Abstract
Background
The objective of this study was to investigate the role of clinical factors together with FOXO1 fusion status in patients with nonmetastatic rhabdomyosarcoma (RMS) to develop a predictive model for event‐free survival and provide a rationale for risk stratification in future trials.
Methods
The authors used data from patients enrolled in the European Pediatric Soft Tissue Sarcoma Study Group (EpSSG) RMS 2005 study (EpSSG RMS 2005; EudraCT number 2005‐000217‐35). The following baseline variables were considered for the multivariable model: age at diagnosis, sex, histology, primary tumor site, Intergroup Rhabdomyosarcoma Studies group, tumor size, nodal status, and FOXO1 fusion status. Main effects and significant second‐order interactions of candidate predictors were included in a multiple Cox proportional hazards regression model. A nomogram was generated for predicting 5‐year event‐free survival (EFS) probabilities.
Results
The EFS and overall survival rates at 5 years were 70.9% (95% confidence interval, 68.6%–73.1%) and 81.0% (95% confidence interval, 78.9%–82.8%), respectively. The multivariable model retained five prognostic factors, including age at diagnosis interacting with tumor size, tumor primary site, Intergroup Rhabdomyosarcoma Studies clinical group, and FOXO1 fusion status. Based on each patient's total score in the nomogram, patients were stratified into four groups. The 5‐year EFS rates were 94.1%, 78.4%, 65.2%, and 52.1% in the low‐risk, intermediate‐risk, high‐risk, and very‐high‐risk groups, respectively, and the corresponding 5‐year overall survival rates were 97.2%, 91.5%, 74.3%, and 60.8%, respectively.
Conclusions
The results presented here provide the rationale to modify the EpSSG stratification, with the most significant change represented by the replacement of histology with fusion status. This classification was adopted in the new international trial launched by the EpSSG.
Keywords: FOXO1 protein, human; nomograms; pediatrics; proportional hazards models; rhabdomyosarcoma; survival analysis
Short abstract
Traditional clinical factors, together with FOXO1 fusion status, in nonmetastatic rhabdomyosarcoma were investigated to develop a predictive model for event‐free survival and provide a rationale for risk stratification in future trials. The most important result was the replacement of histology with fusion status, and this model was used for patient stratification in the new European Pediatric Soft Tissue Sarcoma Study Group Frontline and Relapse Rhabdomyosarcoma trial (ClinicalTrials.gov identifier NCT04625907).
INTRODUCTION
The survival of patients with rhabdomyosarcoma (RMS) has improved in the past 30 years because of the application of a multimodality approach that includes chemotherapy with surgery and/or radiotherapy. Clinical trials coordinated by national and international cooperative groups have helped to refine the treatment and to identify the most active multidrug regimens through randomized studies. A major advance has been the capacity to tailor the treatment strategy according to a series of prognostic factors found to be associated with different levels of the risk of treatment failure. 1 , 2 , 3 The most powerful adverse risk factor for patients with RMS is the presence of metastases at diagnosis. In this group, the outcome tends to be much poorer, with only one third of patients surviving 3 years after diagnosis. 4 , 5
In the absence of metastatic dissemination, the search for prognostic factors is made difficult by the clinical and biologic heterogeneity of RMS: patients vary in age (with two peaks of incidence: those younger than 6 years and adolescents), the primary tumor arises in many different sites across the body, and its disease extent and involvement of nearby organs and lymph nodes show considerable variation, with consequences for accessibility to local therapy. Two main histologic subtypes are distinguished: embryonal RMS (70% of all RMS) and, with a poorer prognosis, alveolar RMS (ARMS; 20%–30% of all RMS), characterized by the presence of PAX3/7‐FOXO1 translocations.
All this information was used by the European Pediatric Soft Tissue Sarcoma Study Group (EpSSG) to elaborate a risk‐stratification system that has been used in the RMS 2005 protocol for nonmetastatic RMS. The EpSSG risk‐stratification system was based on six prognostic factors: histology, postsurgical stage according to Intergroup Rhabdomyosarcoma Studies (IRS) grouping, primary tumor site, nodal involvement, tumor size, and patient age, as reported in Table 1.
TABLE 1.
Risk grouping stratification and therapy in the European Pediatric Sarcoma Study Group Rhabdomyosarcoma 2005 study.
| Risk group | Subgroups | Pathology | Postsurgical stage (IRS group | Site | Node stage | Size and age | Chemotherapy | Delayed surgery | Radiation therapy |
|---|---|---|---|---|---|---|---|---|---|
| Low risk | A | Favorable | I | Any | N0 | Favorable | VA × 8 cycles | Not necessary | No |
| Standard risk | B | Favorable | I | Any | N0 | Unfavorable | IVA × 4 cycles + VA × 5 cycles | Not necessary | No |
| C | Favorable | II, III | Favorable | N0 | Any | IVA × 9 cycles or IVA × 5 cycles + VA × 4 cycles if radiotherapy | Yes, if not mutilating | Optional | |
| D | Favorable | II, III | Unfavorable | N0 | Favorable | IVA × 9 cycles | Yes, if not mutilating | Yes | |
| High risk | E | Favorable | II, III | Unfavorable | N0 | Unfavorable | IVA × 9 cycles vs. IVADo × 4 cycles + IVA × 5 cycles with or without maintenance × 6 cycles | Yes | Yes |
| F | Favorable | II, III | Any | N1 | Any | ||||
| G | Unfavorable | I, II, III | Any | N0 | Any | ||||
| Very high risk | H | Unfavorable | II, III | Any | N1 | Any | IVADo × 4 cycles + IVA × 5 cycles + maintenance × 6 cycles | Yes | Yes |
Note: Pathology (histology): Favorable indicates all embryonal cells, spindle cells, botryoid rhabdomyosarcoma (RMS); unfavorable, all alveolar RMS (including the solid‐alveolar variant). Postsurgical stage (according to the Intergroup Rhabdomyosarcoma Studies [IRS] grouping): Group I indicates primary complete resection (R0); group II, microscopic residual (R1) or primary complete resection but N1; group III, macroscopic residual (R2). Site: Favorable indicates orbit, genitourinary nonbladder prostate (i.e., paratesticular and vagina/uterus), and nonparameningeal head and neck; unfavorable, all other sites (parameningeal, extremities, genitourinary bladder‐prostate, and other sites). Node stage (according to the tumor‐node‐metastasis [TNM] classification): N0, no clinical or pathologic node involvement; N1, clinical or pathologic nodal involvement. Size and age: Favorable, tumor size (maximum dimension) ≤5cm and age younger than 10 years; unfavorable, all others (i.e., tumor size >5 cm or age 10 years and older).
Abbreviations: IVA, ifosfamide, vincristine, and dactinomycin; IVADo, ifosfamide, vincristine, and dactinomycin plus doxorubicin; VA, vincristine and dactinomycin.
Since the design of the RMS 2005 protocol, the association between PAX3/7‐FOXO1 translocation and poorer prognosis has been recognized, overruling the impact of histologic classification and leading to the replacement of histology by FOXO1 fusion status in the current risk‐stratification system used by the Children’s Oncology Group (COG) 3 and the new EpSSG Frontline and Relapse Rhabdomyosarcoma (FaR‐RMS) study (ClinicalTrials.gov identifier NCT04625907).
The objective of the current study presented was to investigate the role of clinical factors together with FOXO1 fusion status in patients with nonmetastatic RMS treated on the RMS 2005 protocol to develop a predictive model for event‐free survival (EFS) and provide a rationale for risk stratification in future trials.
MATERIALS AND METHODS
Patients and treatments
For this study, we used data from 1733 patients with nonmetastatic rhabdomyosarcoma who were enrolled in the EpSSG RMS 2005 study (EudraCT number: 2005‐000217‐35) from October 1, 2005, to December 31, 2016. The data cutoff for last follow‐up was November 15, 2022. Only patients who had complete data were eligible for the analyses. Because FOXO1 status was not always investigated a priori for patients who had favorable histology (botryoid, embryonal, spindle cells/leiomiomatous), it was assumed to be negative, and we performed a clinical and fixed imputation considering FOXO1 fusion status as negative for the 361 patients who had favorable histology RMS without FOXO1 fusion data. 6 Patients who had unfavorable histology (ARMS, solid alveolar, and not otherwise specified) without FOXO1 fusion data (n = 47) and those who had missing clinical data (n = 9 without a record of nodal involvement and n = 16 with missing tumor size) were excluded, yielding 1661 evaluable patients (see Table S1). Ninety‐four of 362 patients (26%) who had ARMS were fusion‐negative, a result that is consistent with the literature.
The protocol encouraged histology to be centrally reviewed, and 73% of patients had their diagnosis reviewed by a national reference pathologist and/or by the international EpSSG Pathology Panel. FOXO1 fusion status assessment was performed in different laboratories according to national arrangements. It was not mandatory, and treatment usually was given based on the histopathology diagnosis. The analysis presented here was performed according to the final diagnosis; i.e., the diagnosis reviewed centrally or, if this was missing, the local diagnosis.
Patients were assigned to one of the four RMS 2005 risk groups and were treated according to the protocol guidelines that were previously described in detail 7 , 8 , 9 , 10 and are summarized in Table 1. The protocol included two randomized trials for high‐risk patients that evaluated: (1) two regimens of chemotherapy in the first part of treatment: ifosfamide, vincristine, and dactinomycin plus doxorubicin (IVA) versus the IVA plus doxorubicin (IVADo) regimen (IVA plus doxorubicin 30 mg/m2 on days 1 and 2 in the initial four cycles of chemotherapy followed by five cycles of IVA); and (2) the addition of a maintenance treatment with low‐dose cyclophosphamide and vinorelbine for patients who were in clinical complete remission after initial standard treatment.
Delayed surgery and/or radiotherapy were planned after assessing tumor response to the three initial cycles of chemotherapy. When a residual mass was identified, surgical resection was encouraged if clear margins were achievable without organ or functional impairment. Marginal resection was acceptable at sites where complete resection was deemed unfeasible provided it was followed by radiotherapy. Radiotherapy was the only local treatment for patients who were not suitable for secondary surgery because of the tumor's location (i.e., parameningeal RMS). Radiotherapy doses were delivered according to histology, chemotherapy response, and surgical results: 41.4 grays (Gy) were given to patients who had ARMS in IRS group I or II, to patients in IRS group III who achieved complete remission after secondary surgery, and to patients with embryonal RMS who achieved complete remission with initial chemotherapy; and 50.4 Gy for patients with incomplete or unfeasible secondary resection. A boost of 5.4 Gy to the residual tumor was recommended for large tumors that responded poorly to chemotherapy. Radiotherapy to the involved lymph node sites was recommended at a dose of 41.4 Gy independent of histology and surgical resection. Treatment was delivered with megavoltage photons, one fraction per day, 5 days per week, with conventional fraction sizes of 1.8 Gy per day.
This study was approved by the ethics committees of the participating centers, and informed consent was obtained from all patients according to the Declaration of Helsinki.
Statistical analysis
The primary end point was EFS, which was assessed by the investigator at each center and defined as the time from the date of study enrollment to the date of the first event, including death from any cause, disease progression (for patients who never achieved complete tumor remission), relapse after previous complete remission, the appearance of a new tumor, or the time of the latest follow‐up.
The following baseline variables were considered for the multivariable model: age at diagnosis, sex, histology, primary tumor site, IRS group, tumor size, nodal status, and FOXO1 fusion status. For the purpose of this analysis, histology was maintained with its original clinical classification as either favorable (embryonal, spindle cell) or unfavorable (ARMS and not otherwise specified), whereas categorization for age, tumor size, and primary tumor site was re‐evaluated to confirm or to establish new groups with favorable and unfavorable prognoses. The treatment received was not included as an independent prognostic factor because it was administered according to clinical patient characteristics that were considered in the multivariable model.
No formal sample size was calculated because we used an event‐per‐candidate variable for the derivation of the model. 11 Patient characteristics were summarized as the median and interquartile range for continuous variables, or as the count and percentage for categorical variables. To evaluate the functional form of age and tumor size, these continuous variables were plotted against martingale residuals of a null Cox proportional hazards model. Cutoff values were determined based both on a visual evaluation of martingale residual distribution and on cutoff points corresponding to the most significant relation with the risk of event, estimated with a maximally selected log‐rank statistic for values between the 10% and 90% quantiles using the upper bound of the p value, as described by Hothorn and Lausen, 12 as well as on optimal equal‐hazard ratio method to discretize a continuous variable that has a U‐shaped relationship with log relative hazards in survival data. 13 The classification for primary tumor site was defined both on a visual evaluation of the martingale residual distribution and on a pairwise log‐rank test with Benjamini–Hochberg correction.
Median follow‐up was computed using the reverse Kaplan–Meier method. The occurrence of second‐order interactions was verified using a likelihood ratio test comparing models with and without the interaction terms.
Main effects and significant second‐order interactions of candidate predictors were included in a multiple Cox proportional hazards regression model. No deviation from the proportional hazards assumption was found by the test statistic of Grambsch and Therneau. 14 A backward elimination with the Akaike information criterion was applied for selecting all independent prognostic variables. A nomogram of the final reduced Cox regression model was generated for predicting 3‐year and 5‐year EFS probabilities. Model performance was evaluated by examining measures of discrimination and calibration. Discrimination (i.e., the ability of the model to differentiate between high‐risk and low‐risk patients) was calculated using the Harrell concordance (C) index, adjusted through 1000 bootstrap resamples. Bias‐corrected calibration plots at 3‐year and 5‐year EFS rates were produced by using a bootstrap procedure (1000 resamples) to account for consistency between observed and estimated survival probabilities.
Patients were stratified into four risk groups based on their individual score in the nomogram corresponding to optimal cutoff points, 12 and the log‐rank test was used to compare groups. The survival probabilities were estimated using the Kaplan–Meier method and were reported with their 95% confidence interval (CI), which was calculated according to log‐log transformation.
Statistical analyses were performed using R version 4.0.2 (R Foundation for Statistical Analysis) and the R packages rms, survival, survminer, and ggplot2.
RESULTS
Patient characteristics and outcome
Patient characteristics are summarized in Table 2. The median follow‐up was 6.3 years (interquartile range, 4.5–8.6 years). During follow‐up, 491 patients had an event, and 326 died. Overall, 245 (50%) locoregional relapses, 128 (26%) metastatic progressions, 84 (17%) progressive diseases, 24 (5%) second malignancies, five (1%) deaths from disease, and five (1%) failures because of toxicity were registered. The pattern of treatment failures appeared different across age groups, with higher local failures in younger children: 24% in patients younger than 3 years, 18% in the group aged 3–10 years, and 19% in the older patients (p = .0156; see Table S2).
TABLE 2.
Patient characteristics.
| Variable | Categories, No. (%) | Total, N = 1661 |
|---|---|---|
| Age at diagnosis, years | Median [Q1, Q3] | 5.3 [2.7, 10.1] |
| Sex | Female | 655 (39) |
| Male | 1006 (61) | |
| Histology | Favorable | 1286 (77) |
| Embryonal/botryoid | 1218 (73) | |
| Spindle cells | 68 (4) | |
| Unfavorable | 375 (23) | |
| Alveolar | 362 (22) | |
| Not otherwise specified | 13 (1) | |
| Tumor site | Extremities | 184 (11) |
| Bile ducts | 26 (2) | |
| Bladder/prostrate | 196 (12) | |
| Genitourinary–nonbladder/prostate | 322 (19) | |
| Head and neck–nonparameningeal | 159 (10) | |
| Parameningeal | 393 (24) | |
| Orbit | 179 (11) | |
| Other sites | 202 (12) | |
| IRS group | I | 204 (12) |
| II | 204 (12) | |
| III | 1253 (75) | |
| Tumor size | ≤5 cm | 815 (49) |
| >5 cm | 846 (51) | |
| Tumor size, cm a | Median [Q1, Q3] | 5.2 [3.4, 7.2] |
| Lymph node status | N0 | 1392 (84) |
| N1 | 269 (16) | |
| FOXO1 fusion status | Negative | 1393 (84) |
| Positive | 268 (16) |
Abbreviations: CI, confidence interval; HR, hazard ratio; IRS, Intergroup Rhabdomyosarcoma Studies; Q, quartile.
Data were available for 1445 patients.
The EFS and overall survival rates at 5 years were 71% (95% CI, 69–73 years) and 81% (95% CI, 79–83 years), respectively.
Continuous variable categorization
Considering age as a continuous variable, the risk of an event was greater for patients younger than 3 years, it decreased moving toward age 10 years, plateaued from ages 10 to 14 years, and was higher again in older patients (see Figure S1). Therefore, we categorized age at diagnosis as follows: younger than 3 years, 3–9 years, and 10 years or older.
When tumor size was considered as a continuous variable, the risk of an event increased when the greatest tumor dimension was >5 cm (see Figure S1). Similarly, the single estimated cutoff point that corresponded to the most significant relationship with outcome was 5 cm.
The analysis of the primary tumor site identified three groups with different risks of an event: (1) bile duct and genitourinary nonbladder/prostate sites; (2) orbit, head and neck nonparameningeal, and bladder/prostrate sites; and 3) extremity, parameningeal, and other sites (see Figure S1).
Multivariable model
Interactions involving age at diagnosis with tumor primary site, tumor size and FOXO1 fusion status, sex with tumor primary site, and IRS group with tumor primary site (see Table S3) were identified as significant and were included in the multivariable Cox regression model. A backward elimination procedure based on Akaike information criterion (6924.83) in the multivariable modeling retained five prognostic factors, including age at diagnosis interacting with tumor size, tumor primary site, IRS group, and FOXO1 fusion status (Table 3). The nomogram predicting 3‐year and 5‐year EFS is presented in Figure 1.
TABLE 3.
Multiple Cox regression model for event‐free survival and nomogram coefficients.
| Variable | E/No. | HR (95% CI) | p | HR (95%CI) after bootstrapping | p | Points | |
|---|---|---|---|---|---|---|---|
| Tumor size | ≤5 cm | ||||||
| Age at diagnosis birth to 3 years | 76/233 | 1.44 (1.06–1.97) | .0201 | 1.41 (1.04–1.92) | .0294 | 70 | |
| Age at diagnosis 3–10 years | 86/394 | Ref | 25 | ||||
| Age at diagnosis 10 years and older | 33/188 | 0.81 (0.54–1.22) | .3122 | 0.82 (0.55–1.23) | .3438 | 0 | |
| >5 cm | |||||||
| Age at diagnosis birth to 3 years | 76/234 | 1.15 (0.86–1.54) | .3381 | 57 | |||
| Age at diagnosis 3–10 years | 124/377 | Ref | 40 | ||||
| Age at diagnosis 10 years and older | 96/235 | 1.58 (1.19–2.09) | .0014 | 96 | |||
| Primary site | Extremities/HNPM/other sites | 312/779 | Ref | Ref | 75 | ||
| GUBP/HNnoPM/orbit | 125/534 | 0.60 (0.48–0.75) | < .0001 | 0.62 (0.49–0.77) | < .0001 | 12 | |
| GUnoBP/bile ducts | 54/348 | 0.54 (0.38–0.77) | .0006 | 0.56 (0.40–0.80) | .0014 | 0 | |
| IRS group | I | 24/204 | Ref | Ref | 0 | ||
| II | 37/204 | 1.29 (0.76–2.21) | .3512 | 1.27 (0.74–2.17) | .3825 | 31 | |
| III | 430/1253 | 2.27 (1.41–3.66) | .0008 | 2.15 (1.33–3.48) | .0017 | 100 | |
| Fusion status | Negative | 367/1393 | Ref | Ref | 0 | ||
| Positive | 124/268 | 1.45 (1.16, 1.80) | .0011 | 1.41 (1.13–1.76) | .0022 | 45 | |
Abbreviations: CI, confidence interval; E, events; GUBP, genitourinary bladder‐prostate; HNnoPM, head and neck nonparameningeal; HNPM, head and neck parameningeal; HR, hazard ratio; IRS, Intergroup Rhabdomyosarcoma Studies; Ref, reference category.
FIGURE 1.
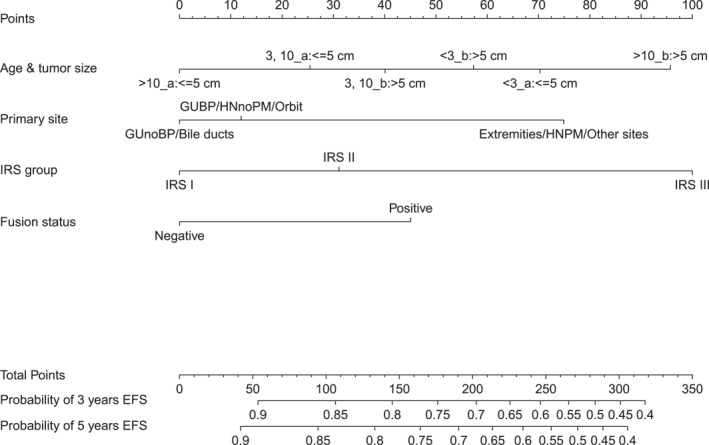
A nomogram for predicting 3‐year and 5‐year EFS probabilities. EFS indicates event‐free survival; GUBP, genitourinary bladder‐prostate; HNnoPM, head and neck nonparameningeal; IRS, Intergroup Rhabdomyosarcoma Studies Group.
The calibration plot for internal validation (see Figure S2) showed good agreement of 3‐year and 5‐year EFS probabilities between the estimated outcomes and actual observations. The C‐index was 0.66 in the original data, and the optimistic‐corrected C statistic with 1000 bootstrap replications was 0.65.
Based on each patient's total score in the nomogram, patients were stratified into four groups: a low‐risk group (176 of 1661 patients [11%]; total score, <68), an intermediate‐risk group (701 of 1661 patients [42%]; total score ≥68 and total score <182.4), a high‐risk group (423 of 1661 patients [26%]; total score ≥182.4 and total score <232), and a very‐high‐risk group (361 of 1661 patients [22%]; total score, ≥232). The 5‐year EFS rates were 94%, 78%, 65%, and 52%, respectively, in the low‐risk, intermediate‐risk, high‐risk, and very‐high‐risk groups (Table 4 and Figure 2A); and the corresponding 5‐year overall survival rates were 97%, 92%, 74%, and 61% (Figure 2B).
TABLE 4.
Patients’ characteristics according to risk group.
| No. (%) | |||||
|---|---|---|---|---|---|
| Characteristic | Low risk, N = 176 | Medium risk, N = 701 | High risk, N = 423 | Very high risk, N = 361 | |
| Age at diagnosis | Birth to 3 years | 6 (3) | 256 (36) | 23 (5) | 182 (50) |
| 3–10 years | 102 (58) | 277 (40) | 331 (78) | 61 (17) | |
| 10 years and older | 68 (39) | 168 (24) | 69 (17) | 118 (33) | |
| Tumor size | ≤5 cm | 147 (83) | 438 (62) | 144 (34) | 86 (24) |
| >5 cm | 29 (17) | 263 (38) | 279 (66) | 275 (76) | |
| Primary site | Extremities/HNPM/other sites | — | 80 (11) | 346 (82) | 353 (98) |
| GUBP/HNnoPM/orbit | 21 (12) | 451 (64) | 54 (13) | 8 (2) | |
| GUnoBP/bile ducts | 155 (88) | 170 (24) | 23 (5) | — | |
| IRS group | I | 128 (73) | 73 (10) | 3 (1) | — |
| II | 48 (27) | 142 (20) | 12 (3) | 2 (1) | |
| III | — | 486 (69) | 408 (96) | 359 (99) | |
| Fusion status | Negative | 176 (100) | 661 (94) | 360 (85) | 196 (54) |
| Positive | 40 (6) | 63 (15) | 165 (46) | ||
| Age/tumor size | Birth to 3 years/>5 cm | 6 (3) | 112 (16) | 3 (1) | 113 (31) |
| Birth to 3 years/≤5 cm | — | 144 (20) | 20 (5) | 69 (19) | |
| 3–10 years/≤5 cm | 79 (45) | 194 (28) | 104 (25) | 17 (5) | |
| 3–10 years/>5 cm | 23 (13) | 83 (12) | 227 (54) | 44 (12) | |
| 10 years and older/≤5 cm | 68 (39) | 100 (14) | 20 (5) | — | |
| 10 years and older/>5 cm | — | 68 (10) | 49 (12) | 118 (33) | |
| EFS probability, % | 3 years [95% CI] | 94.1 [89.4–96.8] | 80.6 [77.4–83.3] | 68.4 [63.7–72.6] | 54.3 [48.9–59.3] |
| 5 years [95% CI] | 94.1 [89.4–96.8] | 78.4 [75.1–81.3] | 65.2 [60.4–69.6] | 52.1 [46.8–57.2] | |
| Type of event | Dead | — | 2 (1) | 3 (2) | — |
| Local‐regional | 6 (55) | 96 (64) | 66 (44) | 77 (43) | |
| Metastases progression | 4 (36) | 24 (16) | 40 (26) | 60 (34) | |
| Other | 1 (9) | 10 (7) | 13 (9) | 5 (3) | |
| Progressive disease | — | 19 (13) | 29 (19) | 36 (20) | |
| OS probability | 3 years [95% CI] | 98.8 [95.2–99.7] | 94.0 [92.0–95.6] | 78.7 [74.5–82.4] | 72.5 [67.5–76.8] |
| 5 years [95% CI] | 97.2 [92.7–99.0] | 91.5 [89.0–93.4] | 74.3 [69.7–78.3] | 60.8 [55.4–65.9] | |
Abbreviations: CI, confidence interval; EFS, event‐free survival; GUBP, genitourinary bladder‐prostate; HNnoPM, head and neck nonparameningeal; HNPM, head and neck parameningeal; IRS, Intergroup Rhabdomyosarcoma Studies; OS, overall survival.
FIGURE 2.
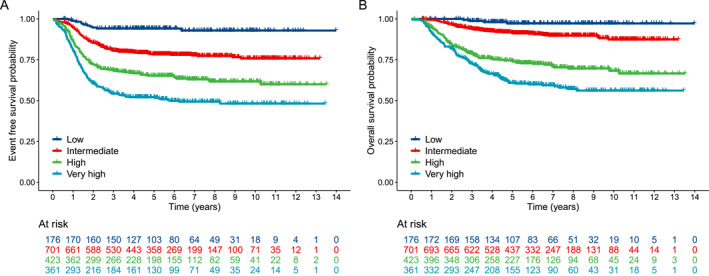
Kaplan–Meier curves for (A) event‐free survival and (B) overall survival stratified by risk group.
DISCUSSION
There is a continuous need to refine risk classification for pediatric tumors to confirm the prognostic variables used in the past and incorporate new findings as they are discovered and to help choose the best possible treatment for each patient. This analysis represents an effort to review the EpSSG classification, which has been in use since 2005 and has served as a basis for the current EpSSG FAR‐RMS trial. It is particularly important also to try to incorporate molecular findings into a classification system that, to date, has been based essentially on clinical factors.
A recent study validated the clinicopathologic factors used in the COG studies and confirmed that patients older than 10 years, unfavorable tumor site, and unfavorable tumor size (>5 cm) are associated with an inferior outcome. Clinical group, nodal involvement, and histology were also confirmed as prognostic factors. 3
The EpSSG adopted the same factors in the RMS 2005 study, but they were combined in a different way and determined a different treatment allocation for at least a proportion of patients with RMS. Our analysis confirms the prognostic value of most of the factors we used previously, but it also presents important new information.
The role of patient age as a prognostic variable is difficult to establish because the biologic characteristics and treatment modalities applied may change depending on age. 15 The unfavorable fusion‐positive ARMS is more common in older children, and the favorable spindle cell VGLL2/NCOA2‐positive RMS is typical of infants. Conversely, the treatment of younger children is challenging, and the RMS 2005 protocol recommended age–dose adaptation of chemotherapy for infants and a case‐by‐case discussion to decide on the use of radiotherapy in children younger than 3 years. Therefore, it is not surprising to find that children younger than 3 years have a relatively poorer prognosis. The relatively higher proportion of local failures in this group may be determined by difficulties with implementing an aggressive local treatment, and particularly radiotherapy, because of concerns about late sequelae.
The upper cutoff of 10 years and older is currently used both in EpSSG and COG trials, and it is confirmed by our analysis. Because the risk of failure is similar in patients aged 10–14 years, an age limit of 14 years could also be considered to identify patients at higher risk.
We confirmed that a tumor size of 5 cm in greatest dimension is the optimal cutoff to separate children with differing risk. This variable is easy to use, and it is not clear whether considering two or three dimensions of the tumor may be more appropriate (but it is certainly more complicated). 16
However, in our analysis, and as shown previously, age and tumor size outcomes were interdependent, confirming that older children who have large tumors represent the population at higher risk of treatment failure.
Compared with patients who have RMS arising in the extremities, parameningeal sites, or in the so‐called other sites, those located in the bladder/prostate and bile ducts had better outcomes. The latter sites were included in the unfavorable group in the RMS 2005 study; however, in light of the good results obtained in RMS 2005, we decided to move them into the more favorable standard‐risk group in the FaR‐RMS trial. For RMS arising in a biliary site, this is in contrast with the results presented by the COG, which recently decided to include biliary RMS in the unfavorable category because of the suboptimal outcomes of patients treated in low‐risk studies. 17 This difference may be explained in part by the different dose of alkylating agents administered to this group of patients in the EpSSG and COG studies and demonstrates the necessity of a common analysis and classification.
Clinical group has been identified as a major prognostic determinant since the initial cooperative studies on RMS. 2 and it has always retained its value.
FOXO1 fusion status has been identified as an independent prognostic factor in several retrospective studies. In a recent analysis published by the COG, only the presence of metastases surpassed FOXO1 fusion status as a prognostic factor. 6 This led the COG to include FOXO1 status in their stratification system. We present similar results in this report, further supporting the use of FOXO1 status rather than histology to assign treatment. The inclusion of PAX3/7‐FOXO1 fusion status in risk stratification in place of histology in the FaR‐RMS protocol represents a first attempt to include tumor molecular characteristics in a risk‐stratification system. Additional prognostic biologic factors have been identified in RMS. MYOD1 and TP53 mutations have been associated with a worse prognosis, whereas NCOA2/VGLL2‐associated gene fusions have a very good prognosis. The RMS 2005 study was not designed to collect these data in our population, so their inclusion in the EpSSG stratification system is under debate. These and other new biologic factors could have a very important role in stratifying patients.
The independent role of nodal involvement has been controversial. A lower survival rate has been reported both in patients with ARMS and in those with node‐positive embryonal RMS included in the RMS 2005 study. 8 , 18 It is possible, however, that other tumor characteristics may be more important when a more intensive treatment is adopted. In addition, the gradual introduction of more sensitive imaging methods like fluorodeoxyglucose‐positron emission tomography may have changed the evaluation of nodal involvement, possibly upstaging patients who have a lower tumor load and a better prognosis.
The impact of treatment has not been included in our model, and this represents a limitation of our study. However, treatment is determined by the risk group assigned to the patient on the basis of the initial disease and patient characteristics. Therefore, we mainly aimed to identify prognostic factors that can stratify patients at diagnosis.
In addition to identifying the role of different prognostic factors, the merit of this analysis is the production of a nomogram that may be used to calculate the prognosis for each patient based on currently known risk factors. This methodology is in use for adult patients with sarcoma. Validated nomograms can be used to predict overall survival and distant metastases in patients after surgical resection of soft tissue sarcoma of the extremities. 19 A nomogram has been developed to estimate the chance of salvage for individual children with relapsed RMS treated according to the International Society of Pediatric Oncology Malignant Mesenchymal Tumor protocols to direct therapy appropriately toward cure, use of experimental therapies, and/or palliation. 20
The nomogram we propose is based on a study analyzing d a large, international, multicenter population that was treated homogeneously and had data prospectively collected. It can be used at diagnosis to assist clinicians in guiding treatment. At the same time, it should be recognized that we have not yet undertaken an external validation, which could be facilitated through an international collaboration. Moreover, it should be noted that this analysis used data from a prospective study in which the assessment of fusion status was not mandatory.
In conclusion, the results presented provide the rationale to modify the EpSSG stratification for adoption in the current FaR‐RMS trial, confirming the adverse prognostic value of an age of 10 years and a tumor size of 5 cm. It also supports reconsideration of the role of primary tumor site. Bladder/prostate and biliary tree RMS are now included in the favorable category in the FaR‐RMS trial. The most significant change is probably represented by the replacement of histology with fusion status. This makes the EpSSG stratification more similar to that of the COG system and will facilitate data comparison in the future.
In the meantime, the international community has recognized the need to adopt a common stratification system. This represents the main goal of the recently established INSTRuCT consortium 21 and will help establishing a common language and, hopefully, risk stratification in RMS treatment and research.
AUTHOR CONTRIBUTIONS
Gian Luca De Salvo: Conceptualization, writing–original draft, writing–review and editing, and methodology. Paola Del Bianco: Conceptualization, methodology, formal analysis, writing–original draft, and writing–review and editing. Veronique Minard‐Colin: Conceptualization, investigation, funding acquisition, writing–review and editing, and data curation. Julia Chisholm: Conceptualization, data curation, investigation, funding acquisition, and writing–review and editing. Meriel Jenney: Conceptualization, data curation, investigation, writing–review and editing, and funding acquisition. Gabriela Guillen: Data curation and investigation. Christine Devalck: Data curation and investigation. Rick Van Rijn: Investigation. Janet Shipley: Investigation. Daniel Orbach: Data curation and investigation. Anna Kelsey: Investigation. Timothy Rogers: Investigation. Florent Guerin: Investigation. Giovanni Scarzello: Investigation. Andrea Ferrari: Investigation and data curation. Maja Cesen Mazic: Data curation and investigation. Johannes H. M. Merks: Conceptualization, data curation, investigation, and writing–review and editing. Gianni Bisogno: Conceptualization, data curation, investigation, writing–original draft, writing–review and editing, and funding acquisition.
CONFLICT OF INTEREST STATEMENT
Veronique Minard‐Colin reports grants from F. Hoffmann‐La Roche outside the submitted work. Julia Chisholm reports service on a Children's Oncology Group Data Safety and Monitoring Board and support for other professional activities from the National Cancer Institute outside the submitted work. Meriel Jenney reports personal/consulting fees from the Cardiff and Vale University Health Board outside the submitted work. Daniel Orbach reports personal/consulting fees from Bayer Healthcare, EUSA Pharma (US) LLC, and F. Hoffmann‐La Roche; and service on a Data Safety and Monitoring Board for Eli Lilly and Company outside the submitted work. Johannes H. M. Merks reports personal/consulting fees from Bayer, GlaxoSmithKline, and Merck outside the submitted work. Gianni Bisogno reports personal/consulting fees from GlaxoSmithKline and support for other professional activities from Bayer outside the submitted work. The remaining authors disclosed no conflicts of interest.
Supporting information
Supporting Information S1
Supporting Information S2
Figure S1
Figure S2
ACKNOWLEDGMENTS
This project was conducted thanks to the support of the KickCancer Foundation and the King Baudouin Foundation. The overall organization of the EpSSG RMS 2005 study was supported by the Fondazione Città della Speranza (Padua, Italy). In France, it was supported by Association Léon Berard Enfant Cancéreux (Grant 2005). In the United Kingdom, the study was supported by Cancer Research UK. Julia Chisholm is supported by the Giant Pledge through the Royal Marsden Cancer Charity, and this independent research is supported by the National Institute for Health Research Biomedical Research Center at the Royal Marsden National Health Service Foundation Trust and the Institute of Cancer Research, London. The views expressed are those of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care.
Open access funding provided by BIBLIOSAN.
De Salvo GL, Del Bianco P, Minard‐Colin V, et al. Reappraisal of prognostic factors used in the European Pediatric Soft Tissue Sarcoma Study Group RMS 2005 study for localized rhabdomyosarcoma to optimize risk stratification and generate a prognostic nomogram. Cancer. 2024;130(13):2351‐2360. doi: 10.1002/cncr.35258
The first two authors contributed equally to this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Rodary C, Flamant F, Donaldson SS. An attempt to use a common staging system in rhabdomyosarcoma: a report of an international workshop initiated by the International Society of Pediatric Oncology (SIOP). Med Pediatr Oncol. 1989;17(3):210‐215. doi: 10.1002/mpo.2950170308 [DOI] [PubMed] [Google Scholar]
- 2. Raney RB, Maurer HM, Anderson JR, et al. The Intergroup Rhabdomyosarcoma Study Group (IRSG): major lessons from the IRS‐I through IRS‐IV studies as background for the current IRS‐V treatment protocols. Sarcoma. 2001;5(1):9‐15. doi: 10.1080/13577140120048890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haduong JH, Heske CM, Allen‐Rhoades W, et al. An update on rhabdomyosarcoma risk stratification and the rationale for current and future Children's Oncology Group clinical trials. Pediatr Blood Cancer. 2022;69(4):e29511. doi: 10.1002/pbc.29511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oberlin O, Rey A, Lyden E, et al. Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J Clin Oncol. 2008;26(14):2384‐2389. doi: 10.1200/jco.2007.14.7207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schoot RA, Chisholm JC, Casanova M, et al. Metastatic rhabdomyosarcoma: results of the European Paediatric Soft Tissue Sarcoma Study Group MTS 2008 study and pooled analysis with the concurrent BERNIE study. J Clin Oncol. 2022;40(32):3730‐3740. doi: 10.1200/jco.21.02981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hibbitts E, Chi YY, Hawkins DS, et al. Refinement of risk stratification for childhood rhabdomyosarcoma using FOXO1 fusion status in addition to established clinical outcome predictors: a report from the Children's Oncology Group. Cancer Med. 2019;8(14):6437‐6448. doi: 10.1002/cam4.2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bergeron C, Jenney M, De Corti F, et al. Embryonal rhabdomyosarcoma completely resected at diagnosis: the European Paediatric Soft Tissue Sarcoma Study Group RMS2005 experience. Eur J Cancer. 2021;146:21‐29. doi: 10.1016/j.ejca.2020.12.025 [DOI] [PubMed] [Google Scholar]
- 8. Gallego S, Chi YY, De Salvo GL, et al. Alveolar rhabdomyosarcoma with regional nodal involvement: results of a combined analysis from two cooperative groups. Pediatr Blood Cancer. 2021;68(3):e28832. doi: 10.1002/pbc.28832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bisogno G, Jenney M, Bergeron C, et al. Addition of dose‐intensified doxorubicin to standard chemotherapy for rhabdomyosarcoma (EpSSG RMS 2005): a multicentre, open‐label, randomised controlled, phase 3 trial. Lancet Oncol. 2018;19(8):1061‐1071. doi: 10.1016/s1470-2045(18)30337-1 [DOI] [PubMed] [Google Scholar]
- 10. Bisogno G, De Salvo GL, Bergeron C, et al. Vinorelbine and continuous low‐dose cyclophosphamide as maintenance chemotherapy in patients with high‐risk rhabdomyosarcoma (RMS 2005): a multicentre, open‐label, randomised, phase 3 trial. Lancet Oncol. 2019;20(11):1566‐1575. doi: 10.1016/s1470-2045(19)30617-5 [DOI] [PubMed] [Google Scholar]
- 11. Moons KG, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1‐W73. doi: 10.7326/m14-0698 [DOI] [PubMed] [Google Scholar]
- 12. Hothorn T, Lausen B. On maximally selected rank statistics. R News. 2002;2(1):3‐5. [Google Scholar]
- 13. Chen Y, Huang J, He X, et al. A novel approach to determine two optimal cut‐points of a continuous predictor with a U‐shaped relationship to hazard ratio in survival data: simulation and application. BMC Med Res Methodol. 2019;19(1):96. doi: 10.1186/s12874-019-0738-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515‐526. [Google Scholar]
- 15. Joshi D, Anderson JR, Paidas C, Breneman J, Parham D, Crist W. Age is an independent prognostic factor in rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Pediatr Blood Cancer. 2004;42(1):64‐73. doi: 10.1002/pbc.10441 [DOI] [PubMed] [Google Scholar]
- 16. Ferrari A, Miceli R, Meazza C, et al. Comparison of the prognostic value of assessing tumor diameter versus tumor volume at diagnosis or in response to initial chemotherapy in rhabdomyosarcoma. J Clin Oncol. 2010;28(8):1322‐1328. doi: 10.1200/jco.2009.25.0803 [DOI] [PubMed] [Google Scholar]
- 17. Aye JM, Xue W, Palmer JD, et al. Suboptimal outcome for patients with biliary rhabdomyosarcoma treated on low‐risk clinical trials: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2021;68(4):e28914. doi: 10.1002/pbc.28914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ben‐Arush M, Minard‐Colin V, Scarzello G, et al. Therapy and prognostic significance of regional lymph node involvement in embryonal rhabdomyosarcoma: a report from the European Paediatric Soft Tissue Sarcoma Study Group. Eur J Cancer. 2022;172:119‐129. doi: 10.1016/j.ejca.2022.05.033 [DOI] [PubMed] [Google Scholar]
- 19. Callegaro D, Miceli R, Bonvalot S, et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft‐tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol. 2016;17(5):671‐680. doi: 10.1016/s1470-2045(16)00010-3 [DOI] [PubMed] [Google Scholar]
- 20. Chisholm JC, Marandet J, Rey A, et al. Prognostic factors after relapse in nonmetastatic rhabdomyosarcoma: a nomogram to better define patients who can be salvaged with further therapy. J Clin Oncol. 2011;29(10):1319‐1325. doi: 10.1200/jco.2010.32.1984 [DOI] [PubMed] [Google Scholar]
- 21. Hawkins DS, Bisogno G, Koscielniak E. Introducing INSTRuCT: an international effort to promote cooperation and data sharing. Pediatr Blood Cancer. 2023;70(3):e28701. doi: 10.1002/pbc.28701 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Supporting Information S2
Figure S1
Figure S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


