Abstract
Background
Atopic dermatitis (AD) is one of the most common inflammatory skin diseases worldwide and Staphylococcus aureus colonization and secondary infections occur in the majority of AD patients. Allergic sensitizations against microbial antigens have been discussed as possible trigger factors of AD. Recently, we reported IgE sensitization against fibronectin‐binding protein 1 (FBP1), an essential virulence component in S. aureus, in a subgroup of patients suffering from AD. To expand these findings by investigating delayed‐type immune reactions, the objective of this study was to detect and phenotypically characterize FBP1‐specific T cells as possible trigger factors in AD.
Methods
Immunodominant T‐cell epitopes were mapped by proliferation testing of patient‐derived FBP1‐specific T‐cell lines after stimulation with single 15mer peptides, which were derived from different functional domains of the FBP1 sequence. Major histocompatibility complex class II tetramers carrying immunodominant epitopes successfully stained T helper cells in 8 out of 8 HLA‐matched, IgE‐sensitized AD patients.
Results
Cytokine profiling of multimer‐sorted cells revealed that predominantly the type 2 cytokines IL‐13 and IL‐4 were secreted by these cells. In contrast, IL‐17, the marker cytokine for response to extracellular pathogens, was scarcely detectable.
Conclusions
We demonstrate that FBP1 contains immunodominant peptides that induce a specific pro‐inflammatory T helper cell response with increased Th2 levels that can drive an allergic inflammation in sensitized AD patients.
Keywords: antigen‐specific T cells, atopic dermatitis, fibronectin‐binding protein 1, staphylococcus aureus
By means of MHC multimer staining, we describe quantity and quality of FBP1‐specific T cells in patients suffering from atopic dermatitis. We demonstrate that FBP1‐specific T cells drive an allergic type 2 response in atopic dermatitis patients. Our study highlights the role of FBP1 as a microbial allergen and its potential to aggravate atopic dermatitis.

Abbreviations: FACS, fluorescence‐activated cell sorting; FBP1, fibronectin‐binding protein 1; HLA‐DRB1, major histocompatibility complex, class II, DR beta 1; MHC, major histocompatibility complex, Th, T helper cell; S. aureus, Staphylococcus aureus.

1. INTRODUCTION
Atopic dermatitis (AD) is a common inflammatory skin disease with a prevalence of 13% in children and up to 7% in adults. 1 , 2 A hallmark of AD is T‐cell infiltration into the skin, and T cells have been shown to be predominantly of a Th2 phenotype, while also Th1, Th17, and Th22 polarized T cells play important roles. 3 , 4 , 5
The most common complication of AD is secondary Staphylococcus aureus infection. 6 Considering that S. aureus colonizes the lesional skin of nearly 90% of AD patients, knowledge on S. aureus factors that may trigger the disease represents an urgent need. 7 With technologies such as the 16S ribosomal sequencing, Kong et al. 8 showed a significant skin microbiome shift toward S. aureus dominance on lesional AD skin in AD pediatric patients that was restored after the inflammation was resolved. A positive correlation with disease severity has been suggested, and bacterial diversity diminishes with S. aureus prevalence. 8 , 9 Simpson et al. 10 analyzed a cohort of 96 AD patients and found S. aureus skin colonization to be associated with more severe clinical symptoms of AD, less type 2 inflammation, and weaker barrier disruption. The pathological role of S. aureus in exacerbating inflammation has been studied most intensively regarding toxins which act as superantigens (SAs) by causing non‐specific activation of T cells and a subsequent inflammatory cytokine release. 11 , 12 , 13 Many IgE‐reactive SAs among bacterial enterotoxins have been described such as Staphylococcal enterotoxin A, B, and E. However, IgE sensitization also occurs to antigens without superantigen capacities. We identified earlier fibronectin‐binding protein 1 (FBP1) and other S. aureus antigens by binding to patients IgE followed by mass spectrometry. 14 , 15 FBP1 is a S. aureus MSCRAMM surface protein (acronym for "microbial surface components recognizing adhesive matrix molecules") and is essential in invasion and adhesion to epithelial cells. This is achieved by binding to fibronectin, an extracellular matrix protein present on host cells. 16 , 17 , 18 S. aureus strains carrying a mutated FBP1 showed remarkably reduced colonization rate and decrease in mortality upon infected mice. 19 Following presentation by antigen‐presenting cells, FBP1 triggers T‐cell proliferation in AD patients in comparable levels to classical allergens, accompanied by a high release of pro‐inflammatory cytokines. 14 We showed earlier that PBMC (Peripheral Blood Mononuclear Cells) from sensitized AD patients released the cytokines IFN‐γ, IL‐6, and TNF‐α in response to FBP1. These cytokines have been described in response to S. aureus 20 also by others and may contribute to a cytokine milieu that favors development of Th17 cells. Th17 cells have been shown to be present in the acute phase of AD whenever TGF‐β and IL‐1 are also present at the site of inflammation. Our in vivo experiments revealed that mice sensitized to FBP1 show a specific T‐cell proliferation and a mixed Th2/Th1 FBP1‐specific T‐cell phenotype, along with basophil degranulation. 14
To characterize FBP1‐specific T cells in patients suffering from AD in a state‐of‐the‐art manner, we identified FBP1 immunodominant epitopes in this study which led to the synthesis of MHC (major histocompatibility complex) class II tetramers.
2. MATERIALS AND METHODS
2.1. Study patients
Adult patients suffering from AD were consecutively recruited from the Hannover Medical School Department of Dermatology and Allergy's outpatient clinic. All patients recruited gave written informed consent, and this study was approved by the Hannover Medical School ethics committee (7565). The patients included were diagnosed with mild to very severe atopic dermatitis according to the Hanifin and Rajka Criteria for AD. 21 Disease severity was assessed by SCOring Atopic Dermatitis (SCORAD). Investigators global assessment (IGA) was performed, ranging from 0 (clear), over 1 (mild), 2 (moderate) to 3 (severe). All patients recruited were not under systemic therapy. Patients selected for tetramer staining were HLA‐typed for HLA‐DRB1*15 via specific PCR primers as described below.
2.2. Proteins and peptides
Recombinant FBP1 was produced as [His]6‐tagged fusion protein in Escherichia coli and purified by means of Ni2+ affinity chromatography, as described previously. 14 LPS contamination was determined by LAL test (Limulus Amebocyte Lysate, Pyrochrome) to be virtually absent (<5 EU/ml). Single peptides were synthesized at Peptides and Elephants, Hennigsdorf, Germany.
2.3. IgE sensitization testing
To identify patients with an IgE sensitization to FBP1, a semi‐quantitative ELISA was performed. Therefore, ELISA plates (Nunc maxisorb, 442404) were coated overnight with 5 µg/ml recombinant human FBP1 in PBS. Unspecific binding was inhibited by 1h incubation with 1% bovine serum albumin (BSA) in PBS. Patients' sera were applied undiluted in duplicates for 16 h at 4°C. After washing, bound IgE was detected by 2 µg/ml mouse‐anti‐human IgE (BectonDickinson, clone G7‐26) in PBS/1%BSA, followed by Streptavidin‐horseradish peroxidase (Biotechne) in PBS/1%BSA and tetramethylbenzidine/hydrogen peroxide (substrate reagent pack, Biotechne). Patients were regarded positive if the observed value exceeded the mean of all samples measured by three standard deviations. Levels of staphylococcal enterotoxin B (SEB)‐specific IgE were quantified by ImmunoCAP FEIA (Thermo Fisher). Statistical test performed in Figure 1A was Mann‐Whitney test.
FIGURE 1.
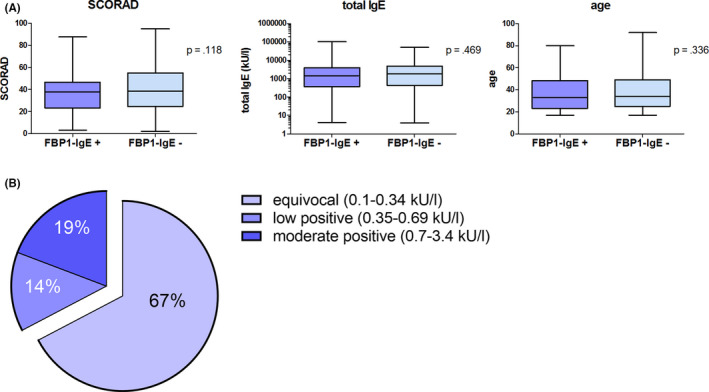
IgE sensitization toward FBP1 in AD patients. A, Comparison between FBP1‐sensitized (FBP1‐IgE+, n = 133) vs. non‐sensitized (FBP1‐IgE‐, n = 300) AD patients regarding disease severity (Scoring AD, SCORAD), total IgE level (kU/L) and age of subjects. p‐values were calculated by Mann‐Whitney test. B, SEB sensitization according to IgE levels (kU/L) in FBP1‐sensitized AD patients
2.4. Generation of T‐cell lines
Fresh human PBMC were isolated from the blood of 21 patients with a positive result in the FBP1‐ELISA by density‐gradient centrifugation and cultured in presence or absence of 2.5 μg/ml antigen at a density of 1 × 106/ml in Iscove's medium supplemented with 4% human heat‐inactivated AB serum, 2 mM glutamine, 50 mg/ml of gentamicin, 100 mg/ml penicillin/streptomycin, and nonessential amino acids (referred to as cell culture medium, CCM). After 7 days, rhIL‐2 (10 U/ml) was added and after 14 days, cells were expanded with allogeneic, irradiated PBMC (55 Gy) as feeder cells in the presence of phytohemagglutinin (10 μg/ml) and rhIL‐2 (10 U/ml).
2.5. Proliferation assay
The resulting cell lines were submitted to re‐stimulation testing after 3 weeks. Therefore, autologous PBMC (7.5 × 104) were irradiated (55 Gy) and incubated with T‐cell lines (5 × 103) to present recombinant FBP1 (2.5 μg/ml) in a total volume of 200 μl cell culture medium. Experiments were performed in triplicates. After 5–7 days, 3H‐thymidine (18,500 Bq/well) was added to the culture and incubated overnight to measure incorporation using a beta counter (Microbeta2, PerkinElmer). A stimulation index following FBP1 stimulation of greater than 1.5 was regarded as positive, and in those cell lines, the proliferative capacity toward putative epitope peptides was assessed by 3H‐thymidine incorporation.
2.6. HLA‐typing
HLA‐DRB1 genotype of 22 FBP1‐sensitized AD patients enrolled in the epitope mapping was assessed by PCR with sequence‐specific primers. To enlarge the subgroup of FBP1‐sensitized AD patients that express the HLA type HLA‐DRB1*15, further donors were screened for HLA‐DRB1*15 in a targeted manner applying sequence‐specific primers following a published protocol. 22
2.7. Tetramer staining and cell sorting
Fresh PBMC were isolated from 8 FBP1‐sensitized AD patients that express the HLA type HLA‐DRB1*15 as mentioned above. Cells were cultured in the presence or absence of 10 μg/ml peptide 12, 16, and 17, respectively. As controls, three healthy donors expressing HLA‐DR1*15 as well as three healthy donors not expressing HLA‐DR1*15 were included. At days three, five, eight, and eleven, rhIL‐2 (20 U/ml) was added to the culture. At day 14, the peptide‐stimulated cells were stained for 1 h at room temperature with MHC class II tetramers harboring peptide epitopes 12, 16, or 17, respectively. MHC tetramers were supplied by the Tetramer Core Facility of the Benaroya Research Institute. Following that, cells were stained by anti‐CD4‐allophycocyanin (Beckman Coulter), anti‐CD14‐brilliant violet 510 (Biolegend), and anti‐CD19‐brilliant violet 510 (Biolegend). MHC tetramer+/CD4+/CD14−/CD19− cells were sorted on a FACSAria™ Fusion in the Hannover Medical School Research Facility Cell Sorting. The sorted cells were stimulated using 10 μl of concanavalin A (Sigma‐Aldrich) and after 2 days of incubation, the supernatants were collected. Of each donor, corresponding cells were cultured under the same conditions but in absence of peptides. These cultures served as controls in our experiments to substract background cytokine levels after ConA stimulation.
2.8. Luminex multiplex assay
The resulting cell culture supernatants were analyzed by the Millipore hu Th17 13‐Plex (Bio‐Rad) assay, which was performed according to manufacturer's instructions to analyze IL‐17F, IFNγ, IL‐17A, IL‐9, IL‐2, IL‐4, IL‐5, IL‐31, IL‐10, IL‐13, IL‐21, IL‐17E/IL‐25, and TNF‐α.
3. RESULTS
3.1. In silico prediction of immunodominant FBP1 peptides
15mer peptides were chosen by applying two T‐cell epitope prediction algorithms, SYFPEITHI 23 and the consensus algorithm of the immune epitope database (IEDB) 24 , to the FBP1 sequence S. aureus (GeneBank HM245235, strain RN450). Relevant test values are depicted in Table 1. MHC binding to HLA‐DRB1*01:01, DRB1*04:01, and DRB1*15:01 was taken into account since those occur with comparably high frequencies in the German population. FBP1 contains two known functional domains: The FbpA domain is conserved among pathogenic proteins that bind fibronectin to enter host cells, the DUF418 domain is of so far unknown function. Peptides 1‐14 are located in the FbpA domain, peptides 15 and 16 in the DUF418 domain, and peptide 17 at the C‐terminus (compare 20).
TABLE 1.
In silico prediction of immunodominant FBP1 peptides
| No | Position | Sequence | DRB1*01:01 | DRB1*04:01 | DRB1*15:01 | ||||
|---|---|---|---|---|---|---|---|---|---|
| SYFPEITHI | IEDB | SYFPEITHI | IEDB | SYFPEITHI | IEDB | ||||
| 1 | 12 | 27 | VESLQFLTTGRVHKI | 31 | 10.00 | 26 | 6.15 | 18 | 4.38 |
| 2 | 45 | 60 | HQLLLSIHPNFSRLQ | 28 | 9.82 | 20 | 1.64 | 18 | 2.68 |
| 3 | 115 | 130 | RTVILEIMGKHSNLI | 31 | 15.82 | 20 | 7.96 | 18 | 1.18 |
| 4 | 126 | 141 | SNLILVDENRKIIEG | 26 | 3.79 | ||||
| 5 | 138 | 153 | IEGFKHLTPNTNHYR | 25 | 6.34 | 22 | 0.99 | ||
| 6 | 148 | 163 | TNHYRTVMPGFNYEA | 22 | 15.38 | ||||
| 7 | 169 | 184 | INPYDITGAEVLKYI | 30 | 16.56 | ||||
| 8 | 179 | 194 | VLKYIDFNAGNIAKQ | 26 | 30.98 | 22 | 7.96 | 18 | 9.93 |
| 9 | 198 | 213 | FEGFSPLITNEIVSR | 32 | 12.77 | 28 | 2.15 | ||
| 10 | 212 | 227 | RRQFMTSSTLPEAFD | 16 | 1.06 | ||||
| 11 | 222 | 237 | PEAFDEVMAETKLPP | 22 | 16.71 | ||||
| 12 | 298 | 313 | QQQLHKYQNKLAKLI | 20 | 12.1 | 28 | 1.76 | ||
| 13 | 324 | 339 | EQLYGELITANIYRI | 32 | 12.43 | 28 | 2.19 | ||
| 14 | 354 | 369 | EEVVIPLNPTKSPSA | 32 | 19.54 | 20 | 2.83 | ||
| 15 | 490 | 505 | VVIFNDAPSDTTIKE | 28 | 4.1 | ||||
| 16 | 522 | 537 | PVDYTLIKNVHKPSG | 28 | 0.96 | ||||
| 17 | 539 | 554 | PGFVTYDNQKTLYAT | 22 | 2.11 | 26 | 8.28 | ||
Test values from SYFPEITHI and IEDB (consensus algorithm) are depicted for the HLA‐types as indicated. Only relevant values are depicted.
3.2. In vitro identification of immunodominant FBP1 epitopes
Patients were included in the epitope mapping studies upon positive testing for FBP1‐specific serum IgE by ELISA (n = 21). No association between patient severity and response to FBP1 was observed. Further information on those patients can be found in Table S1. Among testing a wider patient cohort, we observed that IgE sensitization frequency to FBP1 was not associated with age, disease severity as determined by SCORAD or total IgE levels (Figure 1A). IgE sensitization to SEB was detectable in several (33%) but not the majority of the FBP1‐sensitized AD patient subgroup (Figure 1B), as reported for AD patients in general by Leung et al. 1993. 25
T‐cell lines were generated from FBP1‐sensitized patients' PBMCs in the presence of recombinant FBP1 according to an established protocol. 26 After 21 days, proliferative capacity to recombinant FBP1 was tested by re‐stimulation with autologous irradiated PBMCs as antigen‐presenting cells. T‐cell lines from 10 patients (out of 21 investigated patients) displayed distinct proliferation to recombinant FBP1 and were therefore considered as FBP1‐specific. In these T‐cell lines, the proliferative capacity toward each of the seventeen FBP1 15mer peptides was measured by 3H‐thymidine incorporation. Top proliferation indices were observed for the peptides 12, 16, and 17 (Figure 2). Since HLA‐typing of reactive T‐cell lines revealed a relative high proportion of HLA‐DRB1*15:01‐positive donors and prediction algorithms displayed strong values regarding HLA‐DRB1*15:01 (Table 1), this HLA was chosen for generating MHC class II tetramers.
FIGURE 2.
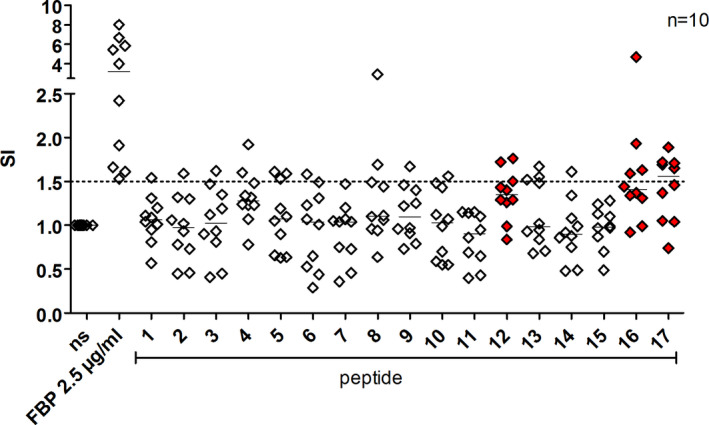
T helper cell epitope mapping of FBP1. Seventeen 15mer peptides of FBP1 that were predicted candidate binders by T‐cell epitope prediction algorithms were synthesized and tested for T‐cell proliferation by 3H‐thymidine intake in FBP1‐reactive T‐cell lines derived from FBP1‐IgE‐sensitized AD patients. ns, non‐stimulated control cells. Stimulation index (SI) is calculated as the ratio between protein or peptide‐stimulated and unstimulated control cells. The three peptide epitopes displaying the highest proliferative capacity are depicted in red. Horizontal lines represent the median values
Patients that displayed a T‐cell proliferation response toward FBP1 did not differ from those who did not in terms of SCORAD, total IgE, or IGA (Figure S1).
3.3. Direct effects of S. aureus FBP1 on leukocytes
Allergens often possess intrinsic capacities to interact with the immune system, which push the immune response to Th2, for example, due to homology to parasites. 27 Furthermore, several allergens function as proteases, a mechanism shared with parasites, or act as adjuvants by activating the innate immune system. 28 , 29 To investigate whether FBP1 is capable of inducing immune responses by intrinsic properties, cytokine levels were measured in supernatants of patient‐derived PBMC after 48 h incubation with recombinant FBP1 with or without IL‐2. In our set of experiments, there was a trend of a higher median of secreted IL‐13 but also of IL‐17, IL‐22, and IFN‐γ upon stimulation of the cells with FBP1 in combination with IL‐2 (Figure S2).
3.4. Presence of T cells specific for immunodominant FBP1 epitopes in sensitized AD patients
Applying MHC tetramers engineered on the basis of the three immunodominant epitopes, respective peptide‐specific T cells were detected, quantified, and sorted from the blood of 8 FBP1‐sensitized HLA‐DRB1*15:01 matched AD patients. Figure 3 shows staining of FBP1‐specific CD4+ T‐cell lines generated in the presence or absence of the corresponding peptide. Without peptide stimulation prior to tetramer staining, only low frequencies of positive cells were observed that did often not exceed background levels. After peptide stimulation for 3 weeks, distinct populations of positive cells became detectable (frequencies within CD4+ cells: tetramer 12: 1.34% ± 1.55%; tetramer 16: 0.44% ± 0.33%; tetramer 17: 0.32% ± 0.28%; n = 8 for all three tetramers). Detailed information on the responsiveness of each donor is given in Table S2.
FIGURE 3.
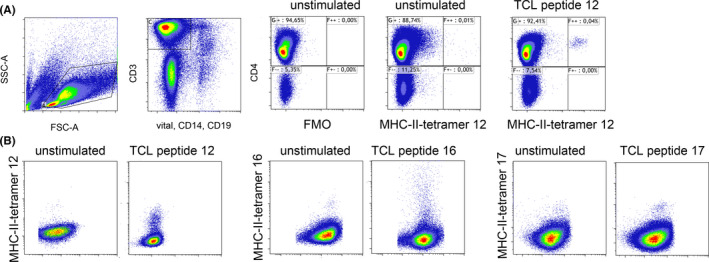
Major histocompatibility complex II tetramer staining of FBP1‐specific T helper cells. A, MHC II tetramers harboring FBP1 peptide12 stained specifically a subgroup of CD4+ T cells in a 21‐day T‐cell line (TCL peptide 12) grown from a sensitized DRB1*15:01 AD patient's PBMC in the presence of peptide12. Without stimulation, no distinct population is detectable (unstimulated). FMO, fluorescence minus one control lacking MHC II tetramer. B, exemplary MHC II tetramers staining of 3‐week T‐cell lines generated from PBMC of DRB1*15:01 AD subjects in presence or absence of FBP1 peptide12, peptide16, and peptide17 followed by staining with their respective tetramer reagents
3.5. Fibronectin‐binding protein 1 peptide‐specific T cells show a distinct pro‐inflammatory profile
After sorting of the FBP1 peptide‐specific T cells from the 21 days T‐cell lines of the 8 FBP1‐sensitized AD patients, we aimed to characterize the T‐cell polarization based on their cytokine expression by Luminex multiplex ELISA. Detected amounts of target cytokines in the cell culture supernatants of sorted and mitogen‐activated peptide‐specific T cells are shown in Figure 4. Interestingly, the overall cytokine profile resembled a type 2/type 1 response. The highest levels were reached by type 2 cytokines IL‐13 and IL‐4, while IL‐17 was barely measurable. Further on, a considerable stimulation of IFN‐γ could be observed. In healthy HLA‐matched subjects, we were not able to detect sufficient amounts of FBP1‐specific T cells to perform similar measurements (data not shown).
FIGURE 4.
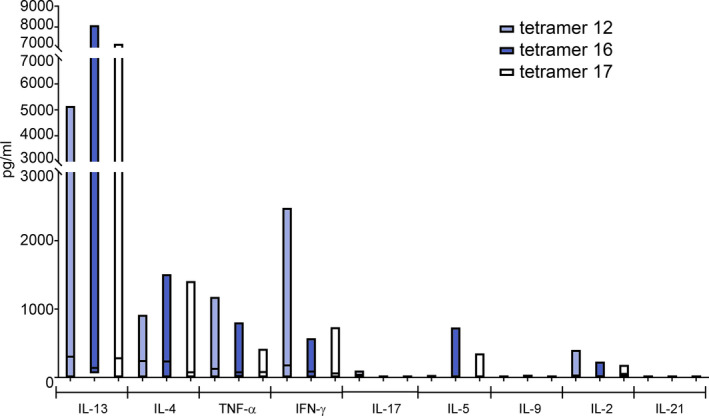
Cytokine expression capacities of FBP1‐specific T cells derived from FBP1‐sensitized AD patients. TCLs were grown in presence of respective peptide epitopes for 2 weeks. FBP1‐specific T cells were sorted after MHC‐tetramer staining from TCLs and activated with concanavalin A (ConA). Cytokine levels of corresponding cells cultured in absence of peptides before ConA activation were subtracted. Tetramer12 n = 7; tetramer16 n = 5; tetramer17 n = 6
4. DISCUSSION
It is generally accepted that allergen‐specific T cells are a major contributor to the allergic response in atopic patients, particularly in those suffering from late phase and delayed‐type allergic reactions such as AD, certain forms of asthma and chronic allergic rhinitis. 30
While individuals usually mount a type 1/type 3 immune response to antigens derived from microbial pathogens, 31 patients suffering from atopic diseases also display hypersensitivity reactions toward bacterial antigens. 32 In bronchial asthma, S. aureus SAs specific IgEs levels could be detected in serum and were associated with disease severity. It has been also demonstrated that S. aureus SAs specific IgE can predict the development of severe asthma with exacerbations over a long duration. 33 , 34 , 35 In allergic rhinitis (AR), it has been demonstrated that specific IgE to several S. aureus SAs including SEA, SEB, and TSST‐1 was detected in patients' serum contributing to the chronic disease course. 36
The type 2 inflammation bias is induced by S. aureus virulence factors, like enterotoxins, serine protease‐like proteins, and FBP1 as described here. Underlying mechanisms include the release of IL‐33 from the epithelium, activation of Th2 cells and innate lymphoid cells with subsequent release of type 2 cytokines eventually leading to epithelial damage via mast cell degranulation, B‐cell activation, and airway eosinophilia. 37 Studies in mice suggest that this is of pathological relevance, since a pre‐existing allergy led to diminished pathogen responses. 38 Interestingly, a recent study showed that S. aureus proteins were among the most frequent underdiagnosed allergens proposing that the pathogenesis of non‐allergic compared to allergic asthma is more similar than what was previously assumed. 39 This would advocate for including S. aureus SA proteins among the standard allergens tested by allergists to improve patients' outcome.
State of the art in detecting allergen‐specific T cells is the MHC tetramer staining technology. High sensitivity, epitope specificity, and cell viability are believed to be the main advantages of this technology compared to other methods. 40 The characterization of fine specificity and functions of FBP1‐specific T cells shown here for the first time confirms and extends our previous observations made on AD patients and a mouse model of FBP1 hypersensitivity, both depicting a type 1/type 2 response to this S. aureus protein. Type 1 cytokines have been described together with type 3 cytokines to represent the natural immune response to S. aureus in healthy donors, 31 while also type 2 responses have been described to single proteins. 31 , 41 Besides type 2 responses, also type 1 cytokines have been reported in AD inflammation, which have been linked to disease chronicity. 3 , 42 The overweight of type 2 over type 1 cytokines observed here is of interest, since it may explain the frequent IgE sensitizations to S. aureus and give an explanation on the findings that S. aureus even appears to precede AD 43 or allergy in general. 41 Further, IgE sensitization to bacterial antigens may be caused through house dust mites who carry bacteria with them. 44
While in AD the predominant role of Th2‐polarized T cells is well established, additional T‐cell subtypes such as Th17 and Th22 T cells have been described to play important roles. 4 , 5 , 26 Since type 3 cytokines play key roles in fighting extracellular pathogens and S. aureus, 31 it is a current matter of debate if microbial allergens might contribute to the Th17 polarization in AD. Specific T cells targeting microbial allergens have been described, for example, to Malassezia ssp., where IgE levels to Mala s 13 correlate with disease severity. 45 , 46 We described earlier that Mala s 13 bears the potential to cross‐react with a human homologous protein (thioredoxin), inducing an immune response containing IL‐17. 47 In this context, it is of interest that induction of TNF‐a and IL‐6 by FBP‐1 was reported before, 14 a combination known to induce Th17 cells. However, IL‐17 was only scarcely detectable, which might be explained by the overweight of type 1/type 2 cytokines. We show here that IgE and T‐cell sensitizations to allergens include bacterial antigens and contribute to the immune response driving the disease. Whether these sensitizations occur early in life or later as a consequence of an ongoing type 2 inflammation cannot be answered by this kind of study.
Targeting further pro‐inflammatory mediators may be the solution for non‐responders, since patients' allergen‐specific T cells show a range of diverse phenotypes. A limitation that we encountered in our patient cohort was that different topical medication including steroids was not assessed in the study patients, in addition to that different experiments were performed with different number of study patients.
Taken together, we describe a type 2/type 1 response to the S. aureus antigen FBP1 in sensitized AD patients. While the type 2 immune response is well‐known for its functions in parasite defense, it is most probably suboptimal in controlling S. aureus. It may be hypothesized that by inducing a type 2 immune response via FBP1 in this subset of sensitized patients, S. aureus evades the immune system and in parallel aggravates ongoing inflammation, and thereby facilitates further self‐growth and subsequent pathogenicity. Increased numbers of S. aureus on the skin lead in turn to an increased load of virulence factors that may further promote the skin inflammation combined with skin barrier defects. As a response, an increased suprabasal epidermal fibronectin deposition can be observed, 48 which may lead to a vicious circle (Figure 5). Type 1 cytokines like IFN‐γ are a substantial part of the immune response to S. aureus in healthy subjects 31 and appear to be outnumbered by type 2 responses in our measurements on FBP1. Targeted therapy that blocks type 2 immune responses has been shown recently to reduce S. aureus abundance and to increase bacterial diversity. 49 It may thus be assumed that interfering with S. aureus type 2‐mediated pathology is indeed beneficial for AD, and especially for FBP1‐sensitized AD patients.
FIGURE 5.
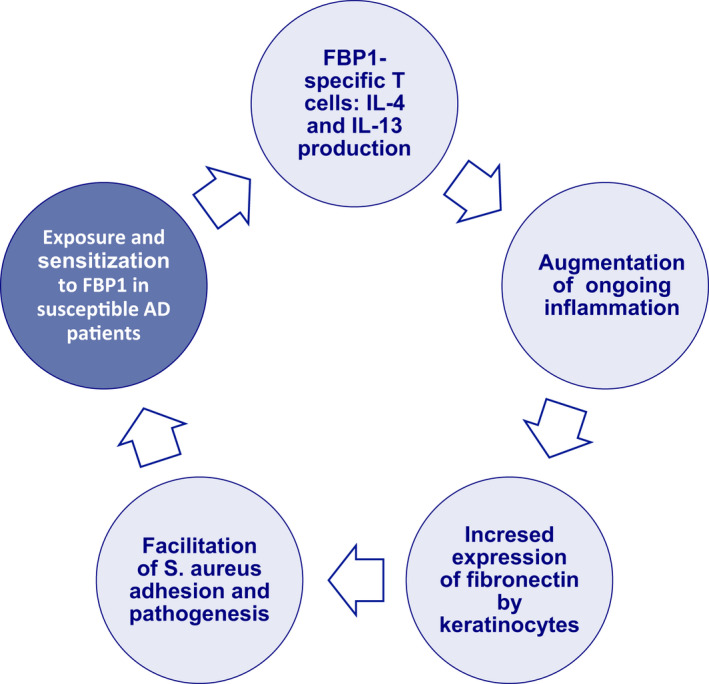
Immune response to S. aureus FBP1 may facilitate S. aureus skin colonization in FBP1‐sensitized AD patients. We hypothesize that the Th2‐dominated and therefore bona fide suboptimal immune response to FBP1 leads to a facilitation of S. aureus adhesion and invasion of lesional AD skin epithelium. S. aureus virulence factors lead to inflammation that mediates increased expression of fibronectin by human keratinocytes, which again supports further S. aureus skin infection
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Conceptualization: TW, LMR, SW, AH, RV. Methodology: AKF, LMR, SW, BEV, WWK. Investigation: AKF, LMR, SW. Visualization: LMR, AKF, SW. Writing – original draft: AKF, LMR. Writing – review & editing: TW, RV, BEV, WWK, AH.
Supporting information
Fig S1
Fig S2
Table S1‐S2
ACKNOWLEDGEMENTS
We would like to acknowledge Gabriele Begemann and Petra Kienlin for excellent technical assistance and in addition the assistance of the Cell Sorting Core Facility of the Hannover Medical School supported in part by Braukmann‐Wittenberg‐Herz‐Stiftung and Deutsche Forschungsgemeinschaft. Rudolf Valenta was supported by grant F4605 from the Austrian Science Fund (FWF) and is a recipient of a Megagrant of the Government of the Russian Federation, grant No 14.W03.31.0024. Parts of the study were funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy – EXC 2155 – Project ID 390874280. Biorender was used for designing the illustration in the graphical abstract.
Farag AK, Roesner LM, Wieschowski S , et al. Specific T cells targeting Staphylococcus aureus fibronectin‐binding protein 1 induce a type 2/type 1 inflammatory response in sensitized atopic dermatitis patients. Allergy. 2022;77:1245–1253. doi: 10.1111/all.15120
Ahmed K. Farag and Lennart M. Roesner contributed equally to this work.
Funding information
Braukmann‐Wittenberg‐Herz‐Stiftung and Deutsche Forschungsgemeinschaft. Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy – EXC 2155 – Project ID 390874280. Austrian Science Fund (FWF) F4605. Megagrant of the Government of the Russian Federation 14.W03.31.0024. The country of Lower Austria, Danuber ARC
REFERENCES
- 1. Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross‐sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583‐590. [DOI] [PubMed] [Google Scholar]
- 2. Lee HH, Patel KR, Singam V, Rastogi S, Silverberg JI. A systematic review and meta‐analysis of the prevalence and phenotype of adult‐onset atopic dermatitis. J Am Acad Dermatol. 2019;80(6):1526‐1532 e1527. [DOI] [PubMed] [Google Scholar]
- 3. Werfel T, Morita A, Grewe M, et al. Allergen specificity of skin‐infiltrating T cells is not restricted to a type‐2 cytokine pattern in chronic skin lesions of atopic dermatitis. J Invest Dermatol. 1996;107(6):871‐876. [DOI] [PubMed] [Google Scholar]
- 4. Eyerich K, Pennino D, Scarponi C, et al. IL‐17 in atopic eczema: linking allergen‐specific adaptive and microbial‐triggered innate immune response. J Allergy Clin Immunol. 2009;123(1):59‐66 e54. [DOI] [PubMed] [Google Scholar]
- 5. Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Investig. 2009;119(12):3573‐3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alexander H, Paller AS, Traidl‐Hoffmann C, et al. The role of bacterial skin infections in atopic dermatitis: expert statement and review from the international eczema council skin infection group. Br J Dermatol. 2020;182(6):1331‐1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361(9352):151‐160. [DOI] [PubMed] [Google Scholar]
- 8. Kong HH, Oh J, Deming C, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22(5):850‐859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Byrd AL, Deming C, Cassidy SKB, et al. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med. 2017;9(397): eaal4651. 10.1126/scitranslmed.aal4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simpson EL, Villarreal M, Jepson B, et al. Patients with atopic dermatitis colonized with staphylococcus aureus have a distinct phenotype and endotype. J Invest Dermatol. 2018;138(10):2224‐2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langer K, Breuer K, Kapp A, Werfel T. Staphylococcus aureus‐derived enterotoxins enhance house dust mite‐induced patch test reactions in atopic dermatitis. Exp Dermatol. 2007;16(2):124‐129. [DOI] [PubMed] [Google Scholar]
- 12. Niebuhr M, Gathmann M, Scharonow H, et al. Staphylococcal alpha‐toxin is a strong inducer of interleukin‐17 in humans. Infect Immun. 2011;79(4):1615‐1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niebuhr M, Scharonow H, Gathmann M, Mamerow D, Werfel T. Staphylococcal exotoxins are strong inducers of IL‐22: a potential role in atopic dermatitis. J Allergy Clin Immunol. 2010;126(6):1176‐1183 e1174. [DOI] [PubMed] [Google Scholar]
- 14. Reginald K, Westritschnig K, Linhart B, et al. Staphylococcus aureus fibronectin‐binding protein specifically binds IgE from patients with atopic dermatitis and requires antigen presentation for cellular immune responses. J Allergy Clin Immunol. 2011;128(1):82‐91.e88. [DOI] [PubMed] [Google Scholar]
- 15. Reginald K, Westritschnig K, Werfel T, et al. Immunoglobulin E antibody reactivity to bacterial antigens in atopic dermatitis patients. Clin Exp Allergy. 2011;41(3):357‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Josse J, Laurent F, Diot A. Staphylococcal adhesion and host cell invasion: fibronectin‐binding and other mechanisms. Front Microbiol. 2017;8:2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards AM, Potter U, Meenan NA, Potts JR, Massey RC. Staphylococcus aureus keratinocyte invasion is dependent upon multiple high‐affinity fibronectin‐binding repeats within FnBPA. PLoS One. 2011;6(4):e18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cho SH, Strickland I, Boguniewicz M, Leung DY. Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. J Allergy Clin Immunol. 2001;108(2):269‐274. [DOI] [PubMed] [Google Scholar]
- 19. Shinji H, Yosizawa Y, Tajima A, et al. Role of fibronectin‐binding proteins A and B in in vitro cellular infections and in vivo septic infections by Staphylococcus aureus . Infect Immun. 2011;79(6):2215‐2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fournier B, Philpott DJ. Recognition of Staphylococcus aureus by the innate immune system. Clin Microbiol Rev. 2005;18(3):521‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta dermato‐venereologica. 1980;92:44‐47. [Google Scholar]
- 22. D'Alfonso S, Bolognesi E, Guerini FR, et al. A sequence variation in the MOG gene is involved in multiple sclerosis susceptibility in Italy. Genes Immun. 2008;9(1):7‐15. [DOI] [PubMed] [Google Scholar]
- 23. Chowell D, Krishna S, Becker PD, et al. TCR contact residue hydrophobicity is a hallmark of immunogenic CD8+ T cell epitopes. Proc Natl Acad Sci USA. 2015;112(14):E1754‐E1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moutaftsi M, Peters B, Pasquetto V, et al. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)‐cell responses to vaccinia virus. Nat Biotechnol. 2006;24(7):817‐819. [DOI] [PubMed] [Google Scholar]
- 25. Leung DY, Harbeck R, Bina P, et al. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest. 1993;92(3):1374‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roesner LM, Heratizadeh A, Begemann G, et al. Der p1 and Der p2‐specific T cells display a Th2, Th17, and Th2/Th17 phenotype in atopic dermatitis. J Invest Dermatol. 2015;135(9):2324‐2327. [DOI] [PubMed] [Google Scholar]
- 27. Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen‐induced T helper type 2 responses. Nat Immunol. 2008;9(3):310‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hradetzky S, Werfel T, Rosner LM. Autoallergy in atopic dermatitis. Allergo J Int. 2015;24(1):16‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jacquet A, Robinson C. Proteolytic, lipidergic and polysaccharide molecular recognition shape innate responses to house dust mite allergens. Allergy. 2020;75(1):33‐53. [DOI] [PubMed] [Google Scholar]
- 30. Valenta R, Karaulov A, Niederberger V, et al. Molecular aspects of allergens and allergy. Adv Immunol. 2018;138:195‐256. [DOI] [PubMed] [Google Scholar]
- 31. Kolata JB, Kuhbandner I, Link C, et al. The fall of a dogma? unexpected high T‐cell memory response to staphylococcus aureus in humans. J Infect Dis. 2015;212(5):830‐838. [DOI] [PubMed] [Google Scholar]
- 32. Muluk NB, Altin F, Cingi C. Role of superantigens in allergic inflammation: their relationship to allergic rhinitis, chronic rhinosinusitis, asthma, and atopic dermatitis. Am J Rhinol Allergy. 2018;32(6):502‐517. [DOI] [PubMed] [Google Scholar]
- 33. Liu JN, Shin YS, Yoo HS, et al. The prevalence of serum specific IgE to superantigens in asthma and allergic rhinitis patients. Allergy Asthma Immunol Res. 2014;6(3):263‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Losol P, Kim SH, Hwang EK, Shin YS, Park HS. IL‐5 promoter polymorphism enhances IgE responses to staphylococcal superantigens in adult asthmatics. Allergy Asthma Immunol Res. 2013;5(2):106‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tomassen P, Jarvis D, Newson R, et al. Staphylococcus aureus enterotoxin‐specific IgE is associated with asthma in the general population: a GA(2)LEN study. Allergy. 2013;68(10):1289‐1297. [DOI] [PubMed] [Google Scholar]
- 36. Rossi RE, Monasterolo G. Prevalence of serum IgE antibodies to the Staphylococcus aureus enterotoxins (SAE, SEB, SEC, SED, TSST‐1) in patients with persistent allergic rhinitis. Int Arch Allergy Immunol. 2004;133(3):261‐266. [DOI] [PubMed] [Google Scholar]
- 37. Bachert C, Humbert M, Hanania NA, et al. Staphylococcus aureus and its IgE‐inducing enterotoxins in asthma: current knowledge. Eur Respir J. 2020;55(4): 1901592. 10.1183/13993003.01592-2019 [DOI] [PubMed] [Google Scholar]
- 38. Totten AH, Xiao L, Luo D, et al. Allergic airway sensitization impairs antibacterial IgG antibody responses during bacterial respiratory tract infections. J Allergy Clin Immunol. 2019;143(3):1183‐1197 e1187. [DOI] [PubMed] [Google Scholar]
- 39. Schreiber J, Broker BM, Ehmann R, Bachert C. Nonatopic severe asthma might still be atopic: sensitization toward Staphylococcus aureus enterotoxins. J Allergy Clin Immunol. 2019;143(6):2279‐2280 e2272. [DOI] [PubMed] [Google Scholar]
- 40. Wambre E, James EA, Kwok WW. Characterization of CD4+ T cell subsets in allergy. Curr Opin Immunol. 2012;24(6):700‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stentzel S, Teufelberger A, Nordengrun M, et al. Staphylococcal serine protease‐like proteins are pacemakers of allergic airway reactions to Staphylococcus aureus . J Allergy Clin Immunol. 2016;139(2): 492‐500. [DOI] [PubMed] [Google Scholar]
- 42. Thepen T, Langeveld‐Wildschut EG, Bihari IC, et al. Biphasic response against aeroallergen in atopic dermatitis showing a switch from an initial TH2 response to a TH1 response in situ: an immunocytochemical study. J Allergy Clin Immunol. 1996;97(3):828‐837. [DOI] [PubMed] [Google Scholar]
- 43. Meylan P, Lang C, Mermoud S, et al. Skin colonization by staphylococcus aureus precedes the clinical diagnosis of atopic dermatitis in infancy. J Invest Dermatol. 2017;137(12):2497‐2504. [DOI] [PubMed] [Google Scholar]
- 44. Dzoro S, Mittermann I, Resch‐Marat Y, et al. House dust mites as potential carriers for IgE sensitization to bacterial antigens. Allergy. 2018;73(1):115‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johansson C, Ahlborg N, Andersson A, et al. Elevated peripheral allergen‐specific T cell response is crucial for a positive atopy patch test reaction. Int Arch Allergy Immunol. 2009;150(1):51‐58. [DOI] [PubMed] [Google Scholar]
- 46. Glatz M, Buchner M, von Bartenwerffer W, et al. Malassezia spp.‐specific immunoglobulin E level is a marker for severity of atopic dermatitis in adults. Acta Derm Venereol. 2015;95(2):191‐196. [DOI] [PubMed] [Google Scholar]
- 47. Balaji H, Heratizadeh A, Wichmann K, et al. Malassezia sympodialis thioredoxin‐specific T cells are highly cross‐reactive to human thioredoxin in atopic dermatitis. J Allergy Clin Immunol. 2011;128(1):92‐99 e94. [DOI] [PubMed] [Google Scholar]
- 48. Cho SH, Strickland I, Tomkinson A, Fehringer AP, Gelfand EW, Leung DY. Preferential binding of Staphylococcus aureus to skin sites of Th2‐mediated inflammation in a murine model. J Invest Dermatol. 2001;116(5):658‐663. [DOI] [PubMed] [Google Scholar]
- 49. Callewaert C, Nakatsuji T, Knight R, et al. IL‐4Ralpha Blockade by dupilumab decreases staphylococcus aureus colonization and increases microbial diversity in atopic dermatitis. J Invest Dermatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1‐S2


