Abstract
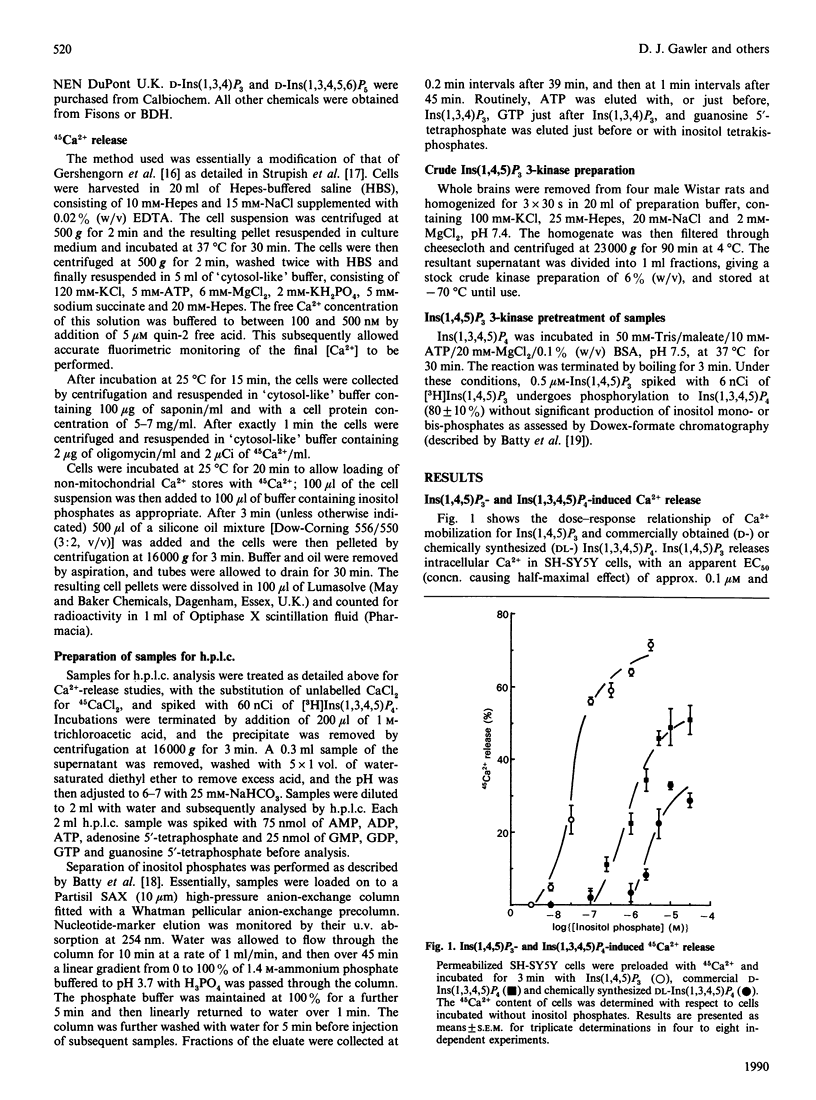
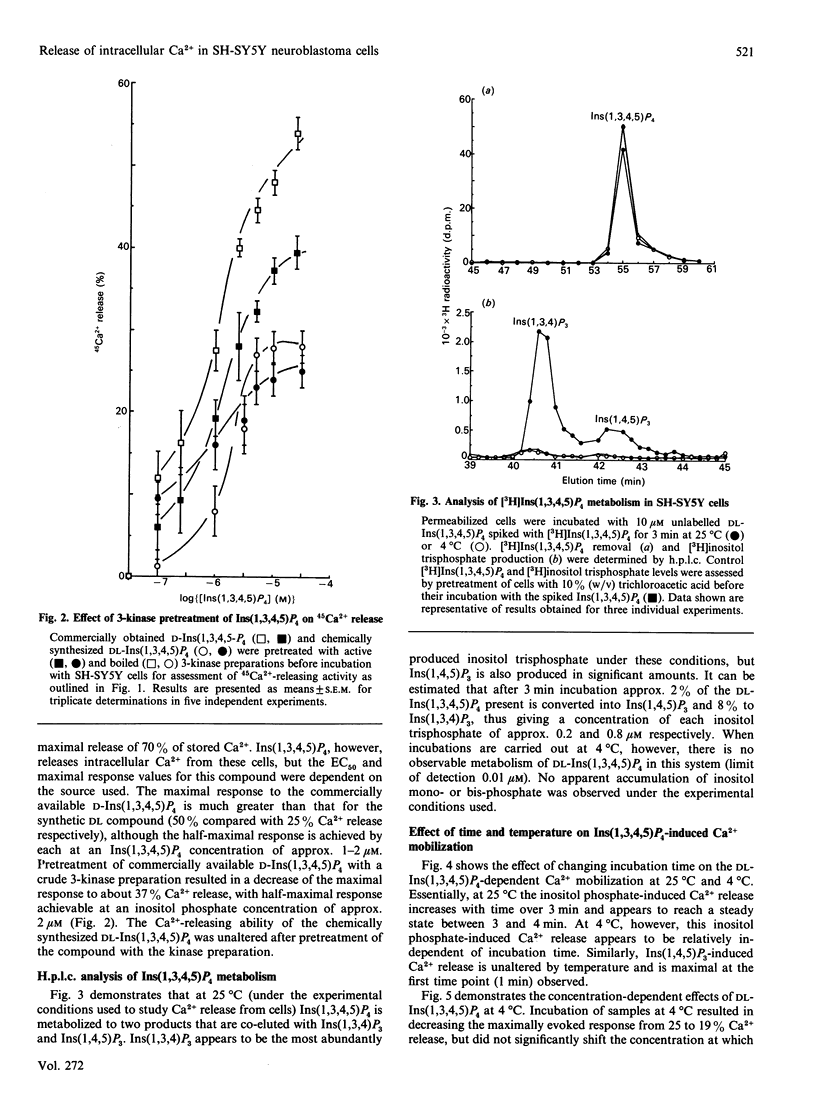
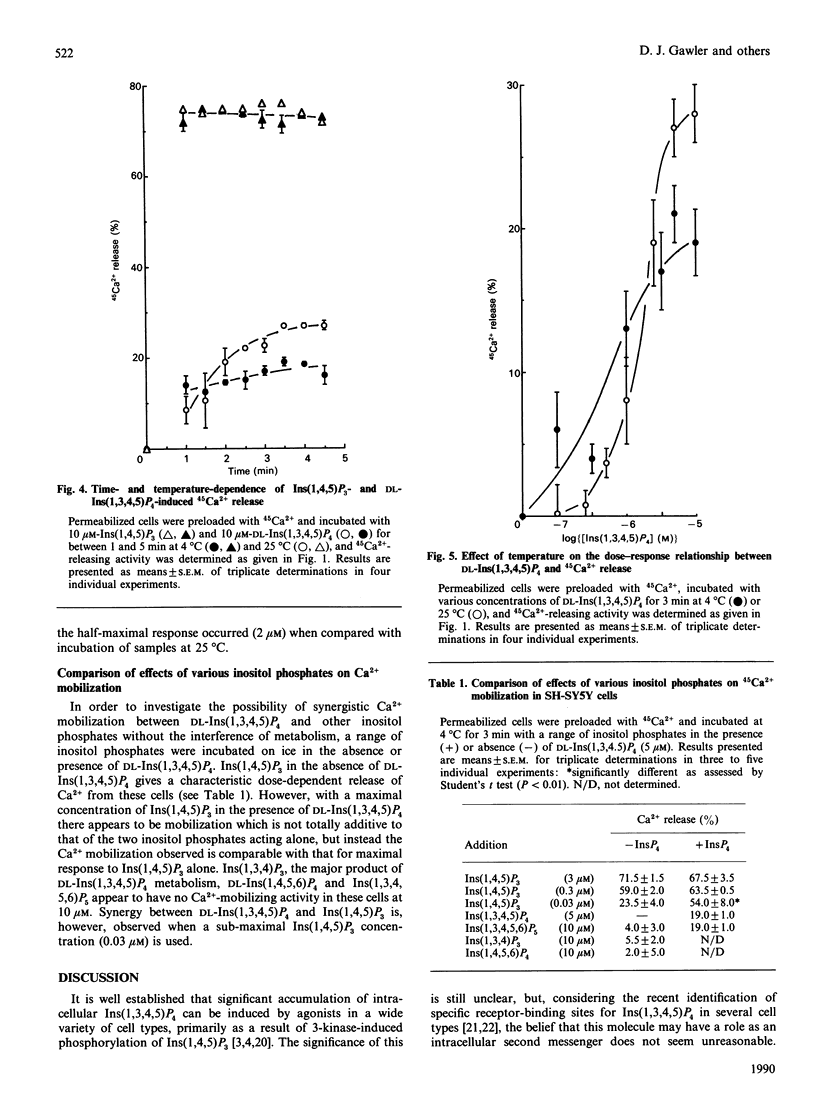
Inositol-polyphosphate-induced Ca2+ mobilization was investigated in saponin-permeabilized SH-SY5Y human neuroblastoma cells. Ins(1,4,5)P3 induced a dose-related release from intracellular Ca2+ stores with an EC50 (concn. giving half-maximal effect) of 0.1 microM and a maximal release of 70%. Ins(1,3,4)P3, DL-Ins(1,4,5,6)P4 and Ins(1,3,4,5,6)P5 did not evoke Ca2+ mobilization in these cells when used at concentrations up to 10 microM. However, Ins(1,3,4,5)P4 was found to release Ca2+ in a dose-related manner, but the response was dependent on the source of Ins(1,3,4,5)P4 used. When commercially available D-Ins(1,3,4,5)P4 was used, the EC50 and maximal response values were 1 microM and 50% respectively, compared with values for chemically synthesized DL-Ins(1,3,4,5)P4 of 2 microM and 25%. The enhanced maximal response of commercial D-Ins(1,3,4,5)P4 was decreased by pretreatment with rat brain crude Ins(1,4,5)P3 3-kinase and was therefore concluded to be indicative of initial Ins(1,4,5)P3 contamination of the Ins(1,3,4,5)P4 preparation. When metabolism of DL-Ins(1,3,4,5)P4 (10 microM) in these cells at 25 degrees C was investigated by h.p.l.c., substantial amounts of Ins(1,4,5)P3 (0.2 microM) and Ins(1,3,4)P3 (0.8 microM) were found to be produced within 3 min. Analysis of DL-Ins(1,3,4,5)P4 incubation with cells at 4 degrees C, however, indicated that metabolism had been arrested ([3H]Ins(1,4,5)P3 detection limits were estimated to be approx. 0.01 microM). When chemically synthesized DL-Ins(1,3,4,5)P4 and incubation conditions of low temperature were used, the Ca2(+)-releasing properties of this compound were established to be 1 microM and 19% for the EC50 and maximal response values respectively. The results obtained strongly suggest that Ins(1,3,4,5)P4 alone has the ability to release intracellular Ca2+. However, in the presence of sub-maximal concentrations of Ins(1,4,5)P3, Ca2+ release appears to be synergistic with Ins(1,3,4,5)P4, but at supramaximal concentrations not even additive effects are observed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balla T., Baukal A. J., Hunyady L., Catt K. J. Agonist-induced regulation of inositol tetrakisphosphate isomers and inositol pentakisphosphate in adrenal glomerulosa cells. J Biol Chem. 1989 Aug 15;264(23):13605–13611. [PubMed] [Google Scholar]
- Batty I. H., Letcher A. J., Nahorski S. R. Accumulation of inositol polyphosphate isomers in agonist-stimulated cerebral-cortex slices. Comparison with metabolic profiles in cell-free preparations. Biochem J. 1989 Feb 15;258(1):23–32. doi: 10.1042/bj2580023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Irvine R. F. Specific binding sites for [3H]inositol(1,3,4,5)tetrakisphosphate on membranes of HL-60 cells. Biochem Biophys Res Commun. 1987 Dec 16;149(2):680–685. doi: 10.1016/0006-291x(87)90421-9. [DOI] [PubMed] [Google Scholar]
- Crossley I., Swann K., Chambers E., Whitaker M. Activation of sea urchin eggs by inositol phosphates is independent of external calcium. Biochem J. 1988 May 15;252(1):257–262. doi: 10.1042/bj2520257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P. J., Comerford J. G., Dawson A. P. Heparin inhibits the inositol 1,4,5-trisphosphate-induced Ca2+ release from rat liver microsomes. FEBS Lett. 1988 Feb 8;228(1):57–59. doi: 10.1016/0014-5793(88)80584-2. [DOI] [PubMed] [Google Scholar]
- Cullen P. J., Irvine R. F., Drøbak B. K., Dawson A. P. Inositol 1,3,4,5-tetrakisphosphate causes release of Ca2+ from permeabilized mouse lymphoma L1210 cells by its conversion into inositol 1,4,5-trisphosphate. Biochem J. 1989 May 1;259(3):931–933. doi: 10.1042/bj2590931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donié F., Reiser G. A novel, specific binding protein assay for quantitation of intracellular inositol 1,3,4,5-tetrakisphosphate (InsP4) using a high-affinity InsP4 receptor from cerebellum. FEBS Lett. 1989 Aug 28;254(1-2):155–158. doi: 10.1016/0014-5793(89)81029-4. [DOI] [PubMed] [Google Scholar]
- Ely J. A., Hunyady L., Baukal A. J., Catt K. J. Inositol 1,3,4,5-tetrakisphosphate stimulates calcium release from bovine adrenal microsomes by a mechanism independent of the inositol 1,4,5-trisphosphate receptor. Biochem J. 1990 Jun 1;268(2):333–338. doi: 10.1042/bj2680333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi P., Williams G. H. Heterogenous inositol tetrakisphosphate binding sites in the adrenal cortex. J Biol Chem. 1988 Jun 15;263(17):7940–7942. [PubMed] [Google Scholar]
- Föhr K. J., Scott J., Ahnert-Hilger G., Gratzl M. Characterization of the inositol 1,4,5-trisphosphate-induced calcium release from permeabilized endocrine cells and its inhibition by decavanadate and p-hydroxymercuribenzoate. Biochem J. 1989 Aug 15;262(1):83–89. doi: 10.1042/bj2620083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershengorn M. C., Geras E., Purrello V. S., Rebecchi M. J. Inositol trisphosphate mediates thyrotropin-releasing hormone mobilization of nonmitochondrial calcium in rat mammotropic pituitary cells. J Biol Chem. 1984 Sep 10;259(17):10675–10681. [PubMed] [Google Scholar]
- Guillemette G., Lamontagne S., Boulay G., Mouillac B. Differential effects of heparin on inositol 1,4,5-trisphosphate binding, metabolism, and calcium release activity in the bovine adrenal cortex. Mol Pharmacol. 1989 Mar;35(3):339–344. [PubMed] [Google Scholar]
- Hill T. D., Dean N. M., Boynton A. L. Inositol 1,3,4,5-tetrakisphosphate induces Ca2+ sequestration in rat liver cells. Science. 1988 Nov 25;242(4882):1176–1178. doi: 10.1126/science.2847317. [DOI] [PubMed] [Google Scholar]
- Hirata M., Watanabe Y., Ishimatsu T., Ikebe T., Kimura Y., Yamaguchi K., Ozaki S., Koga T. Synthetic inositol trisphosphate analogs and their effects on phosphatase, kinase, and the release of Ca2+. J Biol Chem. 1989 Dec 5;264(34):20303–20308. [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Berridge M. J. Specificity of inositol phosphate-stimulated Ca2+ mobilization from Swiss-mouse 3T3 cells. Biochem J. 1986 Nov 15;240(1):301–304. doi: 10.1042/bj2400301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Inositol(1,3,4,5)tetrakisphosphate-induced activation of sea urchin eggs requires the presence of inositol trisphosphate. Biochem Biophys Res Commun. 1987 Jul 15;146(1):284–290. doi: 10.1016/0006-291x(87)90723-6. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M., Pollock W. K., Smith P. M., Wreggett K. A. Inositol phosphates: proliferation, metabolism and function. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):281–298. doi: 10.1098/rstb.1988.0077. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Hansen C. A., Williamson J. R. Inositol 1,3,4,5-tetrakisphosphate increases the duration of the inositol 1,4,5-trisphosphate-mediated Ca2+ transient. FEBS Lett. 1987 Jul 13;219(1):125–129. doi: 10.1016/0014-5793(87)81203-6. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Hansen C. A., Williamson J. R. Inositol tetrakisphosphate mobilizes calcium from cerebellum microsomes. Mol Pharmacol. 1989 Sep;36(3):391–397. [PubMed] [Google Scholar]
- Joseph S. K., Williamson J. R. Inositol polyphosphates and intracellular calcium release. Arch Biochem Biophys. 1989 Aug 15;273(1):1–15. doi: 10.1016/0003-9861(89)90156-2. [DOI] [PubMed] [Google Scholar]
- Michell B. Inositol phosphates. Profusion and confusion. Nature. 1986 Jan 16;319(6050):176–177. doi: 10.1038/319176a0. [DOI] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Nahorski S. R. Inositol polyphosphates and neuronal calcium homeostasis. Trends Neurosci. 1988 Oct;11(10):444–448. doi: 10.1016/0166-2236(88)90196-8. [DOI] [PubMed] [Google Scholar]
- Nahorski S. R., Potter B. V. Molecular recognition of inositol polyphosphates by intracellular receptors and metabolic enzymes. Trends Pharmacol Sci. 1989 Apr;10(4):139–144. doi: 10.1016/0165-6147(89)90165-x. [DOI] [PubMed] [Google Scholar]
- Shears S. B. Metabolism of the inositol phosphates produced upon receptor activation. Biochem J. 1989 Jun 1;260(2):313–324. doi: 10.1042/bj2600313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder P. M., Krause K. H., Welsh M. J. Inositol trisphosphate isomers, but not inositol 1,3,4,5-tetrakisphosphate, induce calcium influx in Xenopus laevis oocytes. J Biol Chem. 1988 Aug 15;263(23):11048–11051. [PubMed] [Google Scholar]
- Stephens L., Hawkins P. T., Carter N., Chahwala S. B., Morris A. J., Whetton A. D., Downes P. C. L-myo-inositol 1,4,5,6-tetrakisphosphate is present in both mammalian and avian cells. Biochem J. 1988 Jan 1;249(1):271–282. doi: 10.1042/bj2490271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strupish J., Cooke A. M., Potter B. V., Gigg R., Nahorski S. R. Stereospecific mobilization of intracellular Ca2+ by inositol 1,4,5-triphosphate. Comparison with inositol 1,4,5-trisphosphorothioate and inositol 1,3,4-trisphosphate. Biochem J. 1988 Aug 1;253(3):901–905. doi: 10.1042/bj2530901. [DOI] [PMC free article] [PubMed] [Google Scholar]


