Abstract
Background
Secondary oligo/amenorrhoea occurs in 3%–5% of women of reproductive age. The two most common causes are polycystic ovary syndrome (PCOS) (2%–13%) and functional hypothalamic amenorrhoea (FHA) (1%–2%). Whilst both conditions have distinct pathophysiology and their diagnosis is supported by guidelines, in practice, differentiating these two common causes of menstrual disturbance is challenging. Moreover, both diagnoses are qualified by the need to first exclude other causes of menstrual disturbance.
Aim
To review clinical, biochemical and radiological parameters that could aid the clinician in distinguishing PCOS and FHA as a cause of menstrual disturbance.
Results
FHA is uncommon in women with BMI > 24 kg/m2, whereas both PCOS and FHA can occur in women with lower BMIs. AMH levels are markedly elevated in PCOS; however, milder increases may also be observed in FHA. Likewise, polycystic ovarian morphology (PCOM) is more frequently observed in FHA than in healthy women. Features that are differentially altered between PCOS and FHA include LH, androgen, insulin, AMH and SHBG levels, endometrial thickness and cortisol response to CRH. Other promising diagnostic tests with the potential to distinguish these two conditions pending further study include assessment of 5‐alpha‐reductase activity, leptin, INSL3, kisspeptin and inhibin B levels.
Conclusion
Further data directly comparing the discriminatory potential of these markers to differentiate PCOS and FHA in women with secondary amenorrhoea would be of value in defining an objective probability for PCOS or FHA diagnosis.
Keywords: functional hypothalamic amenorrhoea, oligo/amenorrhoea, polycystic ovary syndrome (PCOS)
1. INTRODUCTION
Secondary oligo/amenorrhoea occurs in 3%–5% of women of reproductive age. The two commonest causes are polycystic ovary syndrome (PCOS) 1 (2%–13%) and functional hypothalamic amenorrhoea (FHA) (1%–2%). 1 Whilst both conditions have distinct pathophysiology and their diagnosis is supported by guidelines 1 , 2 in practice, differentiating these two common causes of menstrual disturbance is challenging. Moreover, both are diagnoses of exclusion qualified by the prerequisite to exclude other causes of menstrual disturbance. 1 , 2
PCOS is diagnosed by the presence of at least 2 of hyperandrogenism, oligo/amenorrhoea and polycystic ovarian morphology on ultrasound (PCOM). 2 , 3 The Endocrine Society recommends the following criteria for diagnosis of FHA: menstrual cycle length persistently >45 days or amenorrhoea >3 months, history of weight loss, vigorous exercise or stress, and the presence of hypogonadotrophic hypo‐oestrogenism (typically <184 pmol/L). 1 A negative progestogen challenge test and normal MRI pituitary are also recommended to confirm FHA. 1
Furthermore, two related conditions to FHA are anorexia nervosa (AN) and the ‘female athlete triad’ (FAT). AN is diagnosed by the presence of restricted energy intake leading to low body weight (albeit a BMI threshold is no longer defined in Diagnostic and Statistical Manual of Mental Disorders, DSMV, although moderate severity AN has BMI < 17 kg/m2), disturbed self‐body image, intense fear of weight gain or lack of recognition of the seriousness of low body weight. 4 Hypogonadotrophic hypogonadism is no longer requisite in DSMV for the diagnosis of AN; however, amenorrhoea is present in up to 89% of AN. 4 The prevalence of secondary amenorrhoea in athletes is as high as 69%. 1 , 5 FAT is comprised of menstrual disturbance, insufficient energy availability (dietary energy minus energy expended through exercise) and reduced bone mineral density (BMD) (Z score <−1.0). 6 Overt signs of low energy availability in FAT include BMI ≤ 17.5 kg/m2 or <85% of expected body weight. 6
In women with menstrual disturbance, where PCOS and FHA are the most likely differential diagnoses, a diagnostic conundrum is often encountered. For example, a cardinal feature of PCOS is polycystic ovarian morphology (PCOM) (present in 80%–88%), but PCOM is commonly found in healthy women (20%–30%), and even more so in FHA (30%–45%), such that its presence could be incidental. Thus, even the clinician with specialist expertise may be faced with diagnostic uncertainty in women presenting with oligo/amenorrhoea, with the potential for mis‐categorization difficult to fully circumvent. Moreover, as both conditions are common, it is feasible that they may co‐exist, further complicating matters. Herein, we aim to review clinical, biochemical and radiological parameters that could aid the clinician in distinguishing between PCOS and FHA as a cause of menstrual disturbance.
2. HISTORY AND EXAMINATION
2.1. Menstrual disturbance
Women with FHA typically present with amenorrhoea, whereas oligomenorrhoea is more common in PCOS. Thresholds for cycle length used to signify menstrual disturbance vary from 35, 2 to 38, 7 to 45 days. 1 Due to cycle variability, oligomenorrhoea can also be defined by the number of cycles per year (4–9 cycles per year), whereas amenorrhoea is defined as absent menses for ≥3 months or ≤3 cycles per year. 1 Most (80%–90%) women with oligomenorrhoea have PCOS, as compared to only 40% of women with amenorrhoea. 8 Thus, amenorrhoea is more likely to associate with FHA and oligomenorrhoea with PCOS. Menarche in PCOS can be early if BMI is high, or late if BMI is low. 9 By contrast, women with FHA or FAT have a propensity to late menarche, especially if body fat percentage is low. 10
2.2. Body mass index (BMI)
PCOS is more common and FHA less common with increasing BMI (Table 1 and Table S1). Women with PCOS are almost twice as likely to be overweight (12% of PCOS have BMI 25–30 kg/m2) and three times as likely to be obese (49% of PCOS have BMI ≥ 30 kg/m2) than controls. 11 A BMI threshold of 26.6 kg/m2 discriminated Chinese women with PCOS (n = 300) from controls (n = 110) with an area under the ROC (AUROC) curve of 0.78 (sensitivity: 54.5%; specificity: 98%). 12
TABLE 1.
Discriminatory potential of parameters
| Parameter | Controls | FHA | PCOS | Area under the ROC curve to differentiate FHA from PCOS | Optimal threshold to differentiate FHA from PCOS | References |
|---|---|---|---|---|---|---|
| Body mass index (kg/m2) | 21.2 (20.3 22.4) | 19.2 (18.8, 20.0) | 25.0 (24.4, 27.3) | 98.3% |
100% sensitivity and 88.9% specificity at BMI 21.2 kg/m2 84.6% sensitivity and 100% specificity at BMI 23.7 kg/m2 |
22, 23, 65, 69, 105, 129, 130, 131, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142 |
| Resting energy expenditure (REE, kJ) | 5786 (5304, 6643) | 4300 (4300, 4300) | 6519 (5297, 6796) | 100% |
4798 kJ (same threshold when only including BMI < 25 kg/m2) |
28, 30, 32 |
| Luteinising hormone (LH, IU/L) | 5.14 (3.69, 6.40) | 2.10 (0.84, 3.22) | 9.58 (7.50, 10.68) | 100% | 5.36 IU/L | 60, 61, 65, 67, 68 |
| Anti‐Müllerian hormone (AMH, pmol/L) | 20.4 (14.7, 24.0) | 27.9 (27.0, 35.4) | 53.6 (47.1, 66.4) | 100% | 41.3 pmol/L | 65, 74, 83, 143, 144, 145 |
| Antral follicle count (AFC) | 10 (7.3, 17.8) | 16 (6.5 20.5) | 24 (16.6, 36.0) | 88.9% |
100% sensitivity and 66.7% Specificity at AFC > 16.3 66.7% sensitivity and 100% Specificity at AFC > 22.3 |
65, 68, 81, 144, 145 |
| Proportion with PCOM | 24% | 31.4% | 87.5% | n/a |
PCOM was associated with an increased odds of PCOS diagnosis by 9.6‐fold (95%CI 6.2‐14.8) compared to FHA |
65 |
| Inhibin B (pg/ml) | 88.5 (75.2, 96.5) | 46.5 (45.0, 48.0) | 105.5 (64.2, 140.1) | 100% | 50.1 pg/ml | 83, 85, 146, 147, 148, 149 |
| Fasting glucose (mmol/L) | 4.87 (4.50, 5.14) | 3.95 (3.80, 4.10) | 4.90 (4.74, 5.51) | 100% | 4.3 mmol/L | 24, 102, 104, 106 |
| Fasting insulin (pmol/L) | 54.3 (36.3, 93.3) | 86.1 (84.5, 193.7) | 48.7 (30.4, 63.5) | 100% | 74.5 pmol/L | 65, 69, 103, 105 |
| SHBG (nmol/L) | 67.4 (63.3, 71.4) | 61.4 (60.0, 62.9) | 45.3 (44.0, 46.7) | 100% | 53.3 nmol/L | 65, 107 |
| Total testosterone (nmol/L) | 1.11 (1.12, 1.20) | 0.92 (0.78, 1.06) | 1.81 (1.49, 2.94) | 100% | 1.26 nmol/L | 24, 25, 30, 65, 109 |
| Total testosterone (nmol/L) BMI < 25 kg/m2 only | 1.15 (1.13, 1.79) | 0.92 (0.78, 1.06) | 2.45 (1.50, 3.4) | 100% | 1.26 nmol/L | 24, 25, 30, 65, 109 |
| Basal morning cortisol (nmol/L) | 203 (144, 233) | 255 (210, 300) | 236 (227, 245) | 50% | 100% sensitivity & 50% specificity at cortisol 219 nmol/L | 24, 116, 118 |
Summary of clinical, biochemical and radiological parameters that could be used to differentiate FHA and PCOS. Aggregated summary statistics (median, IQR) are presented for healthy controls, women with functional hypothalamic amenorrhoea (FHA) and women with polycystic ovary syndrome (PCOS). The data presented were aggregated from the references indicated (see Tables [Link], [Link], [Link], [Link], [Link] for more details on the included studies). Sensitivities and specificities for thresholds to differentiate FHA and PCOS are provided; a single threshold indicates 100% sensitivity and specificity. However, these thresholds should be interpreted with caution, as they were calculated from summary statistics that is unable to fully capture the variability in individual patient data, thus over‐estimating their discriminatory capability. Therefore, these thresholds should be regarded as indicative pending further study in which these parameters are directly compared in women with amenorrhoea in the same study. Polycystic ovarian morphology (PCOM) was defined as having at least one ovary with 12 or more follicles per ovary and odds of PCOS diagnosis calculated by univariate logistic regression. 66
Abbreviations: AFC, total antral follicle count on ultrasound; AMH, anti‐Müllerian hormone; BMI, body mass index; LH, luteinizing hormone; SHBG, sex hormone–binding globulin.
FHA is due to one of, or a combination of low body weight, excessive exercise and psychological stress, such that some have proposed subtypes of FHA if one feature predominates. However, BMI is low (19.7 ± 1.4 kg/m2) even in ‘stress‐induced’ FHA, 13 perhaps as some women may respond to stress with caloric restriction or excessive exercise. As women with FHA have a relative energy deficit, one would expect weight loss and low BMI if sustained. However, adaptation to energy deficit can maintain body weight at the expense of energy‐consuming physiological functions such as reproduction. 14 Indeed, BMI was ‘normal’ at the time of first amenorrhoea in 38.5% of 69 women with AN, 15 highlighting the need to quantify weight loss in addition to only BMI at presentation. 16 Furthermore, one third of athletes had amenorrhoea despite ‘normal’ BMI (21.3 ± 1.6 kg/m2), 17 whereas two thirds of women with AN had amenorrhoea at a BMI between 17 and 18.9 kg/m2. 4 Although the set point for body weight at which menses are lost varies between individuals (generally BMI < 24 kg/m2, Table 1), intriguingly recovery of menses may require ~2 kg more than this set point and take up to 2 years after regain of normal body weight. 18 Recovery of menstrual function was observed in 86% of adolescents with AN who reached ≥90% of their ideal body weight, 19 and in 78% of women with FHA who gained weight (BMI 22 kg/m2 in recovered vs 20 kg/m2 in unrecovered). 20
In summary, FHA is uncommon in overweight women, whereas both PCOS and FHA can occur in women with low/normal BMI's (Figure 1). Although some women with FHA have ‘normal’ BMIs, typically these are in the lower half of the reference range (Table 1 and Table S1).
FIGURE 1.
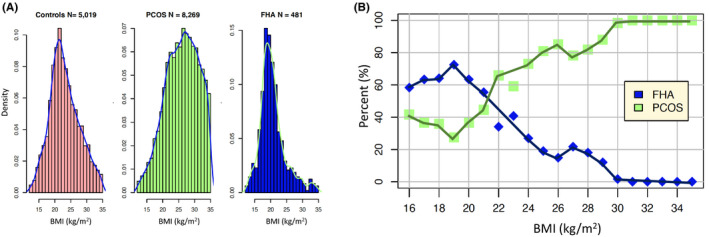
(A) The summary statistics of the studies given in Table S1 were used to simulate truncated normal data in the 12–35 kg/m2 range for BMI using a Ferguson‐Klass type algorithm for posterior normalized random measures. Instance sizes, histograms and densities of the combined data are shown for controls (left panel), PCOS (centre panel) and FHA (right panel). The y‐axes are density centiles, so 0.15 represents 15% of the total instances. (B) The 481 simulated FHA values are combined with 1000 sampled values from the simulated PCOS data so as to match the proportions expected. For each BMI in the range 16–35 kg/m2 the percentage of FHA and PCOS instances are shown. The blue and green percentages sum to 100 for each BMI. The distributions presented are simulations of summative statistics from Table S1 that reflect the same underlying populations but are not exact representations of the distribution of the original data
2.3. Body composition
Body fat percentage is higher in lean PCOS than in weight‐matched controls, 21 and fat distribution is more typically android, that is ‘fat distribution index’ (FDI = upper body fat mass/lower body fat mass) is >1.1, rather than gynoid (FDI < 0.9). 21 Conversely, women with FHA have lower body fat mass than BMI‐matched controls, 22 with 18%–28% body fat required for resumption of menses. 4 Evaluation of body composition in lean women with either PCOS, healthy controls or AN found that body fat percentage was 35.7%, 27.1% or 19.9% and FDI was 1.03, 0.70 or 0.58, respectively. 23 A meta‐analysis reported that women with PCOS are almost twice as likely to have central obesity with a pooled estimate of 54%. 11 Conversely, waist circumference and waist‐to‐hip ratios did not differ between PCOS and healthy controls in a large well‐conducted study. 24 Whether markers that correlate with body weight such as ghrelin, amylin, orexin A and adiponectin can provide additional discriminatory potential over knowledge of BMI alone remains a subject for further study.
2.4. Bone mineral density (BMD)
Women with FHA have reduced BMD for chronological age, due to hypo‐oestrogenism, nutritional deficits and other endocrine abnormalities (eg increased cortisol/decreased IGF‐1 levels). As a result, FHA guidelines recommend that a DEXA scan be conducted in all women with amenorrhoea of at least 6 months duration. 1 Spinal Z score was −1.7 in AN, −0.6 in FHA and +0.3 in controls. 25 As BMD is increased in athletes by 5%–30%, primarily due to increased weight‐bearing, a Z score of −1.0 rather than −2.0 is deemed sufficient to indicate low BMD in FAT. In FAT, lumbar BMD was reduced with T scores between −0.88 and −2.1. 26 Lumbar BMD is marginally reduced in women with PCOS and BMI < 27 kg/m2 with a pooled mean difference of −0.07, with no difference when BMI ≥ 27 kg/m2. 27 Taken together, these data demonstrate that low BMD is more consistent with FHA than PCOS.
2.5. Energy expenditure
Although resting energy expenditure (REE) was unchanged in PCOS, 28 adjusted basic metabolic rate (ie REE at rest >12 h after eating) was reduced in PCOS with insulin resistance (1116 vs 1868 kcal/day). 29 Moreover, post‐prandial thermogenesis was reduced in both obese (45.4 kJ vs 86.5 kJ) and lean (79.4 kJ vs 89.9 kJ) PCOS. 30
Amenorrhoeic athletes had lower REE than normo‐ovulatory athletes or sedentary controls. 31 REE was lower in AN (n = 28) than controls (n = 49), even after adjustment for BMI and fat‐free mass. 32 Additionally, REE was associated with Triiodothyronine (T3) levels in AN, and weight regain coincided with increases in both T3 and REE. 32 Women with FHA may have slightly lower fT3 than controls (3.1 vs 4.2 pmol/L) 22 ; however, PCOS may also be associated with mild thyroid dysfunction. 33
2.6. Psychological disorders
Psychological stress is one of the main aetiological factors contributing to FHA. 1 Women with FHA had more life events than those with PCOS (FHA 59.8%, PCOS 26.6%), and tended to perceive them more negatively. 34 When faced with a task designed to induce psychological stress, women with FHA were less able to complete the task compared with PCOS or healthy controls. 35 Both women with FHA or PCOS had increased cortisol levels during the task compared with controls. 35 Specific personality traits such as higher levels of perfectionism are also noted in FHA. 36 Cognitive behavioural therapy (CBT) increases the chance of recovery in FHA (87.5% vs 25%). 37
Depression is more common in PCOS, independently of BMI. 38 PCOS increased the odds of depression by 3.8‐fold and anxiety by 5.6‐fold. 39 One third of women with PCOS reported anxiety, with acne and subfertility being identified as risk factors. 40 CBT resulted in weight loss and improved quality‐of‐life scores in PCOS. 41
2.7. Eating disorders
Rates of eating disorder behaviours are increased in FHA, but most women do not have a formal diagnosis 42 ; almost half of FHA had disordered eating behaviours including dieting, bulimia, food and overweight preoccupation compared with 11% of controls. 42 Eating disorders, especially binge‐eating disorder, were more frequent in PCOS (11%) than controls (7.6%). 43 Women with PCOS (n = 24,385) have an increased odds of bulimia (OR 1.21), but reduced odds of AN (OR 0.72). 44 Thus, whilst both women with FHA and PCOS may display bulimic traits, restrictive eating patterns are more typically associated with FHA.
2.8. Genetic predisposition
PCOS is genetically predisposed, with ~70% heritability in monozygotic twins versus 38% in dizygotic twins/sisters. 45 A GWAS meta‐analysis identified 14 polymorphisms associated with PCOS, including genes implicated in insulin regulation, obesity and ovarian function. 46
In FHA, 7 of 55 (13%) women had heterozygous variants in genes encoding for GnRH neuronal function or migration. 47 Using whole‐exome sequencing, 55% (58/106) of women with FHA had at least one rare sequence variant (RSV) identified, as compared to 42% (200/477) of controls, and women with FHA were more likely to have 3 RSVs than controls (4.7% vs 1.2%). 48 Thus, women with FHA may be genetically predisposed to menstrual disturbance after an environmental stimulus such as weight loss. 47 There is also a high degree of heritability in eating disorders such as AN (5%–60% in twin studies), specifically in genes regulating metabolism. 49
3. BIOCHEMICAL/RADIOLOGICAL INVESTIGATIONS
3.1. GnRH pulsatility
Hypothalamic GnRH pulsatility is increased in PCOS and reduced in FHA. Increased GnRH pulsatility favours LH secretion from the pituitary gland, whereas reduced pulsatility favours FSH secretion. 50 LH pulse frequency (a surrogate of GnRH pulse frequency) was reduced from 8 to15 pulses per 24 h in controls to 1–6 pulses in long‐distance runners with secondary amenorrhoea. 51 LH pulsatility is disrupted at an energy availability <30 kcal/kg, with each unit decrease in energy availability reducing LH pulse frequency by 0.017 pulses/h 4 . LH pulse amplitude was reduced from 2.9 iU/L in controls to 0.7 iU/L in FHA. 52 Although reduced LH pulsatility is considered a hallmark feature of FHA, in fact only 8% of 49 women with FHA were completely apulsatile. 53 Overall, 78% had low pulse frequency (<9 pulses per 24 h) and 43% had low pulse amplitude (<4 iU/L). 53 BMI in those with ‘low frequency’ alone was 20.4 kg/m2, compared with 23.2 kg/m2 in ‘low amplitude and low frequency’, and 26.3 kg/m2 in ‘low amplitude’ alone. 53 Oestradiol levels were lower in those with reduced LH pulse amplitude. 53 Whilst most healthy women have reduced LH pulsatility during sleep, it was increased in 45% of women with FHA. 53 Notably, most women had significant variability in LH pulsatility on repeated measurement, suggesting that a one‐off assessment is unreliable. 53
Women with PCOS have an inherent abnormality in the GnRH pulse generator that is independent of sex steroids 54 with higher pulse frequency than controls (~22–24 vs ~16 pulses per 24 h). 55 LH pulse amplitude is higher in lean (BMI < 23 kg/m2) PCOS (13.3 iU/L) than obese (BMI > 30 kg/m2) PCOS (6.4 iU/L), or healthy controls (5.3 iU/L). 55 Together, LH pulse frequency is increased in all women with PCOS by ~40% and reduced in 78% of women with FHA. As LH pulse amplitude is not increased in obese PCOS 56 and reduced in only 43% of FHA, LH pulse frequency is likely to have greater discriminatory potential in differentiating PCOS and FHA than LH pulse amplitude.
3.2. GnRH test
LH responses to GnRH correlated with basal LH levels in 37 women with FHA. 57 LH rises after 50 μg GnRH were similar in FHA (3.1 ≫ 8.6 iU/L; n = 8) as in healthy women (3.9 ≫ 6.0 iU/L; n = 6). 58 Likewise, LH responses to GnRH (1.56–6.25 μg) were similar in 14 women with secondary ‘stress’ amenorrhoea as in 8 healthy women. 59 In 40 women with AN (BMI 15.1 kg/m2), although basal LH (3.2 ± 3.4 vs 7.2 ± 2.3 iU/L) and FSH (3.6 ± 2.5 vs 5.0 ± 1.4 iU/L) levels were lower than in controls, LH rises following 100 μg GnRH were similar (17.9 ± 17.0 vs 20.7 ± 13.4 iU/L); albeit FSH rises were higher (10.9 ± 7.5 vs 3.3 ± 1.5 iU/L). 60
Absolute LH rises after GnRH (2–20 μg) were two‐ to three‐fold greater in PCOS (BMI 34.7 kg/m2; n = 13) than in healthy women (BMI 26.8 kg/m2, n = 13). 61 Notably, LH rises after GnRH positively correlated with basal LH values but negatively with BMI. 61 Therefore, due to higher basal LH values in PCOS (7.5 ± 1.2 vs 3.6 ± 0.4 iU/L), the percentage rise in LH after GnRH was not increased. 61 Overall, although absolute LH rises to GnRH were higher in PCOS, this may not necessarily indicate heightened pituitary sensitivity per se but rather reflect increased basal LH values. 61 Mean (±SD) LH rose from 9.0 ± 5.8 to 35.4 ± 31.3 iU/L at 30 min after 100 μg of GnRH in PCOS (n = 121), compared with from 4.8 ± 1.7 to 16.3 ± 6.7 iU/L in healthy controls (n = 32), but FSH‐rise did not significantly differ. 62 An LH/FSH ratio at 30 min after GnRH of >2.11 had a 78.5% sensitivity and 87.5% specificity for the diagnosis of PCOS. 62 The response to GnRH in PCOS did not differ by androgen status. 63
3.3. Basal gonadotrophin values
LH levels were raised to >95th percentile of healthy women in 40%–60% of PCOS. 64 In PCOS (n = 3640), FHA (n = 159) (defined by gonadotrophins <2 iU/L) and healthy controls (n = 83), LH was 9.6, 0.8 and 3.8 iU/L, FSH was 5.6, 3.1 and 6.3 iU/L and oestradiol was 279, 72 and 170 pmol/L, respectively. 65 Notably, LH was more commonly raised in PCOS if associated with menstrual disturbance. 66
An LH:FSH ratio of 1.33 had an AUROC curve of 0.87 (95% CI 0.84–0.89) to distinguish PCOS from healthy controls, with a one unit increase in the ratio increasing the odds of PCOS by 14‐fold. 67 Baseline LH levels were lower in 28 women with FHA than in 30 controls (2.1 ± 1.2 vs 5.6 ± 1.5 iU/L), whereas FSH levels did not differ (5.6 ± 2.1 vs 5.4 ± 1.6 iU/L). 68 Thus, LH levels (more reflective of alterations in GnRH pulsatility) can better aid in distinguishing FHA and PCOS than FSH levels.
3.4. Growth hormone axis
Women with FHA (n = 8) had higher nocturnal growth hormone (GH) levels than eumenorrhoeic controls (5.21 ± 0.89 vs 3.06 ± 0.33 μg/L), although 24‐h GH values did not differ. 69 Reduced nutritional intake results in GH resistance characterised by increased GH and reduced IGF‐1. 70 GH pulse amplitude is higher in lean PCOS than lean controls (9.1 vs 5.9 μg/L), but reduced with obesity (1.6–2.2 μg/L). 55 GH pulse frequency and IGF‐1 levels did not differ in PCOS in most, 55 but not all studies. 71 Thus, IGF‐1 levels could have potential to differentiate FHA and PCOS pending further study.
3.5. Anti‐Müllerian hormone (AMH)
AMH is produced by granulosa cells of growing antral follicles in the ovary and thus levels correlate with total antral follicle count (AFC). 72 AMH is increased in PCOS corresponding to the number of PCOS features and better predicts menstrual disturbance than AFC (AUROC 0.77 vs 0.67). 72 A meta‐analysis identified an AMH threshold of 33.6 pmol/L to have 79.4% sensitivity and 82.8% specificity for PCOS diagnosis. 73
In FHA, AMH levels are mildly increased compared with healthy controls (Table 1 and Table S2). In 40 women with FHA, AMH levels fell from 42.1 to 27.1 pmol/L at 1 year after resumption of menstrual cycles. 74 Notably, this was not accompanied by a corresponding change in AFC or ovarian volume. 74 Furthermore, in 61 women with AN, higher AMH at diagnosis predicted 1‐year recovery of menstrual cyclicity (43.6 vs 31.4 pmol/L). 75
AMH was higher in age‐matched women with PCOS (47.9 pmol/L), than in FHA (27.1 pmol/L) and in healthy women (13.6 pmol/L). 65 In 1843 women with oligo/amenorrhoea, AMH was higher in the 69% with PCOS (65 pmol/L) than in the 7% with FHA (24.3 pmol/L). 76 Collectively, AMH levels are markedly elevated in PCOS; however, milder increases are also seen in FHA. Thus, whilst AMH has discriminatory potential to distinguish PCOS from healthy women, its performance could be tempered in women with menstrual disturbance.
3.6. PCO morphology on ultrasound
PCOM is a cardinal feature of PCOS; morphologically, PCOM ovaries have a central stroma surrounded by peripherally located follicles, 77 whereas increased follicle number per ovary (FNPO) without this typical peripheral follicular distribution has been described as multicystic ovarian morphology (MCOM). 78 Women with FHA have increased rates of MCOM 78 ; however due to the subjective nature of assessing follicular distribution, PCOM is now more typically defined by FNPO. Thus, MCOM ovaries are classified as PCOM in diagnostic criteria even in the absence of peripheral follicular distribution, such that PCOM by FNPO is reported in 30%–45% of FHA and 20%–30% of healthy women. 79 , 80
Recently, the recommended threshold for FNPO to denote PCOM has increased from 12 81 to 20 2 to reflect improved ultrasound resolution. The impact of this was assessed in a study of 1390 women, in which 861 (62%) qualified for the diagnosis of PCOS due to having both oligo/amenorrhoea and hyperandrogenism. 83 Of the remainder, 67% (306/529) qualified as PCOS using an FNPO threshold >12, compared with only 9% (101/529) if using a threshold of >20. 82 Thus, diagnosis of PCOS can significantly vary by the criteria used to define PCOM. Although AMH correlates with AFC, AMH may be disproportionately increased in FHA and PCOS (Table 1 and Tables S2 and S3). AMH levels in FHA correlate with the number of smaller antral follicles (2–5 mm), but not larger follicles (6–9 mm), which could contribute to discrepancy between AFC and AMH. 83
3.7. Inhibin‐B
Inhibin B is produced by granulosa cells and peaks during the follicular phase. Although some studies have reported higher inhibin B in PCOS, 84 others have found similar levels to healthy controls 85 (Table 1 and Table S4). Inhibin B negatively correlates with BMI in both PCOS and healthy women, 85 which could explain some of this incongruity. 85 Inhibin B levels are lower in FHA than controls, 83 suggesting that it has the potential to differentiate lean PCOS from FHA.
3.8. Circulating kisspeptin levels
Kisspeptin neurons regulate GnRH neuronal pulsatility; if circulating kisspeptin levels are assumed to reflect central GnRH neuronal pulsatility, one would expect kisspeptin levels to be increased in PCOS and reduced in FHA. Kisspeptin levels corresponded to LH pulses in women with FHA and were lower in women with FHA and LH ≤ 3 iU/L at 1.7 ng/ml than in women with FHA and LH > 3 IU/L at 2.6 ng/ml. 86 Kisspeptin levels were not significantly lower in AN (0.20 ± 0.07 ng/ml) compared with controls (0.3 ± 0.36 ng/ml). 87 However, in women with AN (BMI 14.8 kg/m), kisspeptin levels positively correlated with BMI. 88 Most studies have reported higher kisspeptin levels in PCOS 89 and a meta‐analysis determined that circulating kisspeptin levels have an AUROC curve of 0.84 for PCOS diagnosis. 90
3.9. Progesterone withdrawal test and endometrial thickness (ET)
FHA is a low oestradiol state, whereas oestradiol is typically preserved in PCOS. Historically, oestradiol assays performed poorly at low levels, and thus, alternative assessments of functional oestradiol exposure were used, for example cervical mucus examination or the progesterone withdrawal test. 91 Accordingly, women with PCOS are expected to have a withdrawal bleed after a short course of progesterone, whereas women with FHA are not.
Originally, oestradiol levels were reported to be higher in amenorrhoeic women who had a withdrawal bleed after a course of progesterone (297.4 vs 135.8 pmol/L), 92 with a threshold of 146.9 pmol/L predicting those who bled. 93 However, 40%–57% of women with FHA have a withdrawal bleed after progesterone withdrawal 93 , 94 and the response does not always relate to oestradiol levels. 95 Thus, despite its widespread adoption and inclusion in FHA guidelines, there remain concerns regarding the discriminatory ability of this test.
Indeed, endometrial thickness (ET) better predicts the response to progesterone withdrawal than oestradiol level. 94 Most (93%, 50/54) amenorrhoeic women with ET > 1.5 mm have a withdrawal bleed, compared with only 6% (1/16) of those with thinner ET. 96 ET is higher in PCOS than controls both on cycle days 3–5 (4.8 vs 3.4 mm) 97 and on days 6–10 (11.1 vs 6.2 mm). 98 ET on a random cycle day is lower in lean amenorrhoeic (n = 43) than oligomenorrhoeic women (n = 18) (5.1 vs 8.2 mm). 99 Furthermore, ET on days 10–11 of the cycle is thinner in athletes than sedentary age‐matched controls (10 vs 13 mm). 100 Thus, it is unclear that the progesterone withdrawal test provides any additional information over estimation of ET.
3.10. Insulin resistance
Women with PCOS have a three‐ to five‐fold increased risk of impaired glucose tolerance (IGT), depending on ethnicity. 101 Insulin resistance was more common in women with PCOS, whether overweight (BMI ≥ 27 kg/m2, 95%) or lean (BMI < 27 kg/m2, 75%), than in overweight controls (62%). 102 Both glucose and basal/stimulated insulin levels were higher in PCOS than weight/age‐matched controls after oral glucose tolerance test (OGTT). 103 Of 208 women with PCOS, 10% had T2DM by OGTT, but only 35% of these women had an HbA1c ≥ 6.5%. 104 In lean (BMI < 25 kg/m2) PCOS, fasting plasma glucose did not significantly differ from controls (median 4.72 vs 4.83 mmol/L); however, 2‐h glucose after an OGTT (6.30 vs 5.42 mmol/L) and HOMA IR were increased (1.91 vs 1.47). 24
In contrast, FHA is typically associated with hypoinsulinaemia and hypoglycaemia. 69 Basal insulin was lower in FHA (n = 88) than age/BMI‐matched controls (n = 65). 105 Insulin sensitivity following OGTT 106 or IVGTT was not significantly altered. In a direct comparison, fasted insulin levels were higher in PCOS (84.2 ± 76.7 pmol/L) than FHA (60.7 ± 67.5 pmol/L) or controls (54.3 ± 27.3). 65
3.11. Sex hormone–binding globulin (SHBG)
Insulin inhibits hepatic SHBG synthesis, such that hyperinsulinaemia is associated with reduced SHBG levels. Although SHBG is inversely correlated with BMI, SHBG levels are lower in PCOS even accounting for BMI (lean healthy 74 ± 24, lean PCOS 55 ± 29, overweight healthy 50 ± 24, overweight PCOS 32 ± 18 nmol/L). 107 In FHA (n = 15), SHBG levels did not differ from age/weight‐matched controls (n = 14). 108 In a direct comparison, SHBG levels were 47, 63 and 71 nmol/L in PCOS, healthy controls and FHA, respectively. 65 Thus, free androgen index (FAI) is likely to better differentiate FHA and PCOS than unadjusted testosterone levels.
3.12. Androgens
Insulin resistance is tissue‐specific; such that whilst there is resistance to insulin's hypoglycaemic action, ovarian thecal androgen production remains sensitive to elevated insulin levels and contribute to hyperandrogenism in PCOS. 109 Women with lean PCOS (BMI < 25 kg/m2) had higher median testosterone (1.5 vs 1.1 nmol/L), DHEAS (3.2 vs 2.7 μmol/L), androstenedione (8.0 vs 6.4 nmol/L) and FAI (1.37 vs 0.55) than lean controls. 24 Notably, adrenally derived androgens, for example DHEAS, 17‐hydroxypregnenolone and 17‐hydroxyprogesterone, were more often raised in lean PCOS (CAH excluded), whereas FAI was more often elevated in women with higher BMIs. 24
Women with AN had reduced testosterone levels (0.8 vs 1.1 nmol/L), but SHBG or DHEAS levels did not differ. 25 Testosterone was higher in PCOS (1.95 ± 1.04) than in controls (1.15 ± 0.5), or FHA (0.78 ± 0.42). 65 Similarly, androstenedione levels were 6.14, 3.48 and 3.05 nmol/L and the Ferriman‐Gallwey scores were 7.29, 3.45 and 4.64, respectively. 65 Women with AN have reduced 5‐alpha‐reductase activity, 110 whereas women with PCOS have increased 5‐alpha‐reductase activity, 111 such that pending further data, a ‘DHEA challenge test’ could potentially be used to distinguish these conditions. 112
3.13. Leptin
Leptin levels correlate with fat mass and therefore would be expected to be increased in PCOS and reduced in FHA. Fasting leptin levels are also increased by oestradiol, which may also contribute to a difference in leptin levels between FHA and PCOS. Leptin levels are highest at midnight and lowest in the morning. A meta‐analysis found that leptin was 1.62‐fold higher in 991 women with PCOS than 898 controls. 113 Leptin was also higher in overweight (BMI > 23 kg/m2) than lean controls (17 vs 13 μg/L), and overweight than lean PCOS (27 vs 19 μg/L). 114
Conversely, women with FHA have lower leptin levels than expected for their BMI. 105 Leptin was ~40%–60% lower in FHA than BMI‐matched controls. 105 In FHA, each 1 kg/m2 BMI increase was associated with an 0.89 μg/ml elevation in leptin levels, which is half of that seen in controls (1.8 μg/L). 105 Although pre‐treatment leptin levels were similar in FHA and controls, 2 weeks of recombinant leptin treatment increased LH levels (2.8–4.8 iU/L) and LH pulse frequency (2.4–5.0 pulses per 12 h) but not LH pulse amplitude (3.2–3.6 iU/L). 115
3.14. Cortisol
Cortisol levels were higher in FHA than healthy women, and normalised after recovery of menstrual cyclicity. 116 Although cortisol pulse frequency is unchanged, cortisol pulse amplitude is increased, 117 with higher 8PM and 24‐h cortisol levels (Table 1 and Table S5). In female athletes, 24‐h cortisol levels negatively correlated with LH pulse frequency. 118 Early morning cortisol values were higher in amenorrhoeic athletes (230 nmol/L) than ovulatory athletes (180 nmol/L), or sedentary ovulatory women (170 nmol/L). 118 Cortisol levels after CRH were lower in FHA than healthy controls (320 vs 440 nmol/L), 116 whereas ACTH and cortisol rises after CRH were increased in PCOS compared with controls. 119 In 350 lean women with PCOS, morning cortisol levels were higher (245 nmol/L) than in 203 healthy controls (203 nmol/L). 24 Consequently, whilst basal cortisol levels were elevated in both conditions, the cortisol response to CRH is reduced in FHA but increased in PCOS and thus could have discriminatory potential.
3.15. Insulin‐like factor 3 (INSL3)
INSL3 is produced by ovarian theca cells and the corpus luteum. INSL3 levels were higher in 134 women with PCOS (0.18 ng/ml) than in 344 healthy controls (0.079 ng/ml). 120 Specifically, INSL3 levels were higher in lean PCOS than in controls, but not in overweight PCOS (n = 22 per group). 121 Notably, INSL3 was only raised if PCOS was associated with oligo/amenorrhoea. 28 INSL3 levels correlated with follicle number, LH levels, hirsutism and androgen levels in PCOS. 121 One would anticipate that INSL3 levels would be lower in FHA given the reduced gonadotrophin stimulus to theca cells; thus, data assessing whether INSL3 has discriminatory potential in lean PCOS/FHA would be of interest.
4. OVERLAP BETWEEN FHA AND PCOS
PCOS and FHA are common conditions, and thus, it is eminently feasible for both diagnoses to co‐exist. By definition, women with FHA have menstrual disturbance, and thus, the additional presence of PCOM would be sufficient to qualify a diagnosis of PCOS. Thus, it is noteworthy that PCOM 83 , 122 and raised AMH levels are more frequent in FHA. Of 159 women with FHA, 36% also met criteria for PCOS (25% PCOM, 6.9% hyperandrogenism, 5% both). 65 Of 122 women with FHA, 41 ‘suspected of having underlying PCOS’ (although specific criteria for this suspicion were unclear), had higher LH (7.7 vs 3.1 iU/L), testosterone (1.9 vs 1.1 nmol/L) and lumbar T scores (−1.1 vs −1.9). 123 Of 100 ‘exercising’ (~6 h per week) women with oligo/amenorrhoea (who might be presumed to have FHA), 17 had hyperandrogenism which would be more consistent with PCOS 124 ; these women had greater BMI (22.3 vs 20.6 kg/m2), body fat (27.3% vs 24.4%), fat mass (16.2 vs 13.8 kg), insulin (5.8 vs 4.2 IU/L), leptin (12.2 vs 6.6 ng/ml), FAI (6.1 vs 1.7) and LH:FSH ratio (1.9 vs 1.3). 124 In a further study, 13 of 40 (32.5%) women with FHA had high AMH levels (>33.6 pmol/L), and 4 (10%) had increased ovarian volume (≥10 cm3), and these women were more likely to have elevated androgen levels. 74 Likewise, 10% of 58 women with FHA had PCOS‐range AMH levels and high ovarian area, 79 although LH levels were not altered in these women. 68 Of 65 Australian lean women with AMH > 40 pmol/L and BMI < 20 kg/m2, 22 were diagnosed with FHA and 43 with PCOS. 125 Notably, 86% of women diagnosed as FHA also met the diagnostic criteria for PCOS. 125 Almost all (95%) women with FHA had PCOM and all were Caucasian (vs 35% of PCOS). Whereas all women with FHA were amenorrhoeic and had an ET of <4 mm, those with PCOS had either oligomenorrhoea or regular cycles. 125 Median SHBG in FHA vs PCOS was 111 vs 56 nmol/L, LH (1.6 vs 5.5 iU/L), FSH (4.2 vs 6.4 iU/L), and FAI (0.7 vs 1.6). 125
Six women with ‘FHA and PCOM’ were found to be more likely to develop hyperandrogenism after gonadotrophin stimulation, and 5 women with ‘FHA and PCOM’, who increased their BMI by 5%–18% to restore menses, developed oligomenorrhoea and hyperandrogenism. 126 Likewise, 6 of 120 women with FHA developed PCOS features following pulsatile GnRH therapy. 127 Conversely, a study compared 40 women with ‘FHA and PCOM’ to 27 women with ‘FHA alone’ but did not find any differences in androgen or gonadotrophin levels (FHA with PCOM: LH 1.7, FSH 4.9 vs FHA alone: LH 1.1, FSH 5.0 iU/L), nor in the response to pulsatile GnRH therapy. 128 A further study reported that only one of twelve FHA women with features of PCOS such as raised AMH, or raised androgens, went on to develop PCOS following menstrual recovery. 68
Together, these data confirm that a diagnostic dilemma is often encountered when assessing patients presenting with secondary amenorrhoea, with many women meeting criteria for both conditions. Additionally, women with ‘FHA and PCOM’ may have ovaries that are prone to producing androgens, but that this may be masked by the low LH levels that are characteristic of FHA. Thus, FHA predominates when both conditions co‐exist; however in some women, PCOS may be revealed following restoration of body weight or gonadotrophin therapy.
5. CONCLUSION
Despite FHA and PCOS having contrasting pathophysiology, in practice, differentiating these two common causes of menstrual disturbance is challenging. In the literature, there is wide variation in the criteria used to define both PCOS and FHA, and the performance of many of the factors evaluated in this review heavily depends on how the initial categorization was reached. Although diagnostic criteria for both PCOS and FHA recommend the exclusion of other causes of menstrual disturbance, in practice, there is no test capable of doing so, and many women meet the criteria for both diagnoses. Indeed, even characteristic features of PCOS, such as PCOM, are more frequently observed in FHA than in healthy women. Moreover, PCOM is also a common incidental finding in healthy women, such that its presence does not have sufficient specificity to identify ‘underlying PCOS’ in women with FHA. Furthermore, PCOM does not affect the clinical presentation in FHA, and the FHA phenotype predominates when both diagnoses co‐exist. However, in a proportion of women with ‘FHA and PCOM’, the presence of ‘underlying PCOS’ can be revealed following weight gain, or gonadotrophin treatment, suggesting that some women have ovaries that are prone to secreting androgens once stimulated. Thus, PCOM can be regarded as a sensitive but not specific indicator for underlying PCOS and may be incidental in many women with FHA.
Overall, the diagnosis in uncertain cases could be concluded based on a theoretical demarcation of the essence of each condition. A fundamental pathophysiological difference between PCOS and FHA is the change in GnRH pulsatility, being increased in PCOS but reduced in FHA. In women with PCOS and hyperandrogenism, use of the oral contraceptive pill can attenuate LH levels and moderate hyperandrogenism. Accordingly, it is logical that in a woman with PCOS who enters a state of energy deficiency such as to also develop FHA and have reduced GnRH pulsatility, the resultant low LH levels would diminish androgen secretion. Therefore, raised LH/androgen levels would indicate that PCOS is the predominant phenotype, whereas low LH/androgen levels would denote FHA.
The challenges in correctly assigning each diagnosis lead to the potential for misclassification. In many studies that investigate the performance of a factor to identify FHA or PCOS, the initial categorization is often influenced by the presence of that factor. For example, one could arbitrarily define ‘FHA with underlying PCOS’ as being women with ‘FHA and PCOM’, and then subsequently report that PCOM is more common in ‘FHA with underlying PCOS’. Although understandable, this approach is flawed in that it predisposes to the realization of a self‐fulfilling prophecy. Furthermore, although PCOS and FHA are regarded as life‐long conditions with an underlying genetic basis, modifying factors such as BMI and age can affect the phenotypic presentation. Thus, it is perfectly feasible for a woman to drift between diagnoses as she, for example, gains or looses weight and functionally behaves ‘as PCOS’ or ‘as FHA’ at different times in her life.
In this review, features that were differentially altered between FHA and PCOS included GnRH pulsatility, BMI, levels of LH, androgens, insulin, AMH and SHBG, ET, BMD and cortisol response to CRH. Other tests with discriminatory potential pending further study included assessment of 5‐alpha‐reductase activity, leptin, INSL3, IGF‐1, kisspeptin and inhibin B levels (Table 1, Figure 2). Future studies measuring all potential discriminatory clinical, biochemical and radiological markers in all women presenting with secondary oligo/amenorrhoea would be of value in order that we may attempt to objectively classify these two common causes of menstrual disturbance.
FIGURE 2.
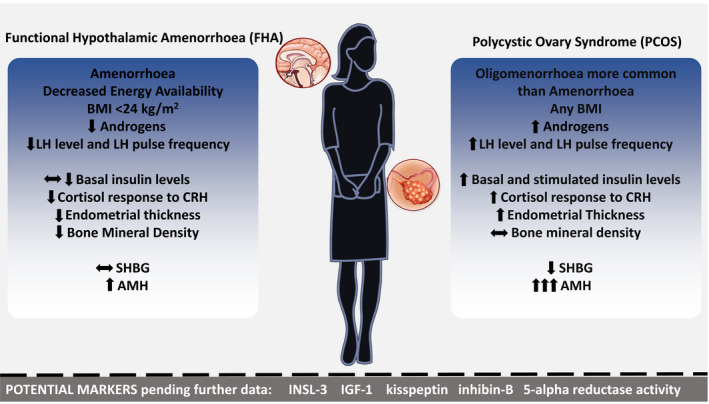
Clinical, biochemical and radiological factors that may aid the clinician in distinguishing functional hypothalamic amenorrhoea (FHA) and polycystic ovarian syndrome (PCOS) in women with secondary oligo/amenorrhoea. OGTT, oral glucose tolerance test; CRH, corticotrophin‐releasing hormone; AMH, anti‐Müllerian Hormone; SHBG, sex hormone binding globulin; LH, luteinising hormone; BMI, body mass index
CONFLICT OF INTEREST
The research was conducted in the absence of any personal, professional, commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
MP, SC, PB CB, CNJ, AC, WSD, AA designed the study, analysed the data, prepared the manuscript and designed the figures and tables. MP, SC, BP, CB, AA conducted data collection. MP, SC, BP, TK, AA performed the statistical analysis. AA was the project supervisor, reviewed and edited the manuscript and is the guarantor of this research project. All authors have made a substantial, direct and intellectual contribution to the work and approved the manuscript prior to its submission.
Supporting information
Table S1
Table S2
Table S3
Table S4
Table S5
ACKNOWLEDGEMENTS
This work is supported by grants from the National Institute of Health Research (NIHR) and supported by the NIHR/Wellcome Trust Imperial Clinical Research Facility and the NIHR Imperial Biomedical Research Centre. The Section of Endocrinology and Investigative Medicine was funded by grants from the MRC, BBSRC and NIHR and was supported by the NIHR Biomedical Research Centre Funding Scheme. The views expressed are those of the author(s) and not necessarily those of the MRC, BBSRC, the NHS, the NIHR or the Department of Health. SAC was supported an NIHR Academic Clinical Lectureship. CNJ was supported by an NIHR Post‐Doctoral Fellowship. ANC was supported by the NHS. WSD was supported by an NIHR Research Professorship NIHR‐RP‐2014‐05‐001. AA was supported by an NIHR Clinician Scientist Award CS‐2018‐18‐ST2‐002.
Phylactou M, Clarke SA, Patel B, et al. Clinical and biochemical discriminants between functional hypothalamic amenorrhoea (FHA) and polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf). 2021;95:239–252. 10.1111/cen.14402
Maria Phylactou and Sophie A. Clarke should be considered joint first authors.
Funding information
This work is supported by grants from the National Institute for Health Research (NIHR) and supported by the NIHR/Wellcome Trust Imperial Clinical Research Facility and the NIHR Imperial Biomedical Research Centre. The Section of Endocrinology and Investigative Medicine was funded by grants from the MRC, BBSRC and NIHR and was supported by the NIHR Biomedical Research Centre Funding Scheme. The views expressed are those of the author(s) and not necessarily those of the MRC, BBSRC, the NHS, the NIHR or the Department of Health. SAC was supported by an NIHR Academic Clinical Lectureship. CNJ was supported by an NIHR Post‐Doctoral Fellowship. ANC was supported by the NHS. WSD was supported by an NIHR Research Professorship NIHR‐RP‐2014‐05‐001. AA was supported by an NIHR Clinician Scientist Award CS‐2018‐18‐ST2‐002.
Footnotes
In this review, ‘PCOS’ is used as short‐form to denote ‘women with PCOS’, ‘HA’ to denote ‘women with HA’, and ‘healthy controls’ to denote ‘women with regular menstrual cycles unaffected by PCOS or HA’.
DATA AVAILABILITY STATEMENT
The data sets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Gordon CM, Ackerman KE, Berga SL, et al. Functional hypothalamic amenorrhea: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(5):1413–1439. [DOI] [PubMed] [Google Scholar]
- 2. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence‐based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):364–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fauser BCJM, Tarlatzis F, Chang A, et al. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome. Hum Reprod. 2004;19:41–47. [DOI] [PubMed] [Google Scholar]
- 4. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Health Disorders: DSM‐5, 5th ed. Washington, DC: DSM; 2015. [Google Scholar]
- 5. Meczekalski B, Katulski K, Czyzyk A, Podfigurna‐Stopa A, MacIejewska‐Jeske M. Functional hypothalamic amenorrhea and its influence on women's health. Acad Psychiatry. 2014;37(11):1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot‐Borgen J, Warren MP. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867–1882. [DOI] [PubMed] [Google Scholar]
- 7. Munro MG, Critchley HOD, Fraser IS, et al. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstetr. 2018;143(3):393–408. [DOI] [PubMed] [Google Scholar]
- 8. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carroll J, Saxena R, Welt CK. Environmental and genetic factors influence age at menarche in women with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2012;25(5–6):459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klentrou P, Plyley M. Rhythmic gymnasts compared with normal controls. Br J Sports Med. 2003;37:490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta‐analysis. Hum Reprod Update. 2012;18(6):618–637. [DOI] [PubMed] [Google Scholar]
- 12. Dou P, Ju H, Shang J, et al. Application of receiver operating characteristic curve in the assessment of the value of body mass index, waist circumference and percentage of body fat in the Diagnosis of Polycystic Ovary Syndrome in childbearing women. J Ovarian Res. 2016;9(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bruni V, Dei M, Morelli C, Schettino MT, Balzi D, Nuvolone D. Body composition variables and leptin levels in functional hypothalamic amenorrhea and amenorrhea related to eating disorders. J Pediatr Adolesc Gynecol. 2011;24(6):347–352. [DOI] [PubMed] [Google Scholar]
- 14. Wade GN, Schneider JE, Li HY. Control of fertility by metabolic cues. Am J Physiol ‐ Endocrinol Metab. 1996;270(1):E1–E19. [DOI] [PubMed] [Google Scholar]
- 15. Berner LA, Feig EH, Witt AA, Lowe MR. Menstrual cycle loss and resumption among patients with anorexia nervosa spectrum eating disorders: is relative or absolute weight more influential? Int J Eat Disord. 2017;50(4):442–446. [DOI] [PubMed] [Google Scholar]
- 16. Berner LA, Shaw JA, Witt AA, Lowe MR. The relation of weight suppression and body mass index to symptomatology and treatment response in anorexia nervosa. J Abnorm Psychol. 2013;122(3):694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Souza MJ, Toombs RJ, Scheid JL, O'Donnell E, West SL, Williams NI. High prevalence of subtle and severe menstrual disturbances in exercising women: confirmation using daily hormone measures. Hum Reprod. 2010;25(2):491–503. [DOI] [PubMed] [Google Scholar]
- 18. Pape J, Herbison AE, Leeners B. Recovery of menses after functional hypothalamic amenorrhoea: if, when and why. Hum Reprod Update. 2020;1–24. [DOI] [PubMed] [Google Scholar]
- 19. Golden NH, Jacobson MS, Schebendach J, Solanto MV, Hertz SM, Shenker IR. Resumption of menses in anorexia nervosa. Arch Pediatr Adolesc Med. 1997;151(1):16. [DOI] [PubMed] [Google Scholar]
- 20. Perkins RB, Hall JE, Martin KA. Aetiology, previous menstrual function and patterns of neuro‐endocrine disturbance as prognostic indicators in hypothalamic amenorrhoea. Hum Reprod. 2001;16(10):2198–2205. [DOI] [PubMed] [Google Scholar]
- 21. Kirchengast S, Huber J. Fat distribution patterns and hormone levels among lean and overweight PCOS patients. Endocrinol Diabetes Res. 2017;03(01). [Google Scholar]
- 22. Couzinet B, Young J, Brailly S, Le Bouc Y, Chanson P, Schaison G. Functional hypothalamic amenorrhoea: a partial and reversible gonadotrophin deficiency of nutritional origin. Clin Endocrinol. 1999;50(2):229–235. [DOI] [PubMed] [Google Scholar]
- 23. Kirchengast S, Huber J. Body composition characteristics and fat distribution patterns in young infertile women. Fertil Steril. 2004;81(3):539–544. [DOI] [PubMed] [Google Scholar]
- 24. Deng Y, Zhang Y, Li S, et al. Steroid hormone profiling in obese and nonobese women with polycystic ovary syndrome. Sci Rep. 2017;7(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller KK, Lawson EA, Mathur V, et al. Androgens in women with anorexia nervosa and normal‐weight women with hypothalamic amenorrhea. J Clin Endocrinol Metab. 2007;92(4):1334–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khan KM, Liu‐Ambrose T, Sran MM, Ashe MC, Donaldson MG, Wark JD. New criteria for female athlete triad syndrome? As osteoporosis is rare, should osteopenia be among the criteria for defining the female athlete triad syndrome? Br J Sports Med. 2002;36(1):10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piovezan JM, Premaor MO, Comim FV. Negative impact of polycystic ovary syndrome on bone health: a systematic review and meta‐analysis. Hum Reprod Update. 2019;25:634–646. [DOI] [PubMed] [Google Scholar]
- 28. Larsson I, Hulthén L, Landén M, Pålsson E, Janson PO, Stener‐Victorin E. Dietary intake, resting energy expenditure, and eating behavior in women with and without polycystic ovary syndrome. Clin Nutr. 2016;35(1):213–218. [DOI] [PubMed] [Google Scholar]
- 29. Georgopoulos NA, Saltamavros AD, Vervita V, et al. Basal metabolic rate is decreased in women with polycystic ovary syndrome and biochemical hyperandrogenemia and is associated with insulin resistance. Fertil Steril. 2009;92(1):250–255. [DOI] [PubMed] [Google Scholar]
- 30. Robinson S, Chan S‐P, Spacey S, Anyaoku V, Johnston DG, Franks S. Postprandial thermogenesis is reduced in polycystic ovary syndrome and is associated with increased insulin resistance. Clin Endocrinol. 1992;36(6):537–543. [DOI] [PubMed] [Google Scholar]
- 31. Scheid JL, Williams NI, West SL, VanHeest JL, De SMJ. Elevated PYY is associated with energy deficiency and indices of subclinical disordered eating in exercising women with hypothalamic amenorrhea. Appetite. 2009;52(1):184–192. [DOI] [PubMed] [Google Scholar]
- 32. Onur S, Haas V, Bosy‐Westphal A, et al. L‐tri‐iodothyronine is a major determinant of resting energy expenditure in underweight patients with anorexia nervosa and during weight gain. Eur J Endocrinol. 2005;152(2):179–184. [DOI] [PubMed] [Google Scholar]
- 33. Janssen OE, Mehlmauer N, Hahn S, Öffner AH, Gärtner R. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur J Endocrinol. 2004;150(3):363–369. [DOI] [PubMed] [Google Scholar]
- 34. Fioroni L, Fava M, Genazzani AD, Facchinetti F, Genazzani AR. Life events impact in patients with secondary amenorrhoea. J Psychosom Res. 1994;38(6):617–622. [DOI] [PubMed] [Google Scholar]
- 35. Gallinelli A, Matteo ML, Volpe A, Facchinetti F. Autonomic and neuroendocrine responses to stress in patients with functional hypothalamic secondary amenorrhea. Fertil Steril. 2000;73(4):812–816. [DOI] [PubMed] [Google Scholar]
- 36. Pentz I, Nakić RS. Functional hypothalamic amenorrhea and its psychological correlates: a controlled comparison. J Reprod Infant Psychol. 2017;35(2):137–149. [DOI] [PubMed] [Google Scholar]
- 37. Berga SL, Marcus MD, Loucks TL, Hlastala S, Ringham R, Krohn MA. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertil Steril. 2003;80(4):976–981. [DOI] [PubMed] [Google Scholar]
- 38. Dokras A, Clifton S, Futterweit W, Wild R. Increased risk for abnormal depression scores in women with polycystic ovary syndrome: a systematic review and meta‐analysis. Obstet Gynecol. 2011;117(1):145–152. [DOI] [PubMed] [Google Scholar]
- 39. Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta‐analysis. Hum Reprod. 2017;32(5):1075–1091. [DOI] [PubMed] [Google Scholar]
- 40. Benson S, Hahn S, Tan S, et al. Prevalence and implications of anxiety in polycystic ovary syndrome: results of an internet‐based survey in Germany. Hum Reprod. 2009;24(6):1446–1451. [DOI] [PubMed] [Google Scholar]
- 41. Cooney LG, Milman LW, Hantsoo L, et al. Cognitive‐behavioral therapy improves weight loss and quality of life in women with polycystic ovary syndrome: a pilot randomized clinical trial. Fertil Steril. 2018;110(1):161–171.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tranoulis A, Soldatou A, Georgiou D, Mavrogianni D, Loutradis D, Michala L. Adolescents and young women with functional hypothalamic amenorrhoea: is it time to move beyond the hormonal profile? Arch Gynecol Obstet. 2020;301(4):1095–1101. [DOI] [PubMed] [Google Scholar]
- 43. Tay CT, Teede HJ, Hill B, Loxton D, Joham AE. Increased prevalence of eating disorders, low self‐esteem, and psychological distress in women with polycystic ovary syndrome: a community‐based cohort study. Fertil Steril. 2019;112(2):353–361. [DOI] [PubMed] [Google Scholar]
- 44. Cesta CE, Månsson M, Palm C, Lichtenstein P, Iliadou AN, Landén M. Polycystic ovary syndrome and psychiatric disorders: co‐morbidity and heritability in a nationwide Swedish cohort. Psychoneuroendocrinology. 2016;73:196–203. [DOI] [PubMed] [Google Scholar]
- 45. Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin‐family study. J Clin Endocrinol Metab. 2006;91(6):2100–2104. [DOI] [PubMed] [Google Scholar]
- 46. Day F, Karaderi T, Jones MR, et al. Large‐scale genome‐wide meta‐analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018;14(12):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caronia LM, Martin C, Welt CK, et al. A genetic basis for functional hypothalamic amenorrhea. N Engl J Med. 2011;364(3):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Delaney A, Burkholder AB, Lavender CA, et al. Increased burden of rare sequence variants in GnRH‐associated genes in women with hypothalamic amenorrhea. J Clin Endocrinol Metab. 2020. 10.1210/clinem/dgaa609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thornton LM, Mazzeo SE, Bulik CM. The heritability of eating disorders: methods and current findings. Curr Top Behav Neurosci. 2011;6(1):141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsutsumi R, Webster NJG. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J. 2009;56(6):729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Veldhuis JD, Evans WS, Demers LM, Thorner MO, Wakat D, Rogol AD. Altered neuroendocrine regulation of gonadotropin secretion in women distance runners. J Clin Endocrinol Metab. 1985;61(3):557–563. [DOI] [PubMed] [Google Scholar]
- 52. Genazzani AD, Petraglia F, Fabbri G, Monzani A, Montanini V, Genazzani AR. Evidence of luteinizing hormone secretion in hypothalamic amenorrhea associated with weight loss. Fertil Steril. 1990;54(2):222–226. [DOI] [PubMed] [Google Scholar]
- 53. Perkins RB, Hall JE, Martin KA. Neuroendocrine abnormalities in hypothalamic amenorrhea: spectrum, stability, and response to neurotransmitter modulation. J Clin Endocrinol Metab. 1999;84(6):1905–1911. [DOI] [PubMed] [Google Scholar]
- 54. Cheung AP, Lu JKH, Chang RJ. Pulsatile gonadotrophin secretion in women with polycystic ovary syndrome after gonadotrophin‐releasing hormone agonist treatment. Hum Reprod. 1997;12(6):1156–1164. [DOI] [PubMed] [Google Scholar]
- 55. Morales AJ, Laughlin GA, Bützow T, Maheshwari H, Baumann G, Yen SS. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab. 1996;81(8):2854–2864. [DOI] [PubMed] [Google Scholar]
- 56. Arroyo A, Laughlin GA, Morales AJ, Yen SSC. Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity 1. J Clin Endocrinol Metab. 1997;82(11):3728–3733. [DOI] [PubMed] [Google Scholar]
- 57. Vandekerckhove D, Dhont M, Van Eyck J. Diagnostic value of the LH releasing hormone stimulation test in functional amenorrhea. Acta Endocrinol. 1975;78(4):625–633. [DOI] [PubMed] [Google Scholar]
- 58. Koninckx P, Dehertogh R, Heyns W, Meulepas E, Brosens I, Demoor P. Secretion rates of LH and FSH during infusion of LH‐FSH/RH in normal women and in patients with secondary amenorrhea: suggestive evidence for two pools of LH and FSH. J Clin Endocrinol Metab. 1976;43(1):159–167. [DOI] [PubMed] [Google Scholar]
- 59. Newton JR, Kilpatrick MJ, Pike JM, Collins WP. An evaluation of the diagnostic value of synthetic luteinizing hormone releasing hormone. Acta Endocrinol. 1975;80(3):417–428. [DOI] [PubMed] [Google Scholar]
- 60. Tomova A, Makker K, Kirilov G, Agarwal A, Kumanov P. Disturbances in gonadal axis in women with anorexia nervosa. Eat Weight Disorders. 2007;12(4):e92–e97. [DOI] [PubMed] [Google Scholar]
- 61. Patel K, Coffler MS, Dahan MH, Malcom PJ, Deutsch R, Chang RJ. Relationship of GnRH‐stimulated LH release to episodic LH secretion and baseline endocrine‐metabolic measures in women with polycystic ovary syndrome. Clin Endocrinol. 2004;60(1):67–74. [DOI] [PubMed] [Google Scholar]
- 62. Lewandowski KC, Cajdler‐Łuba A, Salata I, Bieńkiewicz M, Lewiński A. The utility of the gonadotrophin releasing hormone (GnRH) test in the diagnosis of polycystic ovary syndrome (PCOS). Endokrynol Polska. 2011;62(2).120–128. [PubMed] [Google Scholar]
- 63. Lewandowski KC, Cajdler‐łuba A, Bienḱiewicz M, Lewinśki A. Women with oligo‐/amenorrhoea and polycystic ovaries have identical responses to GnRH stimulation regardless of their androgen status: comparison of the Rotterdam and Androgen excess society diagnostic criteria. Neuroendocrinol Lett. 2011;32(6):847–856. [PubMed] [Google Scholar]
- 64. Balen A. The pathophysiology of polycystic ovary syndrome: trying to understand PCOS and its endocrinology. Best Pract Res Clin Obstetr Gynaecol. 2004;18(5):685–706. [DOI] [PubMed] [Google Scholar]
- 65. Alemyar A, van der Kooi ALLF, Laven JSE. Anti‐Müllerian hormone and ovarian morphology in women with hypothalamic hypogonadism. J Clin Endocrinol Metab. 2020;105(5):e2008–e2014. [DOI] [PubMed] [Google Scholar]
- 66. Panidis D, Tziomalos K, Chatzis P, et al. Association between menstrual cycle irregularities and endocrine and metabolic characteristics of the polycystic ovary syndrome. Eur J Endocrinol/European Federation of Endocrine Societies. 2013;168(2):145–152. [DOI] [PubMed] [Google Scholar]
- 67. Le MT, Le VNS, Le DD, Nguyen VQH, Chen C, Cao NT. Exploration of the role of anti‐Mullerian hormone and LH/FSH ratio in diagnosis of polycystic ovary syndrome. Clin Endocrinol. 2019;90(4):579–585. [DOI] [PubMed] [Google Scholar]
- 68. Carmina E, Fruzzetti F, Lobo RA. Features of polycystic ovary syndrome (PCOS) in women with functional hypothalamic amenorrhea (FHA) may be reversible with recovery of menstrual function. Gynecol Endocrinol. 2018;34(4):301–304. [DOI] [PubMed] [Google Scholar]
- 69. Laughlin GA, Dominguez CE, Yen SSC. Nutritional and endocrine‐metabolic aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1998;83(1):25–32. [DOI] [PubMed] [Google Scholar]
- 70. Fazeli PAK. Determinants of growth hormone resistance in malnutrition. J Endocrinol. 2015;220(3):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu XK, Sallinen K, Zhou SY, Su YH, Pöllänen P, Erkkola R. Androgen excess contributes to altered growth hormone/insulin‐like growth factor‐1 axis in nonobese women with polycystic ovary syndrome. Fertil Steril. 2000;73(4):730–734. [DOI] [PubMed] [Google Scholar]
- 72. Abbara A, Eng PC, Phylactou M, et al. Anti‐müllerian hormone (AMH) in the diagnosis of menstrual disturbance due to polycystic ovarian syndrome. Front Endocrinol. 2019;10(SEP):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can anti‐Müllerian hormone predict the diagnosis of polycystic ovary syndrome? A systematic review and meta‐analysis of extracted data. J Clin Endocrinol Metab. 2013;98(8):3332–3340. [DOI] [PubMed] [Google Scholar]
- 74. Carmina E, Fruzzetti F, Lobo RA. Increased anti‐Mullerian hormone levels and ovarian size in a subgroup of women with functional hypothalamic amenorrhea: further identification of the link between polycystic ovary syndrome and functional hypothalamic amenorrhea. Am J Obstet Gynecol. 2016;214(6):714.e1–714.e6. [DOI] [PubMed] [Google Scholar]
- 75. van Elburg AA, Eijkemans MJC, Kas MJH, et al. Predictors of recovery of ovarian function during weight gain in anorexia nervosa. Fertil Steril. 2007;87(4):902–908. [DOI] [PubMed] [Google Scholar]
- 76. Lie Fong S, Schipper I, Valkenburg O, De Jong FH, Visser JA, Laven JSE. The role of anti‐Müllerian hormone in the classification of anovulatory infertility. Eur J Obstetr Gynecol Reprod Biol. 2015;186:75–79. [DOI] [PubMed] [Google Scholar]
- 77. Zhu RY, Wong YC, Yong EL. Sonographic evaluation of polycystic ovaries. Best Pract Res Clin Obstetr Gynaecol. 2016;37:25–37. [DOI] [PubMed] [Google Scholar]
- 78. Adams J, Polson DW, Abdulwahid N, et al. Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. The Lancet. 1985;326(8469‐8470):1375–1379. [DOI] [PubMed] [Google Scholar]
- 79. Robin G, Gallo C, Catteau‐Jonard S, et al. Polycystic ovary‐like abnormalities (PCO‐L) in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 2012;97(11):4236–4243. [DOI] [PubMed] [Google Scholar]
- 80. Polson DW, Wadsworth J, Adams J, Franks S. Polycystic ovaries‐a common finding in normal women. The Lancet. 1988;331(8590):870–872. [DOI] [PubMed] [Google Scholar]
- 81. Jonard S, Robert Y, Dewailly D. Revisiting the ovarian volume as a diagnostic criterion for polycystic ovaries. Hum Reprod. 2005;20(10):2893–2898. [DOI] [PubMed] [Google Scholar]
- 82. Kim JJ, Hwang KR, Chae SJ, Yoon SH, Choi YM. Impact of the newly recommended antral follicle count cutoff for polycystic ovary in adult women with polycystic ovary syndrome. Hum Reprod. 2020;35(3):652–659. [DOI] [PubMed] [Google Scholar]
- 83. Jonard S, Pigny P, Jacquesson L, Demerle‐Roux C, Robert Y, Dewailly D. The ovarian markers of the FSH insufficiency in functional hypothalamic amenorrhoea. Hum Reprod. 2005;20(1):101–107. [DOI] [PubMed] [Google Scholar]
- 84. Anderson RA, Groome NP, Baird DT. Inhibin A and inhibin B in women with polycystic ovarian syndrome during treatment with FSH to induce mono‐ovulation. Clin Endocrinol. 1998;48(5):577–584. [DOI] [PubMed] [Google Scholar]
- 85. Pigny P, Cortet‐Rudelli C, Decanter C, et al. Serum levels of inhibins are differentially altered in patients with polycystic ovary syndrome: effects of being overweight and relevance to hyperandrogenism. Fertil Steril. 2000;73(5):972–977. [DOI] [PubMed] [Google Scholar]
- 86. Podfigurna A, Maciejewska‐Jeske M, Meczekalski B, Genazzani AD. Kisspeptin and LH pulsatility in patients with functional hypothalamic amenorrhea. Endocrine. 2020;70(3):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Katulski K, Podfigurna A, Czyzyk A, Meczekalski B, Genazzani AD. Kisspeptin and LH pulsatile temporal coupling in PCOS patients. Endocrine. 2018;61(1):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hofmann T, Elbelt U, Haas V, et al. Plasma kisspeptin and ghrelin levels are independently correlated with physical activity in patients with anorexia nervosa. Appetite. 2017;108:141–150. [DOI] [PubMed] [Google Scholar]
- 89. Tang R, Ding X, Zhu J. Kisspeptin and polycystic ovary syndrome. Front Endocrinol. 2019;10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Varikasuvu SR, Prasad VS, Vamshika VC, Satyanarayana MV, Panga JR. Circulatory metastin/kisspeptin‐1 in polycystic ovary syndrome: a systematic review and meta‐analysis with diagnostic test accuracy. Reprod BioMed Online. 2019;39(4):685–697. [DOI] [PubMed] [Google Scholar]
- 91. Schlaff WD, Coddington CC. Use of the progestin challenge test in diagnosing amenorrhea: the time has come to say goodbye. Fertil Steril. 2020;113(1):51–52. [DOI] [PubMed] [Google Scholar]
- 92. Rarick LD, Shangold MM, Ahmed SW. Cervical mucus and serum estradiol as predictors of response to progestin challenge. Fertil Steril. 1990;54(2):353–355. [DOI] [PubMed] [Google Scholar]
- 93. Oscar A, Kletzky MD, Val Davajan MD, et al. Clinical categorization of patients with secondary amenorrhea using progesterone‐induced uterine bleeding and measurement of serum gonadotropin levels. Am J Obstet Gynecol. 1975;121:695–703. [DOI] [PubMed] [Google Scholar]
- 94. Nakamura S, Douchi T, Oki T, Ijuin H, Yamamoto SNY. Relationship between sonographic endometrial thickness and progestin‐induced withdrawal bleeding. Obstetr Gynaecol. 1996;87:722–725. [DOI] [PubMed] [Google Scholar]
- 95. Shangold M, Tomai T, Cook J, et al. Factors associated with withdrawal bleeding after administration of oral micronized progesterone in women with secondary amenorrhea. Gynecol Endocrinol. 1991;56(6):1040–1047. [PubMed] [Google Scholar]
- 96. Morcos RN, Leonard MD, Smith M, Bourguet C, Makii M, Khawli O. Vaginosonographic measurement of endometrial thickness in the evaluation of amenorrhea. Fertil Steril. 1991;55(3):543–546. [DOI] [PubMed] [Google Scholar]
- 97. Eryilmaz OG, Sarikaya E, Gulerman C, Akar S, Cicek N. Endometrial thickness measurement throughout a menstrual cycle in non‐obese infertile patients with polycystic ovary syndrome. Arch Gynecol Obstet. 2012;286(6):1597–1600. [DOI] [PubMed] [Google Scholar]
- 98. Iatrakis G, Tsionis C, Adonakis G, et al. Polycystic ovarian syndrome, insulin resistance and thickness of the endometrium. Eur J Obstetr Gynecol Reprod Biol. 2006;127(2):218–221. [DOI] [PubMed] [Google Scholar]
- 99. Tsuda H, Ito YM, Todo Y, et al. Measurement of endometrial thickness in premenopausal women in office gynecology. Reprod Med Biol. 2018;17(1):29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Genç A, Güven D, Acar H, Tutkun E. Investigation of the endometrial thickness and estrogen level in athletes and sedentaries. Clin Exp Obstet Gynecol. 2019;46(1):123–126. [Google Scholar]
- 101. Kakoly NS, Khomami MB, Joham AE, et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta‐regression. Hum Reprod Update. 2018;24(4):455–467. [DOI] [PubMed] [Google Scholar]
- 102. Stepto NK, Cassar S, Joham AE, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic‐hyperinsulaemic clamp. Hum Reprod. 2013;28(3):777–784. [DOI] [PubMed] [Google Scholar]
- 103. Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A. Characterization of groups of hyperandrogenic women. J Clin Endocrinol Metab. 1987;65(3):499–507. [DOI] [PubMed] [Google Scholar]
- 104. Velling Magnussen L, Mumm H, Andersen M, Glintborg D. Hemoglobin A1c as a tool for the diagnosis of type 2 diabetes in 208 premenopausal women with polycystic ovary syndrome. Fertil Steril. 2011;96(5):1275–1280. [DOI] [PubMed] [Google Scholar]
- 105. Andrico S. Leptin in functional hypothalamic amenorrhoea. Hum Reprod. 2002;17(8):2043–2048. [DOI] [PubMed] [Google Scholar]
- 106. Takeuchi T, Kawana T. Oral glucose challenge effects on growth and sex steroid hormones in normal women and women with hypothalamic amenorrhea. Int J Gynecol Obstetr. 1998;61(2):171–178. [DOI] [PubMed] [Google Scholar]
- 107. Bernasconi D, Del MP, Meozzi M, et al. The impact of obesity on hormonal parameters in hirsute and nonhirsute women. Metabolism. 1996;45(1):72–75. [DOI] [PubMed] [Google Scholar]
- 108. Brundu B, Loucks TL, Adler LJ, Cameron JL, Berga SL. Increased cortisol in the cerebrospinal fluid of women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 2006;91(4):1561–1565. [DOI] [PubMed] [Google Scholar]
- 109. Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in poly cystic ovarian disease. J Clin Endocrinol Metab. 1980;50(1):113–116. [DOI] [PubMed] [Google Scholar]
- 110. Wassif WS, McLoughlin DM, Vincent RP, Conwy S, Russell GFM, Taylor NF. Steroid metabolism and excretion in severe anorexia nervosa: effects of refeeding. Am J Clin Nutr. 2011;93(5):911–917. [DOI] [PubMed] [Google Scholar]
- 111. Wu C, Wei K, Jiang Z. 5α‐reductase activity in women with polycystic ovary syndrome: a systematic review and meta‐analysis. Reprod Biol Endocrinol: RB&E. 2017;15(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fassnacht M, Schlenz N, Schneider SB, Wudy SA, Allolio B, Arlt W. Beyond adrenal and ovarian androgen generation: increased peripheral 5 alpha‐reductase activity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(6):2760–2766. [DOI] [PubMed] [Google Scholar]
- 113. Zheng SH, Du DF, Li XL. Leptin levels in women with polycystic ovary syndrome: a systematic review and a meta‐analysis. Reprod Sci. 2017;24(5):656–670. [DOI] [PubMed] [Google Scholar]
- 114. Pusalkar M, Meherji P, Gokral J, Savardekar L, Chinnaraj S, Maitra A. Obesity and polycystic ovary syndrome: association with androgens, leptin and its genotypes. Gynecol Endocrinol. 2010;26(12):874–882. [DOI] [PubMed] [Google Scholar]
- 115. Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351(10):987–997. [DOI] [PubMed] [Google Scholar]
- 116. Biller BMK, Federoff HJ, Koenig JI, Klibanski A. Abnormal cortisol secretion and responses to corticotropin‐releasing hormone in women with hypothalamic amenorrhea. J Clin Endocrinol Metab. 1990;70(2):311–317. [DOI] [PubMed] [Google Scholar]
- 117. Suh BY, Liu JH, Berga SL, Quigley ME, Laughlin GA, Yen SS. Hypercortisolism in patients with functional hypothalamic‐amenorrhea. J Clin Endocrinol Metab. 1988;66(4):733–739. [DOI] [PubMed] [Google Scholar]
- 118. Loucks AB, Mortola JF, Girton L, Yen SSC. Alterations in the hypothalamic‐pituitary‐ovarian and the hypothalamic‐pituitary‐adrenal axes in athletic women. J Clin Endocrinol Metab. 1989;68(2):402–411. [DOI] [PubMed] [Google Scholar]
- 119. Lanzone A, Petraglia F, Fulghesu AM, Ciampelli M, Caruso A, Mancuso S. Corticotropin‐releasing hormone induces an exaggerated response of adrenocorticotropic hormone and cortisol in polycystic ovary syndrome. Fertil Steril. 1995;63(6):1195–1199. [DOI] [PubMed] [Google Scholar]
- 120. Anand‐Ivell R, Dai Y, Ivell R. Neohormones as biomarkers of reproductive health. Fertil Steril. 2013;99:1153–1160. [DOI] [PubMed] [Google Scholar]
- 121. Gambineri A, Patton L, De Iasio R, Palladoro F, Pagotto U, Pasquali R. Insulin‐like factor 3: a new circulating hormone related to luteinizing hormone‐dependent ovarian hyperandrogenism in the polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(6):2066–2073. [DOI] [PubMed] [Google Scholar]
- 122. Futterweit W, Yeh HC, Mechanick JI. Ultrasonographic study of ovaries of 19 women with weight loss‐related hypothalamic oligo‐amenorrhea. Biomed Pharmacother. 42(4):279–283. [PubMed] [Google Scholar]
- 123. Sum M, Warren MP. Hypothalamic amenorrhea in young women with underlying polycystic ovary syndrome. Fertil Steril. 2009;92(6):2106–2108. [DOI] [PubMed] [Google Scholar]
- 124. Koltun KJ, Williams NI, Scheid JL, De Souza MJ. Discriminating hypothalamic oligomenorrhea/amenorrhea from hyperandrogenic oligomenorrhea/amenorrhea in exercising women. Appl Physiol Nutr Metab. 2020;45(7):707–714. [DOI] [PubMed] [Google Scholar]
- 125. Bradbury RA, Lee P, Smith HC. Elevated anti‐Mullerian hormone in lean women may not indicate polycystic ovarian syndrome. Aust N Z J Obstet Gynaecol. 2017;57(5):552–557. [DOI] [PubMed] [Google Scholar]
- 126. Wang JG, Lobo RA. The complex relationship between hypothalamic amenorrhea and polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(4):1394–1397. [DOI] [PubMed] [Google Scholar]
- 127. Mattle V, Bilgyicildirim A, Hadziomerovic D, et al. Polycystic ovarian disease unmasked by pulsatile GnRH therapy in a subgroup of women with hypothalamic amenorrhea. Fertil Steril. 2008;89(2):404–409. [DOI] [PubMed] [Google Scholar]
- 128. Dumont A, Dewailly D, Plouvier P, Catteau‐Jonard S, Robin G. Does polycystic ovarian morphology influence the response to treatment with pulsatile GnRH in functional hypothalamic amenorrhea? Reprod Biol Endocrinol: RB&E. 2016;14(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chae SJ, Kim JJ, Choi YM, et al. Clinical and biochemical characteristics of polycystic ovary syndrome in Korean women. Hum Reprod. 2008;23(8):1924–1931. [DOI] [PubMed] [Google Scholar]
- 130. Chen MJ, Chiu HM, Chen CL, Yang WS, Yang YS, Ho HN. Hyperandrogenemia is independently associated with elevated alanine aminotransferase activity in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2010;95(7):3332–3341. [DOI] [PubMed] [Google Scholar]
- 131. Ferk P, Teran N, Gersak K. The (TAAAA)n microsatellite polymorphism in the SHBG gene influences serum SHBG levels in women with polycystic ovary syndrome. Hum Reprod. 2007;22(4):1031–1036. [DOI] [PubMed] [Google Scholar]
- 132. Hsu MI, Liou TH, Chou SY, Chang CY, Sen HC. Diagnostic criteria for polycystic ovary syndrome in Taiwanese Chinese women: comparison between Rotterdam 2003 and NIH 1990. Fertil Steril. 2007;88(3):727–729. [DOI] [PubMed] [Google Scholar]
- 133. Liou TH, Yang JH, Hsieh CH, Lee CY, Sen HC, Hsu MI. Clinical and biochemical presentations of polycystic ovary syndrome among obese and nonobese women. Fertil Steril. 2009;92(6):1960–1965. [DOI] [PubMed] [Google Scholar]
- 134. Mukherjee S, Shaikh N, Khavale S, et al. Genetic variation in exon 17 of INSR is associated with insulin resistance and hyperandrogenemia among lean Indian women with polycystic ovary syndrome. Eur J Endocrinol. 2009;160(5):855–862. [DOI] [PubMed] [Google Scholar]
- 135. Pinola P, Puukka K, Piltonen TT, et al. Normo‐ and hyperandrogenic women with polycystic ovary syndrome exhibit an adverse metabolic profile through life. Fertil Steril. 2017;107(3):788–795.e2. [DOI] [PubMed] [Google Scholar]
- 136. Vrbikova J, Dvorakova K, Grimmichova T, et al. Prevalence of insulin resistance and prediction of glucose intolerance and type 2 diabetes mellitus in women with polycystic ovary syndrome. Clin Chem Lab Med. 2007;45(5):639–644. [DOI] [PubMed] [Google Scholar]
- 137. Wang K, Wang L, Zhao Y, Shi Y, Wang L, Chen ZJ. No association of the Arg51Gln and Leu72Met polymorphisms of the ghrelin gene and polycystic ovary syndrome. Hum Reprod. 2009;24(2):485–490. [DOI] [PubMed] [Google Scholar]
- 138. Colombo O, Pinelli G, Comelli M, et al. Dietary intakes in infertile women a pilot study. Nutr J. 2009;8(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Podfigurna‐Stopa A, Pludowski P, Jaworski M, Lorenc R, Genazzani AR, Meczekalski B. Skeletal status and body composition in young women with functional hypothalamic amenorrhea. Gynecol Endocrinol. 2012;28(4):299–304. [DOI] [PubMed] [Google Scholar]
- 140. Schneider LF, Warren MP. Functional hypothalamic amenorrhea is associated with elevated ghrelin and disordered eating. Fertil Steril. 2006;86(6):1744–1749. [DOI] [PubMed] [Google Scholar]
- 141. Falsetti L, Gambera A, Barbetti L. Long‐term follow‐up of functional hypothalamic. J Endocrinol Metab. 2002;87(2):500–505. [DOI] [PubMed] [Google Scholar]
- 142. Kirchengast S, Huber J. Body composition characteristics, sex hormone levels and circadian gonadotropin fluctuations in infertile young women. Collegium Antropol. 1999;23:407–423. [PubMed] [Google Scholar]
- 143. La Marca A, Volpe A. Anti‐Müllerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol. 2006;64(6):603–610. [DOI] [PubMed] [Google Scholar]
- 144. Pigny P, Merlen E, Robert Y, et al. Elevated serum level of anti‐mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88(12):5957–5962. [DOI] [PubMed] [Google Scholar]
- 145. Laven JSE, Mulders AGMGJ, Visser JA, Themmen AP, De Jong FH, Fauser BCJM. Anti‐Mullerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab. 2004;1:318–323. [DOI] [PubMed] [Google Scholar]
- 146. Anderson RA, Wallace EM, Groome NP, Bellis AJ, Wu FCW. Physiological relationships between inhibin B, follicle stimulating hormone secretion and spermatogenesis in normal men and response to gonadotrophin suppression by exogenous testosterone. Hum Reprod. 1997;12(4):746–751. [DOI] [PubMed] [Google Scholar]
- 147. Torgac M, Kokcu A, Cetinkaya MB, Alper T, Malatyalioglu E. Do basal inhibin A and inhibin B levels have value in the diagnosis of polycystic ovary syndrome? Gynecol Endocrinol. 2005;20(6):322–326. [DOI] [PubMed] [Google Scholar]
- 148. Shayya RF, Rosencrantz MA, Chuan SS, et al. Decreased inhibin B responses following recombinant human chorionic gonadotropin administration in normal women and women with polycystic ovary syndrome. J Invest Dermatol. 2014;101(1):275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Luisi S, Ciani V, Podfigurna‐Stopa A, et al. Serum anti‐Müllerian hormone, inhibin B, and total inhibin levels in women with hypothalamic amenorrhea and anorexia nervosa. Gynecol Endocrinol. 2012;28(1):34–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Table S5
Data Availability Statement
The data sets generated and/or analysed during the current study are available from the corresponding author on reasonable request.


