Abstract
Previously isolated lysosomal alpha-glucosidase cDNA clones were ligated to full-length constructs for expression in vitro and in mammalian cells. One of these constructs (pSHAG1) did not code for functional enzyme, due to an arginine residue instead of a tryptophan residue at amino acid position 402. The mutation does not affect the rate of enzyme synthesis, but interferes with post-translational modification and intracellular transport of the acid alpha-glucosidase precursor. Using immunocytochemistry it is demonstrated that the mutant precursor traverses the endoplasmic reticulum and the Golgi complex, but does not reach the lysosomes. Pulse-chase experiments suggest premature degradation. The Trp-402-containing enzyme (encoded by construct pSHAG2) is processed properly, and has catalytic activity. A fraction of the enzyme is localized at the plasma membrane. It is hypothesized that membrane association of the acid alpha-glucosidase precursor, as demonstrated by Triton X-114 phase separation, is responsible for transport to this location. Transiently expressed acid alpha-glucosidase also enters the secretory pathway, since a catalytically active precursor is found in the culture medium. This precursor has the appropriate characteristics for use in enzyme replacement therapy. Efficient uptake via the mannose 6-phosphate receptor results in degradation of lysosomal glycogen in cultured fibroblasts and muscle cells from patients with glycogenosis type II.
Full text
PDF





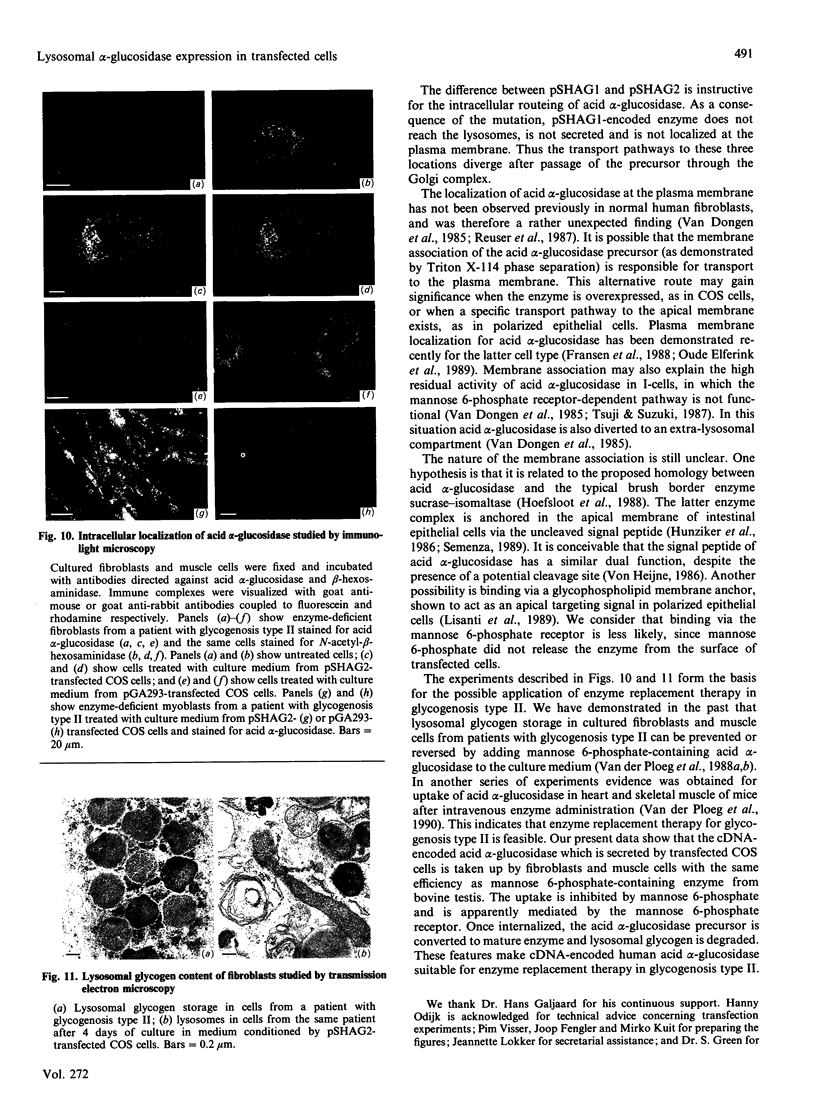

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Hidaka K., Siminovitch L. Expression of bacterial beta-galactosidase in animal cells. Mol Cell Biol. 1982 Dec;2(12):1628–1632. doi: 10.1128/mcb.2.12.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beratis N. G., LaBadie G. U., Hirschhorn K. Acid alpha-glucosidase: kinetic and immunologic properties of enzyme variants in health and disease. Isozymes Curr Top Biol Med Res. 1983;11:25–36. [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Fransen J. A., Ginsel L. A., Cambier P. H., Klumperman J., Oude Elferink R. P., Tager J. M. Immunocytochemical demonstration of the lysosomal enzyme alpha-glucosidase in the brush border of human intestinal epithelial cells. Eur J Cell Biol. 1988 Oct;47(1):72–80. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERS H. G. alpha-Glucosidase deficiency in generalized glycogenstorage disease (Pompe's disease). Biochem J. 1963 Jan;86:11–16. doi: 10.1042/bj0860011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980 May 25;255(10):4946–4950. [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hoefsloot L. H., Hoogeveen-Westerveld M., Kroos M. A., van Beeumen J., Reuser A. J., Oostra B. A. Primary structure and processing of lysosomal alpha-glucosidase; homology with the intestinal sucrase-isomaltase complex. EMBO J. 1988 Jun;7(6):1697–1704. doi: 10.1002/j.1460-2075.1988.tb02998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W., Spiess M., Semenza G., Lodish H. F. The sucrase-isomaltase complex: primary structure, membrane-orientation, and evolution of a stalked, intrinsic brush border protein. Cell. 1986 Jul 18;46(2):227–234. doi: 10.1016/0092-8674(86)90739-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Caras I. W., Davitz M. A., Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989 Nov;109(5):2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima A., Nolan C. M., Kyle J. W., Grubb J. H., Sly W. S. The human cation-independent mannose 6-phosphate receptor. Cloning and sequence of the full-length cDNA and expression of functional receptor in COS cells. J Biol Chem. 1988 Feb 15;263(5):2553–2562. [PubMed] [Google Scholar]
- Oude Elferink R. P., Fransen J., Klumperman J., Ginsel L. A., Tager J. M. Secretion of a precursor form of lysosomal alpha-glucosidase from the brush border of human kidney proximal tubule cells. Eur J Cell Biol. 1989 Dec;50(2):299–303. [PubMed] [Google Scholar]
- Oude Elferink R. P., Van Doorn-Van Wakeren J., Strijland A., Reuser A. J., Tager J. M. Biosynthesis and intracellular transport of alpha-glucosidase and cathepsin D in normal and mutant human fibroblasts. Eur J Biochem. 1985 Nov 15;153(1):55–63. doi: 10.1111/j.1432-1033.1985.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Reuser A. J., Kroos M., Oude Elferink R. P., Tager J. M. Defects in synthesis, phosphorylation, and maturation of acid alpha-glucosidase in glycogenosis type II. J Biol Chem. 1985 Jul 15;260(14):8336–8341. [PubMed] [Google Scholar]
- Reuser A. J., Kroos M., Willemsen R., Swallow D., Tager J. M., Galjaard H. Clinical diversity in glycogenosis type II. Biosynthesis and in situ localization of acid alpha-glucosidase in mutant fibroblasts. J Clin Invest. 1987 Jun;79(6):1689–1699. doi: 10.1172/JCI113008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld E. L. Alpha-glucosidases (gamma-amylases) in human and animal organisms. Pathol Biol (Paris) 1975 Jan;23(1):71–84. [PubMed] [Google Scholar]
- Semenza G. The J.E. Purkyne lecture: the insertion of stalked proteins of the brush border membranes: the state of the art in 1988. Biochem Int. 1989 Jan;18(1):15–33. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji A., Suzuki Y. The precursor of acid alpha-glucosidase is synthesized as a membrane-bound enzyme. Biochem Int. 1987 Nov;15(5):945–952. [PubMed] [Google Scholar]
- Van Dongen J. M., Barneveld R. A., Geuze H. J., Galjaard H. Immunocytochemistry of lysosomal hydrolases and their precursor forms in normal and mutant human cells. Histochem J. 1984 Sep;16(9):941–954. doi: 10.1007/BF01003850. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg A. T., Loonen M. C., Bolhuis P. A., Busch H. M., Reuser A. J., Galjaard H. Receptor-mediated uptake of acid alpha-glucosidase corrects lysosomal glycogen storage in cultured skeletal muscle. Pediatr Res. 1988 Jul;24(1):90–94. doi: 10.1203/00006450-198807000-00021. [DOI] [PubMed] [Google Scholar]
- de Bruijn W. C. Glycogen, its chemistry and morphologic appearance in the electron microscope. I. A modified OsO 4 fixative which selectively contrasts glycogen. J Ultrastruct Res. 1973 Jan;42(1):29–50. doi: 10.1016/s0022-5320(73)80004-8. [DOI] [PubMed] [Google Scholar]
- de Jonge A. J., de Smit S., Kroos M. A., Reuser A. J. Cotransfer of syntenic human genes into mouse cells using isolated metaphase chromosomes or cellular DNA. Hum Genet. 1985;69(1):32–38. doi: 10.1007/BF00295526. [DOI] [PubMed] [Google Scholar]
- van Dongen J. M., Willemsen R., Ginns E. I., Sips H. J., Tager J. M., Barranger J. A., Reuser A. J. The subcellular localization of soluble and membrane-bound lysosomal enzymes in I-cell fibroblasts: a comparative immunocytochemical study. Eur J Cell Biol. 1985 Nov;39(1):179–189. [PubMed] [Google Scholar]
- van der Horst G. T., Hoefsloot E. H., Kroos M. A., Reuser A. J. Cell-free translation of human lysosomal alpha-glucosidase: evidence for reduced precursor synthesis in an adult patient with glycogenosis type II. Biochim Biophys Acta. 1987 Nov 20;910(2):123–129. doi: 10.1016/0167-4781(87)90064-9. [DOI] [PubMed] [Google Scholar]
- van der Ploeg A. T., Bolhuis P. A., Wolterman R. A., Visser J. W., Loonen M. C., Busch H. F., Reuser A. J. Prospect for enzyme therapy in glycogenosis II variants: a study on cultured muscle cells. J Neurol. 1988 Sep;235(7):392–396. doi: 10.1007/BF00314479. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]