Abstract
Nutrition and exercise metabolism are vibrant physiological fields, yet at times it feels as if greater progress could be made by better integrating these disciplines. Exercise is advocated for improving metabolic health, in part by increasing peripheral insulin sensitivity and glycaemic control. However, when a modest‐to‐high carbohydrate load is consumed before and/or during each exercise bout within a training programme, increases in oral glucose insulin sensitivity can be blunted in both men of a healthy weight and those with overweight/obesity. Exercise training‐induced adaptation in the energy sensing AMP‐activated protein kinase (AMPK) and the insulin‐sensitive glucose transporter GLUT4 protein levels are sensitive to pre‐exercise feeding status in both healthy individuals and individuals classified as overweight or obese. Increased lipid oxidation may, in part, explain the enhanced adaptive responses to exercise training performed before (i.e. fasted‐state exercise) versus after nutrient ingestion. Evidence in individuals with type 2 diabetes currently shows no effect of altering nutrient–exercise timing for measured markers of metabolic health, or greater reductions in glycated haemoglobin (HbA1c) concentrations with exercise performed after versus before nutrient provision. Since the metabolic inflexibility associated with type 2 diabetes diminishes differences in lipid oxidation between the fasted and fed states, it is plausible that pre‐exercise feeding status does not alter adaptations to exercise when metabolic flexibility is already compromised. Current evidence suggests restricting carbohydrate intake before and during exercise can enhance some health benefits of exercise, but in order to establish clinical guidelines, further research is needed with hard outcomes and different populations.
Keywords: energy balance, exercise, fasting, metabolic disease
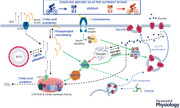
Abstract figure legend Candidate mechanisms linking nutrient–exercise timing to insulin sensitivity. Exercise performed in an overnight‐fasted state (before nutrient intake), increases fatty acid availability for skeletal muscle, and also increases intramuscular triglyceride utilisation. An increase in skeletal muscle lipid turnover may result in phospholipid remodelling, with a relative reduction in saturated fatty acids within skeletal muscle phospholipids. Increased fatty acid availability can also increase AMPK activity and this increase PGC‐1α levels. Exercise before versus after nutrient intake can also result in an increase in skeletal muscle GLUT4 and CHC22 levels, which are involved in insulin‐stimulated glucose transport. Akt, protein kinase B; AMPK, adenosine monophosphate‐activated protein kinase; CD36, fatty acid translocase; CHC22, clathrin heavy chain 22; CPT1, carnitine palmitoyltransferase 1; GLUT4, glucose transporter 4; GSV, GLUT4 storage vesicle; IMTG, intramuscular triglyceride; MUFA, monounsaturated fatty acid; OXPHOS, oxidative phosphorylation proteins; PGC‐1α, peroxisome proliferator‐activated receptor γ coactivator 1‐α; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid; TBC1D1/D4, TBC1 domain family member 1/family member 4 (AS160).

Introduction
Nutritional intake cannot be fully understood without accounting for physical activity status, and similarly, knowledge of exercise metabolism requires consideration of nutrient status. From an evolutionary perspective, it might be considered that humans would be well‐adapted to eat after exercise (versus before exercise) due to the need to perform physical activity in order to hunt or forage (Lieberman, 2015). However, it is also possible that humans could have evolved under conditions in which they ate carbohydrates whilst hunting and/or needed to run shortly after eating to avoid predation. Therefore, it is impossible to know with certainty under which conditions humans evolved under with respect to nutrient timing and physical activity. Nevertheless, it seems that nutrient timing does play an important role in regulating the acute and chronic effects of exercise. The role of physical activity (Bird & Hawley, 2017; Sylow & Richter, 2019) and nutrient–exercise timing (Haxhi et al. 2013; Wallis & Gonzalez, 2019; Aqeel et al. 2020; Mancilla et al. 2020) for improving aspects of metabolic health have been reviewed before. However, for nutrient–exercise timing, these reviews focused almost exclusively on acute metabolic responses to exercise. These reviews also called for further investigation into the role of exercise–nutrient interactions during training studies in populations at risk of, or diagnosed with, metabolic diseases. Several key studies have now been published, providing novel evidence on the role of nutrient–exercise timing in mediating health‐related exercise adaptations in humans. The aims of this review are therefore to highlight (1) the latest research on nutrient–exercise timing and adaptations to exercise with implications for metabolic health and (2) candidate mechanisms underpinning adaptations to nutrient–exercise interactions.
Responses to endurance exercise training
Skeletal muscle, adipose tissue and the liver play roles in regulating the postprandial storage and oxidation of dietary carbohydrates (Kelley et al. 1988; Randle, 1998). A reduced peripheral insulin sensitivity to glucose uptake underpins many of the defects associated with metabolic diseases, and helps to account for an association between obesity and type 2 diabetes (Reaven, 1988). Although exercise training results in adaptations across many physiological systems, the main focus of this review will be on skeletal muscle, due to the key role of this tissue in regulating postprandial glycaemia (DeFronzo et al. 1985; Baron et al. 1988). In addition, the role of nutrient–exercise timing for adaptations in adipose tissue and the liver during exercise training remains to be studied, but relevant acute studies will be highlighted.
Skeletal muscle responses to exercise which underlie improvements in blood glucose control can be attributed to two main effects (Sylow & Richter, 2019). First, there is an increase in skeletal muscle glucose uptake to help satisfy the higher energy demands of the activity. Each bout of exercise performed activates AMP‐activated protein kinase (AMPK), Akt substrate of 160 kDa (AS160/TBC1D4) (Treebak et al. 2009; Herzig & Shaw, 2018), Rac1 (Gliemann et al. 2017) and Ca2+/calmodulin‐dependent protein kinase II (Witczak et al. 2010) pathways within skeletal muscle, which induces the translocation of the glucose transporter type 4 (GLUT4) protein to the plasma membrane and the t‐tubules to facilitate increased transmembrane glucose transport (Hawley & Lessard, 2008). Together with increased microvascular perfusion, these mechanisms contribute to increased peripheral insulin sensitivity and skeletal muscle glucose uptake in the acute post‐exercise period (Sjøberg et al. 2017; Hingst et al. 2018). However, it should be noted that while it was, until recently, accepted that AMPK is central for increasing glucose uptake during exercise via GLUT4 translocation, emerging animal studies report that this might not be the case and that it regulates glucose uptake and enhances insulin sensitivity only post‐exercise (Kjøbsted et al. 2019; Hingst et al. 2020). Secondly, regular exercise training increases the time spent in this acute phase, but also induces longer‐term adaptations across multiple tissues that can enhance blood glucose control (Sylow & Richter, 2019). The skeletal muscle responses to exercise training include a greater capacity to store glycogen and increased GLUT4 protein content and mitochondrial content and function (McGee & Hargreaves, 2006; Hawley et al. 2014; Marcelo et al. 2020). These adaptations are thought to largely result from the repeated stimuli from each bout of exercise, which then culminate in a change in phenotype. For example, acute metabolic responses to exercise produce a transient (<24 h) burst in the expression of transcriptional proteins such as peroxisome proliferator activated receptor γ coactivator 1‐α (PGC1α) (Pilegaard et al. 2003; Perry et al. 2010). These mRNA bursts may be followed by an increase in protein levels after ∼24 h (Perry et al. 2010), which coincides with increases in skeletal muscle mitochondrial protein synthesis (Di Donato et al. 2014). With subsequent bouts of matched‐intensity exercise, the magnitude of the mRNA bursts can be diminished, but the PGC1α protein accumulates in skeletal muscle, followed by an increase in the mitochondrial protein content (Perry et al. 2010). However, despite exercise being widely advocated as a means to improve aspects of metabolic health, many people fail to achieve physical activity recommendations, partly due to a perceived lack of time (Reichert et al. 2007; Korkiakangas et al. 2009). Therefore, strategies to maximise benefits of any exercise that is performed could be important. There is also evidence of inter‐individual variability to exercise training for postprandial insulinaemic responses (de Lannoy et al. 2017). This may be accounted for, at least in part, by the feeding status under which exercise is performed.
Nutrient–exercise timing (acute studies)
The acute responses to nutrient–exercise timing have been reviewed for substrate utilisation (Vieira et al. 2016), glycaemia and lipidaemia following a single pre‐ or post‐exercise meal (Haxhi et al. 2013), and for recovery from exercise (Arent et al. 2020). However, the primary focus of this review will be on adaptations to exercise training interventions. Acute responses will, however, be discussed briefly to provide context. Pre‐exercise carbohydrate consumption decreases whole‐body lipid oxidation in untrained and trained individuals, an effect mostly seen at moderate (40–60% ) exercise intensities (Bergman & Brooks, 1999; Vieira et al. 2016). Nutrient–exercise timing also alters the metabolic responses to the exercise session. For people with or without type 2 diabetes, the glycaemic response to a single meal can be attenuated to a greater extent with post‐ versus pre‐meal exercise, partly through a greater oxidation of the ingested carbohydrates (Poirier et al. 2000, 2001). As such, it has been proposed that moderate‐intensity physical activity could be undertaken between 30 and 120 min after carbohydrate‐rich meals are consumed, to lower blood glucose excursions from that particular meal (Haxhi et al. 2013; Chacko, 2017). However, caution should be taken when extrapolating acute glycaemic responses to longer‐term changes in blood glucose control. For example, if exercise is performed three times a week and when three meals are consumed per day, any glucose lowering effect of post‐ versus pre‐meal exercise would apply to only 3 out of 15 meals (20%) consumed for that week.
Nutrient–exercise timing (training studies)
Whilst post‐ versus pre‐meal exercise may offer additional benefits for blood glucose control at a single meal, regularly exercising before versus after nutrient (particularly carbohydrate) consumption may be a greater stimulus for more enduring adaptations relating to oral glucose insulin sensitivity. In a seminal paper investigating nutrient–exercise timing for metabolic health, physically active men consumed a hyper‐caloric (+ ∼30% kcal), high‐fat diet (50% of kcal) and completed exercise for 300 min per week over 6 weeks (Van Proeyen et al. 2010). They consumed a carbohydrate‐rich breakfast 90 min before any exercise and 1 g kg−1 of carbohydrate during exercise, or exercised in an overnight fasted state consuming the breakfast which they missed in the morning, and maltodextrin omitted during exercise, mid‐afternoon. Relative to the control group, the glycaemic response to a 75 g oral glucose tolerance test (OGTT) decreased if exercise was performed in the fasted state, but not when carbohydrate was consumed before and during exercise training. The Matsuda insulin sensitivity index also increased only if the exercise was performed in the fasted state. However, feeding versus fasting can exert different physiological responses in lean people compared to individuals classified as overweight or obese, or with type 2 diabetes. For example, regularly extending the morning fast until noon upregulates the expression of genes involved in lipid turnover in the adipose tissue compared to the consumption of breakfast in lean humans, but not in individuals with obesity (Gonzalez et al. 2018). Also, the blunting of exercise adaptations resulting from carbohydrate consumption before and during exercise may not translate to the case in which carbohydrates are only consumed before exercise. The suppression of fatty acid availability in the latter scenario is less pronounced and there is even evidence that consuming carbohydrates before exercise can augment increases in skeletal muscle AMPK activity (Edinburgh et al. 2018).
A fully supervised exercise training intervention was recently completed in individuals classified as overweight or obese, but without type 2 diabetes (Edinburgh et al. 2020), with the aim of establishing whether the proof‐of‐principle in lean individuals extends to people with increased risk of metabolic disease. Moderate‐intensity cycling exercise was performed before or after a carbohydrate‐rich breakfast for 6 weeks. In the breakfast before exercise group, breakfast was consumed 2 h prior to exercise and in the exercise before breakfast group, breakfast was consumed at least 2 h post‐exercise. Increases in oral glucose insulin sensitivity were reported with exercise before versus after breakfast, which was driven by reductions in postprandial insulinaemia. These measures were derived from a pre‐ and post‐intervention OGTT with the follow‐up OGTT completed between 48 and 72 h after the last exercise training session to reduce any residual effects of the last exercise bout performed on these measurements. The carbohydrate dose consumed was lower (∼120 g) than in a study in healthy men (Van Proeyen et al. 2010) and no carbohydrate was consumed during exercise. The exercise training also adhered to current physical activity guidelines, by the completion of moderate intensity cycling, three times a week, for 50 min per session. That study demonstrated that it is possible for people classified as overweight or obese to start exercise training but not improve postprandial glycaemic control or oral glucose insulin sensitivity if a carbohydrate‐rich breakfast is consumed before exercise sessions. These responses to exercise before versus after breakfast occurred despite no differences between groups for the change in whole‐body aerobic capacity, skeletal muscle citrate synthase activity, body mass, average daily self‐reported energy intake and/or average daily physical activity energy expenditure. In that study, net skeletal muscle glycogen utilisation and acute skeletal muscle mRNA responses were largely unaffected by the same exercise performed before versus after breakfast. This is important because muscle glycogen availability can regulate adaptations to training (Burke & Hawley, 2018). Lower muscle glycogen concentrations are therefore unlikely to have driven the training responses observed in that particular study. Other studies also report changes in aerobic fitness (Van Proeyen et al. 2010, 2011; Gillen et al. 2013; Verboven et al. 2020) and body mass (Gillen et al. 2013; Schoenfeld et al. 2014; Escalante & Barakat, 2020) in response to exercise training to be independent of the pre‐exercise feeding status.
Two exercise training studies have also recently been published with altered nutrient–exercise timing in individuals with type 2 diabetes. In one study, 30 patients experienced similar decreases in glycated haemoglobin (HbA1c) and serum insulin concentrations after high‐intensity cycling (30 min) and strength training, performed three times a week for 8 weeks either before or after breakfast (Brinkmann et al. 2019). In a similar study, 25 patients completed a 12 week exercise intervention, with exercise (25 min walking and 20 min cycling per session) performed three times a week before or after breakfast (Verboven et al. 2020). In that study the breakfast was standardised within participant and self‐selected, containing 375 ± 72 kcal (60 ± 4% carbohydrate (CHO)) for the exercise after breakfast group, which was consumed 1 h before exercise, and 479 ± 73 kcal (58 ± 4% CHO) for the exercise before breakfast group, which was consumed within 1 h of exercise completion. HbA1c concentrations decreased after exercise training, but there was a greater reduction when exercise was performed after versus before breakfast. One explanation for the differences between that study and research which used OGTTs (Van Proeyen et al. 2010; Edinburgh et al. 2020) is that HbA1c concentrations do not reflect glycaemic control per se, but rather average blood glucose concentrations over time. Whilst the study in individuals with type 2 diabetes has good ecological validity as breakfast and food intake was self‐selected, it is possible that the HbA1c results were not reflective of glycaemic control alone, but also the glycaemic load of the diet chosen by the patients throughout the training. Nonetheless, the OGTT also has limitations for assessing peripheral insulin sensitivity (Muniyappa et al. 2008) and there is now a need for a training study to alter nutrient–exercise timing and use a hyperinsulinaemic–euglycaemic clamp to measure peripheral insulin sensitivity. Other differences between studies include the exercise mode, the length of training and population studied (Table 1). For the latter point, if increased lipid oxidation alters adaptation to exercise with altered nutrient–exercise timing (discussed subsequently), a lack of effect of exercise before breakfast on markers of metabolic health in people with type 2 diabetes could be related to metabolic inflexibility, which blunts metabolic differences between fasting and feeding (Kelley & Simoneau, 1994; Kelley et al. 1999; Goodpaster & Sparks, 2017). In addition, in rodents, exercise‐induced remodelling of skeletal muscle can be blunted by chronic hyperglycaemia, a common characteristic of diseases such as type 2 diabetes (MacDonald et al. 2020).
Table 1.
Overview of exercise training studies examining metabolic responses with manipulation of nutrient timing
| First author (year) | Nutritional intervention | Exercise mode | Exercise intensity | Exercise frequency | Participants | Fasting glucose | Fasting insulin | Postprandial glucose | Postprandial insulin | Muscle adaptation |
|---|---|---|---|---|---|---|---|---|---|---|
| De Bock (2005) | CHO before and during exercise, vs after exercise | Cycling | 75% | 3 days/week, 6 weeks | Lean (M) | = | = | N/A | N/A (↑ * exercise‐induced reduction) |
=: HKII, GLUT4, FAT/CD36, UCP3, IMCL utilisation ↑ † : FABPm |
| Van Proeyen (2010) | CHO before and during exercise, vs. after exercise | Cycling | 70–75% | 4 days/week, 6 weeks | Lean (M), high‐fat diet | = | = | ↓ † | ↓ † |
↑ * : COX ↑ † : glycogen, GLUT4, p‐AMPK, FAT/CD36, CPT1 =: CS, β‐HAD |
| Van Proeyen (2011) | CHO before and during exercise, vs. after exercise | Cycling | ∼70% | 4 days/week, 6 weeks | Lean (M), high‐CHO diet | = | N/A | N/A | N/A |
↑ * : CS ↑ † : β‐HAD, type IIa IMCL utilisation |
| Gillen (2013) | Breakfast before exercise, vs. after exercise | Cycling intervals | ∼90% HRmax | 3 days/week, 6 weeks | OW/OB (F) | = | = | = | = | =: CS, β‐HAD, GLUT4 |
| Brinkmann (2019) | Breakfast before exercise, vs. 12 h fast | Resistance/cycling | ∼70‐80% HRpeak | 3 days/week, 8 weeks | T2D (M&F) | = | = | N/A | N/A | N/A |
| Verboven (2020) | Breakfast before exercise, vs. after exercise | Walking/cycling | 65% | 3 days/week, 12 weeks | T2D (M) | = | = | N/A | N/A | =: mRNA |
| Edinburgh (2020) | CHO before exercise, vs. after exercise | Cycling | 50–55% W peak | 3 days/week, 6 weeks | OW/OB (M) | = | = | = | ↓ † |
↑ * : AMPK, CHC22 ↑ † : GLUT4 =: Akt, AS160, CPT1, FAT/CD36, OXPHOS, CS |
*Difference between fasted‐exercise and fed‐exercise group (P < 0.05); †difference between fasted‐exercise and control group (P < 0.05). Akt, protein kinase B; AMPK, adenosine monophosphate‐activated protein kinase; AS160, Akt substrate of 160 kDa (TBC1D4); β‐HAD, 3‐hydroxyacyl‐CoA dehydrogenase; CHC22, clathrin heavy chain 22; CHO, carbohydrate; CPT1, carnitine palmitoyltransferase 1; COX, cytochrome c oxidase; CS, citrate synthase; F, females; FAT/CD36, fatty acid translocase; FABPm, fatty acid binding protein muscle; GLUT4, glucose transporter 4; HKII, hexokinase II; HRmax, maximal heart rate; HRpeak, peak heart rate, IMCL, intramuscular lipid; M, males; N/A, Not applicable/measured; OB, obese; OW, overweight; OXPHOS, oxidative phosphorylation proteins; p‐AMPK, phosphorylated AMPK; T2D, type 2 diabetes; UCP3, uncoupling protein 3; , peak aerobic capacity; W peak, peak power; =, no change.
Candidate mechanisms for enhanced adaptations from exercise in a fasted state
Increased lipid oxidation
It is well established that exercising in an overnight‐fasted state increases whole‐body lipid oxidation, and decreases carbohydrate oxidation, compared to exercise in the fed state (Vieira et al. 2016). In men classified as overweight or obese, there are no clear differences in muscle glycogen utilisation during a single bout of moderate‐intensity exercise performed before versus after a mixed‐macronutrient breakfast, but net intramuscular triglyceride (IMTG) utilisation is blunted and plasma non‐esterified fatty acid (NEFA) and glycerol concentrations are lower (likely due to adipose tissue NEFA re‐esterification; Enevoldsen et al. 2004) during exercise after breakfast (Edinburgh et al. 2020). Fatty acids are ligands for peroxisome proliferated activator receptors (PPARs) which can mediate the expression of lipid metabolism proteins (Ehrenborg & Krook, 2009). For example, PPAR‐δ helps regulate CPT1 expression (Dressel et al. 2003) and fatty acid oxidation enzymes (Luquet et al. 2003). In rodents, increases in PPAR‐δ activity can be augmented by exercising with a low carbohydrate availability (Philp et al. 2013). Together with increased AMPK activation (as discussed subsequently) this may augment some adaptations (Handschin & Spiegelman, 2006). In support of a possible role for increased lipid flux in mediating metabolic adaptations to exercise training, a positive correlation between changes in oral glucose insulin sensitivity and cumulative whole‐body lipid utilisation during exercise training has been reported (Edinburgh et al. 2020). This highlights the possibility that for improving key aspects of metabolic health, exercise training may be more effective when the activity stimulates an increased utilisation of endogenous lipid stores, a concept which has recently been highlighted (Gemmink et al. 2020). In support of this theory is evidence that in individuals classified as overweight or obese, altering nutrient–exercise timing does not alter changes in postprandial glycaemia or markers of insulin sensitivity when the training is high‐intensity (Gillen et al. 2013). Differences in lipid utilisation between exercise in the fasted versus fed state are less apparent at high exercise intensities, where carbohydrate is the predominant fuel source, irrespective of prior feeding status (Bergman & Brooks, 1999). The greater utilisation of IMTG with fasted versus fed exercise may be especially important as this is associated with increases in peripheral insulin sensitivity, potentially by alleviating any accumulation of the lipid metabolites (e.g. fatty acyl‐CoA, ceramide or diacylglycerol) that can interfere with insulin signalling (Hulver & Dohm, 2004). Moreover, IMTG degradation during a subsequent bout of exercise can also be enhanced by prior exercise training in the fasted state but not in the fed state (Van Proeyen et al. 2011). Despite this, that the lipid store being utilised (i.e. IMTG versus adipose tissue‐derived fatty acids) may explain the effect of nutrient–exercise timing for metabolic adaptations to exercise remains to be fully elucidated.
AMP‐activated protein kinase
Of the molecular mechanisms that drive exercise adaptations, a protein with a key role is AMPK, an enzyme sensitive to the cellular energy status (Mounier et al. 2015). AMPK is a heterotrimeric protein complex consisting of α, β and γ subunits. Increases in the kinase activity of AMPK results in the phosphorylation of downstream targets (e.g. TBC1D1, TBC1D4 and acetyl coenzyme A carboxylase (ACC)) and nuclear transcription factors which regulate the expression of genes involved in glucose uptake, mitochondrial biogenesis and autophagy (Herzig & Shaw, 2018). In response to exercise, AMPK can be activated by an increased AMP:ATP ratio via the γ‐subunit, and reductions in glycogen concentrations via the β‐subunit (Pilegaard et al. 2002; Frøsig et al. 2004; McBride et al. 2009; Yeo et al. 2010). Recent in vitro research has demonstrated that long‐chain fatty acyl‐CoA esters can activate the β‐subunit of AMPK and promote increased lipid oxidation (Pinkosky et al. 2020), a finding that is discussed elsewhere (Hardie, 2020). This supports earlier observations that skeletal muscle AMPK can be activated by increased fatty acid availability, independent of muscle glycogen or AMP concentrations (Watt et al. 2006). AMPK activation may contribute to regulating fuel metabolism during and immediately post‐exercise, and this is partly mediated by increasing skeletal muscle glucose uptake via regulation of TBC1D1 and TBC1D4 activity (Szekeres et al. 2012; Richter & Hargreaves, 2013; Chadt et al. 2015; Stöckli et al. 2015) and lipid oxidation via the inhibition of ACC (Hardie, 1989; O'Neill et al. 2014). It has recently been reported that TBC1D1 and TBC1D4 activity can regulate long chain fatty acid uptake in skeletal muscle via the SLC27A4/FATP4 pathway, which is an additional mechanism by which exercise and nutrient status via AMPK may regulate lipid uptake and metabolism (Benninghoff et al. 2020). Skeletal muscle AMPK activation also stimulates enduring adaptations to exercise including increases in the GLUT4 protein levels and mitochondrial biogenesis via transcription factors including PGC1α (Wojtaszewski et al. 2000; Ojuka et al. 2002). Enhanced AMPK activation in response to exercise commenced with reduced muscle glycogen concentrations (35–45% of basal) may explain increased mitochondrial adaptations compared to the same exercise started with higher muscle glycogen concentrations (Morton et al. 2009; Hulston et al. 2010; Bartlett et al. 2013; Andrade‐Souza et al., 2020). This may be important as obesity and peripheral insulin resistance have been associated with a lower mitochondrial oxidative capacity (Kelley et al. 1999; Simoneau et al. 1999). However, these studies used high exercise volumes and often asked participants to exercise multiple times over 24 h, which may not be sustainable for people exercising primarily for health. Nonetheless, in physically active healthy men, altering breakfast timing in relation to exercise (i.e. fasted exercise) can increase the content/expression of lipid metabolism proteins in skeletal muscle such as cluster of differentiation 36 (CD36) or carnitine palmitoyltransferase (CPT‐1) over 6–8 weeks (De Bock et al. 2008; Nybo et al. 2009; Van Proeyen et al. 2011). In people classified as overweight or obese, the protein content of AMPK in skeletal muscle also increases by ∼3‐fold with exercise before breakfast, whereas exercise after breakfast did not increase AMPK levels (Edinburgh et al. 2020). In that study, this AMPK response did not translate into changes in proteins involved in fatty acid transport or fatty acid utilisation (CD36 or CPT‐1) or markers of mitochondrial oxidative capacity (OXPHOS complex protein levels or citrate synthase activity), but there was a 2‐fold increase in the protein levels of GLUT4 in skeletal muscle and proteins in the GLUT4 biosynthetic pathway (i.e. CHC22) were also increased with exercise training before versus after breakfast (Abstract Figure). This AMPK and GLUT4 response to pre‐meal versus post‐meal exercise supports results reported in healthy men (Van Proeyen et al. 2010). As prior feeding status did not alter muscle glycogen utilisation during exercise in that study, increases in GLUT4 levels and oral glucose insulin sensitivity that were reported with exercise before versus after breakfast (Edinburgh et al. 2020) could be due to increased fatty acid flux driving skeletal muscle AMPK activation (Pinkosky et al. 2020) (Abstract Figure).
Phospholipid remodelling
There is also some suggestion of increased skeletal muscle phospholipid remodelling in people with obesity after exercise training performed before versus after breakfast (Edinburgh et al. 2020). An increased phospholipid saturated fatty acid (SFA) content and a decreased polyunsaturated fatty acid (PUFA) content has been associated with obesity and peripheral insulin resistance (Harayama & Riezman, 2018). Increasing the PUFA content increases membrane fluidity, insulin receptor number and insulin action and this is reversed if the saturated fatty acid content of the membranes is increased (Ginsberg et al. 1982; Field et al. 1988; Yorek et al. 1989). Increased PGC1α activity may regulate changes to the phospholipid content of exercise‐trained skeletal muscle (Senoo et al. 2015). As exercising with a low carbohydrate availability increasingly activates proteins upstream of PGC1α such as AMPK, an increased PUFA: SFA ratio in skeletal muscle phospholipids (via AMPK) could also help to link nutrient–exercise timing to oral glucose insulin sensitivity (Abstract Figure). In support of this, reductions in the SFA content of skeletal muscle phospholipids has been shown to correlate with changes in postprandial insulinaemia after exercise (Edinburgh et al. 2020).
Future research
In men classified as overweight, feeding versus fasting before a single bout of exercise leads to a decreased expression of several genes involved in lipid metabolism, insulin signalling and glucose uptake in adipose tissue (Chen et al. 2017). Whether these acute responses to altered nutrient–exercise timing in adipose tissue are important for chronic metabolic health needs to be investigated with intervention studies. Glucose ingestion during exercise can also abolish reductions in hepatic glycogen content seen with a single bout of exercise (Gonzalez et al. 2015), which could blunt longer‐term hepatic adaptations to exercise via AMPK activity. A single bout of exercise in the fasted state also leads to increased intrahepatic lipid content compared to exercise performed with glucose consumption (Bilet et al. 2015). However, it is unclear how acute changes in the hepatic lipid content reflect longer term responses, as exercise training lowers the hepatic lipid content (Brouwers et al. 2016). More work is required to establish a role of nutrient–exercise timing on adipose tissue and the liver. The timing of the first post‐exercise meal may also be important for adaptations in response to altered nutrient–exercise timing. No clear differences were observed in the mRNA expression of many genes implicated in metabolic adaptations to exercise (e.g. AMPK, PGC1α and GLUT4) in overweight or obese individuals after a single bout of exercise before versus after breakfast, when the breakfast was consumed immediately post‐exercise (Edinburgh et al. 2020). Other studies that have provided the breakfast within 60 min of exercise completion in exercise before breakfast groups also observe no differences in protein levels of GLUT4 (Gillen et al. 2013) or the mRNA expression of genes involved in exercise adaptation (Verboven et al. 2020) with exercise before versus after breakfast. However, if the time window between exercise completion and breakfast consumption is extended (>2 h), augmented adaptations in skeletal muscle are reported with exercise before versus after breakfast (Van Proeyen et al. 2010; Edinburgh et al. 2020). This is in line with research showing that extended periods of fasting may have benefits for metabolic health (Parr et al. 2020). It is also possible that a lower insulinaemia in the immediate post‐exercise period could contribute to these discordant results between studies. The studies in this review also investigated high‐carbohydrate provision and recruited only men. As carbohydrate is the macronutrient which most potently regulates fat oxidation rates and muscle glycogen availability (Acheson et al. 2011), it is likely that low‐carbohydrate pre‐exercise meals produce outcomes more similar to the overnight fasted state than the carbohydrate‐fed state, yet this would need confirming with empirical data. Omitting versus consuming nutrients prior to exercise increases lipid utilisation in both sexes, so it is plausible that the results discussed apply to women, but the magnitude of the response could differ (Wallis et al. 2006) and warrants investigation. Further research is also needed to better establish the potential effects of nutrient timing on other aspects of glucose metabolism and insulin availability, such as gastrointestinal absorption rate and hepatic insulin extraction. Current evidence would suggest that consuming a carbohydrate‐rich meal prior to a single exercise bout increases exogenous glucose appearance rates post‐exercise (reflecting a more rapid digestion and absorption of ingested glucose) (Edinburgh et al. 2018) and the lower insulinaemia seen with chronic exercise training in an overnight fasted state is most likely due to lower insulin secretion rates rather than increase hepatic insulin extraction, based on C‐peptide data (Edinburgh et al. 2020). Finally, as compliance is a determinant of training effectiveness, future studies could investigate nutrient–exercise interactions in conditions where compliance to an exercise intervention could differ.
Summary
Exercising has many health benefits, such as increasing aerobic fitness independent of nutrient–exercise timing. However, recent research has shown that increases in oral glucose insulin sensitivity can depend on the nutrient‐status in which exercise is performed. Specifically, exercise training after an extended, overnight‐fast augments skeletal muscle adaptations such as GLUT4 and AMPK protein levels, and reductions in postprandial insulinaemia, even when energy balance is unaffected. This may occur to the extent to which it is possible for people with obesity to start exercise training, but achieve no clear benefits for oral glucose insulin sensitivity when carbohydrate‐rich meals are consumed before exercise. The mechanisms underlying the adaptive response to exercise in a fasted state versus fed state are likely to include increased lipid oxidation, and greater remodelling of skeletal muscle relating to glucose uptake and metabolism, such as glucose transporters, energy sensing proteins and membrane phospholipid composition. Further research is needed with hard outcomes (e.g. fasting or 2 h glucose concentrations, HbA1c, LDL‐cholesterol concentrations) and in different populations (e.g. females and people with cardiovascular disease or type 2 diabetes) in order to establish clinical guidelines. Nevertheless, the current evidence indicates that performing some exercise sessions in an overnight fasted state (i.e. before consumption of carbohydrate) may provide additional metabolic health benefits to exercise performed after high‐carbohydrate meals.
Additional information
Competing interests
None of the authors declare any conflicts of interest in relation to this work.
Author contributions
All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The main study that this review highlights was supported by The Physiological Society (UK), The Rank Prize Funds (UK), the Allen Foundation Inc. (USA) and the Medical Research Council (MR/P002927/1 and MR/S008144/1).
Acknowledgements
The authors would like to acknowledge all of the researchers that contributed to the research that is highlight and discussed in this review.
Biographies
Rob Edinburgh recently completed a PhD at the University of Bath, having also gained an undergraduate degree in Sport and Exercise Science. His research to date has focused on the effects of nutrient–exercise timings on substrate utilisation during and after exercise, metabolism, and health‐related adaptations to exercise. He is passionate about ways we can make exercise more efficacious for health.

Francoise Koumanov is a Lecturer (Assistant Professor) at the University of Bath. Her research focuses on molecular signalling processes of glucose and insulin in physiological and pathophysiological states and aims to elucidate the roles of exercise and GLUT4 trafficking in the pathophysiology of insulin resistance.

Javier Gonzalez is Reader (Associate Professor) at the University of Bath. His research focuses on interactions between nutrition and exercise in health and disease and explores the role of carbohydrate availability in the regulation of energy balance, metabolic health and sports performance.

Edited by: Ian Forsythe & Paul Greenhaff
This review was an accepted presentation for the American College of Sports Medicine 2020 annual meeting “Novel dietary approaches to appetite regulation, health and performance”, which was rescheduled for 2021.
References
- Acheson KJ, Blondel‐Lubrano A, Oguey‐Araymon S, Beaumont M, Emady‐Azar S, Ammon‐Zufferey C, Monnard I, Pinaud S, Nielsen‐Moennoz C & Bovetto L (2011). Protein choices targeting thermogenesis and metabolism. Am J Clin Nutr 93, 525–534. [DOI] [PubMed] [Google Scholar]
- Andrade‐Souza VA, Ghiarone T, Sansonio A, Santos Silva KA, Tomazini F, Arcoverde L, Fyfe J, Perri E, Saner N & Kuang J (2020). Exercise twice‐a‐day potentiates markers of mitochondrial biogenesis in men. FASEB J 34, 1602–1619. [DOI] [PubMed] [Google Scholar]
- Aqeel M, Forster A, Richards EA, Hennessy E, McGowan B, Bhadra A, Guo J, Gelfand S, Delp E & Eicher‐Miller HA (2020). The effect of timing of exercise and eating on postprandial response in adults: a systematic review. Nutrients 12, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arent SM, Cintineo HP, McFadden BA, Chandler AJ & Arent MA (2020). Nutrient timing: a garage door of opportunity? Nutrients 12, 1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A, Brechtel G, Wallace P & Edelman S (1988). Rates and tissue sites of non‐insulin‐ and insulin‐mediated glucose uptake in humans. Am J Physiol Endocrinol Metab 255, E769–E774. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Louhelainen J, Iqbal Z, Cochran AJ, Gibala MJ, Gregson W, Close GL, Drust B & Morton JP (2013). Reduced carbohydrate availability enhances exercise‐induced p53 signaling in human skeletal muscle: implications for mitochondrial biogenesis. Am J Physiol Regul Integr Comp Physiol 304, R450–R458. [DOI] [PubMed] [Google Scholar]
- Benninghoff T, Espelage L, Eickelschulte S, Zeinert I, Sinowenka I, Müller F, Schöndeling C, Batchelor H, Cames S, Zhou Z, Kotzka J, Chadt A & Al‐Hasani H (2020). The RabGAPs TBC1D1 and TBC1D4 control uptake of long‐chain fatty acids into skeletal muscle via fatty acid transporter SLC27A4/FATP4. Diabetes 69, 2281–2293. [DOI] [PubMed] [Google Scholar]
- Bergman BC & Brooks GA (1999). Respiratory gas‐exchange ratios during graded exercise in fed and fasted trained and untrained men. J Appl Physiol 86, 479–487. [DOI] [PubMed] [Google Scholar]
- Bilet L, Brouwers B, Van Ewijk P, Hesselink M, Kooi M, Schrauwen P & Schrauwen‐Hinderling V (2015). Acute exercise does not decrease liver fat in men with overweight or NAFLD. Sci Rep 5, 9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird SR & Hawley JA (2017). Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport & Exercise Medicine 2, e000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers B, Hesselink MK, Schrauwen P & Schrauwen‐Hinderling VB (2016). Effects of exercise training on intrahepatic lipid content in humans. Diabetologia 59, 2068–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann C, Weh‐Gray O, Brixius K, Bloch W, Predel H‐G & Kreutz T. (2019). Effects of exercising before breakfast on the health of T2DM patients—A randomized controlled trial. Scand J Med Sci Sports 29, 1930–1936. [DOI] [PubMed] [Google Scholar]
- Burke LM & Hawley JA (2018). Swifter, higher, stronger: What's on the menu? Science 362, 781–787. [DOI] [PubMed] [Google Scholar]
- Chacko E (2017). A time for exercise: the exercise window. J Appl Physiol 122, 206–209. [DOI] [PubMed] [Google Scholar]
- Chadt A, Immisch A, De Wendt C, Springer C, Zhou Z, Stermann T, Holman GD, Loffing‐Cueni D, Loffing J & Joost H‐G (2015). Deletion of both Rab‐GTPase–activating proteins TBC14KO and TBC1D4 in mice eliminates insulin‐ and AICAR‐stimulated glucose transport. Diabetes 64, 746–759. [DOI] [PubMed] [Google Scholar]
- Chen Y‐C, Travers RL, Walhin J‐P, Gonzalez JT, Koumanov F, Betts JA & Thompson D (2017). Feeding influences adipose tissue responses to exercise in overweight men. Am J Physiol Endocrinol Metab 313, E84–E93. [DOI] [PubMed] [Google Scholar]
- De Bock K, Derave W, Eijnde BO, Hesselink MK, Koninckx E, Rose AJ, Schrauwen P, Bonen A, Richter EA & Hespel P (2008). Effect of training in the fasted state on metabolic responses during exercise with carbohydrate intake. J Appl Physiol 104, 1045–1055. [DOI] [PubMed] [Google Scholar]
- De Bock K, Ricter EA, Russell AP, Eijnde BO, Derace W, Ramaekers M, Koninckx E, Leger B, Verhaeghe J & Hespel P. (2005). Exercise in the fasted state facilitates fibre type‐specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J Physiol 564, 649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Gunnarsson R, Björkman O, Olsson M & Wahren J (1985). Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin‐dependent (type II) diabetes mellitus. J Clin Invest 76, 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lannoy L, Clarke J, Stotz PJ & Ross R (2017). Effects of intensity and amount of exercise on measures of insulin and glucose: Analysis of inter‐individual variability. PLoS One 12, e0177095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Donato DM, West DW, Churchward‐Venne TA, Breen L, Baker SK & Phillips SM (2014). Influence of aerobic exercise intensity on myofibrillar and mitochondrial protein synthesis in young men during early and late postexercise recovery. Am J Physiol Endocrinol Metab 306, E1025–E1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P & Muscat GE (2003). The peroxisome proliferator‐activated receptor β/δ agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol 17, 2477–2493. [DOI] [PubMed] [Google Scholar]
- Edinburgh RM, Bradley HE, Abdullah N‐F, Robinson SL, Chrzanowski‐Smith OJ, Walhin J‐P, Joanisse S, Manolopoulos KN, Philp A & Hengist A (2020). Lipid metabolism links nutrient‐exercise timing to insulin sensitivity in men classified as overweight or obese. J Clin Endocrinol Metab 105, 660–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinburgh RM, Hengist A, Smith HA, Travers RL, Koumanov F, Betts JA, Thompson D, Walhin J‐P, Wallis GA & Hamilton DL (2018). Preexercise breakfast ingestion versus extended overnight fasting increases postprandial glucose flux after exercise in healthy men. Am J Physiol Endocrinol Metab 315, E1062–E1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenborg E & Krook A (2009). Regulation of skeletal muscle physiology and metabolism by peroxisome proliferator‐activated receptor δ. Pharmacol Rev 61, 373–393. [DOI] [PubMed] [Google Scholar]
- Enevoldsen L, Simonsen L, Macdonald I & Bülow J (2004). The combined effects of exercise and food intake on adipose tissue and splanchnic metabolism. J Physiol 561, 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante G & Barakat C (2020). Fasted versus nonfasted aerobic exercise on body composition: considerations for physique athletes. Strength Cond J 42, 71–78. [Google Scholar]
- Field CJ, Ryan E, Thomson A & Clandinin M (1988). Dietary fat and the diabetic state alter insulin binding and the fatty acyl composition of the adipocyte plasma membrane. Biochem J 253, 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøsig C, Jørgensen SB, Hardie DG, Richter EA & Wojtaszewski JF (2004). 5′‐AMP‐activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab 286, E411–E417. [DOI] [PubMed] [Google Scholar]
- Gemmink A, Schrauwen P & Hesselink MK (2020). Exercising your fat (metabolism) into shape: a muscle‐centred view. Diabetologia 63, 1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen JB, Percival ME, Ludzki A, Tarnopolsky MA & Gibala MJ (2013). Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity 21, 2249–2255. [DOI] [PubMed] [Google Scholar]
- Ginsberg BH, Jabour J & Spector AA (1982). Effect of alterations in membrane lipid unsaturation on the properties of the insulin receptor of Ehrlich ascites cells. Biochimica et Biophysica Acta 690, 157–164. [DOI] [PubMed] [Google Scholar]
- Gliemann L, Rytter N, Lindskrog M, Slingsby MHL, Åkerström T, Sylow L, Richter EA & Hellsten Y (2017). Endothelial mechanotransduction proteins and vascular function are altered by dietary sucrose supplementation in healthy young male subjects. J Physiol 595, 5557–5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JT, Fuchs CJ, Smith FE, Thelwall PE, Taylor R, Stevenson EJ, Trenell MI, Cermak NM & Van Loon LJ (2015). Ingestion of glucose or sucrose prevents liver but not muscle glycogen depletion during prolonged endurance‐type exercise in trained cyclists. Am J Physiol Endocrinol Metab 309, E1032–E1039. [DOI] [PubMed] [Google Scholar]
- Gonzalez JT, Richardson JD, Chowdhury EA, Koumanov F, Holman GD, Cooper S, Thompson D, Tsintzas K & Betts JA (2018). Molecular adaptations of adipose tissue to 6 weeks of morning fasting vs. daily breakfast consumption in lean and obese adults. J Physiol 596, 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster BH & Sparks LM (2017). Metabolic flexibility in health and disease. Cell Metab 25, 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C & Spiegelman BM (2006). Peroxisome proliferator‐activated receptor γ coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27, 728–735. [DOI] [PubMed] [Google Scholar]
- Harayama T & Riezman H (2018). Understanding the diversity of membrane lipid composition. Nat Ecol Evol 19, 281–296. [DOI] [PubMed] [Google Scholar]
- Hardie DG (1989). Regulation of fatty acid synthesis via phosphorylation of acetyl‐CoA carboxylase. Prog Lipid Res 28, 117–146. [DOI] [PubMed] [Google Scholar]
- Hardie DG (2020). AMPK as a direct sensor of long‐chain fatty acyl‐CoA esters. Nat Metab 2, 799–800. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Hargreaves M, Joyner MJ & Zierath JR (2014). Integrative biology of exercise. Cell 159, 738–749. [DOI] [PubMed] [Google Scholar]
- Hawley JA & Lessard SJ (2008). Exercise training‐induced improvements in insulin action. Acta Physiologica 192, 127–135. [DOI] [PubMed] [Google Scholar]
- Haxhi J, Scotto di Palumbo A & Sacchetti M (2013). Exercising for metabolic control: is timing important? Ann Nutr Metab 62, 14–25. [DOI] [PubMed] [Google Scholar]
- Hengist A, Koumanov F & Gonzalez JT (2019). Fructose and metabolic health: governed by hepatic glycogen status? J Physiol 597, 3573–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S & Shaw RJ (2018). AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19, 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingst JR, Bruhn L, Hansen MB, Rosschou MF, Birk JB, Fentz J, Foretz M, Viollet B, Sakamoto K & Færgeman NJ (2018). Exercise‐induced molecular mechanisms promoting glycogen supercompensation in human skeletal muscle. Mol Metab 16, 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingst JR, Kjøbsted R, Birk JB, Jørgensen NO, Larsen MR, Kido K, Larsen JK, Kjeldsen SAS, Fentz J, Frøsig C, Holm S, Fritzen AM, Dohlmann TL, Larsen S, Foretz M, Viollet B, Schjerling P, Overby P, Halling JF, Pilegaard H, Hellsten Y & Wojtaszewski JFP (2020). Inducible deletion of skeletal muscle AMPKα reveals that AMPK is required for nucleotide balance but dispensable for muscle glucose uptake and fat oxidation during exercise. Mol Metab 40, 101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulston CJ, Venables MC, Mann CH, Martin C, Philp A, Baar K & Jeukendrup AE (2010). Training with low muscle glycogen enhances fat metabolism in well‐trained cyclists. Med Sci Sports Exercise 42, 2046–2055. [DOI] [PubMed] [Google Scholar]
- Hulver MW & Dohm GL (2004). The molecular mechanism linking muscle fat accumulation to insulin resistance. Proc Nutr Soc 63, 375–380. [DOI] [PubMed] [Google Scholar]
- Kelley D, Mitrakou A, Marsh H, Schwenk F, Benn J, Sonnenberg G, Arcangeli M, Aoki T, Sorensen J & Berger M (1988). Skeletal muscle glycolysis, oxidation, and storage of an oral glucose load. J Clin Invest 81, 1563–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster B, Wing RR & Simoneau J‐A (1999). Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab 277, E1130–E1141. [DOI] [PubMed] [Google Scholar]
- Kelley DE & Simoneau J‐A (1994). Impaired free fatty acid utilization by skeletal muscle in non‐insulin‐dependent diabetes mellitus. J Clin Invest 94, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjøbsted R, Roll JLW, Jørgensen NO, Birk JB, Foretz M, Viollet B, Chadt A, Al‐Hasani H & Wojtaszewski JFP (2019). AMPK and TBC1D1 regulate muscle glucose uptake after, but not during, exercise and contraction. Diabetes 68, 1427–1440. [DOI] [PubMed] [Google Scholar]
- Korkiakangas EE, Alahuhta MA & Laitinen JH (2009). Barriers to regular exercise among adults at high risk or diagnosed with type 2 diabetes: a systematic review. Health Promot Int 24, 416–427. [DOI] [PubMed] [Google Scholar]
- Lieberman DE (2015). Human locomotion and heat loss: an evolutionary perspective. Compr Physiol 5, 99–117. [DOI] [PubMed] [Google Scholar]
- Luquet S, Lopez‐Soriano J, Holst D, Fredenrich A, Melki J, Rassoulzadegan M & Grimaldi PA (2003). Peroxisome proliferator‐activated receptor δ controls muscle development and oxydative capability. FASEB J 17, 2299–2301. [DOI] [PubMed] [Google Scholar]
- MacDonald TL, Pattamaprapanont P, Pathak P, Fernandez N, Freitas EC, Hafida S, Mitri J, Britton SL, Koch LG & Lessard SJ (2020). Hyperglycaemia is associated with impaired muscle signalling and aerobic adaptation to exercise. Nature Metabolism, 2, 902–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancilla R, Krook A, Schrauwen P & Hesselink MK (2020). Diurnal regulation of peripheral glucose metabolism: Potential effects of exercise timing. Obesity 28, S38–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelo F‐O, Sean LM & Mark H (2020). Exercise and GLUT4. Exerc Sport Sci Rev 48, 110–118. [DOI] [PubMed] [Google Scholar]
- McBride A, Ghilagaber S, Nikolaev A & Hardie DG (2009). The glycogen‐binding domain on the AMPK β subunit allows the kinase to act as a glycogen sensor. Cell Metab 9, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee SL & Hargreaves M (2006). Exercise and skeletal muscle glucose transporter 4 expression: molecular mechanisms. Clin Exp Pharmacol Physiol 33, 395. [DOI] [PubMed] [Google Scholar]
- Morton JP, Croft L, Bartlett JD, MacLaren DP, Reilly T, Evans L, McArdle A & Drust B (2009). Reduced carbohydrate availability does not modulate training‐induced heat shock protein adaptations but does upregulate oxidative enzyme activity in human skeletal muscle. J Appl Physiol 106, 1513–1521. [DOI] [PubMed] [Google Scholar]
- Mounier R, Théret M, Lantier L, Foretz M & Viollet B (2015). Expanding roles for AMPK in skeletal muscle plasticity. Trends Endocrinol Metab 26, 275–286. [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Lee S, Chen H & Quon MJ (2008). Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 294, E15–E26. [DOI] [PubMed] [Google Scholar]
- Nybo L, Pedersen K, Christensen B, Aagaard P, Brandt N & Kiens B (2009). Impact of carbohydrate supplementation during endurance training on glycogen storage and performance. Acta Physiologica 197, 117–127. [DOI] [PubMed] [Google Scholar]
- Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M & Holloszy JO (2002). Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca2+ . Am J Physiol Endocrinol Metab 282, E1008–E1013. [DOI] [PubMed] [Google Scholar]
- O'Neill HM, Lally JS, Galic S, Thomas M, Azizi PD, Fullerton MD, Smith BK, Pulinilkunnil T, Chen Z & Samaan MC (2014). AMPK phosphorylation of ACC2 is required for skeletal muscle fatty acid oxidation and insulin sensitivity in mice. Diabetologia 57, 1693–1702. [DOI] [PubMed] [Google Scholar]
- Parr EB, Heilbronn LK & Hawley JA (2020). A time to eat and a time to exercise. Exerc Sport Sci Rev 48, 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A & Spriet LL (2010). Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588, 4795–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp A, MacKenzie MG, Belew MY, Towler MC, Corstorphine A, Papalamprou A, Hardie DG & Baar K (2013). Glycogen content regulates peroxisome proliferator activated receptor‐∂ (PPAR‐∂) activity in rat skeletal muscle. PLoS One 8, e77200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilegaard H, Keller C, Steensberg A, Wulff Helge J, Klarlund Pedersen B, Saltin B & Neufer PD (2002). Influence of pre‐exercise muscle glycogen content on exercise‐induced transcriptional regulation of metabolic genes. J Physiol 541, 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilegaard H, Saltin B & Neufer PD (2003). Exercise induces transient transcriptional activation of the PGC‐1α gene in human skeletal muscle. J Physiol 546, 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkosky SL, Scott JW, Desjardins EM, Smith BK, Day EA, Ford RJ, Langendorf CG, Ling NXY, Nero TL, Loh K, Galic S, Hoque A, Smiles WJ, Ngoei KRW, Parker MW, Yan Y, Melcher K, Kemp BE, Oakhill JS & Steinberg GR (2020). Long‐chain fatty acyl‐CoA esters regulate metabolism via allosteric control of AMPK β1 isoforms. Nature Metabolism 2, 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier P, Mawhinney S, Grondin L, Tremblay A, Broderick T, Cléroux J, Catellier C, Tancrède G & Nadeau A (2001). Prior meal enhances the plasma glucose lowering effect of exercise in type 2 diabetes. Med Sci Sports Exerc 33, 1259–1264. [DOI] [PubMed] [Google Scholar]
- Poirier P, Tremblay A, Catellier C, Tancrède G, Garneau C & Nadeau A (2000). Impact of time interval from the last meal on glucose response to exercise in subjects with type 2 diabetes. J Clin Endocrinol Metab 85, 2860–2864. [DOI] [PubMed] [Google Scholar]
- Randle PJ (1998). Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 14, 263–283. [DOI] [PubMed] [Google Scholar]
- Reaven GM (1988). Role of insulin resistance in human disease. Diabetes 37, 1595–1607. [DOI] [PubMed] [Google Scholar]
- Reichert FF, Barros AJ, Domingues MR & Hallal PC (2007). The role of perceived personal barriers to engagement in leisure‐time physical activity. Am J Public Health 97, 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter EA & Hargreaves M (2013). Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 93, 993–1017. [DOI] [PubMed] [Google Scholar]
- Schoenfeld BJ, Aragon AA, Wilborn CD, Krieger JW & Sonmez GT (2014). Body composition changes associated with fasted versus non‐fasted aerobic exercise. J Int Soc Sports Nutr 11, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo N, Miyoshi N, Goto‐Inoue N, Minami K, Yoshimura R, Morita A, Sawada N, Matsuda J, Ogawa Y & Setou M (2015). PGC‐1α‐mediated changes in phospholipid profiles of exercise‐trained skeletal muscle. J Lipid Res 56, 2286–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneau JA, Veerkamp JH, Turcotte LP & Kelley DE (1999). Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J 13, 2051–2060. [DOI] [PubMed] [Google Scholar]
- Sjøberg KA, Frøsig C, Kjøbsted R, Sylow L, Kleinert M, Betik AC, Shaw CS, Kiens B, Wojtaszewski JF & Rattigan S (2017). Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes 66, 1501–1510. [DOI] [PubMed] [Google Scholar]
- Stöckli J, Meoli CC, Hoffman NJ, Fazakerley DJ, Pant H, Cleasby ME, Ma X, Kleinert M, Brandon AE & Lopez JA (2015). The RabGAP TBC1D1 plays a central role in exercise‐regulated glucose metabolism in skeletal muscle. Diabetes 64, 1914–1922. [DOI] [PubMed] [Google Scholar]
- Sylow L & Richter EA (2019). Current advances in our understanding of exercise as medicine in metabolic disease. Curr Opin Physiol 12, 12‐19. [Google Scholar]
- Szekeres F, Chadt A, Tom RZ, Deshmukh AS, Chibalin AV, Björnholm M, Al‐Hasani H & Zierath JR (2012). The Rab‐GTPase‐activating protein TBC1D1 regulates skeletal muscle glucose metabolism. Am J Physiol Endocrinol Metab 303, E524–E533. [DOI] [PubMed] [Google Scholar]
- Treebak JT, Frøsig C, Pehmøller C, Chen S, Maarbjerg SJ, Brandt N, MacKintosh C, Zierath J, Hardie D & Kiens B (2009). Potential role of TBC1D4 in enhanced post‐exercise insulin action in human skeletal muscle. Diabetologia 52, 891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Proeyen K, Szlufcik K, Nielens H, Pelgrim K, Deldicque L, Hesselink M, Van Veldhoven PP & Hespel P (2010). Training in the fasted state improves glucose tolerance during fat‐rich diet. J Physiol 588, 4289–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Proeyen K, Szlufcik K, Nielens H, Ramaekers M & Hespel P (2011). Beneficial metabolic adaptations due to endurance exercise training in the fasted state. J Appl Physiol 110, 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verboven K, Wens I, Vandenabeele F, Stevens A, Celie B, Lapauw B, Dendale P, Loon L, Calders P & Hansen D (2020). Impact of exercise‐nutritional state interactions in patients with type 2 diabetes. Med Sci Sports Exercise 52, 720–728. [DOI] [PubMed] [Google Scholar]
- Vieira AF, Costa RR, Macedo RC, Coconcelli L & Kruel LF (2016). Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: a systematic review and meta‐analysis. Br J Nutr 116, 1153–1164. [DOI] [PubMed] [Google Scholar]
- Walhin JP, Richardson JD, Betts JA & Thompson D (2013). Exercise counteracts the effects of short‐term overfeeding and reduced physical activity independent of energy imbalance in healthy young men. J Physiol 591, 6231–6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis GA, Dawson R, Achten J, Webber J & Jeukendrup AE (2006). Metabolic response to carbohydrate ingestion during exercise in males and females. Am J Physiol Endocrinol Metab 290, E708–E715. [DOI] [PubMed] [Google Scholar]
- Wallis GA & Gonzalez JT (2019). Is exercise best served on an empty stomach? Proc Nutr Soc 78, 110–117. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Holmes AG, Pinnamaneni SK, Garnham AP, Steinberg GR, Kemp BE & Febbraio MA (2006). Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab 290, E500‐E508. [DOI] [PubMed] [Google Scholar]
- Witczak CA, Jessen N, Warro DM, Toyoda T, Fujii N, Anderson ME, Hirshman MF & Goodyear LJ (2010). CaMKII regulates contraction‐ but not insulin‐induced glucose uptake in mouse skeletal muscle. Am J Physiol Endocrinol Metab 298, E1150–E1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA & Kiens B (2000). Isoform‐specific and exercise intensity‐dependent activation of 5′‐AMP‐activated protein kinase in human skeletal muscle. J Physiol 528, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo WK, McGee SL, Carey AL, Paton CD, Garnham AP, Hargreaves M & Hawley JA (2010). Acute signalling responses to intense endurance training commenced with low or normal muscle glycogen. Exp Physiol 95, 351–358. [DOI] [PubMed] [Google Scholar]
- Yorek M, Leeney E, Dunlap J & Ginsberg B (1989). Effect of fatty acid composition on insulin and IGF‐I binding in retinoblastoma cells. Invest Ophthalmol Vis Sci 30, 2087–2092. [PubMed] [Google Scholar]


