Bandiera, T. , Pozzobon, M. , Cancedda, R. , & Bollini, S. (2017). First characterization of human amniotic fluid stem cell extracellular vesicles as a powerful paracrine tool endowed with regenerative potential: Amniotic fluid stem cell extracellular vesicles. Stem Cells Translational Medicine, 6(5), 1340–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei, Y. , Xu, T. , Lv, D. , Yu, P. , Xu, J. , Che, L. , Das, A. , Tigges, J. , Toxavidis, V. , Ghiran, I. , Shah, R. , Li, Y. , Zhang, Y. , Das, S. , & Xiao, J. (2017). Exercise‐induced circulating extracellular vesicles protect against cardiac ischemia–reperfusion injury. Basic Research in Cardiology, 112(4), 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendix, I. , Miller, S. L. , & Winterhager, E. (2020). Editorial: Causes and consequences of intrauterine growth restriction. Frontiers in Endocrinology, 11, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon, R. A. , & Vickers, K. C. (2013). Intercellular transport of microRNAs. Arteriosclerosis, Thrombosis, and Vascular Biology, 33(2), 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, M. A. , Magee, L. A. , Kenny, L. C. , Karumanchi, S. A. , McCarthy, F. P. , Saito, S. , Hall, D. R. , Warren, C. E. , Adoyi, G. , & Ishaku, S. & International Society for the Study of Hypertension in P (2018). The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertension, 13, 291–310. [DOI] [PubMed] [Google Scholar]
- Buca, D. , Bologna, G. , D'Amico, A. , Cugini, S. , Musca, F. , Febbo, M. , D'Arcangelo, D. , Buca, D. , Simeone, P. , Liberati, M. , Vitacolonna, E. , Miscia, S. , D'Antonio, F. , & Lanuti, P. (2020). Extracellular vesicles in feto‐maternal crosstalk and pregnancy disorders. International Journal of Molecular Sciences, 21(6), 2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, G. J. , Redman, C. W. , Roberts, J. M. , & Moffett, A. (2019). Pre‐eclampsia: Pathophysiology and clinical implications. British medical journal, 366, l2381. [DOI] [PubMed] [Google Scholar]
- Cartwright, J. E. , Fraser, R. , Leslie, K. , Wallace, A. E. , & James, J. L. (2010a). Remodelling at the maternal‐fetal interface: Relevance to human pregnancy disorders. Reproduction, 140(6), 803–813. [DOI] [PubMed] [Google Scholar]
- Cartwright, J. E. , Fraser, R. , Leslie, K. , Wallace, A. E. , & James, J. L. (2010b). Remodelling at the maternal–fetal interface: Relevance to human pregnancy disorders. Reproduction, 140(6), 803–813. [DOI] [PubMed] [Google Scholar]
- Chang, G. , Mouillet, J. F. , Mishima, T. , Chu, T. , Sadovsky, E. , Coyne, C. B. , Parks, W. T. , Surti, U. , & Sadovsky, Y. (2017). Expression and trafficking of placental microRNAs at the feto‐maternal interface. The Federation of American Societies of Experimental Biology Journal, 31(7), 2760–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K. , Liang, J. , Qin, T. , Zhang, Y. , Chen, X. , & Wang, Z. (2022). The role of extracellular vesicles in embryo implantation. Frontiers in Endocrinology, 13, 809596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarello, D. I. , Salsoso, R. , Toledo, F. , Mate, A. , Vazquez, C. M. , & Sobrevia, L. (2018). Foetoplacental communication via extracellular vesicles in normal pregnancy and preeclampsia. Molecular Aspects of Medicine, 60, 69–80. [DOI] [PubMed] [Google Scholar]
- Colella, M. , Frerot, A. , Novais, A. R. B. , & Baud, O. (2018). Neonatal and Long‐term consequences of fetal growth restriction. Current Pediatric Reviews, 14(4), 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condrat, C. E. , Varlas, V. N. , Duica, F. , Antoniadis, P. , Danila, C. A. , Cretoiu, D. , Suciu, N. , Cretoiu, S. M. , & Voinea, S. C. (2021). Pregnancy‐related extracellular vesicles revisited. International Journal of Molecular Sciences, 22(8), 3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustan, D. R. (2013). Gestational diabetes mellitus. Clinical Chemistry, 59(9), 1310–1321. [DOI] [PubMed] [Google Scholar]
- D'Arrigo, D. , Roffi, A. , Cucchiarini, M. , Moretti, M. , Candrian, C. , & Filardo, G. (2019). Secretome and extracellular vesicles as new biological therapies for knee osteoarthritis: A systematic review. Journal of Clinical Medicine, 8(11), 1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, L. M. , & Wang, M. Z. (2019). Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells, 8(7), 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta, S. , Lai, A. , Scholz‐Romero, K. , Shiddiky, M. J. A. , Yamauchi, Y. , Mishra, J. S. , Rice, G. E. , Hyett, J. , Kumar, S. , & Salomon, C. (2020). Hypoxia‐induced small extracellular vesicle proteins regulate proinflammatory cytokines and systemic blood pressure in pregnant rats. Clinical Science (London, England: 1979), 134(6), 593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Andaloussi, S. , Mäger, I. , Breakefield, X. O. , & Wood, M. J. A. (2013). Extracellular vesicles: Biology and emerging therapeutic opportunities. Nature Reviews Drug Discovery, 12(5), 347–357. [DOI] [PubMed] [Google Scholar]
- Elfeky, O. , Longo, S. , Lai, A. , Rice, G. E. , & Salomon, C. (2017). Influence of maternal BMI on the exosomal profile during gestation and their role on maternal systemic inflammation. Placenta, 50, 60–69. [DOI] [PubMed] [Google Scholar]
- Evans, J. , Rai, A. , Nguyen, H. P. T. , Poh, Q. H. , Elglass, K. , Simpson, R. J. , Salamonsen, L. A. , & Greening, D. W. (2019). Human endometrial extracellular vesicles functionally prepare human trophectoderm model for implantation: Understanding bidirectional maternal‐embryo communication. Proteomics, 19(23), 1800423. [DOI] [PubMed] [Google Scholar]
- Familari, M. , Cronqvist, T. , Masoumi, Z. , & Hansson, S. R. (2017). Placenta‐derived extracellular vesicles: Their cargo and possible functions. Reproduction, Fertility, and Development, 29(3), 433–447. [DOI] [PubMed] [Google Scholar]
- Ge, Q. , Zhou, Y. , Lu, J. , Bai, Y. , Xie, X. , & Lu, Z. (2014). miRNA in plasma exosome is stable under different storage conditions. Molecules, 19(2), 1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini, E. , Vago, R. , Sanchez, A. M. , Podini, P. , Zarovni, N. , Murdica, V. , Rizzo, R. , Bortolotti, D. , Candiani, M. , & Vigano, P. (2017). Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Scientific Reports, 7(1), 5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet, V. , Ouellet, A. , Stepanov, Y. , Rodosthenous, R. S. , Croft, E. K. , Brennan, K. , Abdelouahab, N. , Baccarelli, A. , & Takser, L. (2019). miRNA profiles in extracellular vesicles from serum early in pregnancies complicated by gestational diabetes mellitus. Journal of Clinical Endocrinology and Metabolism, 104(11), 5157–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg, R. L. , Culhane, J. F. , Iams, J. D. , & Romero, R. (2008). Epidemiology and causes of preterm birth. Lancet, 371(9606), 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordijn, S. J. , Beune, I. M. , & Ganzevoort, W. (2018). Building consensus and standards in fetal growth restriction studies. Best Practice & Research Clinical Obstetrics & Gynaecology, 49, 117–126. [DOI] [PubMed] [Google Scholar]
- Graves, M. , Howse, K. , Pudwell, J. , & Smith, G. N. (2019). Pregnancy‐related cardiovascular risk indicators: Primary care approach to postpartum management and prevention of future disease. Canadian Family Physician, 65(12), 883–889. [PMC free article] [PubMed] [Google Scholar]
- Han, C. , Wang, C. , Chen, Y. , Wang, J. , Xu, X. , Hilton, T. , Cai, W. , Zhao, Z. , Wu, Y. , Li, K. , Houck, K. , Liu, L. , Sood, A. K. , Wu, X. , Xue, F. , Li, M. , Dong, J. F. , & Zhang, J. (2020). Placenta‐derived extracellular vesicles induce preeclampsia in mouse models. Haematologica, 105(6), 1686–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heazell, A. , Moll, S. , Jones, C. , Baker, P. , & Crocker, I. (2007). Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta, 28(Suppl A), S33–S40. [DOI] [PubMed] [Google Scholar]
- Hell, L. , Wisgrill, L. , Ay, C. , Spittler, A. , Schwameis, M. , Jilma, B. , Pabinger, I. , Altevogt, P. , & Thaler, J. (2017). Procoagulant extracellular vesicles in amniotic fluid. Translational Research: The Journal of Laboratory and Clinical Medicine, 184, 12–20.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder, B. , Jones, T. , Sancho Shimizu, V. , Rice, T. F. , Donaldson, B. , Bouqueau, M. , Forbes, K. , & Kampmann, B. (2016). Macrophage exosomes induce placental inflammatory cytokines: A novel mode of Maternal‐placental messaging. Traffic, 17(2), 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder, B. S. , Tower, C. L. , Forbes, K. , Mulla, M. J. , Aplin, J. D. , & Abrahams, V. M. (2012). Immune cell activation by trophoblast‐derived microvesicles is mediated by syncytin 1. Immunology, 136(2), 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino, A. , Costa‐Silva, B. , Shen, T. L. , Rodrigues, G. , Hashimoto, A. , Tesic Mark, M. , Molina, H. , Kohsaka, S. , Di Giannatale, A. , Ceder, S. , Singh, S. , Williams, C. , Soplop, N. , Uryu, K. , Pharmer, L. , King, T. , Bojmar, L. , Davies, A. E. , Ararso, Y. , …, & Lyden, D. (2015). Tumour exosome integrins determine organotropic metastasis. Nature, 527(7578), 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromadnikova, I. , Dvorakova, L. , Kotlabova, K. , & Krofta, L. (2019). The prediction of gestational hypertension, preeclampsia and fetal growth restriction via the first trimester screening of plasma exosomal C19MC microRNAs. International Journal of Molecular Sciences, 20(12), 2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz, B. (2008). Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension, 51(4), 970–975. [DOI] [PubMed] [Google Scholar]
- Jansen, C. , Kastelein, A. W. , Kleinrouweler, C. E. , van Leeuwen, E. , de Jong, K. H. , Pajkrt, E. , & van Noorden, C. J. F. (2020). Development of placental abnormalities in location and anatomy. Acta Obstetricia et Gynecologica Scandinavica, 99(8), 983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayabalan, N. , Lai, A. , Ormazabal, V. , Adam, S. , Guanzon, D. , Palma, C. , Scholz‐Romero, K. , Lim, R. , Jansson, T. , McIntyre, H. D. , Lappas, M. , & Salomon, C. (2018). Adipose tissue exosomal proteomic profile reveals a role on placenta glucose metabolism in gestational diabetes mellitus. The Journal of Clinical Endocrinology & Metabolism, 104(5), 1735–1752. [DOI] [PubMed] [Google Scholar]
- Jia, L. , Zhou, X. , Huang, X. , Xu, X. , Jia, Y. , Wu, Y. , Yao, J. , Wu, Y. , & Wang, K. (2018). Maternal and umbilical cord serum‐derived exosomes enhance endothelial cell proliferation and migration. FASEB Journal, 32(8), 4534–4543. [DOI] [PubMed] [Google Scholar]
- Jiao, X. , Fan, Z. , Chen, H. , He, P. , Li, Y. , Zhang, Q. , & Ke, C. (2017). Serum and exosomal miR‐122 and miR‐199a as a biomarker to predict therapeutic efficacy of hepatitis C patients. Journal of Medical Virology, 89(9), 1597–1605. [DOI] [PubMed] [Google Scholar]
- Jin, J. , & Menon, R. (2018). Placental exosomes: A proxy to understand pregnancy complications. American Journal of Reproductive Immunology, 79(5), e12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra, H. , Drummen, G. P. , & Mathivanan, S. (2016). Focus on extracellular vesicles: Introducing the next small big thing. International Journal of Molecular Sciences, 17(2), 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandzija, N. , Zhang, W. , Motta‐Mejia, C. , Mhlomi, V. , McGowan‐Downey, J. , James, T. , Cerdeira, A. S. , Tannetta, D. , Sargent, I. , Redman, C. W. , Bastie, C. C. , & Vatish, M. (2019). Placental extracellular vesicles express active dipeptidyl peptidase IV; levels are increased in gestational diabetes mellitus. Journal of Extracellular Vesicles, 8(1), 1617000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur, A. , McIntyre, H. D. , Divakar, H. , Di Renzo, G. C. , Kihara, A. B. , McAuliffe, F. , Hanson, M. , Ma, R. C. , & Hod, M. & Pregnancy FWGoHi (2020). Towards a global consensus on GDM diagnosis: Light at the end of the tunnel? International Journal of Gynaecology and Obstetrics, 149(3), 257–261. [DOI] [PubMed] [Google Scholar]
- Kennedy, M. , Cartland, S. , Saravanan, P. , Simpson, N. , Scott, E. , & Forbes, K. (2019). miR‐1‐3p and miR‐133‐3p are altered in maternal serum EVs and placenta in pregnancies complicated by gestational diabetes with large‐for‐gestational age babies. Endocrine Abstracts, 65, P349. [Google Scholar]
- Keshtkar, S. , Azarpira, N. , & Ghahremani, M. H. (2018). Mesenchymal stem cell‐derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Research and Therapy, 9(1), 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdom, J. , Huppertz, B. , Seaward, G. , & Kaufmann, P. (2000). Development of the placental villous tree and its consequences for fetal growth. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 92(1), 35–43. [DOI] [PubMed] [Google Scholar]
- Kohli, S. , Ranjan, S. , Hoffmann, J. , Kashif, M. , Daniel, E. A. , Al‐Dabet, M. M. , Bock, F. , Nazir, S. , Huebner, H. , Mertens, P. R. , Fischer, K. D. , Zenclussen, A. C. , Offermanns, S. , Aharon, A. , Brenner, B. , Shahzad, K. , Ruebner, M. , & Isermann, B. (2016). Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood, 128(17), 2153–2164. [DOI] [PubMed] [Google Scholar]
- Kupper, N. , & Huppertz, B. (2022). The endogenous exposome of the pregnant mother: Placental extracellular vesicles and their effect on the maternal system. Molecular Aspects of Medicine, 87, 100955. [DOI] [PubMed] [Google Scholar]
- Lancaster, G. I. , & Febbraio, M. A. (2005). Exosome‐dependent trafficking of HSP70: A novel secretory pathway for cellular stress proteins. Journal of Biological Chemistry, 280(24), 23349–23355. [DOI] [PubMed] [Google Scholar]
- Lee, Y.‐T. , Tran, B. V. , Wang, J. J. , Liang, I. Y. , You, S. , Zhu, Y. , Agopian, V. G. , Tseng, H.‐R. , & Yang, J. D. (2021). The role of extracellular vesicles in disease progression and detection of hepatocellular carcinoma. Cancers, 13(12), 3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees, C. , Marlow, N. , Arabin, B. , Bilardo, C. M. , Brezinka, C. , Derks, J. B. , Duvekot, J. , Frusca, T. , Diemert, A. , Ferrazzi, E. , Ganzevoort, W. , Hecher, K. , Martinelli, P. , Ostermayer, E. , Papageorghiou, A. T. , Schlembach, D. , Schneider, K. T. M. , Thilaganathan, B. , Toos, T. , …, & Wolf, H. (2013). Perinatal morbidity and mortality in early‐onset fetal growth restriction: Cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound in Obstetrics & Gynecology, 42(4), 400–408. [DOI] [PubMed] [Google Scholar]
- Leon, J. , Acurio, J. , Bergman, L. , Lopez, J. , Karin Wikstrom, A. , Torres‐Vergara, P. , Troncoso, F. , Castro, F. O. , Vatish, M. , & Escudero, C. (2021). Disruption of the Blood‐brain barrier by extracellular vesicles from preeclampsia plasma and hypoxic placentae: Attenuation by magnesium sulfate. Hypertension, 78(5), 1423–1433. [DOI] [PubMed] [Google Scholar]
- Li, H. , Ouyang, Y. , Sadovsky, E. , Parks, W. T. , Chu, T. , & Sadovsky, Y. (2020). Unique microRNA signals in plasma exosomes from pregnancies complicated by preeclampsia. Hypertension, 75(3), 762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T. , Greenblatt, E. M. , Shin, M. E. , Brown, T. J. , & Chan, C. (2021). Cargo small non‐coding RNAs of extracellular vesicles isolated from uterine fluid associate with endometrial receptivity and implantation success. Fertility and Sterility, 115(5), 1327–1336. [DOI] [PubMed] [Google Scholar]
- Lisonkova, S. , & Joseph, K. (2013). Incidence of preeclampsia: Risk factors and outcomes associated with early‐ versus late‐onset disease. American Journal of Obstetrics and Gynecology, 209(6), 544.e1–544.e12. [DOI] [PubMed] [Google Scholar]
- Lotvall, J. , & Valadi, H. (2007). Cell to cell signalling via exosomes through esRNA. Cell Adhesion & Migration, 1(3), 156–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack, L. R. , & Tomich, P. G. (2017). Gestational diabetes: Diagnosis, classification, and clinical care. Obstetrics and Gynecology Clinics of North America, 44(2), 207–217. [DOI] [PubMed] [Google Scholar]
- Malhotra, A. , Allison, B. J. , Castillo‐Melendez, M. , Jenkin, G. , Polglase, G. R. , & Miller, S. L. (2019). Neonatal morbidities of fetal growth restriction: Pathophysiology and impact. Frontiers in Endocrinology, 10, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manier, S. , Liu, C. J. , Avet‐Loiseau, H. , Park, J. , Shi, J. , Campigotto, F. , Salem, K. Z. , Huynh, D. , Glavey, S. V. , Rivotto, B. , Sacco, A. , Roccaro, A. M. , Bouyssou, J. , Minvielle, S. , Moreau, P. , Facon, T. , Leleu, X. , Weller, E. , Trippa, L. , & Ghobrial, I. M. (2017). Prognostic role of circulating exosomal miRNAs in multiple myeloma. Blood, 129(17), 2429–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath, T. F. , Cantonwine, D. E. , Jeyabalan, A. , Doss, R. C. , Page, G. , Roberts, J. M. , Brohman, B. , Zhang, Z. , & Rosenblatt, K. P. (2019). Circulating microparticle proteins obtained in the late first trimester predict spontaneous preterm birth at less than 35 weeks' gestation: A panel validation with specific characterization by parity. American Journal of Obstetrics and Gynecology, 220(5), 488.e1–488.e11. [DOI] [PubMed] [Google Scholar]
- McVey, M. J. , & Kuebler, W. M. (2018). Extracellular vesicles: Biomarkers and regulators of vascular function during extracorporeal circulation. Oncotarget, 9(98), 37229–37251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, R. , Debnath, C. , Lai, A. , Guanzon, D. , Bhatnagar, S. , Kshetrapal, P. , Sheller‐Miller, S. , & Salomon, C. (2020). Protein profile changes in circulating placental extracellular vesicles in term and preterm births: A longitudinal study. Endocrinology, 161(4), bqaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, R. , Debnath, C. , Lai, A. , Guanzon, D. , Bhatnagar, S. , Kshetrapal, P. K. , Sheller‐Miller, S. , & Salomon, C. (2019). Circulating exosomal miRNA profile during term and preterm birth pregnancies: A longitudinal study. Endocrinology, 160(2), 249–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, R. , Dixon, C. L. , Sheller‐Miller, S. , Fortunato, S. J. , Saade, G. R. , Palma, C. , Lai, A. , Guanzon, D. , & Salomon, C. (2019). Quantitative Proteomics by SWATH‐MS of Maternal Plasma Exosomes Determine Pathways Associated With Term and Preterm Birth. Endocrinology, 160(3), 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino‐Gonzalez, C. , Zuniga, F. A. , Escudero, C. , Ormazabal, V. , Reyes, C. , Nova‐Lamperti, E. , Salomon, C. , & Aguayo, C. (2016). Mesenchymal stem Cell‐derived extracellular vesicles promote angiogenesis: Potencial clinical application. Frontiers in Physiology, 7, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda, J. , Paules, C. , Nair, S. , Lai, A. , Palma, C. , Scholz‐Romero, K. , Rice, G. E. , Gratacos, E. , Crispi, F. , & Salomon, C. (2018a). Placental exosomes profile in maternal and fetal circulation in intrauterine growth restriction ‐ Liquid biopsies to monitoring fetal growth. Placenta, 64, 34–43. [DOI] [PubMed] [Google Scholar]
- Miranda, J. , Paules, C. , Nair, S. , Lai, A. , Palma, C. , Scholz‐Romero, K. , Rice, G. E. , Gratacos, E. , Crispi, F. , & Salomon, C. (2018b). Placental exosomes profile in maternal and fetal circulation in intrauterine growth restriction ‐ Liquid biopsies to monitoring fetal growth. Placenta, 64, 34–43. [DOI] [PubMed] [Google Scholar]
- Mishra, A. , Ashary, N. , Sharma, R. , & Modi, D. (2021). Extracellular vesicles in embryo implantation and disorders of the endometrium. American Journal of Reproductive Immunology, 85(2), e13360. [DOI] [PubMed] [Google Scholar]
- Morelli, A. E. , & Sadovsky, Y. (2022). Extracellular vesicles and immune response during pregnancy: A balancing act. Immunological Reviews, 308(1), 105–122, [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (2015). (updated 2020) Nice Guideline. Diabetes in pregnancy: management from preconception to the postnatal period. National Institute for Health and Care Excellence. [PubMed] [Google Scholar]
- Out, H. J. , Kooijman, C. D. , Bruinse, H. W. , & Derksen, R. H. (1991). Histopathological findings in placentae from patients with intra‐uterine fetal death and anti‐phospholipid antibodies. European Journal of Obstetrics & Gynecology and Reproductive Biology, 41(3), 179–186. [DOI] [PubMed] [Google Scholar]
- Palma, C. , McIntyre, H. D. , & Salomon, C. (2022). Extracellular Vesicles‐new players in Cell‐to‐Cell communication in gestational diabetes mellitus. Biomedicines, 10(2), 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomares, K. T. , Parobchak, N. , Ithier, M. C. , Aleksunes, L. M. , Castano, P. M. , So, M. , Faro, R. , Heller, D. , Wang, B. , & Rosen, T. (2021). Fetal exosomal Platelet‐activating factor triggers functional progesterone withdrawal in human placenta. Reproductive Sciences, 28(1), 252–262. [DOI] [PubMed] [Google Scholar]
- Paquette, A. G. , Chu, T. , Wu, X. , Wang, K. , Price, N. D. , & Sadovsky, Y. (2018). Distinct communication patterns of trophoblastic miRNA among the maternal‐placental‐fetal compartments. Placenta, 72–73, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton, A. L. , McCallie, B. , Parks, J. C. , Schoolcraft, W. B. , & Katz‐Jaffe, M. (2015). Exosome bound microRNAs transcriptionally regulate embryo‐endometrial dialogue impacting implantation potential for AMA patients. Fertility and Sterility, 104(3), e308–e308. [Google Scholar]
- Pezzana, C. , Agnely, F. , Bochot, A. , Siepmann, J. , & Menasché, P. (2021). Extracellular vesicles and biomaterial design: New therapies for cardiac repair. Trends in Molecular Medicine, 27(3), 231–247. [DOI] [PubMed] [Google Scholar]
- Powell, J. S. , Gandley, R. E. , Lackner, E. , Dolinish, A. , Ouyang, Y. , Powers, R. W. , Morelli, A. E. , Hubel, C. A. , & Sadovsky, Y. (2022). Small extracellular vesicles from plasma of women with preeclampsia increase myogenic tone and decrease endothelium‐dependent relaxation of mouse mesenteric arteries. Pregnancy Hypertension, 28, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilang, R. , Godinho, E. , Timms, K. , Scott, E. M. , & Forbes, K. (2022). ODP434 Maternally‐derived pancreatic extracellular vesicle encompassed miRNAs influence placental development in pregnancies complicated by gestational diabetes. Journal of the Endocrine Society, 6(Supplement_1), A671–A672. [Google Scholar]
- Quintanilla Rodriguez, B. S. , Mahdy H. Gestational Diabetes. (2022). Sep 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; [2023 Jan]. [PubMed] [Google Scholar]
- Redman, C. W. , Tannetta, D. S. , Dragovic, R. A. , Gardiner, C. , Southcombe, J. H. , Collett, G. P. , & Sargent, I. L. (2012). Review: Does size matter? Placental debris and the pathophysiology of pre‐eclampsia. Placenta, 33(Suppl), S48–S54. [DOI] [PubMed] [Google Scholar]
- Redman, C. W. G. , & Sargent, I. L. (2008). Circulating microparticles in normal pregnancy and Pre‐Eclampsia. Placenta, 29(Suppl A), 73–77. [DOI] [PubMed] [Google Scholar]
- Sáez, T. , de Vos, P. , Sobrevia, L. , & Faas, M. M. (2018). Is there a role for exosomes in foetoplacental endothelial dysfunction in gestational diabetes mellitus? Placenta, 61, 48–54. [DOI] [PubMed] [Google Scholar]
- Saheera, S. , Jani, V. P. , Witwer, K. W. , & Kutty, S. (2021). Extracellular vesicle interplay in cardiovascular pathophysiology. American Journal of Physiology‐Heart and Circulatory Physiology, 320(5), H1749–H1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon, C. , Scholz‐Romero, K. , Kobayashi, M. , Smith, M. , Duncombe, G. , llanes, S. , Mitchell, M. D. , & Rice, G. E. (2015). Oxygen tension regulates glucose‐induced biogenesis and release of different subpopulations of exosome vesicles from trophoblast cells: A gestational age profile of placental exosomes in maternal plasma with gestational diabetes mellitus. Placenta, 36(4), 488–488. [Google Scholar]
- Salomon, C. , Scholz‐Romero, K. , Sarker, S. , Sweeney, E. , Kobayashi, M. , Correa, P. , Longo, S. , Duncombe, G. , Mitchell, M. D. , Rice, G. E. , & Illanes, S. E. (2016). Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placenta‐derived exosomes in maternal circulation across gestation. Diabetes, 65(3), 598–609. [DOI] [PubMed] [Google Scholar]
- Salomon, C. , Torres, M. J. , Kobayashi, M. , Scholz‐Romero, K. , Sobrevia, L. , Dobierzewska, A. , Illanes, S. E. , Mitchell, M. D. , & Rice, G. E. (2014). A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS ONE, 9(6), e98667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon, C. , Yee, S. , Scholz‐Romero, K. , Kobayashi, M. , Vaswani, K. , Kvaskoff, D. , Illanes, S. E. , Mitchell, M. D. , & Rice, G. E. (2014). Extravillous trophoblast cells‐derived exosomes promote vascular smooth muscle cell migration. Frontiers in Pharmacology, 5, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, A. , Sharkey, D. J. , Robertson, S. A. , & Zenclussen, A. C. (2018). Immune cells at the fetomaternal interface: How the microenvironment modulates immune cells to foster fetal development. Journal of Immunology, 201(2), 325–334. [DOI] [PubMed] [Google Scholar]
- Sheller‐Miller, S. , Trivedi, J. , Yellon, S. M. , & Menon, R. (2019). Exosomes cause preterm birth in mice: Evidence for paracrine signaling in pregnancy. Scientific Reports, 9(1), 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone, P. , Bologna, G. , Lanuti, P. , Pierdomenico, L. , Guagnano, M. T. , Pieragostino, D. , Del Boccio, P. , Vergara, D. , Marchisio, M. , Miscia, S. , & Mariani‐Costantini, R. (2020). Extracellular vesicles as signaling mediators and disease biomarkers across biological barriers. International Journal of Molecular Sciences, 21(7), 2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu, N. , Zonda, R. , Petrovici, A. R. , & Georgescu, A. (2021). The multifaceted role of extracellular vesicles in glioblastoma: microRNA nanocarriers for disease progression and gene therapy. Pharmaceutics, 13(7), 988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotland, T. , Sandvig, K. , & Llorente, A. (2017). Lipids in exosomes: Current knowledge and the way forward. Progress in Lipid Research, 66, 30–41. [DOI] [PubMed] [Google Scholar]
- Tannetta, D. , Collett, G. , Vatish, M. , Redman, C. , & Sargent, I. (2017). Syncytiotrophoblast extracellular vesicles ‐ Circulating biopsies reflecting placental health. Placenta, 52, 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry, C. , Witwer, K. W. , Aikawa, E. , Andriantsitohaina, R. , Baharvand, H. , Bauer, N. N. , Baxter, A. A. , Beckham, C. , Bielska, E. , Boireau, W. , Bongiovanni, A. , Brisson, A. , Broekman, M. L. D. , Bryl‐Górecka, P. , Buch, S. , Bussolati, B. , Caruso, S. , Clayton, A. , Cocucci, E. , …, & Zickler, A. M. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7, 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms, K. , Holder, B. , Day, A. , Mclaughlin, J. , Forbes, K. A. , & Westwood, M. (2022). Watermelon‐derived extracellular vesicles influence human ex vivo placental cell behavior by altering intestinal secretions. Molecular Nutrition & Food Research, 66(19), 2200013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, M. , & Chamley, L. W. (2015). Placental extracellular vesicles and feto‐maternal communication. Cold Spring Harbor Perspectives in Medicine, 5(3), a023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, M. , Stanley, J. L. , Chen, Q. , James, J. L. , Stone, P. R. , & Chamley, L. W. (2017). Placental Nano‐vesicles target to specific organs and modulate vascular tone in vivo. Human Reproduction, 32(11), 2188–2198. [DOI] [PubMed] [Google Scholar]
- Truong, G. , Guanzon, D. , Kinhal, V. , Elfeky, O. , Lai, A. , Longo, S. , Nuzhat, Z. , Palma, C. , Scholz‐Romero, K. , Menon, R. , Mol, B. W. , Rice, G. E. , & Salomon, C. (2017). Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells ‐ Liquid biopsies for monitoring complications of pregnancy. PLoS ONE, 12(3), e0174514–e0174514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umapathy, A. , Chamley, L. W. , & James, J. L. (2019). Reconciling the distinct roles of angiogenic/anti‐angiogenic factors in the placenta and maternal circulation of normal and pathological pregnancies. Angiogenesis, 23(2), 105–117. [DOI] [PubMed] [Google Scholar]
- Valadi, H. , Ekström, K. , Bossios, A. , Sjöstrand, M. , Lee, J. J. , & Lötvall, J. O. (2007). Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology, 9(6), 654–659. [DOI] [PubMed] [Google Scholar]
- Warrander, L. K. , Batra, G. , Bernatavicius, G. , Greenwood, S. L. , Dutton, P. , Jones, R. L. , Sibley, C. P. , & Heazell, A. E. (2012). Maternal perception of reduced fetal movements is associated with altered placental structure and function. PLoS ONE, 7(4), e34851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman, G. , Julian, L. , & Olson, M. F. (2012). How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death and Differentiation, 19(5), 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, P. J. , Bulmer, J. N. , Searle, R. F. , Innes, B. A. , & Robson, S. C. (2009). Altered decidual leucocyte populations in the placental bed in pre‐eclampsia and foetal growth restriction: A comparison with late normal pregnancy. Reproduction, 138(1), 177–184. [DOI] [PubMed] [Google Scholar]
- Xu, R. , Rai, A. , Chen, M. , Suwakulsiri, W. , Greening, D. W. , & Simpson, R. J. (2018). Extracellular vesicles in cancer–implications for future improvements in cancer care. Nature Reviews Clinical Oncology, 15(10), 617–638. [DOI] [PubMed] [Google Scholar]
- Yadava, S. M. , Feng, A. , Parobchak, N. , Wang, B. , & Rosen, T. (2021). miR‐15b‐5p promotes expression of proinflammatory cytokines in human placenta by inhibiting Apelin signaling pathway. Placenta, 104, 8–15. [DOI] [PubMed] [Google Scholar]
- Yáñez‐Mó, M. , Siljander, P. R. M. , Andreu, Z. , Bedina Zavec, A. , Borràs, F. E. , Buzas, E. I. , Buzas, K. , Casal, E. , Cappello, F. , Carvalho, J. , Colás, E. , Cordeiro‐da Silva, A. , Fais, S. , Falcon‐Perez, J. M. , Ghobrial, I. M. , Giebel, B. , Gimona, M. , Graner, M. , Gursel, I. , …, De Wever, O. (2015). Biological properties of extracellular vesicles and their physiological functions. Journal of Extracellular Vesicles, 4(1), 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, H. F. , Yan, S. , & Wu, S. F. (2017). MicroRNA‐153‐3p suppress cell proliferation and invasion by targeting SNAI1 in melanoma. Biochemical and Biophysical Research Communications, 487(1), 140–145. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Li, H. , Fan, B. , Xu, W. , & Zhang, X. (2020). Extracellular vesicles in normal pregnancy and pregnancy‐related diseases. Journal of Cellular and Molecular Medicine, 24(8), 4377–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Q. , Ma, Z. , Wang, X. , Liang, M. , Wang, W. , Su, F. , Yang, H. , Gao, Y. , & Ren, Y. (2020). Lipidomic biomarkers of extracellular vesicles for the prediction of preterm birth in the early second trimester. Journal of Proteome Research, 19(10), 4104–4113. [DOI] [PubMed] [Google Scholar]
- Zhao, Y. , & Yang, G. (2021). Potential of extracellular vesicles in the Parkinson's disease – Pathological mediators and biomarkers. Neurochemistry International, 144, 104974–104974. [DOI] [PubMed] [Google Scholar]
- Zheng, S. , Shi, A. , Hill, S. , Grant, C. , Kokkinos, M. I. , Murthi, P. , Georgiou, H. M. , Brennecke, S. P. , & Kalionis, B. (2020). Decidual mesenchymal stem/stromal cell‐derived extracellular vesicles ameliorate endothelial cell proliferation, inflammation, and oxidative stress in a cell culture model of preeclampsia. Pregnancy Hypertension, 22, 37–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peer Review History


