Summary
Background
Emerging evidence suggests an association between common inflammatory skin diseases and chronic kidney disease (CKD).
Objectives
To explore the association between CKD stages 3–5 (CKD3–5) and atopic eczema, psoriasis, rosacea and hidradenitis suppurativa.
Methods
We undertook two complementary analyses; a prevalent case–control study and a cohort study using routinely collected primary care data [UK Clinical Practice Research Datalink (CPRD)]. We matched individuals with CKD3–5 in CPRD in March 2018 with up to five individuals without CKD for general practitioner practice, age and sex. We compared the prevalence of CKD3–5 among individuals with and without each inflammatory skin disease. We included individuals in CPRD with diabetes mellitus (2004–2018) in a cohort analysis to compare the incidence of CKD3–5 among people with and without atopic eczema and psoriasis.
Results
Our study included 56 602 cases with CKD3–5 and 268 305 controls. Cases were more likely than controls to have a history of atopic eczema [odds ratio (OR) 1·14, 99% confidence interval (CI) 1·11–1·17], psoriasis (OR 1·13, 99% CI 1·08–1·19) or hidradenitis suppurativa (OR 1·49, 99% CI 1·19–1·85), but were slightly less likely to have been diagnosed with rosacea (OR 0·92, 99% CI 0·87–0·97), after adjusting for age, sex, practice (matching factors), index of multiple deprivation, diabetes, smoking, harmful alcohol use and obesity. Results remained similar after adjusting for hypertension and cardiovascular disease. In the cohort with diabetes (N = 335 827), there was no evidence that CKD3–5 incidence was associated with atopic eczema or psoriasis.
Conclusions
Atopic eczema, psoriasis and hidradenitis suppurativa are weakly associated with CKD3–5. Future research is needed to elucidate potential mechanisms and the clinical significance of our findings.
Short abstract
What is already known about this topic?
Emerging evidence supports an association between more common inflammatory skin diseases and chronic kidney disease (CKD), but the size and nature of this association remain unclear.
What does this study add?
People with CKD were more likely to have atopic eczema (14%), psoriasis (13%) and hidradenitis suppurativa (49%), compared with those without CKD.
The link between inflammatory skin diseases and CKD did not appear to be mediated through cardiovascular comorbidity, hypertension or nephrotoxic drugs.
A stronger association with CKD among those with severe atopic eczema and psoriasis was consistent with a dose–response association.
There was no evidence of CKD incidence being associated with atopic eczema or psoriasis in the cohort of people with diabetes.
Linked Comment: J. Wan and K. Abuabara. Br J Dermatol 2021; 185:693–694.
Plain language summary available online
Chronic kidney disease (CKD) affects up to 13% of the world’s population, 1 and is associated with death and progression to end‐stage renal disease. 2 , 3 , 4 , 5 Established risk factors for CKD include older age, diabetes and hypertension, but for most cases, the aetiology of CKD remains unknown. 6 , 7
Skin diseases are a leading cause of disability worldwide, 8 and inflammatory skin diseases are associated with a range of comorbidities. 9 , 10 Emerging evidence supports an association between many inflammatory skin diseases and CKD. Longitudinal studies demonstrate increased CKD incidence in people with psoriasis, 11 , 12 , 13 , 14 and cross‐sectional evidence supports associations between reduced kidney function or renal abnormalities, and hidradenitis suppurativa, rosacea and atopic eczema. 15 , 16 , 17 Inflammatory skin diseases may be associated with CKD through increased metabolic syndrome risk and cardiovascular disease in people with skin conditions, 9 , 18 , 19 the use of nephrotoxic medications to manage skin diseases (or associated conditions) 20 or chronic low‐grade inflammation of skin disease. 21 , 22
Exploring the association between inflammatory skin conditions and CKD could help identify at‐risk populations who may benefit from regular renal function monitoring. It could also guide prudent systemic nephrotoxic prescribing for people with inflammatory skin conditions, and provide insight into pathological mechanisms leading to CKD. Elucidating the nature of an association between inflammatory skin diseases and CKD could guide targeted investigations into the possible role of skin conditions as CKD risk factors. Therefore, we aimed to compare the prevalence of specific common skin diseases (i.e. atopic eczema, psoriasis, rosacea and hidradenitis suppurativa) in adults with and without CKD, and assess whether atopic eczema and psoriasis are associated with CKD development in a cohort of people with diabetes mellitus.
Patients and methods
Setting
CKD diagnosis relies on blood and urine tests, which are not recommended as routine screening in the general population. 23 , 24 Therefore, it is difficult to establish the onset of CKD in routinely collected data. 24 However, prevalent CKD stages 3–5 (CKD3–5) based only on blood tests can be reliably detected in the general population 25 and new CKD3–5 cases can be captured among specific at‐risk populations (e.g. people with diabetes), who are more likely to undergo guideline‐recommended routine renal function testing. 24 We conducted two complementary matched analyses [(i) a population‐based prevalent matched case–control study in those with and without CKD3–5 and (ii) a cohort study restricted to people with diabetes mellitus] using routinely collected primary care electronic health record data from the UK Clinical Practice Research Datalink (UK CPRD Gold). Through the case–control study, we were able to include a large population‐based sample of UK primary care (powered to detect small associations). The complementary cohort analysis offered a view of a smaller specific subpopulation (i.e. those with diabetes) where the timing of CKD could be more reliably ascertained. The CPRD includes primary care data, including demographic information, coded diagnoses (Read codes), prescriptions and secondary care referrals. 26 , 27 Analyses of gaps in data entry and recorded deaths in each practice assure the data quality (i.e. ‘up‐to‐standard’ status), and diagnoses recorded in CPRD have been extensively validated. 27 , 28 , 29 The CPRD is nationally representative, covering approximately 7% of the UK population. 27
Study design and population
Case–control study
All people aged 25 years or older, alive and registered in an up‐to‐standard CPRD practice for at least 1 year on 31 March 2018 were eligible for inclusion. We used a validated algorithm to ascertain current CKD status and restricted study participants to individuals aged 25 years or older, as CKD3–5 occurring in people younger than age 25 years is rare. 25 , 30 We matched each individual with CKD3–5 (cases) with up to five individuals without CKD3–5 (controls), for age (i.e. same year of birth), sex and general practice. We compared the proportion of people with an inflammatory skin disease (diagnosed before 31 March 2018) among CKD3–5 cases and controls, repeating the analysis separately for each inflammatory skin condition (i.e. atopic eczema, psoriasis, rosacea and hidradenitis suppurativa, see Outcomes and Exposures sections below). A graphic representation of the study designs is provided in Appendix S1 (see Supporting Information).
Cohort study
All adults with diabetes mellitus in CPRD, who were aged ≥ 25 years (1 April 2004 to 31 March 2018), were eligible for inclusion. Follow‐up began at the latest date of the following: (i) 1 year after practice registration [to allow time for general practitioners (GPs) to record previous medical history, to allow for robust capture of baseline characteristics and so that we could be more confident that we had captured new‐onset CKD3–5], (ii) the date that the general practice reached CPRD quality standards, (iii) the start of the study (1 April 2004) or (iv) the date of the patient’s first record of a diabetes diagnosis. We excluded those with pre‐existing CKD3–5 (see Outcomes) and compared new CKD3–5 incidence among people with and without psoriasis or atopic dermatitis (i.e. the more common of the explored skin conditions) at cohort entry. We followed each participant until incident CKD3–5 (outcome), or the earliest of the following: (i) date of death, (ii) change of practice, (iii) last data collection from practice or (iv) the end of the study (31 March 2018) (diabetes definition is provided in Appendix S2; see Supporting Information).
Outcomes
We defined the primary outcome, CKD3–5, as being on renal replacement therapy (RRT), having received a renal transplant, or having two measurements of estimated glomerular filtration rate (eGFR) < 60 mL min−1 1·73m−2 calculated from serum creatinine test results [using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation] recorded in primary care at least 3 months apart. 31 The CKD‐EPI equation accounts for black ethnicity when estimating glomerular filtration rate. 32 As only 3·7% of the CPRD population is black, we classified individuals with no record of ethnicity as white. 25 Various methods are used in the UK to measure serum creatinine, but most laboratories did not report standardized values up to at least 2013. 33 Following the approach taken in previous CPRD studies, 34 , 35 , 36 we multiplied the recorded values of creatinine by 0·95 to correct for lack of isotope dilution mass spectrometry standardization. 37
For the case–control analysis, we identified all serum creatinine test results recorded in primary care over a 5‐year period (1 April 2013 to 31 March 2018). Using the same approach as a previous validation study, we considered those who did not meet the CKD3–5 criteria during that period as not having CKD3–5. 25 In the cohort analysis, we used the latest of the two creatinine measurements (recorded at least 3 months apart) required to fulfil the diagnostic criteria as the date of incident CKD3–5. Our secondary outcome was CKD stage based on the eGFR recorded on the date of incident CKD diagnosis [by eGFR: stage 3a (45–59 mL min−1 1·73m−2), stage 3b (30–44 mL min−1 1·73m−2), stage 4/5 (< 30 mL min−1 1·73m−2), RRT]. 25 , 38 We excluded patients from the cohort analysis who had at least one low eGFR < 60 mL min−1 1·73m−2 or a diagnostic code compatible with RRT before cohort entry.
Exposures
We used definitions of atopic eczema [positive predictive value (PPV) 86%], psoriasis (PPV = 90%), hidradenitis suppurativa and rosacea that have previously been applied in CPRD. 39 , 40 , 41 , 42 Diagnoses were based on the presence of recorded diagnostic codes (in addition to therapies for atopic eczema). A previous validation study assured the exclusion of nonatopic eczema cases. We chose these specific inflammatory skin diseases as exposures because they are common and predominantly managed in primary care (further details are provided in Appendix S3; see Supporting Information). 41 , 43 , 44
Covariate selection
We used a directed acyclic graph to guide a priori informed selection of covariates and avoid collider bias. 45 , 46 Consequently, we considered the following covariates: age (categorized into 5‐year intervals in the cohort study), sex, GP practice, level of deprivation [using quintiles of the 2015 Index of Multiple Deprivation (IMD)], ethnicity (white/South Asian/black/other/mixed), smoking status (current/ex‐smoker/never), alcohol use, body mass index (BMI) (< 18·5 kg m−2, 20·0–24·0 kg m−2, 25·0–29·0 kg m−2, > 30·0 kg m−2), diabetes, cardiovascular disease (i.e. ischaemic heart disease, chronic heart failure and peripheral arterial disease), hypertension, medications used to treat skin conditions with known renal implications [ciclosporin (nephrotoxic requiring monitoring), methotrexate (contraindicated for advanced CKD), mycophenolate mofetil (associated with anaemia and infection risk but not strictly contraindicated)]. All morbidity code lists used in this study are available to download (https://doi.org/10.17037/DATA.00001205) and additional details are available in Appendix S4 (see Supporting Information).
Statistical analysis
Case–control study
We initially described the characteristics of those with and without CKD3–5. We then used conditional logistic regression to estimate the odds ratios (ORs) comparing odds of CKD3–5 in individuals with each skin condition separately (main analysis) compared with those without. All analyses implicitly accounted for matching factors (age, sex, GP practice). We fitted the following sequential regression models: (i) a minimally adjusted model, additionally including IMD; (ii) a fully adjusted model, additionally including diabetes mellitus, smoking, harmful alcohol use and obesity and (iii) a final model that also included potential mediators, i.e. hypertension and cardiovascular disease. We used complete case analysis. 47 To preserve matching, we excluded entire matched sets if a person with CKD3–5 was excluded (e.g. owing to missing data or restrictions in a sensitivity analysis), or if no individuals without CKD3–5 remained in the set.
We conducted sensitivity analyses to explore potential bias introduced by the algorithm used to define CKD3–5, skin disease definitions, lack of accurate ethnicity data before 2006, 32 information bias owing to more frequent renal function testing among those taking medications requiring frequent blood testing, the inclusion of non‐English CPRD practices and missing BMI data (Appendix S5; see Supporting Information).
We conducted the following secondary analyses: (i) repeating the analyses after redefining individuals with atopic eczema as having mild, moderate or severe disease, and after redefining people with psoriasis as having mild/moderate or severe disease [based on recorded prescriptions for potent or systemic treatments, phototherapy records and referrals (Appendix S5)], (ii) exploring potential effect modification by age and sex and (iii) using multinomial logistic regression to compare the relative risk (RR) of various levels of impaired kidney function among individuals with each skin condition with those who did not have skin disease. To account for matching in the multinomial regression, we additionally adjusted for the matching variables (age group categorized as 45–64 years, 65–69 years, 70–74 years, 75–79 years, 80–84 years, 85–89 years, ≥ 90 years and sex) and calculated 99% confidence intervals (CIs) allowing for intragroup correlation within GP practices by using clustered robust SEs. We estimated the population attributable risk (PAR), the proportion of CKD cases attributable to each skin condition, assuming causality [PAR = P(RR − 1)/RR, where P is the proportion of CKD cases with the inflammatory skin condition (using the calculated matched OR as an estimate for the RR)]. 48
Cohort study
For the cohort analysis of individuals with diabetes, we initially presented baseline characteristics of individuals with and without atopic eczema and psoriasis. We then calculated CKD3–5 incidence rates and used Poisson regression to estimate the rate ratio for CKD3–5 among those with and without atopic eczema and psoriasis, using the same framework of covariate selection described above. As in the case–control study, we conducted sensitivity analyses to explore potential bias (Appendix S6; see Supporting Information).
In all analyses, we used likelihood‐ratio tests to calculate P‐values (unless stated otherwise). We used 99% CIs to reduce the risk of type 1 error in the context of multiple analyses. 49 Statistical analysis was performed using Stata, version 15·1 IC (StataCorp LP, College Station, TX, USA). The study was approved by the CPRD Independent Scientific Advisory Committee (#19_011) and the London School of Hygiene and Tropical Medicine Research Ethics Committee (#16353).
Results
Case–control study
We matched 56 602 adults with CKD3–5 in CPRD on 31 March 2018 with a control group of 268 305 people (Figure 1). The mean age (SD) was 78·6 years (10·4) and 42·3% (137 505) were men. Those with CKD3–5 were more likely to be obese than those without (38·8% vs. 23·5%), and more likely to be current or ex‐smokers (65·0% vs. 59·6%). People with CKD3–5 were more likely to have a cardiovascular disease risk factor (i.e. smoking, hypertension, diabetes, obesity) or cardiovascular disease (Table 1). Characteristics stratified by CKD3–5 category are provided in Appendix S5.
Figure 1.
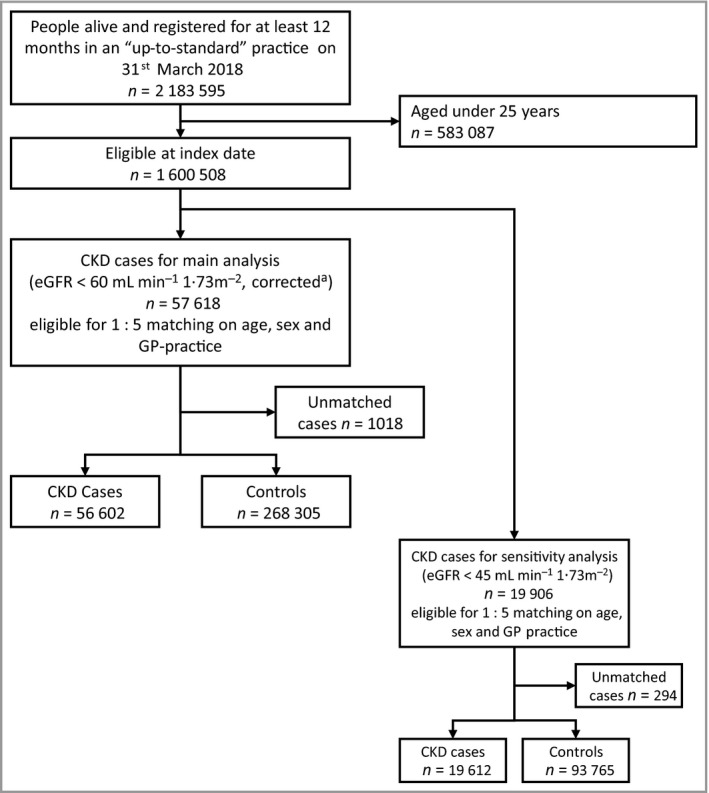
Case–control study. Flowchart illustrating selection of the study population. CKD, chronic kidney disease; CPRD, Clinical Practice Research Datalink; eGFR, estimated glomerular filtration rate aRecorded creatinine values were multiplied by 0·95 to give a conservative estimate, corrected for lack of test standardization. CKD on index date was defined if two consecutive eGFR measurements (including the one closest to the index date) were below 60 mL min−1 1·73m−2 and at least 90 days apart, or if there were diagnostic codes indicating renal replacement therapy or a kidney transplant.
Table 1.
Case–control study. Characteristics of the study population
| CKD3–5 | No CKD3–5 | |
|---|---|---|
| N = 56 602 | N = 268 305 | |
| Age, years, mean (SD) | 79·1 (10·6) | 78·4 (10·4) |
| 25–64 | 4782 (8·4) | 23 910 (8·9) |
| 65–74 | 10 484 (18·5) | 52 401 (19·5) |
| 75–84 | 22 549 (39·8) | 111 696 (41·6) |
| ≥ 85 | 18 787 (33·2) | 80 298 (29·9) |
| Sex, male | 24 039 (42·5) | 113 466 (42·3) |
| IMD | ||
| 1 lowest quintile | 13 288 (23·5) | 65 167 (24·3) |
| 2 | 9151 (16·2) | 44 189 (16·5) |
| 3 | 12 071 (21·3) | 57 086 (21·3) |
| 4 | 12 581 (22·2) | 58 031 (21·6) |
| 5 highest quintile | 9511 (16·8) | 43 832 (16·3) |
| Ethnicitya | ||
| White | 26 237 (94·9) | 117 606 (94·9) |
| South Asian | 742 (2·7) | 2941 (2·4) |
| Black | 354 (1·3) | 1869 (1·5) |
| Other/mixed | 310 (1·1) | 1564 (1·3) |
| Body mass index (BMI),a kg m−2, mean (SD) | 28·4 (5·8) | 26·9 (5·2) |
| Underweight (< 18·5) | 748 (1·7) | 5429 (2·8) |
| Normal (20·0–24·0) | 11 443 (26·6) | 67 144 (35·1) |
| Overweight (25·0–29·0) | 16 260 (37·8) | 73 763 (38·6) |
| Obese (> 30·0) | 14 555 (33·8) | 44 923 (23·5) |
| Current/former smokera | 36 741 (65·0) | 153 407 (59·6) |
| Hypertension | 44 777 (79·1) | 147 648 (55·0) |
| Ischaemic heart disease | 13 209 (23·3) | 37 399 (13·9) |
| Peripheral arterial disease | 3495 (6·2) | 7971 (3·0) |
| Heart failure | 7820 (13·8) | 12 173 (4·5) |
| Diabetes | 16 612 (29·3) | 36 819 (13·7) |
| Harmful alcohol use | 2789 (4·9) | 14 577 (5·4) |
| Methotrexateb | 1184 (2·1) | 4332 (1·6) |
| Ciclosporinb | 428 (0·8) | 206 (0·1) |
| Mycophenolate mofetilb | 906 (1·6) | 206 (0·1) |
| Renal function, mL min−1 1·73m−2 | ||
| eGFR 45–59 (3a) | 32 895 (58·1) | N/A |
| eGFR 30–44 (3b) | 17 418 (30·8) | N/A |
| eGFR < 30 (4/5) | 5601 (9·9) | N/A |
| RRT/renal transplant | 688 (1·2) | N/A |
CKD3–5, chronic kidney disease stages 3–5; eGFR, estimated glomerular filtration rate; IMD, index of multiple deprivation; RRT, renal replacement therapy. aMissing values: ethnicity, N = 173 284; BMI, N = 90 672; smoking status, N = 11 281. bRecord of at least one previous prescription. Data are presented as n (%) unless otherwise stated.
We present the association between all four inflammatory skin diseases and CKD3–5 in Table 2. Individuals with CKD3–5 were more likely to have a history of atopic eczema (OR 1·14, 99% CI 1·11–1·17), psoriasis (OR 1·13, 99% CI 1·08–1·19) or hidradenitis (OR 1·49, 99% CI 1·19–1·85) than those without, after adjusting for age, sex, general practice and IMD, in addition to diabetes, smoking, harmful alcohol use and obesity. CKD3–5 was associated with a lower prevalence of rosacea (OR 0·92, 99% CI 0·87–0·97). After additionally adjusting for hypertension and cardiovascular morbidity (i.e. potential mediators), our results were slightly attenuated for all four skin diseases (Table 2). Appendix S5 includes absolute numbers and crude proportions of people with inflammatory skin diseases among those with and without CKD3–5.
Table 2.
Case–control study. Odds ratios (99% confidence interval) for prevalent chronic kidney disease stages 3–5 among individuals with inflammatory skin diseases
| Model 1: minimally adjusted | Model 2: fully adjusted | Model 3: additionally adjusted for potential mediators | |
|---|---|---|---|
| N = 324 907 | N = 228 812 | N = 228 812 | |
| Atopic eczema | 1·24 (1·21–1·27) | 1·14 (1·11–1·17) | 1·1 (1·07–1·13) |
| Psoriasis | 1·24 (1·19–1·29) | 1·13 (1·08–1·19) | 1·12 (1·07–1·17) |
| Hidradenitis suppurativa | 1·62 (1·34–1·97) | 1·49 (1·19–1·85) | 1·45 (1·16–1·82) |
| Rosacea | 0·96 (0·91–1) | 0·92 (0·87–0·97) | 0·91 (0·86–0·96) |
Odds ratios are derived from conditional logistic regression models, stratified according to the matching variables (i.e. age, sex and general practitioner practice), and presented with 99% confidence intervals. The minimally adjusted model is adjusted for age, sex, general practice and index of multiple deprivation. The fully adjusted model additionally accounts for diabetes status, smoking, harmful alcohol use and obesity. A final model, additionally adjusted for hypertension and cardiovascular disease (ischaemic heart disease, chronic heart failure and peripheral arterial disease) as potential mediators.
Participants with atopic eczema and psoriasis were more likely to have CKD3–5 regardless of atopic eczema or psoriasis severity. The association was stronger in people with more severe skin disease (P trend < 0·001) (Figure 2, Appendix S5). In stratified analyses, there was no evidence of a difference between men and women in the association between the inflammatory skin diseases and CKD3–5, nor was there evidence for effect modification by age. Our multinomial regression results were consistent with the main regression analysis, but CIs were wide. Our results were similar in the sensitivity analyses (Appendix S5). Applying the calculated effect estimates, and assuming a causal association, we found that 2·4% of CKD cases may be attributable to atopic eczema, 0·7% to psoriasis and 1·45% to hidradenitis suppurativa (Appendix S5).
Figure 2.
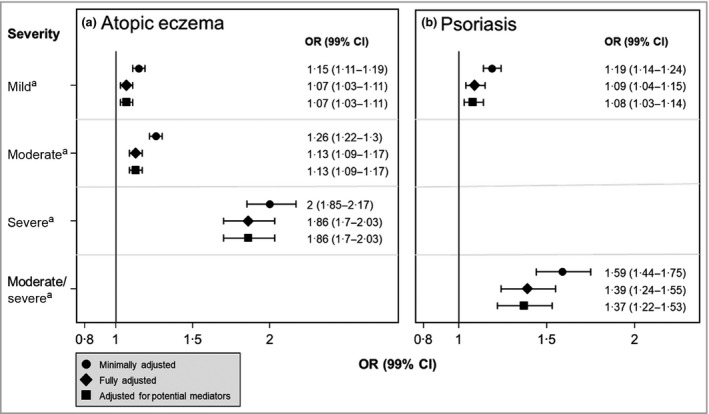
Case–control study. Odds ratios (ORs) [99% confidence intervals (CIs)] for prevalent CKD3–5 among individuals with (a) atopic eczema and (b) psoriasis, by the severity of the skin condition. ORs are derived from conditional logistic regression models, stratified on the matching variables (i.e. age, sex and GP practice) and presented with 99% CIs. All P‐values for trend < 0·001. The minimally adjusted model is adjusted for age, sex, general practice and index of multiple deprivation. The fully adjusted model additionally accounts for diabetes status, smoking, harmful alcohol use and obesity. A final model, additionally adjusted for hypertension and cardiovascular disease (ischaemic heart disease, chronic heart failure and peripheral arterial disease) as potential mediators. aCompared with those without the skin condition.
Cohort study
We identified 448 286 eligible individuals with a diagnosis of diabetes mellitus in CPRD between 1 April 2004 and 31 March 2018. A total of 335 827 individuals remained after we excluded those who had at least one eGFR measurement < 60 mL min−1 1·73m−2 or RRT before cohort entry. The mean age at cohort entry was 58·5 (SD 13·6) years, and 59·7% (200 372) were men. Overall, 9·4% (31 585) had atopic eczema, and 4·6% (15 476) had a diagnosis of psoriasis (Appendix S6). There was no evidence for an association between pre‐existing atopic eczema or psoriasis and new‐onset CKD3–5; this finding did not differ in the sensitivity analyses (Appendix S6).
Discussion
In our case–control study, people with CKD3–5 were more likely to have atopic eczema (14%), psoriasis (13%) and hidradenitis suppurativa (49%), compared with those without CKD3–5. There was no evidence to suggest that the link between inflammatory skin diseases and CKD3–5 was mediated through cardiovascular comorbidity or hypertension. A stronger association with CKD3–5 among those with severe atopic eczema and psoriasis was consistent with a dose–response association. However, those with rosacea were less likely to have CKD3–5, and we did not find an increased incidence of CKD3–5 among those with atopic eczema or psoriasis within a cohort of people with diabetes.
We studied a large, nationally representative, population‐based sample, assuring power and precision in assessing the link between inflammatory skin diseases and CKD3–5. Using real‐world, routinely collected data, we were able to account for a wide range of potential confounders and mediators and conducted extensive sensitivity analyses. We explored four different conditions, following a rigorous pre‐approved protocol, and combined complementary approaches with different potential biases to ‘triangulate’ the association between inflammatory skin diseases and CKD3–5. 50 The integration of diverse data, rather than concentrating on a single disease, supported our attempt to make inferences regarding inflammatory skin conditions in general.
However, our study has several limitations. We regarded those without serum creatinine measurements as not having CKD3–5, as people will only have serum creatinine testing when it is indicated (i.e. in acute illness or incentivized monitoring in specific illnesses, e.g. diabetes) and early disease is often asymptomatic. Thus, we may have misclassified people with undiagnosed silent CKD as being CKD‐free, potentially diluting our effect estimates towards null. However, a previous validation study has shown that the approach we took reliably captures prevalent CKD3–5 in CPRD. 25 Additionally, owing to infrequent urinary testing, we were unable to capture albuminuria, which may have been a more sensitive marker of inflammatory kidney damage. Skin manifestations of late‐stage CKD could have been misclassified as inflammatory skin diseases (e.g. xerosis). This limitation of the case–control design was mitigated by our use of validated algorithms (where available) to reduce the likelihood of misclassifying skin disease. Finally, we were unable to capture potentially relevant covariates, including environmental and genetic risk factors, in routinely collected data. Of note, we were not able to capture information on the use of biological medications, which may have had a mediating role in the development of CKD among people with severe skin conditions.
Longitudinal studies have demonstrated increased CKD incidence in people with psoriasis. 11 , 12 , 13 , 14 Cross‐sectional or smaller‐scale evidence previously suggested an association between CKD and hidradenitis suppurativa, 15 and rosacea. 16 There have been reports of nephrotic syndrome and Henöch–Schonlein purpura in children with atopy, 51 , 52 , 53 , 54 and a Danish analysis suggests that mortality owing to urogenital diseases (International Classification of Diseases 10th revision code groups N00–N99) is more common among those with eczema, although absolute numbers were small. 17 Our findings are consistent with these previous reports, but to the best of our knowledge, the association between atopic eczema and CKD has not been previously explored. Unlike a previous smaller Taiwanese cohort study, 16 we did not demonstrate an association between rosacea and CKD3–5. While GP diagnoses are highly concordant with those made by specialists, 55 a previous report also suggested substantial misclassification of rosacea by GPs, especially regarding acne and seborrhoeic dermatitis. 42 Misclassified rosacea diagnoses are likely to be nondifferential (i.e. to occur regardless of CKD status), and may have therefore diluted the observed association between rosacea and CKD3–5 towards null.
We explored a link between inflammatory skin diseases and CKD. We were able to include a large sample of people with atopic eczema and psoriasis, but relatively few with hidradenitis suppurativa and rosacea. Our case–control analysis supported a positive association between atopic eczema, psoriasis and hidradenitis suppurativa and CKD, but a negative association between rosacea and CKD. A cohort analysis of people with diabetes failed to demonstrate the associations of atopic eczema and psoriasis with CKD3–5. We discuss below potential explanations for our findings and for the discrepancy between the results of the complementary designs.
We cannot ascertain the temporal direction using our prevalent case–control design (owing to its cross‐sectional nature), but the consistent results across multiple skin conditions and the apparent association between increasing atopic eczema and psoriasis severity and increasing CKD3–5 highlight the need for further research to explore a causal link. The underlying mechanism for a potential link between inflammatory skin conditions and CKD remains unknown, but one compelling explanation involves chronic inflammation. 56 Autoimmune inflammation is mediated through abnormal activation of the innate immune system, which plays an important role in common skin diseases, such as psoriasis, atopic eczema, hidradenitis suppurativa and rosacea. 57 , 58 , 59 , 60 Circulating reactive oxygen species and inflammatory cytokines (resulting from the inflammatory process) could impair endothelial function leading to accelerated atherosclerosis (with consequent kidney vasculature damage) or independently modulate kidney damage. 61 However, we observed little change in our results after accounting for potential mediators (i.e. ischaemic heart disease, chronic heart failure and peripheral arterial disease) and conducting sensitivity analyses. Therefore, our findings do not support a mediating role of metabolic syndrome, cardiovascular morbidity, hypertension or nephrotoxic medications in the development of CKD among people with inflammatory skin conditions. We were unable to account for the use of biological medications, which may have mediated the association among those with moderate‐to‐severe skin conditions; further research is needed to elucidate the role of this medication group. Although the results of the cohort study did not mirror those of our main case–control design, we believe that they highlight methodological issues for future research but do not invalidate its results.
In our cohort study of people with diabetes, there was no evidence for an increased CKD3–5 incidence among people with atopic eczema or psoriasis. However, the mean follow‐up for participants in the cohort was 5·3 years, which may have been insufficient to capture differences in CKD3–5 onset between those with and without skin disease. We chose to follow people with diabetes to ensure reliable capture of incident CKD, but this approach may have inadvertently precluded us from observing an association. Diabetes, together with hypertension, is associated with most cases of CKD. 62 Therefore, we speculate that the high baseline risk for developing CKD3–5 in a cohort of people with diabetes may obscure a much smaller contributing effect of an inflammatory skin condition.
There is no firm evidence to support routine population screening for CKD, 38 , 63 but some stakeholders advocate targeted testing of individuals who are at high risk. 7 , 64 If noncommunicable inflammatory skin conditions are indeed risk factors or markers for CKD, future guidelines may consider affected individuals as at‐risk populations for regular renal function monitoring.
We found that atopic eczema, psoriasis and hidradenitis suppurativa were weakly associated with CKD, independent of obesity, cardiovascular morbidity or nonbiological nephrotoxic skin medications. We did not find evidence supporting this association in a cohort of people with diabetes. Further research is needed to elucidate the nature and temporal direction of this link, to account for other potential confounders and to explore whether targeted screening for CKD in people with inflammatory skin diseases is justified.
Author Contribution
Yochai Schonmann : Conceptualization (equal); Funding acquisition (equal); Methodology (equal); Supervision (lead); Writing‐original draft (supporting). Kathryn Mansfield: Conceptualization (supporting); Data curation (supporting); Formal analysis (supporting); Methodology (supporting); Writing‐original draft (supporting). Amy Mulick: Conceptualization (supporting); Formal analysis (supporting); Methodology (supporting); Writing‐original draft (supporting). Amanda Roberts: Conceptualization (supporting); Writing‐original draft (supporting). Liam Smeeth: Conceptualization (supporting); Writing‐original draft (supporting). Sinead Langan: Conceptualization (equal); Funding acquisition (equal); Methodology (equal); Supervision (lead); Writing‐original draft (supporting). Dorothea Nitsch: Conceptualization (equal); Funding acquisition (equal); Methodology (equal); Supervision (lead); Writing‐original draft (supporting).
Supporting information
Appendix S1 Visual representation of the study design.
Appendix S2 Defining diabetes.
Appendix S3 Algorithm definitions for inflammatory skin diseases.
Appendix S4 Covariate selection using a directed acyclic graph (DAG) to conceptualize the relationships of potentially relevant covariates with inflammatory skin conditions and chronic kidney disease.
Appendix S5 Population‐based case–control study details.
Appendix S6 Cohort of people with diabetes.
Funding sources S.M.L. was supported by a Wellcome Trust Senior Research Fellowship in Clinical Science (205039/Z/16/Z). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funders. S.M.L. was also supported by Health Data Research UK (Grant No. LOND1), which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation and Wellcome Trust.
Conflicts of interest The authors declare they have no conflicts of interest.
S.M.L. and D.N. are joint senior authors.
Plain language summary available online
References
- 1. Hill NR, Fatoba ST, Oke JL et al. Global prevalence of chronic kidney disease – a systematic review and meta‐analysis. PLoS One 2016; 11:e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van der Velde M, Matsushita K, Coresh J et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all‐cause and cardiovascular mortality. A collaborative meta‐analysis of high‐risk population cohorts. Kidney Int 2011; 79:1341–52. [DOI] [PubMed] [Google Scholar]
- 3. Matsushita K, van der Velde M, Astor BC et al. Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet 2010; 375:2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nitsch D, Grams M, Sang Y et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta‐analysis. BMJ 2013; 346:f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet 2017; 389:1238–52. [DOI] [PubMed] [Google Scholar]
- 6. Romagnani P, Remuzzi G, Glassock R et al. Chronic kidney disease. Nat Rev Dis Prim 2017; 3:17088. [DOI] [PubMed] [Google Scholar]
- 7. Levin A, Tonelli M, Bonventre J et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 2017; 390:1888–917. [DOI] [PubMed] [Google Scholar]
- 8. Hay RJ, Johns NE, Williams HC et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol 2014; 134:1527–34. [DOI] [PubMed] [Google Scholar]
- 9. Kwa MC, Silverberg JI. Association between inflammatory skin disease and cardiovascular and cerebrovascular co‐morbidities in US adults: analysis of nationwide inpatient sample data. Am J Clin Dermatol 2017; 18:813–23. [DOI] [PubMed] [Google Scholar]
- 10. Narla S, Silverberg JI. Multimorbidity and mortality risk in hospitalized adults with chronic inflammatory skin disease in the United States. Arch Dermatol Res 2020; 312:507–12. [DOI] [PubMed] [Google Scholar]
- 11. Wan J, Wang S, Haynes K et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ 2013; 347:f5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chiu H‐Y, Huang H‐L, Li C‐H et al. Increased risk of glomerulonephritis and chronic kidney disease in relation to the severity of psoriasis, concomitant medication, and comorbidity: a nationwide population‐based cohort study. Br J Dermatol 2015; 173:146–54. [DOI] [PubMed] [Google Scholar]
- 13. Chi C‐C, Wang J, Chen Y‐F et al. Risk of incident chronic kidney disease and end‐stage renal disease in patients with psoriasis: a nationwide population‐based cohort study. J Dermatol Sci 2015; 78:232–8. [DOI] [PubMed] [Google Scholar]
- 14. Parisi R, Rutter MK, Lunt M et al. Psoriasis and the risk of major cardiovascular events: cohort study using the clinical practice research datalink. J Invest Dermatol 2015; 135:2189–97. [DOI] [PubMed] [Google Scholar]
- 15. Miller I, Carlson N, Mogensen U et al. A population‐ and hospital‐based cross‐sectional study of renal function in hidradenitis suppurativa. Acta Derm Venereol 2016; 96:68–71. [DOI] [PubMed] [Google Scholar]
- 16. Chiu H‐Y, Huang W‐Y, Ho C‐H et al. Increased risk of chronic kidney disease in patients with rosacea: a nationwide population‐based matched cohort study. PLoS One 2017; 12:e0180446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thyssen JP, Skov L, Egeberg A. Cause‐specific mortality in adults with atopic dermatitis. J Am Acad Dermatol 2018; 78:506–10. [DOI] [PubMed] [Google Scholar]
- 18. Stefanadi EC, Dimitrakakis G, Antoniou C‐K et al. Metabolic syndrome and the skin: a more than superficial association. Reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr 2018; 10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silverwood RJ, Forbes HJ, Abuabara K et al. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: population based cohort study. BMJ 2018; 361:k1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gisondi P, Pezzolo E, Girolomoni G. Glomerular filtration rate in patients with moderate‐to‐severe psoriasis. J Eur Acad Dermatology Venereol 2019; 33:e244–e246. [DOI] [PubMed] [Google Scholar]
- 21. Fox ER, Benjamin EJ, Sarpong DF et al. The relation of C ‐ reactive protein to chronic kidney disease in African Americans: the Jackson Heart Study. BMC Nephrol 2010; 11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amdur RL, Feldman HI, Gupta J et al. Inflammation and progression of CKD: the CRIC Study. Clin J Am Soc Nephrol 2016; 11:1546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Institute for Health and Care Excellence (NICE) . Chronic kidney disease in adults: assessment and management. Clinical guideline 182. Available at http://nice.org.uk/guidance/cg182 (last accessed 5 April 2021). [PubMed]
- 24. McDonald HI, Shaw C, Thomas SL et al. Methodological challenges when carrying out research on CKD and AKI using routine electronic health records. Kidney Int 2016; 90:943–9. [DOI] [PubMed] [Google Scholar]
- 25. Iwagami M, Tomlinson LA, Mansfield KE et al. Validity of estimated prevalence of decreased kidney function and renal replacement therapy from primary care electronic health records compared with national survey and registry data in the United Kingdom. Nephrol Dial Transplant 2017; 32 (Suppl. 2):ii142–ii150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chisholm J. The Read clinical classification. BMJ 1990; 300:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herrett E, Gallagher AM, Bhaskaran K et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015; 44:827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herrett E, Thomas SL, Schoonen WM et al. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010; 69:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract 2010; 60:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nitsch D, Caplin B, Hull S et al. National Chronic Kidney Disease Audit (Part 1). Available at https://www.hqip.org.uk/wp‐content/uploads/2018/02/HtoEm0.pdf (last accessed on 16 November 2018). [Google Scholar]
- 31. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mathur R, Bhaskaran K, Chaturvedi N et al. Completeness and usability of ethnicity data in UK‐based primary care and hospital databases. J Public Health (Oxf) 2014; 36:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lamb EJ. United Kingdom guidelines for chronic kidney disease. Scand J Clin Lab Invest Suppl 2008; 68:16–22. [DOI] [PubMed] [Google Scholar]
- 34. Iwagami M, Caplin B, Smeeth L et al. Chronic kidney disease and cause‐specific hospitalisation: a matched cohort study using primary and secondary care patient data. Br J Gen Pract 2018; 68:e512–e523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pereg D, Tirosh A, Elis A et al. Mortality and coronary heart disease in euthyroid patients. Am J Med 2012; 125:826.e7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McDonald HI, Thomas SL, Millett ERC, Nitsch D. CKD and the risk of acute, community‐acquired infections among older people with diabetes mellitus: a retrospective cohort study using electronic health records. Am J Kidney Dis 2015; 66:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levey AS, Coresh J, Greene T et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007; 53:766–72. [DOI] [PubMed] [Google Scholar]
- 38. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150. [Google Scholar]
- 39. Abuabara K, Magyari AM, Hoffstad O et al. Development and validation of an algorithm to accurately identify atopic eczema patients in primary care electronic health records from the UK. J Invest Dermatol 2017; 137:1655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huerta C, Rivero E, Rodríguez LAG. Incidence and risk factors for psoriasis in the general population. Arch Dermatol 2007; 143:1559–65. [DOI] [PubMed] [Google Scholar]
- 41. Ingram JR, Jenkins‐Jones S, Knipe DW et al. Population‐based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol 2018; 178:917–24. [DOI] [PubMed] [Google Scholar]
- 42. Spoendlin J, Voegel JJ, Jick SS, Meier CR. A study on the epidemiology of rosacea in the U.K. Br J Dermatol 2012; 167:598–605. [DOI] [PubMed] [Google Scholar]
- 43. Augustin M, Herberger K, Hintzen S et al. Prevalence of skin lesions and need for treatment in a cohort of 90 880 workers. Br J Dermatol 2011; 165:865–73. [DOI] [PubMed] [Google Scholar]
- 44. Sinikumpu S‐P, Huilaja L, Jokelainen J, et al. High prevalence of skin diseases and need for treatment in a middle‐aged population. A Northern Finland Birth Cohort 1966 study. PLoS One 2014; 9:e99533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999; 10:37–48. [PubMed] [Google Scholar]
- 46. Lederer DJ, Bell SC, Branson RD et al. Control of confounding and reporting of results in causal inference studies: guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc 2019; 16:22–8. [DOI] [PubMed] [Google Scholar]
- 47. Hughes RA, Heron J, Sterne JAC, Tilling K. Accounting for missing data in statistical analyses: multiple imputation is not always the answer. Int J Epidemiol 2019; 48:1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health 1998; 88:15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ioannidis JPA. The proposal to lower P‐value thresholds to .005. JAMA 2018; 319:1429–30. [DOI] [PubMed] [Google Scholar]
- 50. Lawlor DA, Tilling K, Smith GD. Triangulation in aetiological epidemiology. Int J Epidemiol 2016; 45:1866–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abdel‐Hafez M, Shimada M, Lee PY et al. Idiopathic nephrotic syndrome and atopy: is there a common link? Am J Kidney Dis 2009; 54:945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wei C‐C, Tsai J‐D, Lin C‐L et al. Increased risk of idiopathic nephrotic syndrome in children with atopic dermatitis. Pediatr Nephrol 2014; 29:2157–63. [DOI] [PubMed] [Google Scholar]
- 53. Berghea EC, Balgradean M, Popa I‐L. Correlation between idiopathic nephrotic syndrome and atopy in children ‐ short review. Maedica (Buchar) 2017; 12:55–8. [PMC free article] [PubMed] [Google Scholar]
- 54. Wei C‐C, Lin C‐L, Shen T‐C et al. Atopic dermatitis and association of risk for Henoch‐Schönlein purpura (IgA vasculitis) and renal involvement among children. Medicine (Baltimore) 2016; 95:e2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Holme SA, Scott‐Lang VE, Ooi ET et al. The south‐east Scotland dermatology workload study: 30 years’ analysis. Br J Dermatol 2012; 167:123–30. [DOI] [PubMed] [Google Scholar]
- 56. Eyerich K, Eyerich S. Immune response patterns in non‐communicable inflammatory skin diseases. J Eur Acad Dermatology Venereol 2018; 32:692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nguyen TV, Cowen EW, Leslie KS. Autoinflammation: from monogenic syndromes to common skin diseases. J Am Acad Dermatol 2013; 68:834–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Murthy AS, Leslie K. Autoinflammatory skin disease: a review of concepts and applications to general dermatology. Dermatology 2016; 232:534–40. [DOI] [PubMed] [Google Scholar]
- 59. de Sá DC, Festa Neto C. Inflammasomes and dermatology. An Bras Dermatol 2016; 91:566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sinikumpu S, Huilaja L, Auvinen J et al. The association between low grade systemic inflammation and skin diseases: a cross‐sectional survey in the Northern Finland Birth Cohort 1966. Acta Derm Venereol 2018; 98:65–9. [DOI] [PubMed] [Google Scholar]
- 61. Impellizzeri D, Esposito E, Attley J, Cuzzocrea S. Targeting inflammation: new therapeutic approaches in chronic kidney disease (CKD). Pharmacol Res 2014; 81:91–102. [DOI] [PubMed] [Google Scholar]
- 62. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA 2019; 322:1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moyer VA, U.S. Preventive Services Task Force . Screening for chronic kidney disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 157:567–70. [DOI] [PubMed] [Google Scholar]
- 64. Komenda P, Ferguson TW, Macdonald K et al. Cost‐effectiveness of primary screening for CKD: a systematic review. Am J Kidney Dis 2014; 63:789–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Visual representation of the study design.
Appendix S2 Defining diabetes.
Appendix S3 Algorithm definitions for inflammatory skin diseases.
Appendix S4 Covariate selection using a directed acyclic graph (DAG) to conceptualize the relationships of potentially relevant covariates with inflammatory skin conditions and chronic kidney disease.
Appendix S5 Population‐based case–control study details.
Appendix S6 Cohort of people with diabetes.


