Abstract
Objectives
Neurocysticercosis (NCC) and human immunodeficiency virus (HIV) have a high disease burden and are prevalent in overlapping low‐ and middle‐income areas. Yet, treatment guidance for people living with HIV/AIDS (PLWH/A) co‐infected with NCC is currently lacking. This study aims to scope the available literature on HIV/AIDS and NCC co‐infection, focusing on epidemiology, clinical characteristics, diagnostics and treatment outcomes.
Methods
The scoping literature review methodological framework, and the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines were followed. A total of 16,969 records identified through database searching, and 45 additional records from other sources were reduced to 52 included studies after a standardised selection process.
Results
Two experimental studies, ten observational studies, 23 case series/case reports and 17 reviews or letters were identified. Observational studies demonstrated similar NCC seroprevalence in PLWH/A and their HIV‐negative counterparts. Of 29 PLWH/A and NCC co‐infection, 17 (59%) suffered from epileptic seizures, 15 (52%) from headaches and 15 (52%) had focal neurological deficits. Eighteen (62%) had viable vesicular cysts, and six (21%) had calcified cysts. Fifteen (52%) were treated with albendazole, of which 11 (73%) responded well to treatment. Five individuals potentially demonstrated an immune‐reconstitution inflammatory syndrome after commencing antiretroviral therapy, although this was in the absence of immunological and neuroimaging confirmation.
Conclusions
There is a paucity of evidence to guide treatment of PLWH/A and NCC co‐infection. There is a pressing need for high‐quality studies in this patient group to appropriately inform diagnostic and management guidelines for HIV‐positive patients with NCC.
Keywords: AIDS, co‐infection, HIV, neurocysticercosis, Taenia solium, taeniosis
INTRODUCTION
Neurocysticercosis (NCC) is caused by the parasitic cestode Taenia solium, which has a complex zoonotic lifecycle. Clinical manifestations usually result from the degeneration of parasites located in the central nervous system, and depend on the host's immune response, and number, size and location of cysts [1]. Signs and symptoms are pleomorphic, ranging from epileptic seizures, headaches, focal neurological deficits and increased intracranial pressure to a range of other manifestations including psychiatric disorders [2, 3].
In endemic areas, NCC accounts for approximately one third of all cases of epilepsy [4] and is ranked as the ‘foodborne parasite of greatest global concerns’, with an estimated burden of 1.37 million Disability Adjusted Life Years (DALYs) in 2019 [5, 6]. According to the most recent endemicity map for T. solium taeniosis/(neuro)cysticercosis of WHO, Latin America, South and South‐East Asia and sub‐Saharan Africa are endemic [7]. These regions are also endemic for human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), indicating potential for interaction between these two diseases.
Pathophysiological interactions between HIV/AIDS and other co‐infections such as malaria, tuberculosis and neglected tropical diseases (including helminthiases) have been previously described [2, 8, 9, 10, 11]. However, to date little is known about NCC‐HIV/AIDS co‐infection [12, 13]. People living with HIV/AIDS (PLWH/A) with asymptomatic NCC may develop an immune‐reconstitution inflammatory syndrome (IRIS) when starting on antiretroviral therapy (ART), converting to symptomatic NCC [14]. There is also a potential risk of drug interactions between anti‐epileptic drugs (AEDs), ART and anthelmintics in PLWH/A with NCC‐associated seizures [15].
We, therefore, conducted a scoping review to map the literature on PLWH/A co‐infected with NCC and their treatment and address the following question: what is known from the existing literature about the treatment of PLWH/A with NCC? This review adds to one recently published by Herrera et al. [16] through additional discussion of observational studies and further investigation regarding treatment of this patient group.
METHODS
Identifying the research question
This work formed part of the WHO Guideline Development Proposal for the Diagnosis and Treatment Guidelines for Taenia solium Neurocysticercosis (AA, ASW) and initially aimed to answer two PICO (population, intervention, control, outcomes) questions (Supplementary File). As preparatory work indicated that high‐quality literature is scarce (Supplementary File), the research question for the scoping review [17, 18] was formulated in a broader way: What is known from the existing literature about the treatment of PLWH/A and symptomatic NCC?
Identifying relevant studies
The following electronic databases were searched: PubMed, Embase, Global Index Medicus (limited to Regional Databases LILACS, AIM, WPRIM; IMSEAR, IMEMR), Global Health (CABI) and Web of Science. The adaptation of the search terms to the different databases, the date of search and the number of publications identified per database as well as further details on the search are presented in Supplementary File. After deduplication, a sub‐search for HIV/AIDS‐related papers was conducted and further corroborated with results of a manual search of the entire identified literature (Supplementary File). Finally, cross‐referencing, personal communications and presentations of the PICO questions at CYSTINET (European Network on Taeniosis/Cysticercosis, COST Action TD 1302, September 2016, Slovenia) were used to complement the literature search (AA, ASW).
Study selection
The inclusion and exclusion criteria, pre‐defined in the study protocol, were formulated broadly to include any type of literature with information on NCC and HIV/AIDS without any further limitations (Supplementary File). Included were publications on PLWH/A and NCC. No further restrictions were applied for intervention, comparator or outcome. Studies reporting on individuals with NCC but without HIV/AIDS, or PLWH/A but without NCC, were excluded. Literature was first screened based on title and abstract against the inclusion and exclusion criteria and then based on full text by one experienced reviewer in the field of NCC literature (AA). Two authors (PDJ and KGB) extracted the data onto a purpose‐built, pre‐defined spreadsheet.
Analysis
Data were analysed using Microsoft Excel (version 1906). Figure 2 was produced using R [17]. Calculation of 95% confidence intervals (95% CI) was performed using the Wilson score interval where appropriate [18].
FIGURE 2.
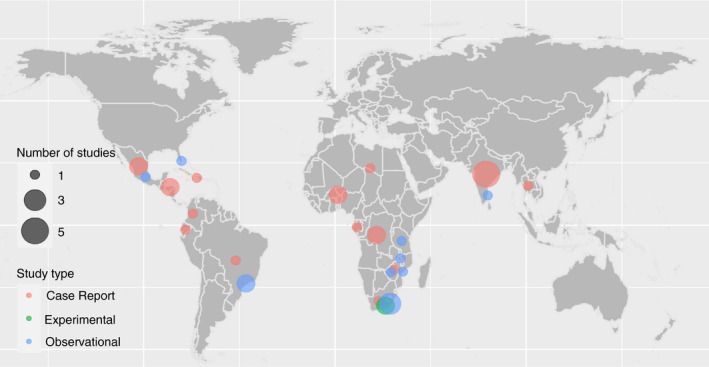
Dot map demonstrating the geographical distribution of included studies. Dots represent geographic locations of study sites. Dot size is proportional to number of studies in a particular area. Dot colour represents the type of study: case series/report, experimental or observational.
RESULTS
Search results
Initially, 16,969 papers were identified by the overall search for NCC literature. After duplicate removal, 11,346 papers remained. An internal search for specific NCC–HIV/AIDS co‐infection identified 494 publications. From the preliminary pilot search, 45 additional records and three additional studies (cross‐referencing and personal communications) were added (Supplementary File). In total, 539 papers entered the screening process and 435 were excluded after screening of title and abstract. The full texts of the remaining 104 studies were assessed for eligibility, and 52 were excluded, mostly because both diseases were mentioned independently. Hence, 52 studies were included in the scoping review. No additional studies were identified after checking against the entire identified NCC literature database. Figure 1 presents the PRISMA [19] flow chart.
FIGURE 1.
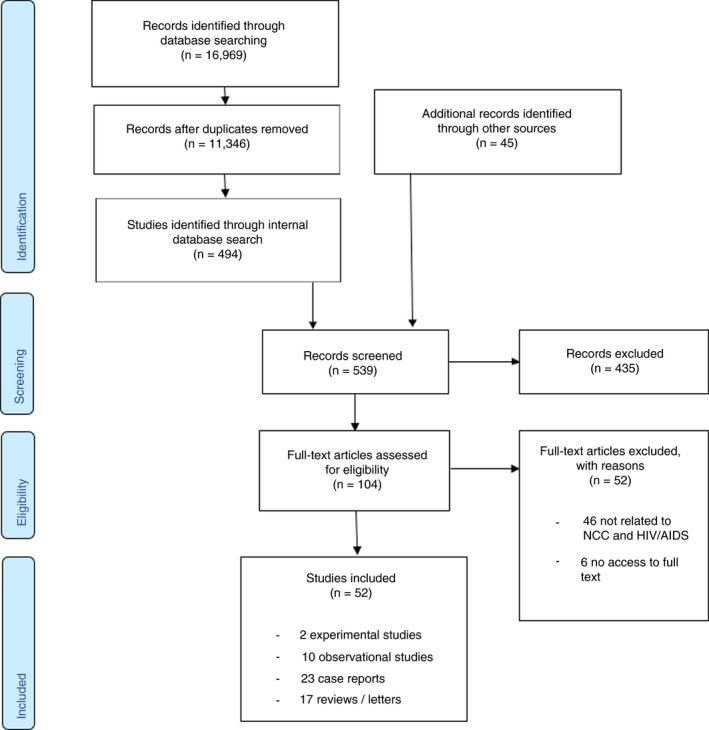
PRISMA flow chart presenting the search for relevant studies
Of the 52 included studies, two experimental studies, ten observational studies (Supplementary File), 23 case series/case reports (Tables 2 and 3) and 17 reviews, letters or interviews relating to the research question were identified (Supplementary File). The geographical distribution of these are displayed in Figure 2.
TABLE 2.
Case reports: signs, symptoms and diagnosis of people living with HIV/AIDS co‐infected with neurocysticercosis
| Case # | Study lead author [Reference a ] | Year | Age (yr) | Sex | Country of origin | CD4 count | Seizures | Headache | Focal neuro.signs | Serology | Histology | Radiology Findings | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Agaba et al. [S14] | 2018 | 49 | M | Namibia | 370 | No | No | No | NA | +ve | +ve | Multiple calcified parenchymal cysts |
| 2 | Anand et al. [S15] | 2015 | 35 | M | India | 530 | Yes | NA | NA | +ve | NA | +ve | Two vesicular parenchymal cysts and calcified cysts |
| 3 | Anand et al. [S15] | 2015 | 40 | M | India | 350 | Yes | NA | Yes | +ve | NA | +ve | Three vesicular parenchymal cysts with one large colloidal cyst |
| 4 | Chianura et al. [S16] | 2006 | 22 | F | Ecuador | 473 | No | Yes | Yes | +ve | NA | +ve | Multiple colloidal parenchymal, intraventricular and subarachnoid cysts |
| 5 | Delobel et al. [S17] | 2003 | 45 | M | Haiti | 351 | No | NA | Yes | +ve | +ve | +ve | Single vesicular parenchymal cyst and spinal epidural racemose cyst |
| 6 | George et al. [S18] | 1999 | 32 | M | India | 23 | No | No | No | NA | NA | −ve | Single subretinal vesicular cyst on direct fundus examination b |
| 7 | Giordani et al. [S19] | 2014 | 24 | M | Burkina Faso | 24 | Yes | NA | NA | −ve | NA | +ve | Two colloidal parenchymal cysts |
| 8 | Gupta et al. [S20] | 2012 | 13 | M | India | 396 | Yes | Yes | NA | +ve | NA | +ve | Multiple vesicular and colloidal parenchymal cysts, with visible scolex |
| 9 | Itani et al. [S21] | 2013 | 39 | F | Thailand | 10 | No | Yes | Yes | −ve | NA | +ve | Multiple vesicular colloidal parenchymal and subarachnoid cysts with visible scolex |
| 10 | Jung et al. [S22] & Lillie et al. [S23] | 2008 | 26 | F | DRC | 378 | Yes | Yes | No | +ve | NA | +ve | Multiple vesicular parenchymal cysts and calcified cysts |
| 11 | Martins et al. [S24] | 2015 | 36 | F | Brazil | NA | Yes | Yes | Yes | NA | NA | +ve | Multiple vesicular and colloidal parenchymal cysts with visible scolex |
| 12 | Millogo et a. [S25] | 2013 | 34 | M | Burkina Faso | NA | Yes | No | No | NA | NA | +ve | Multiple vesicular parenchymal cysts |
| 13 | Motsepe et al. [S26] | 2012 | 46 | F | South Africa | NA | No | NA | Yes | NA | +ve | +ve | Lumbar spinal subarachnoid cysts |
| 14 | Okome‐Nkoumou et al. [S27] | 2010 | 27 | F | Gabon | NA | Yes | Yes | NA | +ve | NA | +ve | Multiple calcified parenchymal cysts |
| 15 | Pandey et al. [S28] | 2005 | 24 | M | India | NA | Yes | NA | NA | NA | NA | +ve | Multiple calcified parenchymal cysts |
| 16 | Prasad et al. [S29] | 2006 | 51 | F | India | 350 | Yes | NA | NA | +ve | +ve | +ve | Multiple colloidal parenchymal cysts with visible scolex |
| 17 | Prasad et al. [S29] | 2006 | 40 | M | Honduras | 32 | Yes | NA | NA | +ve | NA | +ve | Multiple colloidal parenchymal cysts |
| 18 | Prasad et al. [S29] | 2006 | 72 | M | Peru | 105 | No | Yes | Yes | +ve | NA | +ve | Multiple vesicular and colloidal parenchymal cysts with visible scolex |
| 19 | Ramos et al. [S30] | 2007 | 36 | F | Colombia | 13 | No | Yes | NA | +ve | NA | +ve | Multiple colloidal parenchymal cysts with visible scolex |
| 20 | Ruziev et al. [S31] | 2010 | 27 | F | Honduras | 64 | Yes | Yes | NA | NA | NA | +ve | Single vesicular parenchymal cyst with visible scolex |
| 21 | Serpa et al. [S32] | 2007 | 35 | M | Hispanic | 238 | Yes | Yes | Yes | NA | +ve | +ve | Single colloidal parenchymal cyst |
| 22 | Soto‐Hernández et al. [S33] | 1996 | 29 | M | Mexico | 150 | No | Yes | Yes | NA | +ve | +ve | Single giant vesicular parenchymal cyst |
| 23 | Soto‐Hernández et al. [S33] | 1996 | 41 | F | Mexico | NA | NA | Yes | Yes | NA | NA | +ve | Single colloidal parenchymal cyst, and calcified racemose cysticercosis |
| 24 | Taha et al. [S34] | 2013 | 34 | F | DRC | 750 | Yes | Yes | Yes | +ve | NA | +ve | Multiple vesicular and colloidal parenchymal cysts |
| 25 | Thornton et al. [S35] | 1992 | 40 | M | Zimbabwe | NA | Yes | NA | Yes | +ve | NA | +ve | Multiple vesicular parenchymal cysts and racemose cysticercosis |
| 26 | Thornton et al. [S35] | 1992 | 30 | M | Zimbabwe | NA | No | Yes | Yes | +ve | NA | +ve | Multiple vesicular parenchymal cysts |
| 27 | Thornton et al. [S35] | 1992 | 36 | M | Zimbabwe | NA | Yes | NA | Yes | +ve | NA | +ve | Multiple vesicular parenchymal cysts |
| 28 | Thornton et al. [S35] | 1992 | 25 | M | Zimbabwe | NA | Yes | NA | Yes | NA | NA | +ve | Multiple vesicular parenchymal cysts |
| 29 | White et al. [S36] | 1995 | 29 | M | Mexico | NA | NA | Yes | NA | NA | NA | +ve | Multiple vesicular parenchymal cysts with visible scolex |
Abbreviations: DRC, Democratic Republic of Congo; NA, not available.
References as numbered in Supplementary File.
Diagnosis made from direct examination of subretinal cysts, not radiologically.
TABLE 3.
Case reports: treatment and outcomes of people living with HIV/AIDS co‐infected with neurocysticercosis
| Case # | Study lead author [Reference a ] | Year | Age | Sex | Country of origin | Surgery | Anthelmintic, dose, duration (days) | Steroid, dose | Seizures, AED | Antiretroviral therapy | Other therapy | Clinical outcome | Radiological outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Agaba et al. [S14] | 2018 | 49 | M | Namibia | N | ALB | 800 mg/d | 14 | PRED | 30 mg/d | N | N | TDF, FTC, EFV | N | Favourable | Complete resolution |
| 2 | Anand et al. [S15] | 2015 | 35 | M | India | N | ALB | 15/mg/kg/d | 28 | PRED | NK | Y | NK | Y* | N | Favourable | NK |
| 3 | Anand et al. [S15] | 2015 | 40 | M | India | N | ALB | 15/mg/kg/d | 28 | PRED | NK | Y | LEV | Y* | N | Favourable, no further seizures | NK |
| 4 | Chianura et al. [S16] | 2006 | 22 | F | Ecuador | N | ALB | 800 mg/d | 30 | DEX | 8 mg/d | N | PHE | ZDV, 3TC, ABC | N | Favourable | Improvement with calcification of cysts |
| 5 | Delobel et al. [S17] | 2003 | 45 | M | Haiti | Laminectomy and epidural cyst removal | ALB | 800 mg/d | 10 | NK | N | N | ZDV, 3TC, ABC | N | Improved but persistent symptoms | Complete resolution | |
| 6 | George et al. [S18] | 1999 | 32 | M | India | Xenon arc photocoagulation | N | N | N | N | ZDV | Anti‐TB* | Favourable | N/A | |||
| 7 | Giordani et al. [S19] | 2014 | 24 | M | Burkino Faso | N | ALB | 15 mg/kg/d | 14 | DEX | 8 mg/d | Y | NK | NK | Anti‐toxo* | Favourable | Near‐complete resolution |
| 8 | Gupta et al. [S20] | 2012 | 13 | M | India | N | ALB | NK | 14 | Y* | NK | Y | VAL, PHE | NK | N | Favourable | NK |
| 9 | Itani et al. [S21] | 2013 | 39 | F | Thailand | External ventricular drain | ALB | 800 mg/d | NK | PRED | 100 mg/d | N | N | N | N | Died | N/A |
| 10 | Jung et al. [S22] & Lillie et al. [S23] | 2008 | 26 | F | DRC | N | PZQ | 50 mg/kg/d | NK | DEX | NK | Y | Y* | N | AP | Favourable | Improved appearance |
| 11 | Martins et al. [S24] | 2015 | 36 | F | Brazil | N | ALB | 15 mg/kg/d | 8 | NK | Y | PHE | D4T, 3TC, EFV | PYR/SLD | Favourable | NK | |
| 12 | Millogo et a. [S25] | 2013 | 34 | M | Burkina Faso | N | ALB | NK | NK | PRED | NK | Y | CBZ | NK | N | Favourable, no further seizures | NK |
| 13 | Motsepe et al. [S26] | 2012 | 46 | F | South Africa | Laminectomy and epidural cyst removal | ALB | 15 mg/kg/d | NK | DEX | 8 mg/d | N | N | NK | N | Persistent weakness | NK |
| 14 | Okome‐Nkoumou et al. [S27] | 2010 | 27 | F | Gabon | N | ALB | 15 mg/kg/d | NK | PRED | 1 mg/kg/d | Y | PHB | NK | N | Favourable | Complete resolution |
| 15 | Pandey et al. [S28] | 2005 | 24 | M | India | N | PZQ | 50 mg/kg/d | NK | NK | N | N | Y* | AMB | NK | NK | |
| 16 | Prasad et al. [S29] | 2006 | 51 | F | India | N | ALB | NK | NK | Y* | NK | Y | Y* | Y* | Anti‐toxo* | Delayed clinical improvement | Persistent lesions |
| 17 | Prasad et al. [S29] | 2006 | 40 | M | Honduras | N | N | N | Y | Y* | Y* | Anti‐toxo* | Favourable | NK | |||
| 18 | Prasad et al. [S29] | 2006 | 72 | M | Peru | N | ALB | NK | NK | DEX | NK | N | N | N | N | Delayed clinical improvement | NK |
| 19 | Ramos et al. [S30] | 2007 | 36 | F | Colombia | N | ALB | 800 mg/d | NK | DEX | 16 mg/d | N | N | ZDV, 3TC, LOPr | Anti‐toxo* | Died | N/A |
| 20 | Ruziev et al. [S31] | 2010 | 27 | F | Honduras | N | ALB | NK | NK | NK | Y | NK | Y* | Anti‐toxo* GCV | Favourable, no further seizures | NK | |
| 21 | Serpa et al. [S32] | 2007 | 35 | M | Hispanic | Craniotomy and cyst excision | NK | NK | Y | NK | TDF, 3TC, EFV | N | Favourable | NK | |||
| 22 | Soto‐Hernández et al. [S33] | 1996 | 29 | M | Mexico | Craniotomy and cyst excision | ALB | 15 mg/kg/d | NK | NK | N | N | ZDV | N | Favourable | NK | |
| 23 | Soto‐Hernández et al. [S33] | 1996 | 41 | F | Mexico | VP shunt | N | DEX | NK | N | N | NK | PYR, SXT | Favourable | NK | ||
| 24 | Taha et al. [S34] | 2013 | 34 | F | DRC | N | ALB | 15 mg/kg/d | 15 | DEX | 6 mg/d | Y | PHE, CBZ | TDF, FTC, ATV/r | N | Favourable | Complete resolution |
| 25 | Thornton et al. [S35] | 1992 | 40 | M | Zimbabwe | N | ALB | NK | 14 | Y* | NK | Y | N | NK | N | Slight improvement | NK |
| 26 | Thornton et al. [S35] | 1992 | 30 | M | Zimbabwe | N | PZQ | NK | 14 | Y* | NK | N | N | NK | N | No improvement | NK |
| 27 | Thornton et al. [S35] | 1992 | 36 | M | Zimbabwe | N | PZQ | NK | NK | NK | Y | PHE | NK | N | Persistent seizures | NK | |
| 28 | Thornton et al. [S35] | 1992 | 25 | M | Zimbabwe | N | NK | NK | Y | NK | NK | N | Died | N/A | |||
| 29 | White et al. [S36] | 1995 | 29 | M | Mexico | N | N | NK | N | N | NK | AMB | Favourable | NK | |||
Abbreviations: 3TC, lamivudine; ABC, abacavir; AED, anti‐epileptic drug; ALB, albendazole; AMB, amphotericin B; AP, antipsychotic; ATZ/r, atazanavir and ritonavir; CBZ, carbamazepine; d, day; DEX, dexamethasone; DRC, Democratic Republic of Congo; EFV, efavirenz; FTC, emtricitabine; GCV, ganciclovir; LEV, levetiracetam; LOP/r, lopinavir and ritonavir; N, No/none; N/A, not applicable; NK, not known or not specified; PHB, phenobarbital; PHE, phenytoin; PRED, prednisolone; PYR, pyrimethamine; PZQ, Praziquantel; SLD, sulfadiazine; SXT, trimethoprim/sulfamethoxazole;TB, tuberculosis; TDF, tenofovir; VAL, sodium valproate; VP, ventriculoperitoneal; Y, Yes; ZDV, zidovudine.
References as numbered in Supplementary File.
Agent not specified.
Epidemiology, diagnostics and clinical features
In the following, references starting with S are in the Supplementary File. The prevalence of NCC in general populations of PLWH/A, derived from five studies, ranged from 1.1% to 10.2% (Table 1 [S5,S9,S10,S11,S13]). One matched cross‐sectional study in Tanzania, of high methodological quality, demonstrated no significant difference between PLWH/A (n = 170) and their HIV‐negative controls (n = 170) regarding cysticercosis (CC) antibody (Ab) and antigen (Ag) seroprevalence (4/170 = 2.4% (95% CI = 0.6–5.9) CC‐Ab positive in both groups, by electroimmunotransfer blot (EITB); and 1/170 = 0.6% (95% CI = 0.4–2.9) CC‐Ag positive in PLWH/A only, by monoclonal Ab (B158/B60 Ab‐based capture ELISA) and NCC diagnosis based on computed tomography (CT) scan results (3/170 = 1.8% (95% CI = 0.6–5.1) in PLWH/A; CT scans were not performed routinely in HIV‐negative controls due to ethical reasons)[S13]. A study in Beira, Mozambique demonstrated an overall seroprevalence of CC‐Ab of 10.2% (61/601, 95% CI = 8–12.8, by Western blot Immunoglobulin G[IgG] test) in PLWH/A, with neither CD4 count nor ART significantly associated with seroprevalence[S10]. A study in Puducherry, India, reported a seroprevalence of 4% (4/100, 95% CI = 2–10, by Ab‐ELISA test) in PLWH/A, which was lower (albeit not statistically significantly) than 6.1% (88/1442, 95% CI = 5–7.5, by Ab indirect haemagglutination (IHA) test) in a general population (HIV status not specified) in the same area [20][S11]. This should be interpreted with caution given differing study protocols and serological tests used. An autopsy study in Mexico reported a 1.1% (1/94; 95% CI = 0.2–5.8%) prevalence of NCC at autopsy in PLWH/A, not significantly lower than 2.4% (8/335; 95% CI = 1.2–4.6%) in matched HIV‐negative controls; a similar prevalence of NCC in PLWH/A at autopsy of 1.9% (1/52; 95% CI = 0.3–10.1%) was found in a USA study (Table 1 [S5,S9]). The prevalence of NCC based on CT scan results in PLWH/A presenting with specific neurological symptoms (as specified in Table 1) derived from two studies, ranged from 4.2% (2/47, 95% CI = 0.4–11.1) to 34.8% (8/23, 95% CI = 18.8–55.1) and was higher than in their respective HIV‐negative controls with 0% (0/51, 95% CI = 0.0–7.0) and 21.2% (7/33, 95% CI = 10.7–37.8), respectively (Table 1 [S2,S6]).
TABLE 1.
Prevalence of neurocysticercosis in people living with HIV/AIDS and HIV‐negative controls
| Study lead author (Year) [Ref] a | Location, Country | Study participant recruitment | Neurol. Symp‐toms | Sample size (HIV+; HIV−) | Mean age (yr) (HIV+; HIV−) | Female % (HIV+; HIV−) | Measure of NCC prevalence |
Prevalence in HIV+population (%) (95% CI) |
Prevalence in HIV− population (%) (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Benner et al. (2011) [S2] | Eastern Cape, South Africa | Patients with recent onset epileptic seizures or severe chronic progressive headaches consecutively sampled at routine neurology clinic. | Yes | 23; 33 | NA | NA | CT appearance | 34.8 (18.8–55.1) | 21.2 (10.7–37.8) |
|
RR = 1.64 (0.69–3.89) OR = 2.0 (0.59–6.5) | |||||||||
| Jessurun et al. (1992) [S5] | Mexico City, Mexico | All autopsy records from one centre over a 20‐year period analysed. | No | 94; 333 | 31.4; 31.2 | 12.7; 15.3 | Autopsy result | 1.1 (0.2–5.8) | 2.4 (1.2–4.6) |
|
RR = 0.4 (0.05–3.5) OR = 0.4 (0.04–4.0) | |||||||||
| Kumwenda et al. (2005) [S6] | Blantyre, Malawi | All in‐ and outpatients presenting with acute onset of a central neurological deficit included. | Yes | 47; 51 | 37.5; 58.6 | 59.6; 41.2 | CT appearance | 4.2 (0.4–11.1) | 0 (0–7.0) |
| Moskowitz et al. (1984) [S9] | Florida, USA | All autopsy records of PLWH/A from one centre analysed. | No | 52; NA | NA | NA | Autopsy result | 1.9 (0.3–10.1) | NA |
| Noormahomed et al. (2014) [S10] | Beira, Mozambique | Systematic invitation and voluntary participation of PLWH/A from HIV clinic. Symptoms not specified. | No | 601; NA | 39.7 | 62.9 | Ab‐EITB (against T. solium larval stage Ag) | 10.2 (8–12.8) | NA |
| Parija et al. (2009) [S11] | Puducherry, India | Details not specified – samples taken from PLWH/A from outpatient HIV clinic. | No | 100; (1442 b ) | NA | NA | Ab‐ELISA (against T. solium larval stage Ag) | 4 (1.6–9.8) | (6.1 b ) |
| Ab‐EITB (against T. solium larval stage Ag) | 2 (0.6–7) | ||||||||
| Schmidt et al. (2016) [S13] | Manyara region, Tanzania | PLWH/A recruited from HIV clinic. Controls matched for gender, age and village. | No | 170; 170 | 39; 40 c | 50; 50 | Ab‐EITB (LLGP‐EITB and rT24H‐EITB)d | 2.4 (0.6–5.9) | 2.4 (0.6–5.9) |
|
RR = 1 (0.3–3.9) OR = 1 (0.2–4.0) | |||||||||
| Ag‐ELISA | 0.6 (0.4–2.9) | 0 | |||||||
Abbreviations: Ab, antibody; Ag, antigen; CC, cysticercosis; CT, computer tomography; EITB, enzyme‐linked immunoelectrotransfer blot; ELISA, enzyme‐linked immunosorbent assay; HIV−, HIV‐negative controls; HIV+, HIV‐positive individuals or PLWH/A; NA, not available; OR, odds ratio; PLWH/A, people living with HIV/AIDs; RR, risk ratio.
References as numbered in Supplementary File.
6.1% prevalence from different study in Puducherry using an IHA assay [21].
Median age quoted. Both groups were matched for gender, age and residence.
Twenty‐two case series and case reports of PLWH/A co‐infected with NCC were identified, which included a total of 29 cases (Table 2). Eleven cases (38%) were from Latin America, eleven (38%) from Africa and seven (24%) from Asia (Figure 2). Seventeen (59%) individuals suffered from epileptic seizures, 15 (52%) from headaches, 15 (52%) from a focal neurological deficit, nine (31%) of which were hemiparesis and five (17%) had signs/symptoms of raised intracranial pressure. HIV and NCC were reported as being diagnosed at the same time or during the same presentation in 11/29 (38%) of cases. Nine cases (31%) had pre‐existing HIV diagnosis, with a median time between HIV and NCC diagnoses of 14 months. Median CD4 count at time of NCC presentation was 294 (range = 10–750).
Fifteen (of 17 with available results, 88%) individuals had positive T. solium serology: seven were Ab‐ELISA positive (two IgM, one IgG, four not specified), six Ab‐EITB positive, and for two, the test was not specified. Two cases were found to have negative Ab serology for NCC, and diagnosis was confirmed by a visible scolex on brain imaging[S19,S21]. In both individuals, serology was positive for toxoplasmosis. All but one case (28/29, 97%), of subretinal cysticercosis (cysts visible on direct fundus examination), had positive imaging for NCC in the form of CT or magnetic resonance imaging (MRI). Eighteen individuals (62%) had viable vesicular cysts; thirteen (45%) had colloidal cysts, six (21%) had calcified cysts and three (10%) presented racemose cysts. The vast majority had intraparenchymal cysts (26/29, 90%), six (21%) had extra‐parenchymal cysts, two had (7%) spinal cysts[S17,S26], one had subretinal cysticercosis[S18] and one had subcutaneous nodules (histologically confirmed)[S36] (Table 2).
Treatment
The two experimental studies identified were both performed in South Africa and not further considered for this review, due to methodological quality and incomplete data[S3,S4]. Of the 29 PLWH/A summarised in Table 3, seven were already receiving ART at the time of NCC presentation and diagnosis. In four of these cases, patients presented with new‐onset seizures 9–12 months after commencing ART[S15,S21,S24]. In a further case, a patient later deteriorated after commencing treatment with ART plus albendazole [21]. Four cases (14%) received surgery plus albendazole; two (7%) received surgery alone; 15 (52%) received albendazole; four (14%) received praziquantel, and four (14%) did not receive either surgery or anthelmintics (Table 3). Eighteen patients were reported to receive adjuvant steroid therapy (eight dexamethasone, six prednisolone, four not specified). The most commonly used regimen was albendazole 15 mg/kg body weight/day for 14‒28 days, with an adjuvant corticosteroid. Of 19 cases where ART is mentioned, 17 (89%) were taking ART or commenced on ART. Nine of 14 (64%) patients with epileptic seizures received an AED, in the remaining five cases it was not specified, and in one case, a patient without epileptic seizures was given an AED prophylactically.
Eleven of 15 (73%) PLWH/A receiving albendazole and not requiring surgery were reported to have had a favourable response to treatment with symptom resolution (Table 4). Of the seven with available follow‐up imaging (all of whom had received albendazole), complete resolution of cysts was demonstrated in four cases (57%), partial resolution in two (29%) and persistent cysts in one (14%). In one case receiving albendazole and steroids (no details on dosage given), the patient had worsening left‐sided weakness at two‐month follow‐up. Symptoms later resolved after commencing ART[S29]. Similarly, in another case in the same case series, commencing albendazole and dexamethasone resulted in a transient worsening of mental status and ataxia, which later improved by day 12[S29]. One patient treated with albendazole (400 mg twice a day), plus adjuvant dexamethasone and ART commenced at the same time (zidovudine, lamivudine, lopinavir) deteriorated at day 14 after an initial improvement, with reduced level of consciousness, subsequently developing an aspiration pneumonia and later dying in the intensive care unit. Unfortunately, no information is provided as to whether repeat imaging was performed[S30]. One patient with subarachnoid cysticerci with hydrocephalus, treated with albendazole 400 mg twice daily and adjuvant prednisolone 100 mg daily, was readmitted, three days after self‐discharging against medical advice, with progression of hydrocephalus and subsequently died[S21]. Of the three patients receiving praziquantel, one, undergoing a 14‐day course with steroids, was reported to have no response to treatment, and one had persistent seizures at six‐month follow‐up (dose, duration and adjuvant treatment not specified). One patient died of complications of thrombocytopenia shortly after admission, with no detail of any treatment received[S35].
TABLE 4.
Case reports: summary table of treatment, additional medications and adverse outcomes of people living with HIV/AIDS co‐infected with neurocysticercosis
| Treatment | Number of cases | Adjuvant steroid | Anti‐epileptic therapy | Antiretroviral therapy | Favourable outcome | Adverse outcomes |
|---|---|---|---|---|---|---|
| Albendazole | 15 / 29 (52%) | 13 / 15 (87%) | 8 / 15 (53%) | 9 / 15 (60%) | 11 / 15 (73%) |
1 (7%) died 3 (20%) delayed or slight clinical response |
| Praziquantel | 4/ 29 (14%) | 2 / 4 (50%) | 2 / 4 (50%) | 1 / 4 (25%) | 2 / 4 (50%) | 2 (50%) persistent or no improvement in symptoms |
| Surgery +Albendazole | 4 / 29 (15%) | 0 | 0 | 0 | 1 / 4 (25%) |
1 (25%) died 2 (50%) persistent symptoms |
| Surgery alone | 3 / 29 (10%) | N/A | 0 | 2 / 3 (67%) | 3 / 3 (100%) | Nil reported |
| None / not specified | 3 / 29 (10%) | N/A | 1 / 3 (33%) | 1 / 3 (33%) | 2 / 3 (67%) | 1 (33%) died |
Abbreviation: N/A, not applicable.
Seven patients (24%) were treated surgically, four with additional albendazole, including two craniotomies with cyst excision, two laminectomies with removal of spinal cysts, one ventriculoperitoneal (VP) shunt insertion, one external ventricular drain and one case of laser photocoagulation. The two patients with spinal cysticercosis had persistent neurological deficits (hemiparesis and bladder dysfunction). Two of the remaining three patients treated surgically were reported to have had a good outcome on discharge, although no follow‐up data were available. One patient received xenon arc photocoagulation for subretinal cysticercosis to good effect with complete resolution on repeat fundoscopy at two‐week follow‐up (Table 4).
DISCUSSION
This scoping review describes the current range of clinical research in the area of HIV‐NCC co‐infection. The currently available published evidence and data, as highlighted here, are insufficient to allow a rigorous meta‐analysis or undertake a full systematic review. This study complements and expands on a recently published review into HIV and NCC co‐infection [16], through an additional focus on treatment. We identify a pressing need for high‐quality clinical research into the treatment and management of this patient group.
The seroprevalence of NCC has been shown to be similar in general populations of PLWH/A and their HIV‐negative controls, with no statistically significant association with CD4 count, in keeping with other HIV‐helminth co‐infection studies [10, 11]. The reported lower prevalence of NCC in PLWH/A compared with their HIV‐negative controls documented in other studies [20, 22] may be due to impaired CC serodiagnosis due to immunosuppression. Supporting this, negative CC serology was described in two HIV‐NCC co‐infection case reports [unspecified [23] and immunoblot, LDBio [24]], both of which had CD4 counts <50 and NCC diagnosis confirmed radiologically with vesicular and colloidal vesicular cysticerci with visible scoleces [23, 24]. Mason et al. 1992 also described false negative CC serological testing in PLWH/A [25]. Furthermore, the prevalence of NCC by CT radiological diagnosis was higher for PLWH/A presenting with neurological symptoms compared with HIV‐negative controls, further alluding to potentially impaired NCC serodiagnosis in this clinical sub‐group. However, the differences were not statistically significant [26].
Regarding the effect of HIV infection on clinical presentation, in case reports (albeit with very small sample sizes), patients with active stages of the disease with lower CD4 counts were less likely to suffer from epileptic seizures (33%) compared to those with higher CD4 counts (75%), and those reported in studies of HIV‐negative individuals (63%) [1, 3]. This suggests that immunosuppression may alter disease presentation, although this cannot be concluded from case reports alone. Of note, focal neurological deficits, particularly hemiparesis, were described in 52% (and 31% respectively) of PLWH/A co‐infected with NCC, compared with much lower rates described in HIV‐negative studies (11.8% [3]), which again may indicate an association between HIV infection and more atypical NCC presentation. This is consistent with another study, demonstrating higher NCC prevalence in PLWH/A presenting with an acute focal neurological deficit compared with HIV‐negative controls [27]. A study by Carabin et al. reports similar frequency of epileptic seizures (56%) and headaches (52%) compared with HIV‐negative populations [3], which differs slightly from the conclusions by Herrera et al. [16], which may be explained by reporting of single (as opposed to multiple) neurological signs/symptoms. It is important to note that due to the nature of our study, especially when referring to data from case series, differences may also be explained by confounding variables such as differences in performed investigations, especially neuroimaging and intervals of follow‐up, which we were unable to control for.
Regarding radiological findings, including the stage and location of cysts, we found a greater frequency of active cysts (59% vesicular and 48% colloidal) compared with studies in HIV‐negative populations (36% vesicular and 30% colloidal [28]), and similarly fewer cases with inactive calcified cysts (19% vs 64% [28]). This raises a question of whether HIV‐related immunodeficiency delays the degradation and inactivation of cysts. Similarly to Herrera et al. [16], we found an increased frequency of spinal cysts and giant racemose cysts compared with HIV‐negative populations [28].
The majority of patients with HIV‐NCC co‐infection described in case reports responded well to medical treatment, of which albendazole plus corticosteroid therapy was the most commonly used and effective regimen. The importance of appropriate management of raised intracranial pressure needs to be emphasised and warrants different therapeutic approaches based on the underlying cause, that is corticosteroid therapy for cysticercal encephalitis versus an endoscopic approach or shunt for obstructive hydrocephalus [2]. Poor outcomes have been reported when managing intraventricular NCC with VP shunt insertion in PLWH/A [29], which may indicate a role for minimally invasive endoscopic surgery [30], although this is not readily available in resource‐poor settings. Although a high proportion of case reports of HIV‐NCC co‐infection described multiple viable cysts, none were treated with albendazole plus praziquantel combination therapy, which has shown better cyst resolution with a similar side‐effect profile compared with mono‐anthelmintic therapy [30].
This review highlights that IRIS may develop in patients with HIV‐NCC co‐infection after commencing ART. In four cases, patients developed new‐onset seizures nine to 12 months after commencing ART and were subsequently diagnosed with NCC [14, 31, 32], although the time intervals may be too long to be considered IRIS. In a further case, a patient presenting with headache and a CD4 count of 13, simultaneously diagnosed with intraparenchymal NCC and HIV, after an initial improvement, went on to deteriorate with reduced level of consciousness and died in intensive care after treatment with corticosteroids, albendazole and ART had been commenced [21]. Although we have neither immunological nor imaging confirmation of a potential IRIS diagnosis, the possibility cannot be ruled out either. Therefore, the potential for an IRIS‐type reaction should be considered when commencing patients on ART.
Patients with HIV‐NCC co‐infection may require treatment with three different drug classes: ART, anthelmintics and AEDs, yet little is known about risks and drug interactions when used simultaneously in clinical practice. The interaction between drugs used against HIV infection and epilepsy is well described [33]. ART and AEDs have both been demonstrated to decrease the level of anthelmintics. Ritonavir has been demonstrated to decrease plasma concentrations of albendazole due to cytochrome P450 (CYP) induction [34]. Furthermore, first‐generation AEDs can also decrease concentrations of albendazole and praziquantel through CYP induction [35, 36]. However, to the best of our knowledge, a study investigating whether a combination of antiretrovirals and AEDs decreases anthelmintic levels under their therapeutic concentration has yet to be performed and would be necessary to determine whether these drug interactions are clinically relevant. Finally, although anthelmintics are generally considered safe with most antiretrovirals, one case report has described a possible interaction between zidovudine and albendazole resulting in bone marrow suppression [37].
Looking beyond PLWH/A, the behaviour of NCC in other immunosuppressive states is also poorly understood and no formal studies have been conducted. One case report describes an atypical presentation with widespread cardiopulmonary cysticercosis without much local inflammatory reaction and widespread calcifications in the brain on autopsy in a patient with leukaemia [38]. Another case report reviews a patient with occurrence of active NCC with epileptic seizures one month after liver transplant and recurrence of active NCC with loss of consciousness four months after anthelmintic treatment [39]. There was also a report of a meningoencephalitic presentation in a patient starting two weeks after renal transplantation with diffuse neurological signs/symptoms and personality change on clinical examination, later developing neck stiffness and polymorphonuclear pleocytosis on cerebrospinal fluid examination [40]. In addition, we found two cases with active symptomatic NCC with epileptic seizures in another liver transplant patient [41] and a haematopoietic stem cell transplant leukaemia patient [42], four and a half years and two weeks after transplantation, respectively. All four NCC patients showed multiple intraparenchymal cysts with neuroinflammation, for example perilesional oedema was seen on neuroimaging in three cases. Neurological signs/symptoms subsided under anthelmintic therapy in all four patients and cysts regressed or disappeared completely. However, the exact relationship between the exacerbation of symptomatic NCC and the immunosuppressive therapy could not be ascertained, as information on type of medication, dosage and potential change in dosage was not provided consistently.
CONCLUSIONS AND RECOMMENDATIONS
In neurologically asymptomatic PLWH/A, there seems to be no indication of a higher NCC seroprevalence compared to HIV‐negative controls. Conversely, in PLWH/A that present with neurological signs/symptoms, a higher NCC prevalence, although not statistically significant, has been identified. There is some suggestion that clinical and radiological presentations of NCC may be different in PLWH/A compared with their HIV‐negative controls with a higher proportion of symptomatic, multi‐cystic disease. Although current evidence to guide treatment of PLWH/A and NCC co‐infection is lacking, based on our findings and available literature, for patients with simultaneous diagnosis of HIV and NCC an appropriate approach may be to treat NCC as per current guidelines prior to commencing ART, to minimise potential risk of IRIS [30]. Furthermore, for patients from an NCC‐endemic area with a new diagnosis of HIV, clinicians could consider performing CC serology prior to commencing ART, although negative serological results may be unreliable in cases of low CD4 count. Drug interactions between antiretrovirals, anthelmintics and AEDs are not fully understood but should be considered. Overall, at present, there is insufficient evidence to definitively recognise differences in incidence, presentation, treatment or outcomes of NCC in those with or without HIV infection. There is a pressing need for high‐quality studies in PLWH/A co‐infected with NCC, to appropriately inform diagnostic and management guidelines for this patient group.
DISCLAIMER
The views, opinions, assumptions or any other information set out in this article are solely those of the authors and should not be attributed to the funders or any person connected with the funders. The funders had no role in writing the manuscript or in the decision to submit it for publication.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The literature search was part of a wider systematic literature review for the development of WHO guidelines for the management of NCC. In this context, we want to acknowledge the help and contribution of Thomas Allen, librarian; Bernadette Abela‐Ridder, Department of Control of Neglected Tropical Diseases, and Tarun Dua, Department of Mental Health and Substance Abuse, all at WHO headquarters in Geneva. Additionally, publications were shared by Agnès Fleury, whom we want to thank for this.
Jewell PD, Abraham A, Schmidt V, Buell KG, Bustos JA, Garcia HH, et al. Neurocysticercosis and HIV/AIDS co‐infection: A scoping review. Trop Med Int Health. 2021;26:1140–1152. 10.1111/tmi.13652
Sustainable Development Goal: Good health and well‐being
Jewell and Abraham contributed equally to this work.
Basáñez and Winkler are equal senior authors
Funding information
P.D.J. and K.G.B. were funded as part of their Foundation Year 2 (FY2) postgraduate medical training programme, and M.A.D. by the UK Medical Research Council (MRC) doctoral training (non‐clinical) programme at the Faculty of Medicine, Imperial College London. This work was also supported by the German Federal Ministry of Education and Research (BMBF) [CYSTINET‐Africa 01KA1618; V.S., B.J.N., A.S.W.]. A.A. was an unfunded member of CYSTINET‐Africa and contributed with methodological and content‐specific input. M.G.B. acknowledges funding from the MRC Centre for Global Infectious Disease Analysis [grant No. MR/R015600/1], jointly funded by the UK MRC and the UK Foreign, Commonwealth & Development Office (FCDO), under the MRC/FCDO Concordat agreement, and is also part of the European and Developing Countries Clinical Trials Partnership (EDCTP2) programme supported by the European Union. The funders had no influence on study design, collection, analysis and interpretation of the data or the decision to submit the paper for publication. Open Access funding enabled and organized by Projekt DEAL.
REFERENCES
- 1. Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. 2014;13(12):1202–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Winkler AS & Richter H. Landscape analysis: management of neurocysticercosis with an emphasis on low‐ and middle‐income countries. Geneva: World Health Organization; 2015 [62 pp.]. Available from: http://apps.who.int/iris/bitstream/10665/152896/1/WHO_HTM_NTD_NZD_2015.05_eng.pdf.
- 3. Carabin H, Ndimubanzi PC, Budke CM, Nguyen H, Qian Y, Cowan LD, et al. Clinical manifestations associated with neurocysticercosis: a systematic review. PLoS Negl Trop Dis. 2011;5(5):e1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E, et al. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010;4(11):e870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Food and Agriculture Organization/World Health Organization . Multicriteria‐based ranking for risk management of food‐borne parasites. Available from: https://apps.who.int/iris/bitstream/handle/10665/112672/9789241564700_eng.pdf;jsessionid=612553E8EADFF9B1BF984E7DD44EE334?sequence=1.
- 6. GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990‐2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . 10 facts about neurocysticercosis: Neurocysticercosis is prevalent mainly in developing countries [updated Updated April 2017. Available from: https://www.who.int/features/factfiles/neurocysticercosis/en/.
- 8. Alemu A, Shiferaw Y, Addis Z, Mathewos B, Birhan W. Effect of malaria on HIV/AIDS transmission and progression. Parasit Vectors. 2013;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, et al. Prevalence and burden of HCV co‐infection in people living with HIV: a global systematic review and meta‐analysis. Lancet Infect Dis. 2016;16(7):797–808. [DOI] [PubMed] [Google Scholar]
- 10. Simon GG. Impacts of neglected tropical disease on incidence and progression of HIV/AIDS, tuberculosis, and malaria: scientific links. Int J Infect Dis. 2016;42:54–7. [DOI] [PubMed] [Google Scholar]
- 11. Secor WE. The effects of schistosomiasis on HIV/AIDS infection, progression and transmission. Curr Opin HIV AIDS. 2012;7(3):254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abba K, Ramaratnam S, Ranganathan LN. Anthelmintics for people with neurocysticercosis. Cochrane Database Syst Rev. 2010(3):CD000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmidt V, Kositz C, Herbinger KH, Carabin H, Ngowi B, Naman E, et al. Association between Taenia solium infection and HIV/AIDS in northern Tanzania: a matched cross sectional‐study. Infect Dis Poverty. 2016;5(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Serpa JA, Moran A, Goodman JC, Giordano TP, White AC. Neurocysticercosis in the HIV era: a case report and review of the literature. Am J Trop Med Hygiene. 2007;77(1):113–7. [PubMed] [Google Scholar]
- 15. Epilepsy and HIV ‐ a dangerous combination. Lancet Neurol. 2007;6(9):747. [DOI] [PubMed] [Google Scholar]
- 16. Herrera Vazquez O, Romo ML, Fleury A. Neurocysticercosis and HIV Infection: what can we learn from the published literature? Arq Neuropsiquiatr. 2019;77(5):357–65. [DOI] [PubMed] [Google Scholar]
- 17. RStudio Team . RStudio: Integrated Development for R Boston, MA RStudio, PBC; 2020. [updated 2020. Available from: https://rstudio.com/.
- 18. Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16(2):101–117. https://www.jstor.org/stable/2676784. [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parija SC, Sahu PS. A serological study of human cysticercosis in Pondicherry. South India. J Commun Dis. 2003;35(4):283–9. [PubMed] [Google Scholar]
- 21. Ramos JM, Masia M, Padilla S, Bernal E, Martin‐Hidalgo A, Gutiérrez F. Fatal infection due to larval cysts of cestodes (neurocysticercosis and hydatid disease) in human immunodeficiency virus (HIV) infected patients in Spain: report of two cases. Scand J Infect Dis. 2007;39(8):719–23. [DOI] [PubMed] [Google Scholar]
- 22. Parija SC, Gireesh AR. A serological study of cysticercosis in patients with HIV. Rev Inst Med Trop Sao Paulo. 2009;51(4):185–9. [DOI] [PubMed] [Google Scholar]
- 23. Itani MM, Jørgensen GM. Cerebral cysticerc is a rare cause of hydrocephalus. Ugeskr Laeger. 2013;175(23):1651–2. [PubMed] [Google Scholar]
- 24. Giordani MT, Tamarozzi F, Cattaneo F, Brunetti E. Three cases of imported neurocysticercosis in Northern Italy. J Travel Med. 2014;21(1):17–23. [DOI] [PubMed] [Google Scholar]
- 25. Mason P, Houston S, Gwanzura L. Neurocysticercosis: experience with diagnosis by ELISA serology and computerised tomography in Zimbabwe. Central Afr J Med. 1992;38(4):149–54. [PubMed] [Google Scholar]
- 26. Benner CT, Carabin H, Foyaca‐Sibat H, De Fatima I‐V, Cowan LD, Korsman S, et al. Association between HIV infection and the proportion of NCC lesions among patients with neurological disorders in the Eastern Cape Province, South Africa. Am J Trop Med Hyg. 2011;85(6 SUPPL. 1):29–30. [Google Scholar]
- 27. Kumwenda JJ, Mateyu G, Kampondeni S, van Dam AP, van Lieshout L, Zijlstra EE. Differential diagnosis of stroke in a setting of high HIV prevalence in Blantyre, Malawi. Stroke; a journal of cerebral circulation. 2005;36(5):960–4. [DOI] [PubMed] [Google Scholar]
- 28. Marcin Sierra M, Arroyo M, Cadena Torres M, Ramírez Cruz N, García Hernández F, Taboada D, et al. Extraparenchymal neurocysticercosis: Demographic, clinicoradiological, and inflammatory features. PLoS Negl Trop Dis. 2017;11(6):e0005646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Foyaca‐Sibat H, Ibanez‐Valdes L. Intraventricular neurocysticercosis in HIV positive patients. Internet J Neurol. 2003;2:23–31. [Google Scholar]
- 30. White AC, Coyle CM, Rajshekhar V, Singh G, Hauser WA, Mohanty A, et al. Diagnosis and treatment of neurocysticercosis: 2017 Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am J Trop Med Hyg. 2018;98(4):945–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anand KS, Wadhwa A, Garg J, Mahajan RK. HIV‐Associated Neurocysticercosis. J Int Assoc Providers of AIDS Care. 2015;14(2):120–2. [DOI] [PubMed] [Google Scholar]
- 32. Martins JCM, Cruzeiro MM, Pires LA. Neurotoxoplasmosis and neurocysticercosis in patient with AIDS ‐ case report. Revista Neurociencias. 2015;23(3):443–50. [Google Scholar]
- 33. Zaccara G. Neurological comorbidity and epilepsy: implications for treatment. Acta Neurol Scand. 2009;120(1):1–15. [DOI] [PubMed] [Google Scholar]
- 34. Corti N, Heck A, Rentsch K, Zingg W, Jetter A, Stieger B, et al. Effect of ritonavir on the pharmacokinetics of the benzimidazoles albendazole and mebendazole: an interaction study in healthy volunteers. Eur J Clin Pharmacol. 2009;65(10):999–1006. [DOI] [PubMed] [Google Scholar]
- 35. Lanchote VL, Garcia FS, Dreossi SA, Takayanagui OM. Pharmacokinetic interaction between albendazole sulfoxide enantiomers and antiepileptic drugs in patients with neurocysticercosis. Ther Drug Monit. 2002;24(3):338–45. [DOI] [PubMed] [Google Scholar]
- 36. Bittencourt PR, Gracia CM, Martins R, Fernandes AG, Diekmann HW, Jung W. Phenytoin and carbamazepine decreased oral bioavailability of praziquantel. Neurology. 1992;42(3 Pt 1):492–6. [DOI] [PubMed] [Google Scholar]
- 37. Zingg W, Renner‐Schneiter EC, Pauli‐Magnus C, Renner EL, van Overbeck J, Schlapfer E, et al. Alveolar echinococcosis of the liver in an adult with human immunodeficiency virus type‐1 infection. Infection. 2004;32(5):299–302. [DOI] [PubMed] [Google Scholar]
- 38. Mauad T, Battlehner CN, Bedrikow CL, Capelozzi VL, Saldiva PHN. Case report: massive cardiopulmonary cysticercosis in a leukemic patient. Pathol Res Pract. 1997;193(7):527–9. [DOI] [PubMed] [Google Scholar]
- 39. Hoare M, Gelson WT, Antoun N, Alexander GJ. Early recurrence of neurocysticercosis after orthotopic liver transplant. Liver Transpl. 2006;12(3):490–1. [DOI] [PubMed] [Google Scholar]
- 40. Gordillo‐Paniagua G, Muñoz‐Arizpe R, Ponsa‐Molina R. Unusual complication in a patient with renal transplantation cerebral cysticercosis. Nephron. 1987;45(1):65–7. [DOI] [PubMed] [Google Scholar]
- 41. Valencia VB, Elola‐Olaso AM, Suárez YF, Díaz JM, de los Galanes SJ , Saborido BP, et al. editors. Second case of neurocysticercosis in a patient with liver transplantation (first case in Spain): a case report. Transplantation proceedings; 2007: Elsevier. [DOI] [PubMed]
- 42. Illade L, Molina Angulo B, Solís I, Diaz MÁ, Gonzalez‐Vicent M. Neurocysticercosis: an unusual seizure etiology in a hematopoietic stem cell transplanted patient. Pediatr Hematol Oncol. 2018;35(1):20–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


