Abstract
Aims
We examined the efficacy and safety of dapagliflozin, compared with placebo, according to aetiology in patients with heart failure (HF) with reduced ejection fraction (HFrEF) enrolled in the Dapagliflozin And Prevention of Adverse‐outcomes in Heart Failure trial (DAPA‐HF).
Methods and results
Aetiology was investigator‐reported and categorized as ischaemic or non‐ischaemic. The primary outcome was the composite of an episode of worsening HF or cardiovascular death. A total of 4744 patients were randomized in DAPA‐HF, of whom 2674 (56.4%) patients had an ischaemic aetiology. Participants with an ischaemic aetiology had a higher risk of cardiovascular mortality [hazard ratio (HR) 1.35, 95% confidence interval (CI) 1.13–1.63], but lower risk of HF hospitalization (HR 0.83, 95% CI 0.70–0.98) than non‐ischaemic patients. Compared with placebo, dapagliflozin reduced the risk of worsening HF or cardiovascular death to a similar extent in both patients with ischaemic and non‐ischaemic aetiology (HR 0.77, 95% CI 0.65–0.92, and HR 0.71, 95% CI 0.58–0.87, respectively; P for interaction = 0.55). Consistent benefits were observed for the components of the primary outcome and all‐cause mortality. Dapagliflozin, as compared with placebo, increased the proportion of patients with an improvement of Kansas City Cardiomyopathy Questionnaire total symptom score (KCCQ‐TSS) of ≥5 points (P for interaction = 0.32) and decreased the proportion with a deterioration in KCCQ‐TSS of ≥5 points (P for interaction = 0.76), irrespective of aetiology. Study drug discontinuation and serious adverse events were similar according to treatment groups, irrespective of aetiology.
Conclusions
Dapagliflozin reduced the risk of worsening HF and death, and improved symptoms, similarly in patients with ischaemic and non‐ischaemic aetiology. In addition, dapagliflozin was safe and well‐tolerated, irrespective of aetiology.
Keywords: Heart failure, Dapagliflozin, Aetiology, Randomized controlled trial
Effects of dapagliflozin compared with placebo according to aetiology subgroups.
Introduction
Over the past decades, a global transition in the aetiology of heart failure (HF) with reduced ejection fraction (HFrEF) has occurred from hypertension and rheumatic valvular disease to coronary artery disease. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 Thus, in many parts of the world, coronary artery disease has become the most common cause of HFrEF. A distinction between ischaemic and non‐ischaemic aetiology is important for several reasons. First, certain evidence‐based therapies may be indicated in specific aetiologies, for example bypass graft surgery for coronary artery disease. 9 Second, HFrEF of ischaemic origin may portend a worse prognosis than HFrEF due to non‐ischaemic causes. 10 , 11 , 12 , 13 Third, the effectiveness of certain treatments for HFrEF may be modified by aetiology, for example that of the implantable cardioverter‐defibrillator, cardiac resynchronization therapy, and milrinone. 14 , 15 , 16 , 17 It is, therefore, important to evaluate the effectiveness of new HFrEF treatments in both patients with ischaemic and non‐ischaemic aetiology.
In the Dapagliflozin And Prevention of Adverse‐outcomes in Heart Failure trial (DAPA‐HF), the sodium–glucose co‐transporter 2 (SGLT2) inhibitor, dapagliflozin, added to conventional guideline‐recommended therapies reduced the risk of worsening HF events, cardiovascular death, and all‐cause mortality, and improved symptoms, in 4744 patients with HFrEF with and without type 2 diabetes. 18 The proposed improved myocardial energetics with SGLT2 inhibition may suggest more benefit in patients with coronary artery disease causing myocardial ischaemia. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 Likewise, any haemodynamic effects to improve loading conditions might also be more beneficial in a patient with a propensity to myocardial ischaemia. Other proposed mechanisms of action, e.g. improvement in microvascular function and reduced fibrosis, may be more important in patients with coronary disease (and patients with diabetes who are more likely to have coronary disease). 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 In this pre‐specified subgroup analysis of DAPA‐HF, we investigated the efficacy and safety of dapagliflozin compared with placebo according to investigator‐reported HF aetiology.
Methods
DAPA‐HF was a randomized, double‐blind, controlled trial in patients with HFrEF, evaluating the efficacy and safety of dapagliflozin 10 mg once daily compared with matching placebo, in addition to standard care. The design, baseline characteristics, and primary results of the trial are published. 18 , 27 The ethics committee of each of the 410 participating institutions in 20 countries approved the protocol, and all patients gave written informed consent. Drs. McMurray and Jhund had full access to the trial data and take responsibility for its integrity and the data analysis.
Study patients
Men and women aged 18 years or older with a diagnosis of HF for at least 2 months were eligible if they were in New York Heart Association (NYHA) functional class II–IV, had a left ventricular ejection fraction (LVEF) of ≤40%, were optimally treated with pharmacological and device therapy for HF, and had an N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) concentration ≥600 pg/mL (≥400 pg/mL if hospitalized for HF within the previous 12 months; ≥900 pg/mL if atrial fibrillation or atrial flutter, irrespective of history of HF hospitalization). Key exclusion criteria included symptoms of hypotension or systolic blood pressure <95 mmHg, estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 or rapidly declining renal function, type 1 diabetes, and other conditions likely to prevent patient participation in the trial or greatly limit life expectancy. A complete list of exclusion criteria is provided in the design paper. 27 After randomization, follow‐up visits were scheduled at 14 and 60 days, and then at 120, 240, 360 days and every 4 months thereafter.
Investigator‐reported aetiology
Data on HF aetiology were collected from the trial case report form. Investigators were first asked whether the primary aetiology was ischaemic, non‐ischaemic, or unknown. If investigators answered non‐ischaemic, they were then asked to specify from the following options (listed in this order): primary valvular, alcoholic, hypertensive, peripartum, idiopathic, infectious, viral, diabetic, drug‐induced, arrhythmia, and other (please specify). The pre‐specified analysis of aetiology in DAPA‐HF categorized patients as either ischaemic or non‐ischaemic, with unknown aetiology included in the non‐ischaemic category. We also conducted sensitivity analyses excluding patients with ‘unknown aetiology’, i.e. comparing only definite ischaemic aetiology and non‐ischaemic aetiology (without unknown aetiology).
In the present analysis, we further subcategorized patients with an investigator‐reported non‐ischaemic aetiology into hypertensive, idiopathic, other, and unknown non‐ischaemic aetiologies.
Clinical outcomes
The primary outcome was the composite of an episode of worsening HF (HF hospitalization or urgent visit for worsening HF with administration of intravenous treatment for HF) or cardiovascular death, whichever occurred first. In this analysis, secondary outcomes were the occurrence of HF hospitalization or cardiovascular death; HF hospitalization; cardiovascular death; death from any cause; recurrent HF hospitalization or cardiovascular death; and change from baseline to 8 months in the total symptom score of the Kansas City Cardiomyopathy Questionnaire (KCCQ‐TSS). Cause of death was adjudicated by an independent committee using definitions reported in the Appendix published with the primary results manuscript. 18
Pre‐specified safety analyses included adverse events leading to discontinuation of trial treatment and adverse events of interest, including volume depletion, renal adverse events, bone fracture, amputation, major hyperglycaemia, and diabetic ketoacidosis. Safety analyses were performed in patients who had undergone randomization and received at least one dose of either dapagliflozin or placebo (a total of eight randomized patients were excluded from the safety analysis).
Statistical analyses
Patients were divided into two groups according to aetiology. Baseline characteristics were summarized as frequencies with percentages, means with standard deviation, or medians with interquartile ranges. Differences in baseline characteristics were tested using the Chi‐square test for categorical variables and the Wilcoxon test and two‐sample t‐test for non‐normally and normally distributed continuous variables, respectively.
Time‐to‐event data for the primary outcome and secondary clinical outcomes according to aetiology, regardless of treatment allocation, were evaluated using the Kaplan–Meier estimator and cause‐specific Cox proportional‐hazards models, stratified according to diabetes mellitus status, with a history of HF hospitalization and treatment‐group assignment as fixed‐effect factors to calculate hazard ratios (HRs), 95% confidence intervals (CIs), and two‐sided P‐values. The models for all‐cause death were not adjusted for a history of HF hospitalization. In addition, adjusted HRs from models including age, sex, geographical region, heart rate, systolic blood pressure, body mass index, LVEF, NYHA functional class, NT‐proBNP, atrial fibrillation, and eGFR were reported. To address the competing risk of death, Fine–Gray competing risk analyses were performed to compare the risk of outcomes (except for all‐cause death) according to aetiology. For the HF hospitalization outcome, death from any cause was considered a competing risk, while non‐cardiovascular death was considered a competing risk for the rest of the outcomes. Subdistribution HRs, adjusted for the same variables as the cause‐specific Cox regression models, with 95% CIs were reported.
To compare the effects of dapagliflozin vs. placebo on the primary outcome and secondary clinical outcomes in patients with ischaemic and non‐ischaemic aetiology, respectively, time‐to‐event data were evaluated with the Kaplan–Meier estimator and Cox proportional‐hazards models, stratified according to diabetes mellitus status, with a history of HF hospitalization and treatment‐group assignment as fixed‐effect factors. The models for all‐cause death were not adjusted for a history of HF hospitalization. Total, including recurrent events were evaluated with semiparametric proportional‐rates models. 28 The difference between treatment groups in the change in KCCQ‐TSS from baseline to 8 months in surviving patients according to aetiology was analysed using two‐sample t‐test. Responder analyses examining proportions of patients with a deterioration (i.e. decrease in KCCQ‐TSS of ≥5 points) and clinically important improvement (i.e. an increase of KCCQ‐TSS of ≥5 points) in KCCQ at 8 months according to aetiology were conducted with the treatment effect expressed as an odds ratio (OR) using methods previously described. 29
The efficacy of dapagliflozin according to aetiology in patients with and without diabetes separately was also examined; Cox proportional‐hazards models for the time‐to‐event analyses were not stratified according to diabetes mellitus status.
Finally, the effect of dapagliflozin on the primary outcome according to continuous LVEF as a fractional polynomial was examined in patients with and without an ischaemic aetiology separately.
All analyses were conducted using STATA version 16.1 (Stata Corp., College Station, TX, USA) and SAS version 9.4 (SAS Institute, Cary, NC, USA). A P‐value of 0.05 was considered statistically significant.
Sensitivity analyses
In the main analyses, patients with an investigator‐reported unknown aetiology were categorized as non‐ischaemic aetiology. However, to test for the robustness of our findings, we performed sensitivity analyses in which these patients were excluded.
Results
Patient characteristics
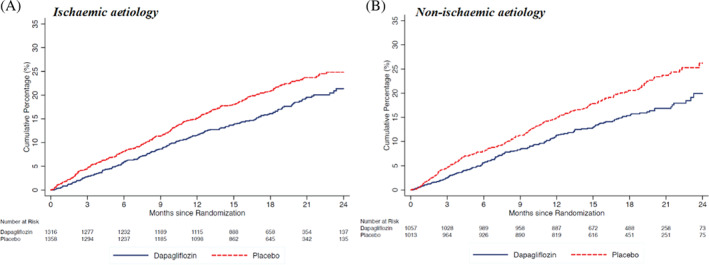
Of the 4744 patients randomized in DAPA‐HF, 2674 patients (56.4%) had an ischaemic aetiology, as reported by investigators, and 2070 (43.6%) had a non‐ischaemic aetiology [1687 (35.6%) with known non‐ischaemic aetiology and 383 (8.1%) with ‘unknown’ aetiology, assumed to be non‐ischaemic, as pre‐specified in the analysis plan]. Baseline characteristics according to aetiology are presented in Table 1 . Compared with patients with non‐ischaemic aetiology, those with ischaemic aetiology were older, more often male and white, more likely to have a previous myocardial infarction, prior coronary revascularization, peripheral artery disease, carotid artery stenosis, angina, hypertension, and type 2 diabetes, but less likely to have a history of atrial fibrillation or prior hospitalization for HF. Patients with ischaemic aetiology had a higher mean systolic blood pressure and ejection fraction, but had a lower mean heart rate and eGFR, as well as worse NYHA functional class overall. With respect to background HF therapy, patients with ischaemic aetiology were less frequently treated with digoxin and angiotensin receptor–neprilysin inhibitors and more often with implantable cardioverter‐defibrillator. A detailed breakdown of aetiology is provided in online supplementary Table S1 , both overall and by geographical region. An ischaemic aetiology was the single most common aetiology reported overall (56.4% of all patients) and was somewhat more common in Europe than other regions. Among the non‐ischaemic aetiologies, idiopathic was the most common overall (17.6% of all patients) and was least common in Europe, compared with other regions. Overall, 8.1% of patients had an ‘unknown aetiology’ and this proportion ranged from 6.0% to 11.0% across regions. Other reported aetiologies individually accounted for <5% of cases with the exception of hypertension (5.5%).
Table 1.
Baseline characteristics of the study population according to aetiology
| Ischaemic (n = 2674) | Non‐ischaemic (n = 2070) | P‐value | |
|---|---|---|---|
| Age (years), mean (SD) | 67.7 (9.8) | 64.6 (11.9) | <0.001 |
| Sex, n (%) | <0.001 | ||
| Female | 538 (20.1) | 571 (27.6) | |
| Male | 2136 (79.9) | 1499 (72.4) | |
| Race, n (%) | <0.001 | ||
| Asian | 541 (20.2) | 575 (27.8) | |
| Black | 66 (2.5) | 160 (7.7) | |
| White | 2043 (76.4) | 1290 (62.3) | |
| Other | 24 (0.9) | 45 (2.2) | |
| Geographic region, n (%) | <0.001 | ||
| Asia/Pacific | 529 (19.8) | 567 (27.4) | |
| Europe | 1404 (52.5) | 750 (36.2) | |
| North America | 351 (13.1) | 326 (15.7) | |
| South America | 390 (14.6) | 427 (20.6) | |
| Physiologic measures | |||
| Systolic blood pressure (mmHg), mean (SD) | 122.9 (15.8) | 120.3 (16.8) | <0.001 |
| Diastolic blood pressure (mmHg), mean (SD) | 73.3 (9.9) | 73.8 (11.2) | 0.12 |
| Heart rate (bpm), mean (SD) | 70.1 (11.0) | 73.4 (12.3) | <0.001 |
| BMI (kg/m2), mean (SD) | 28.1 (5.4) | 28.2 (6.6) | 0.41 |
| Creatinine (µmol/L), mean (SD) | 106.7 (30.2) | 101.6 (30.4) | <0.001 |
| Glycated haemoglobin (%), mean (SD) | 6.6 (1.4) | 6.4 (1.3) | <0.001 |
| eGFR (mL/min/1.73 m2), mean (SD) | 63.7 (18.5) | 68.4 (20.2) | <0.001 |
| eGFR (mL/min/1.73 m2), n (%) | <0.001 | ||
| <60 | 1174 (43.9) | 752 (36.3) | |
| ≥60 | 1498 (56.1) | 1318 (63.7) | |
| NT‐proBNP for AF on ECG (pg/mL), median (IQR) | 2083 (1298–3270) | 1878 (1224–3145) | 0.16 |
| NT‐proBNP for no AF on ECG (pg/mL), median (IQR) | 1264 (766–2353) | 1345 (778–2510) | 0.12 |
| LVEF (%), mean (SD) | 31.7 (6.5) | 30.2 (7.1) | <0.001 |
| NYHA class, n (%) | <0.001 | ||
| II | 1742 (65.1) | 1461 (70.6) | |
| III | 914 (34.2) | 584 (28.2) | |
| IV | 18 (0.7) | 25 (1.2) | |
| KCCQ, mean (SD) | |||
| Total symptom score | 73.2 (21.1) | 74.1 (22.6) | 0.18 |
| Clinical summary score | 70.7 (20.2) | 71.7 (21.5) | 0.10 |
| Overall summary score | 67.9 (20.2) | 68.6 (21.3) | 0.28 |
| Medical history, n (%) | |||
| Hypertension a | 2156 (80.6) | 1367 (66.0) | <0.001 |
| Type 2 diabetes | 1333 (49.9) | 806 (38.9) | <0.001 |
| Atrial fibrillation | 894 (33.4) | 924 (44.6) | <0.001 |
| Hospitalization for HF b | 1232 (46.1) | 1019 (49.2) | 0.03 |
| Previous MI | 1939 (72.5) | 153 (7.4) | <0.001 |
| Previous PCI | 1449 (54.2) | 175 (8.5) | <0.001 |
| Previous CABG | 737 (27.6) | 62 (3.0) | <0.001 |
| Ischaemic stroke | 287 (10.7) | 151 (7.3) | <0.001 |
| Peripheral artery disease | 509 (19.0) | 140 (6.8) | <0.001 |
| Angina | 937 (35.0) | 175 (8.5) | <0.001 |
| Vascular stent | 106 (4.0) | 17 (0.8) | <0.001 |
| Carotid artery stenosis | 205 (7.7) | 46 (2.2) | <0.001 |
| Treatment, n (%) | |||
| ACEI | 1545 (57.8) | 1116 (53.9) | 0.008 |
| ARB | 717 (26.8) | 590 (28.5) | 0.20 |
| ARNI | 260 (9.7) | 248 (12.0) | 0.01 |
| Beta‐blocker | 2567 (96.0) | 1991 (96.2) | 0.74 |
| MRA | 1898 (71.0) | 1472 (71.1) | 0.92 |
| Diuretic | 2250 (84.1) | 1758 (84.9) | 0.46 |
| Digoxin | 381 (14.2) | 506 (24.4) | <0.001 |
| Oral anticoagulant c | 995 (37.2) | 974 (47.1) | <0.001 |
| Antiplatelet d | 1928 (72.1) | 664 (32.1) | <0.001 |
| Statin | 2232 (83.5) | 944 (45.6) | <0.001 |
| ICD | 591 (22.1) | 362 (17.5) | <0.001 |
| ICD/CRT‐D | 749 (28.0) | 493 (23.8) | 0.001 |
| CRT‐P/CRT‐D | 183 (6.8) | 171 (8.3) | 0.07 |
ACE, angiotensin‐converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; BMI, body mass index; CABG, coronary artery bypass grafting; CRT‐D, cardiac resynchronization therapy‐defibrillator; CRT‐P, cardiac resynchronization therapy‐pacemaker; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter‐defibrillator; IQR, interquartile range; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SD, standard deviation.
A history of hypertension.
Any time prior to randomization (a key exclusion criterion was a hospitalization due to decompensated HF <4 weeks prior to enrolment).
Vitamin K antagonists (warfarin/coumadin) and direct oral anticoagulants (dabigatran, rivaroxaban, apixaban, and edoxaban).
Aspirin, ADP receptor inhibitors (clopidogrel, ticagrelor, prasugrel), and adenosine reuptake inhibitors (dipyridamole).
Outcomes according to aetiology
Primary and secondary outcomes according to aetiology
Patients with and without an ischaemic aetiology had a similar risk of worsening HF or cardiovascular death when compared in a multivariable cause‐specific Cox proportional‐hazards model (reference non‐ischaemic group; HR 1.01, 95% CI 0.88–1.15). The risk of HF hospitalization was significantly lower in patients with ischaemic aetiology compared to those with non‐ischaemic aetiology, whereas the risk of cardiovascular death and death from any cause was significantly higher in patients with ischaemic aetiology, even after adjustment for other prognostic variables including NT‐proBNP (online supplementary Table S2 ). Fine–Gray competing risk analyses, accounting for the competing risk of death, yielded similar findings (online supplementary Table S2 ).
Adjudicated causes of death according to aetiology
Adjudicated causes of death according to ischaemic and non‐ischaemic aetiology are shown in Figure 1 . Overall, the two main modes of cardiovascular death were sudden death and death due to worsening HF (‘pump failure’). While the proportion of deaths that were sudden was similar in the ischaemic and non‐ischaemic groups, fewer ischaemic patients, compared to non‐ischaemic patients, had a death attributed to worsening HF. Conversely, the proportion of deaths attributed to myocardial infarction was higher in patients with an ischaemic aetiology, compared to patients with a non‐ischaemic aetiology, although the proportion of deaths due to myocardial infarction was small in both subgroups. The cause of death was undetermined in just under one fifth of cases (these deaths were presumed to be cardiovascular) and this proportion was similar in the ischaemic and non‐ischaemic groups, as was the proportion of deaths attributed to non‐cardiovascular causes (which accounted for about one in seven deaths).
Figure 1.
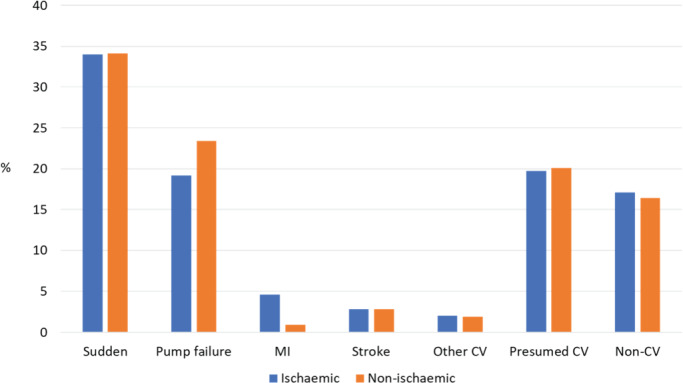
Adjudicated causes of death according to ischaemic and non‐ischaemic aetiology. CV, cardiovascular; MI, myocardial infarction.
Effects of dapagliflozin according to aetiology
Primary composite outcome
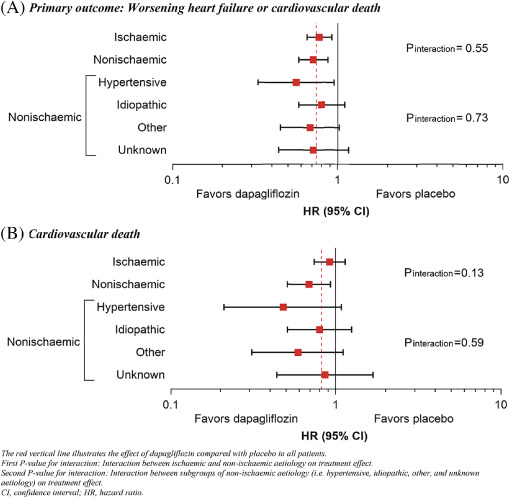
Dapagliflozin reduced the risk of worsening HF or cardiovascular death to the same extent in patients with an ischaemic aetiology (HR 0.77, 95% CI 0.65–0.92) and without an ischaemic aetiology (HR 0.71, 95% CI 0.58–0.87), with no interaction between aetiology and effect of treatment (P for interaction = 0.55) (Table 2 and Figure 2 ).
Table 2.
Effects of dapagliflozin compared with placebo on clinical events according to aetiology
| Outcome | Ischaemic (n = 2674) | Non‐ischaemic (n = 2070) | P‐value for interaction | ||
|---|---|---|---|---|---|
| Placebo (n = 1358) | Dapagliflozin (n = 1316) | Placebo (n = 1013) | Dapagliflozin (n = 1057) | ||
| Worsening HF event or cardiovascular death | 0.55 | ||||
| n (%) | 289 (21.3) | 223 (16.9) | 213 (21.0) | 163 (15.4) | |
| Event rate per 100 person‐years (95% CI) | 15.6 (13.9–17.5) | 11.9 (10.5–13.6) | 15.6 (13.6–17.8) | 11.1 (9.5–12.9) | |
| HR (95% CI) | 0.77 (0.65–0.92) | 0.71 (0.58–0.87) | |||
| HF hospitalization or cardiovascular death | 0.45 | ||||
| n (%) | 284 (20.9) | 222 (16.9) | 211 (20.8) | 160 (15.1) | |
| Event rate per 100 person‐years (95% CI) | 15.2 (13.6–17.1) | 11.9 (10.4–13.5) | 15.4 (13.4–17.6) | 10.9 (9.3–12.7) | |
| HR (95% CI) | 0.78 (0.65–0.93) | 0.70 (0.57–0.86) | |||
| HF hospitalization | 0.73 | ||||
| n (%) | 173 (12.7) | 118 (9.0) | 145 (14.3) | 113 (10.7) | |
| Event rate per 100 person‐years (95% CI) | 9.3 (8.0–10.8) | 6.3 (5.3–7.6) | 10.6 (9.0–12.4) | 7.7 (6.4–9.2) | |
| HR (95% CI) | 0.68 (0.54–0.86) | 0.72 (0.57–0.92) | |||
| Cardiovascular death | 0.13 | ||||
| n (%) | 170 (12.5) | 152 (11.6) | 103 (10.2) | 75 (7.1) | |
| Event rate per 100 person‐years (95% CI) | 8.6 (7.4–10.0) | 7.8 (6.7–9.2) | 7.0 (5.8–8.5) | 4.8 (3.9–6.1) | |
| HR (95% CI) | 0.92 (0.74–1.14) | 0.69 (0.51–0.93) | |||
| All‐cause death | 0.10 | ||||
| n (%) | 206 (15.2) | 185 (14.1) | 123 (12.1) | 91 (8.6) | |
| Event rate per 100 person‐years (95% CI) | 10.4 (9.1–11.9) | 9.5 (8.3–11.0) | 8.4 (7.0–10.0) | 5.9 (4.8–7.2) | |
| HR (95% CI) | 0.92 (0.76–1.12) | 0.70 (0.53–0.92) | |||
| Recurrent HF hospitalization or cardiovascular death | 0.19 | ||||
| No. of events | 407 | 328 | 335 | 239 | |
| RR (95% CI) | 0.82 (0.68–1.00) | 0.67 (0.53–0.85) | |||
| KCCQ‐TSS | |||||
| Change in KCCQ‐TSS score at 8 months | 3.0 (1.9–4.0) | 6.5 (5.4–7.6) | 3.8 (2.4–5.1) | 5.6 (4.4–6.9) | 0.40 |
| ≥5‐point improvement in KCCQ‐TSS at 8 months | 0.32 | ||||
| Proportion of patients | 49.5% | 58.3% | 52.9% | 58.3% | |
| OR (95% CI) | 1.19 (1.09–1.29) | 1.11 (1.01–1.22) | |||
| ≥5‐point decrease in KCCQ‐TSS at 8 months | 0.76 | ||||
| Proportion of patients | 33.3% | 25.2% | 32.3% | 25.5% | |
| OR (95% CI) | 0.83 (0.76–0.90) | 0.85 (0.76–0.94) | |||
CI, confidence interval; HF, heart failure; HR, hazard ratio; KCCQ‐TSS, Kansas City Cardiomyopathy Questionnaire total symptom score; OR, odds ratio; RR, rate ratio.
Figure 2.
Primary outcome (worsening heart failure or cardiovascular death) according to randomized treatment assignment in ischaemic (A) and non‐ischaemic (B) patient subgroups.
Secondary outcomes
Hazard ratios, rate ratios, and ORs for the effect of dapagliflozin compared with placebo on the secondary clinical endpoints are displayed in Table 2 and Figure 3 . The effect of dapagliflozin was consistent in patients with and without an ischaemic aetiology for all secondary endpoints: HF hospitalization or cardiovascular death (P for interaction = 0.45), HF hospitalization (P for interaction = 0.73), cardiovascular death (P for interaction = 0.13), death from any cause (P for interaction = 0.10), and recurrent HF hospitalization or cardiovascular death (P for interaction = 0.19).
Figure 3.
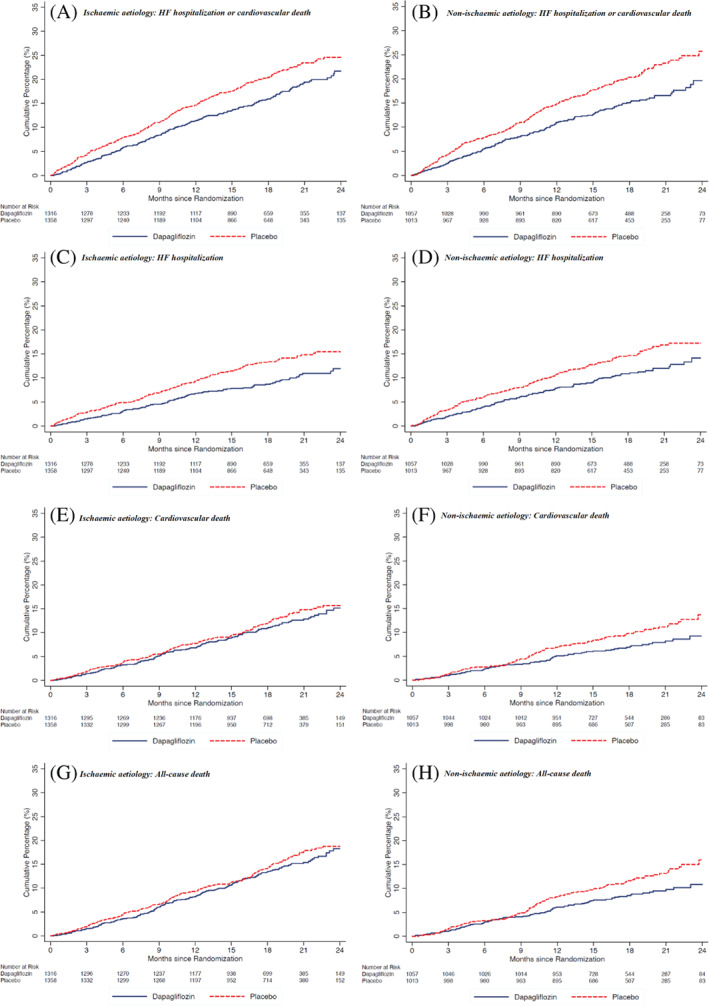
Pre‐specified secondary outcomes according to randomized treatment assignment in ischaemic and non‐ischaemic patient subgroups. HF, heart failure.
The mean increase in KCCQ‐TSS from baseline to 8 months was significantly greater with dapagliflozin in both the ischaemic and non‐ischaemic subgroups (P for interaction = 0.40). The proportion of patients with an improvement of KCCQ‐TSS of ≥5 points was greater with dapagliflozin, compared with placebo, in both patients with an ischaemic aetiology (58.3% vs. 49.5%; OR 1.19, 95% CI 1.09–1.29) and without an ischaemic aetiology (58.3% vs. 52.9%; OR 1.11, 95% CI 1.01–1.22) (P for interaction = 0.32). Conversely, the proportion of patients with a decrease in KCCQ‐TSS of ≥5 points was smaller in those treated with dapagliflozin, compared with placebo, in both patients with an ischaemic (25.2% vs. 33.3%; OR 0.83, 95% CI 0.76–0.90) and a non‐ischaemic aetiology (25.5% vs. 32.3%; OR 0.85, 95% CI 0.76–0.94) (P for interaction = 0.76).
Non‐ischaemic aetiology subgroups
Among the 2070 patients with non‐ischaemic HFrEF, investigators reported the aetiology as hypertensive in 262 (12.7%), idiopathic in 833 (40.2%), other miscellaneous causes in 592 (28.6%), and ‘unknown’ in 383 (18.5%). Data on the effect of dapagliflozin compared with placebo on secondary endpoints and the occurrence of the pre‐specified adverse events according to these non‐ischaemic subgroups are shown in online supplementary Tables S3 and S4 , respectively. In line with the main findings, dapagliflozin, compared with placebo, reduced the risk of worsening HF, cardiovascular death, and all‐cause death, improved symptoms, and was safe and well‐tolerated, across all non‐ischaemic subgroups.
Aetiology and diabetes
Data on the effects of dapagliflozin compared with placebo in patients with ischaemic and non‐ischaemic aetiology according to diabetes status are shown in online supplementary Table S5 . In line with the main findings, dapagliflozin, compared with placebo, reduced the risk of worsening HF, cardiovascular death, and all‐cause death and improved symptoms, irrespective of aetiology, in both patients with and without diabetes.
Aetiology and left ventricular ejection fraction
The benefit of dapagliflozin on worsening HF or cardiovascular death was consistent across the spectrum of LVEF in both patients with and without an ischaemic aetiology (P for interaction = 0.37 and 0.62, respectively) (online supplementary Figure S1 ).
Sensitivity analyses
We excluded patients with aetiology reported as ‘unknown’ by the investigator from the analyses. Baseline characteristics according to aetiology are presented in online supplementary Table S6 . Data on the effect of dapagliflozin compared with placebo on secondary endpoints and the occurrence of the pre‐specified adverse events in patients with ischaemic and non‐ischaemic aetiology are shown in online supplementary Tables S7 and S8 , respectively. In line with the main findings, dapagliflozin, compared with placebo, reduced the risk of worsening HF, cardiovascular death, and all‐cause death, improved symptoms, and was safe and well‐tolerated, irrespective of aetiology.
Safety analyses
The pre‐specified adverse events according to treatment assignment in patients with and without an ischaemic aetiology are shown in Table 3 . In general, the proportions of patients who discontinued trial treatment or experienced adverse events according to treatment assignment were similar, irrespective of aetiology.
Table 3.
Adverse events of dapagliflozin compared with placebo according to aetiology
| Adverse event | Ischaemic (n = 2670) | Non‐ischaemic (n = 2066) | P‐value for interaction | ||
|---|---|---|---|---|---|
| Placebo (n = 1356) | Dapagliflozin (n = 1314) | Placebo (n = 1012) | Dapagliflozin (n = 1054) | ||
| Discontinuation of study drug for any reason | 153 (11.3) | 139 (10.6) | 105 (10.4) | 110 (10.4) | 0.68 |
| Discontinuation of study drug due to adverse event | 64 (4.7) | 67 (5.1) | 52 (5.1) | 44 (4.2) | 0.28 |
| Volume depletion | 87 (6.4) | 100 (7.6) | 75 (7.4) | 78 (7.4) | 0.45 |
| Renal adverse event | 101 (7.4) | 83 (6.3) | 69 (6.8) | 70 (6.6) | 0.52 |
| Fracture | 32 (2.4) | 27 (2.1) | 18 (1.8) | 22 (2.1) | 0.46 |
| Amputation | 10 (0.7) | 11 (0.8) | 2 (0.2) | 2 (0.2) | 0.88 |
| Major hypoglycaemia | 1 (0.1) | 2 (0.2) | 3 (0.3) | 2 (0.2) | 0.44 |
| Diabetic ketoacidosis | 0 (0.0) | 3 (0.2) | 0 (0.0) | 0 (0.0) | N/A |
N/A, not applicable.
A total of eight randomized patients were excluded from the safety analysis, as these were performed in patients who had undergone randomization and received at least one dose of dapagliflozin or placebo.
Discussion
In this pre‐specified analysis of DAPA‐HF, the risk of cardiovascular death and all‐cause death was higher in patients with an ischaemic aetiology compared to those with a non‐ischaemic aetiology, whereas the risk of HF hospitalization was lower in patients with ischaemic aetiology. Furthermore, dapagliflozin, added to conventional guideline‐recommended therapies, reduced the risk of worsening HF events, cardiovascular death, and all‐cause death, and improved symptoms, to a similar extent in both patients with and without an ischaemic aetiology.
Baseline characteristics and outcomes according to aetiology
The proportion of patients with an investigator‐reported ischaemic aetiology was similar to that of four recent HFrEF trials. 13 , 30 , 31 , 32 The present analysis of DAPA‐HF demonstrated substantial differences in the clinical profile between HFrEF patients with and without an ischaemic aetiology, most of which confirmed prior findings. 13 In DAPA‐HF, patients with ischaemic aetiology were older, more often male and white and had a slightly higher ejection fraction and symptom burden than those with non‐ischaemic aetiology. As expected, patients with an ischaemic aetiology were also more likely to have a previous myocardial infarction, prior coronary revascularization, and type 2 diabetes and were more frequently treated with antiplatelet agents, statins, and implantable cardioverter‐defibrillators. An interesting observation was the high proportion of patients with non‐ischaemic aetiology who were treated with antiplatelet agents and statins. Although data on the indications for prescribing these drugs were not available, it most likely reflects that approximately 40% and 7% of patients with non‐ischaemic aetiology had type 2 diabetes and peripheral artery disease, respectively. However, the evidence that statins are beneficial in HF patients with or without coronary artery disease is lacking. 33 , 34 The same is likely true of antiplatelet agents, and other antithrombotic therapies are not beneficial in HFrEF. 35 , 36
Previous studies investigating outcomes according to aetiology found HFrEF of non‐ischaemic origin to be associated with better outcomes than HFrEF due to an ischaemic cause. 10 , 11 , 12 However, in a recent analysis from the Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor With Angiotensin‐Converting‐Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF), aetiology did not appear to significantly modify the risk of the primary composite outcome of HF hospitalization or cardiovascular death. 13 In keeping with PARADIGM‐HF, we found that the risk of the composite of worsening HF or cardiovascular death did not differ by aetiology in a contemporary, globally representative, and well‐treated cohort of patients with HFrEF enrolled in DAPA‐HF. There are several plausible explanations to this difference. First, our analyses were adjusted for important prognostic variables, including natriuretic peptides. Second, patients with ischaemic aetiology enrolled in DAPA‐HF (and PARADIGM‐HF) were more aggressively treated with antiplatelet agents, statins, and beta‐blockers, and more often had undergone coronary revascularization. Third, HFrEF patients with severely reduced kidney function (eGFR <30 mL/min/1.73 m2) more often have HF due to ischaemic causes; as these patients are excluded from most clinical trials (including DAPA‐HF), it is possible that the risk of death and HF hospitalization in patients with ischaemic aetiology may have been underestimated. However, this apparent similarity in the composite outcome in DAPA‐HF masked a directional difference in its HF hospitalization and cardiovascular mortality components. Patients with an ischaemic aetiology had a greater risk of cardiovascular death and all‐cause mortality, but a lower risk of HF hospitalization than those with non‐ischaemic aetiology. While the lower rate of HF hospitalization in ischaemic patients could reflect the competing risk of death, the lower hospitalization rate in ischaemic patients persisted when accounting for the competing risk of death in competing risk analyses. Although the explanations to this finding are not clear, patients with a non‐ischaemic aetiology are a heterogeneous group and it is possible that individuals with certain aetiologies may have a better or worse prognosis than the more common aetiologies.
As expected, the proportion of deaths attributed to myocardial infarction was higher in patients with an ischaemic aetiology compared to patients with a non‐ischaemic aetiology. Perhaps more surprisingly, the proportion of deaths that were sudden was similar in the ischaemic and non‐ischaemic groups. While patients with non‐ischaemic HF may be less prone to sudden death from ventricular tachyarrhythmias than patients with ischaemic HF, electromechanical dissociation, asystole, or a terminal bradyarrhythmia as the cause of sudden death may be relatively more common in patients with a non‐ischaemic cardiomyopathy. 37 , 38 , 39 Our finding of a similar proportion of sudden deaths in the ischaemic and non‐ischaemic groups is in line with previous data, 40 though the proportion was overall lower in the present analysis. The lower proportion of sudden deaths likely reflects the reduction in sudden death in patients with HF due to improved medical and device therapy. 41 Another interesting observation was that fewer ischaemic patients, compared to non‐ischaemic patients, had a death attributed to worsening HF. Although there is no clear explanation, it is possible this reflects the higher ejection fraction in the ischaemic group.
Efficacy and safety of dapagliflozin according to aetiology
While aetiology does not appear to modify the effects of renin–angiotensin system blockers, beta‐blockers, sacubitril/valsartan, or mineralocorticoid receptor antagonists in patients with HFrEF, 13 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 patients with ischaemic aetiology may respond differently to certain HFrEF treatments than those with non‐ischaemic aetiology. Prophylactic implantable cardioverter‐defibrillator implantation has been shown to reduce the rate of sudden cardiac death and all‐cause mortality in HFrEF due to ischaemic heart disease, but not in HFrEF due to non‐ischaemic causes. 14 , 50 , 51 , 52 On the other hand, HFrEF patients with non‐ischaemic aetiology may achieve greater improvements of left ventricular function and remodelling with cardiac resynchronization therapy than those with an ischaemic aetiology. 16 , 17 Likewise, treatment with intravenous milrinone in the acute setting may be associated with better outcomes in patients with non‐ischaemic aetiology and worse outcomes in those with ischaemic aetiology. 15 These differences underline the importance of investigating the efficacy and safety of new HF treatments according to aetiology. In this pre‐specified analysis of DAPA‐HF, dapagliflozin reduced the risk of the primary outcome similarly in patients with both ischaemic and non‐ischaemic aetiology. In addition, the efficacy of dapagliflozin on HF hospitalization (both first and recurrent), cardiovascular death, and all‐cause death was consistent, irrespective of aetiology.
Key goals of management of patients with HFrEF are not only to prevent hospital admissions and reduce mortality, but also to reduce patients' symptom burden and improve their physical function and quality of life. Importantly, this study demonstrated that dapagliflozin considerably increased the mean KSSQ‐TSS at 8 months in both patients with ischaemic and non‐ischaemic aetiology. Moreover, dapagliflozin increased the proportion of patients with a clinically meaningful improvement in symptoms (increase in KCCQ‐TSS of ≥5 points) at 8 months and reduced the proportion with a clinically meaningful deterioration (decrease in KCCQ‐TSS of ≥5 points), irrespective of aetiology. Collectively, these data underline the substantial, and clinically important, benefits of dapagliflozin, irrespective of aetiology, in HFrEF patients receiving optimal guideline‐directed medical therapy and provide further evidence for dapagliflozin as a new treatment option for HFrEF. Interestingly, despite the different mortality and hospitalization profiles of ischaemic and non‐ischaemic patients, their symptom and health status changes over time seemed to be similar.
Data on safety and tolerability in DAPA‐HF were also reassuring. Overall, study drug discontinuation and serious adverse events were generally uncommon with no differences by aetiology. Further, study drug discontinuation and serious adverse events were not more frequently reported in the dapagliflozin group than in the placebo group in both patients with ischaemic and non‐ischaemic aetiology. These data further underline the safety and tolerability of dapagliflozin in patients with HFrEF, irrespective of aetiology.
Limitations
The findings of this study should be viewed in the context of potential limitations. Although aetiology was a pre‐defined subgroup analysis, the assessment of secondary clinical outcomes by aetiology was done post‐hoc. The pre‐specified inclusion and exclusion criteria precluded the enrolment of hospitalized and other very high‐risk patients, which might affect the generalizability of our results. Some degree of misclassification of HF aetiology cannot be precluded as aetiology was investigator‐reported and no specific instructions as to how to identify aetiology were provided. Moreover, 8% of the study population had an undetermined HF aetiology, which may reflect that patients may not have been comprehensively examined for specific causes of HF, but also the difficulties of ascribing an ischaemic aetiology, even with coronary angiography. 53 In addition, it is possible that some patients with HF can have mixed aetiologies, which are not mutually exclusive. Moreover, it was not possible to examine outcomes according to specific groups of non‐ischaemic aetiology other than the idiopathic and hypertensive categories due to the small number of events. Also, while it would have been interesting to evaluate the effect of dapagliflozin on LVEF according to aetiology, echocardiography was not performed routinely during follow‐up in DAPA‐HF. Finally, further studies on sudden deaths according to aetiology in HFrEF are warranted, given the similar proportion of sudden deaths in the ischaemic and non‐ischaemic groups in DAPA‐HF and the lack of detailed data regarding the causes of sudden death.
Conclusions
In DAPA‐HF, dapagliflozin, compared with placebo, reduced the risk of worsening HF events and death, and improved symptoms, similarly in patients with ischaemic and non‐ischaemic aetiology. These findings provide further evidence for dapagliflozin as a new treatment option for HFrEF.
Funding
The DAPA‐HF trial was funded by AstraZeneca. Prof. McMurray is supported by British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217.
Conflict of interest: Dr. Docherty reported personal fees from AstraZeneca and the University of Glasgow during the conduct of the study and personal fees from Eli Lilly outside the submitted work. Dr. Nicolau reported receiving grants from AstraZeneca during the conduct of the study and personal fees from Amgen, Daiichi‐Sankyo, and Servier, grants from AstraZeneca, Bristol‐Myers‐Squibb, CLS Behring, Dalcor, Jansen, Novo Nordisk, and Vifor, and grants and personal fees from Bayer, Novartis, and Sanofi. Dr. Verma received grant support, lecture fees, and advisory board fees from AstraZeneca, Boehringer Ingelheim, Bayer, Janssen, and Merck, lecture fees from Sun Pharmaceutical Industries and EOCI Pharmacomm, grant support and advisory board fees from Amgen, and lecture fees and advisory board fees from Sanofi and Eli Lilly. Dr. Petrie reported grants from AstraZeneca during the conduct of the study; grants from Boehringer Ingelheim, Novo Nordisk, Novartis, and SQ Innovations and personal fees from Boehringer Ingelheim, Takeda, and Bayer outside the submitted work. Dr. Inzucchi reports personal fees and non‐financial support from AstraZeneca, Boehringer Ingelheim, Sanofi/Lexicon, Merck, VTV Therapeutics, and Abbott/Alere, as well as personal fees from AstraZeneca and Zafgen. Dr. Schou reported lecture fees from other from Boehringer Ingelheim, AstraZeneca, and NOVP outside the submitted work. Dr. Kosiborod reported grants from AstraZeneca and Boehringer Ingelheim; personal fees from Amgen, Applied Therapeutics, Astra Zeneca, Bayer, Boehringer‐Ingelheim, Eli Lilly, Janssen, Merck (Diabetes), Novo Nordisk, Sanofi, and Vifor Pharma outside the submitted work. Dr. Langkilde reported being a full‐time employee and shareholder of AstraZeneca during the conduct of the study. Dr. Martinez reported personal fees from AstraZeneca during the conduct of the study. Dr. Ponikowski reported personal fees and other support from AstraZeneca during the conduct of the study; personal fees from Boehringer Ingelheim, Vifor Pharma, Amgen, Servier, Novartis, Berlin Chemie, Bayer, Pfizer, Cibiem, Impulse Dynamics, Renal Guard Solutions, Radcliffe‐Group, BMS, and Respicardia; other support from AbbottVascular, Boehringer Ingelheim, Novartis, Amgen, Vifor, and Bayer outside the submitted work. Dr. Sabatine reported grants from AstraZeneca Institutional research grant to the TIMI Study Group at Brigham and Women's Hospital and personal fees from AstraZeneca during the conduct of the study; personal fees from Althera, Amgen, Antho, Bristol‐Myers Squibb, CVS Caremark, DalCor, Dr Reddy's Laboratories, Dyrnamix, Esperion, IFM, Intarcia, Janssen Research and Development, Medicines Company, Medimmune, Merck, Novartis; grants from Amgen, Bayer, Daiichi‐Sankyo, Eisai, Intarcia, Janssen Research and Development, Medicines Company, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, and Takeda outside the submitted work; and is a member of the TIMI Study Group, which has also received institutional research grant support through Brigham and Women's Hospital from Abbott, Aralez, Regeneron, Roche, and Zora Biosciences. Dr. Sjostrand reported beign an employee and stockholder of AstraZeneca. Dr. Solomon reported grants from AstraZeneca Grants to institution during the conduct of the study; grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Lone Star Heart, Mesoblast, MyoKardia, National Health Institute/National Heart, Lung, and Blood Institute, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos; and personal fees from Abbott, Actelion, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer‐Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi‐Sankyo, Gilead, GSK, Ironwood, Lilly, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Sanofi‐Pasteur, Tenaya, Dinaqor, Tremeau, CellProThera, Moderna, and American Regent outside the submitted work. Dr. Bengtsson reported being an AstraZeneca employee. Dr. Jhund reported other support from AstraZeneca during the conduct of the study; other support from Novartis; and grants and personal fees from Boehringer‐Ingelheim outside the submitted work. Dr. McMurray reported other support from AstraZeneca; non‐financial support from Cytokinetics, Bayer, Theracos, Oxford University, Dalcor, Merck, GlaxoSmithKline, Bristol Myers Squibb, Vifor‐Fresenius, Kidney Research UK, Alnylam, Abbvie, Cyclerion, Cardurion; and personal fees from Amgen, and personal fees from Abbott, Hickma, Sun Pharmaceuticals, Servier lecture fees outside the submitted work. Dr. Køber reported personal fees from speakers honorarium from AstraZeneca during the conduct of the study; personal fees from speakers honorarium from Novo, Boehringer and Novartis outside the submitted work. No other disclosures were reported.
Supporting information
Table S1. Distribution of aetiology subgroups according to geographical region.
Table S2. Time to first event according to aetiology.
Table S3. Effects of dapagliflozin compared with placebo on clinical events according to subgroups of non‐ischaemic aetiology.
Table S4. Adverse events of dapagliflozin compared with placebo according to subgroups of non‐ischaemic aetiology.
Table S5. Effects of dapagliflozin compared with placebo according to aetiology and diabetes status at baseline.
Table S6. Baseline characteristics of the study population according to aetiology (unknown excluded).
Table S7. Effects of dapagliflozin compared with placebo on clinical events according to aetiology (unknown excluded).
Table S8. Adverse events of dapagliflozin compared with placebo according to aetiology (unknown excluded).
Figure S1. Effect of dapagliflozin on worsening heart failure or cardiovascular death according to baseline left ventricular ejection fraction in patients with and without ischaemic aetiology.
References
- 1. Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham study. J Am Coll Cardiol 1993;22:6A–13A. [DOI] [PubMed] [Google Scholar]
- 2. Mahmood SS, Wang TJ. The epidemiology of congestive heart failure: contributions from the Framingham Heart Study. Glob Heart 2013;8:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Y, Zhang J, Butler J, Yang X, Xie P, Guo D, Wei T, Yu J, Wu Z, Gao Y, Han X, Zhang X, Wen S, Anker SD, Filippatos G, Fonarow GC, Gan T, Zhang R; China‐HF Investigators . Contemporary epidemiology, management, and outcomes of patients hospitalized for heart failure in China: results from the China Heart Failure (China‐HF) Registry. J Card Fail 2017;23:868–875. [DOI] [PubMed] [Google Scholar]
- 4. Lam CS, Teng TH, Tay WT, Anand I, Zhang S, Shimizu W, Narasimhan C, Park SW, Yu CM, Ngarmukos T, Omar R, Reyes EB, Siswanto AB, Hung CL, Ling LH, Yap J, MacDonald M, Richards AM. Regional and ethnic differences among patients with heart failure in Asia: the Asian Sudden Cardiac Death in Heart Failure Registry. Eur Heart J 2016;37:3141–3153. [DOI] [PubMed] [Google Scholar]
- 5. Dokainish H, Teo K, Zhu J, Roy A, Alhabib KF, Elsayed A, Palileo‐Villaneuva L, Lopez‐Jaramillo P, Karaye K, Yusoff K, Orlandini A, Sliwa K, Mondo C, Lanas F, Prabhakaran D, Badr A, Elmaghawry M, Damasceno A, Tibazarwa K, Belley‐Cote E, Balasubramanian K, Yacoub MH, Huffman MD, Harkness K, Grinvalds A, McKelvie R, Yusuf S; INTER‐CHF Investigators . Heart failure in Africa, Asia, the Middle East and South America: the INTER‐CHF study. Int J Cardiol 2016;204:133–141. [DOI] [PubMed] [Google Scholar]
- 6. Kumbhani DJ, Fonarow GC, Heidenreich PA, Schulte PJ, Lu D, Hernandez A, Yancy C, Bhatt DL. Association between hospital volume, processes of care, and outcomes in patients admitted with heart failure: insights from Get With The Guidelines‐Heart Failure. Circulation 2018;137:1661–1670. [DOI] [PubMed] [Google Scholar]
- 7. Canepa M, Fonseca C, Chioncel O, Laroche C, Crespo‐Leiro MG, Coats AJS, Mebazaa A, Piepoli MF, Tavazzi L, Maggioni AP; ESC HF Long‐Term Registry Investigators . Performance of prognostic risk scores in chronic heart failure patients enrolled in the European Society of Cardiology Heart Failure Long‐Term Registry. JACC Heart Fail 2018;6:452–462. [DOI] [PubMed] [Google Scholar]
- 8. Callender T, Woodward M, Roth G, Farzadfar F, Lemarie JC, Gicquel S, Atherton J, Rahimzadeh S, Ghaziani M, Shaikh M, Bennett D, Patel A, Lam CS, Sliwa K, Barretto A, Siswanto BB, Diaz A, Herpin D, Krum H, Eliasz T, Forbes A, Kiszely A, Khosla R, Petrinic T, Praveen D, Shrivastava R, Xin D, Macmahon S, McMurray J, Rahimi K. Heart failure care in low‐ and middle‐income countries: a systematic review and meta‐analysis. PLoS Med 2015;11:e1001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velazquez EJ, Lee KL, Jones RH, Al‐Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, Oh JK, She L, Moore VL, Desvigne‐Nickens P, Sopko G, Rouleau JL; STICHES Investigators . Coronary‐artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 2016;374:1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pecini R, Møller DV, Torp‐Pedersen C, Hassager C, Køber L. Heart failure etiology impacts survival of patients with heart failure. Int J Cardiol 2011;149:211–215. [DOI] [PubMed] [Google Scholar]
- 11. Frazier CG, Alexander KP, Newby LK, Anderson S, Iverson E, Packer M, Cohn J, Goldstein S, Douglas PS. Associations of gender and etiology with outcomes in heart failure with systolic dysfunction. A pooled analysis of 5 randomized control trials. J Am Coll Cardiol 2007;49:1450–1458. [DOI] [PubMed] [Google Scholar]
- 12. Martínez‐Sellés M, Doughty RN, Poppe K, Whalley GA, Earle N, Tribouilloy C, McMurray JJ, Swedberg K, Køber L, Berry C, Squire I; Meta‐Analysis Global Group In Chronic Heart Failure (MAGGIC) . Gender and survival in patients with heart failure: interactions with diabetes and aetiology. Results from the MAGGIC individual patient meta‐analysis. Eur J Heart Fail 2012;14:473–479. [DOI] [PubMed] [Google Scholar]
- 13. Balmforth C, Simpson J, Shen L, Jhund PS, Lefkowitz M, Rizkala AR, Rouleau JL, Shi V, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJ. Outcomes and effect of treatment according to etiology in HFrEF: an analysis of PARADIGM‐HF. JACC Heart Fail 2019;7:457–465. [DOI] [PubMed] [Google Scholar]
- 14. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbk L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjr H, Brandes A, Thøgersen AM, Gustafsson F, Egstrup K, Videbk R, Hassager C, Svendsen JH, Høfsten DE, Torp‐Pedersen C, Pehrson S; DANISH Investigators . Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221–1230. [DOI] [PubMed] [Google Scholar]
- 15. Felker GM, Benza RL, Chandler AB, Leimberger JD, Cuffe MS, Califf RM, Gheorghiade M, O'Connor CM; OPTIME‐CHF Investigators . Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME‐CHF study. J Am Coll Cardiol 2003;41:997–1003. [DOI] [PubMed] [Google Scholar]
- 16. Linde C, Abraham WT, Gold MR, Daubert C; REVERSE Study Group . Cardiac resynchronization therapy in asymptomatic or mildly symptomatic heart failure patients in relation to etiology: results from the REVERSE (REsynchronization reVErses Remodeling in Systolic Left vEntricular Dysfunction) study. J Am Coll Cardiol 2010;56:1826–1831. [DOI] [PubMed] [Google Scholar]
- 17. Chen Y, Duan C, Liu F, Shen S, Chen P, Bin J. Impact of etiology on the outcomes in heart failure patients treated with cardiac resynchronization therapy: a meta‐analysis. PLoS One 2014;9:e94614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McMurray JJ, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Lohlavek JB, Bohm M, Chiang CE, Chopra VK, De Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CE, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 19. Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA‐REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care 2016;39:1108–1114. [DOI] [PubMed] [Google Scholar]
- 20. Kappel BA, Lehrke M, Schütt K, Artati A, Adamski J, Lebherz C, Marx N. Effect of empagliflozin on the metabolic signature of patients with type 2 diabetes mellitus and cardiovascular disease. Circulation 2017;136:969–972. [DOI] [PubMed] [Google Scholar]
- 21. Lopaschuk GD, Verma S. Empagliflozin's fuel hypothesis: not so soon. Cell Metab 2016;24:200–202. [DOI] [PubMed] [Google Scholar]
- 22. Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med 2017;104:298–310. [DOI] [PubMed] [Google Scholar]
- 23. Januzzi JL, Butler J, Jarolim P, Sattar N, Vijapurkar U, Desai M, Davies MJ. Effects of canagliflozin on cardiovascular biomarkers in older adults with type 2 diabetes. J Am Coll Cardiol 2017;70:704–712. [DOI] [PubMed] [Google Scholar]
- 24. Packer M, Anker SD, Butler J, Filippatos G, Zannad F. Effects of sodium‐glucose cotransporter 2 inhibitors for the treatment of patients with heart failure – proposal of a novel mechanism of action. JAMA Cardiol 2017;2:1025–1029. [DOI] [PubMed] [Google Scholar]
- 25. Garvey WT, Gaal L Van, Leiter LA, Vijapurkar U, List J, Cuddihy R, Ren J, Davies MJ. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism 2018;85:32–37. [DOI] [PubMed] [Google Scholar]
- 26. Sato T, Aizawa Y, Yuasa S, Kishi S, Fuse K, Fujita S, Ikeda Y, Kitazawa H, Takahashi M, Sato M, Okabe M. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol 2018;17:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McMurray JJ, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD; DAPA‐HF Committees and Investigators . A trial to evaluate the effect of the sodium‐glucose co‐transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA‐HF). Eur J Heart Fail 2019;21:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc Ser B Stat Methodol 2000;62:711–730. [Google Scholar]
- 29. Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, Demets DL, Inzucchi SE, Køber L, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Lindholm D, Niklasson A, Sjöstrand M, Langkilde AM, McMurray JJ. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA‐HF trial. Circulation 2020;141:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McMurray JJ, Krum H, Abraham WT, Dickstein K, Køber LV, Desai AS, Solomon SD, Greenlaw N, Ali MA, Chiang Y, Shao Q, Tarnesby G, Massie BM; ATMOSPHERE Committees Investigators . Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N Engl J Med 2016;374:1521–1532. [DOI] [PubMed] [Google Scholar]
- 31. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 32. Teerlink JR, Diaz R, Felker GM, McMurray JJ, Metra M, Solomon SD, Adams KF, Anand I, Arias‐Mendoza A, Biering‐Sørensen T, Böhm M, Bonderman D, Cleland JG, Corbalan R, Crespo‐Leiro MG, Dahlström U, Diaz R, Echeverria Correa LE, Fang JC, Filippatos G, Fonseca C, Goncalvesova E, Goudev AR, Howlett JG, Lanfear DE, Lund M, Macdonald P, Mareev V, Momomura SI, O'Meara E, Parkhomenko A, Ponikowski P, Ramires FJ, Serpytis P, Sliwa K, Spinar J, Suter TM, Tomcsanyi J, Vandekerckhove H, Vinereanu D, Voors AA, Yilmaz MB, Zannad F, Sharpsten L, Legg JC, Abbasi SA, Varin C, Malik FI, Kurtz CE; GALACTIC‐HF Investigators . Omecamtiv mecarbil in chronic heart failure with reduced ejection fraction: GALACTIC‐HF baseline characteristics and comparison with contemporary clinical trials. Eur J Heart Fail 2020;22:2160–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson Å, Hradec J, Jánosi A, Kamenský G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, Van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J; CORONA Group . Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007;357:2248–2261. [DOI] [PubMed] [Google Scholar]
- 34. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G; GISSI‐HF Investigators . Effect of n‐3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI‐HF trial): a randomised, double‐blind, placebo‐controlled trial. Lancet 2008;372:1223–1230. [DOI] [PubMed] [Google Scholar]
- 35. Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, Mohr JP, Massie BM, Labovitz AJ, Anker SD, Lok DJ, Ponikowski P, Estol CJ, Lip GY, Di Tullio MR, Sanford AR, Mejia V, Gabriel AP, Del Valle ML, Buchsbaum R; WARCEF Investigators . Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med 2012;366:1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zannad F, Anker SD, Byra WM, Cleland JG, Fu M, Gheorghiade M, Lam CS, Mehra MR, Neaton JD, Nessel CC, Spiro TE, Van Veldhuisen DJ, Greenberg B; COMMANDER HF Investigators . Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med 2018;379:1332–1342. [DOI] [PubMed] [Google Scholar]
- 37. Packer M. What causes sudden death in patients with chronic heart failure and a reduced ejection fraction? Eur Heart J 2020;41:1757–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Faggiano P, d'Aloia A, Gualeni A, Gardini A, Giordano A. Mechanisms and immediate outcome of in‐hospital cardiac arrest in patients with advanced heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 2001;87:655–657, A10–1. [DOI] [PubMed] [Google Scholar]
- 39. Luu M, Stevenson WG, Stevenson LW, Baron K, Walden J. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation 1989;80:1675–1680. [DOI] [PubMed] [Google Scholar]
- 40. Cleland JG, Thygesen K, Uretsky BF, Armstrong P, Horowitz JD, Massie B, Packer M, Poole‐Wilson PA, Rydén L; ATLAS Investigators . Cardiovascular critical event pathways for the progression of heart failure; a report from the ATLAS study. Eur Heart J 2001;22:1601–1612. [DOI] [PubMed] [Google Scholar]
- 41. Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JG, Dargie HJ, Granger CB, Kjekshus J, Køber L, Latini R, Maggioni AP, Packer M, Pitt B, Solomon SD, Swedberg K, Tavazzi L, Wikstrand J, Zannad F, Zile MR, McMurray JJ. Declining risk of sudden death in heart failure. N Engl J Med 2017;377:41–51. [DOI] [PubMed] [Google Scholar]
- 42. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS‐HF Study Group . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21.21073363 [Google Scholar]
- 43. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 44. Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN; SOLVD Investigators . Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 45. Eichhorn EJ, Domanski MJ, Krause‐Steinrauf H, Bristow MR, Lavori PW; Beta‐Blocker Evaluation of Survival Trial Investigators . A trial of the beta‐blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001;344:1659–1667. [DOI] [PubMed] [Google Scholar]
- 46. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Lancet 1999;353:2001–2007. [PubMed] [Google Scholar]
- 47. The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- 48. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 49. Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL; Carvedilol Prospective Randomized Cumulative Survival Study Group . Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651–1658. [DOI] [PubMed] [Google Scholar]
- 50. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH; Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators . Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 51. Moss AJ, Zareba W, Jackson Hall W, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML; Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 52. Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators . N Engl J Med 1999;341:1882–1890. [DOI] [PubMed] [Google Scholar]
- 53. Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol 2002;39:210–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Distribution of aetiology subgroups according to geographical region.
Table S2. Time to first event according to aetiology.
Table S3. Effects of dapagliflozin compared with placebo on clinical events according to subgroups of non‐ischaemic aetiology.
Table S4. Adverse events of dapagliflozin compared with placebo according to subgroups of non‐ischaemic aetiology.
Table S5. Effects of dapagliflozin compared with placebo according to aetiology and diabetes status at baseline.
Table S6. Baseline characteristics of the study population according to aetiology (unknown excluded).
Table S7. Effects of dapagliflozin compared with placebo on clinical events according to aetiology (unknown excluded).
Table S8. Adverse events of dapagliflozin compared with placebo according to aetiology (unknown excluded).
Figure S1. Effect of dapagliflozin on worsening heart failure or cardiovascular death according to baseline left ventricular ejection fraction in patients with and without ischaemic aetiology.


