Abstract
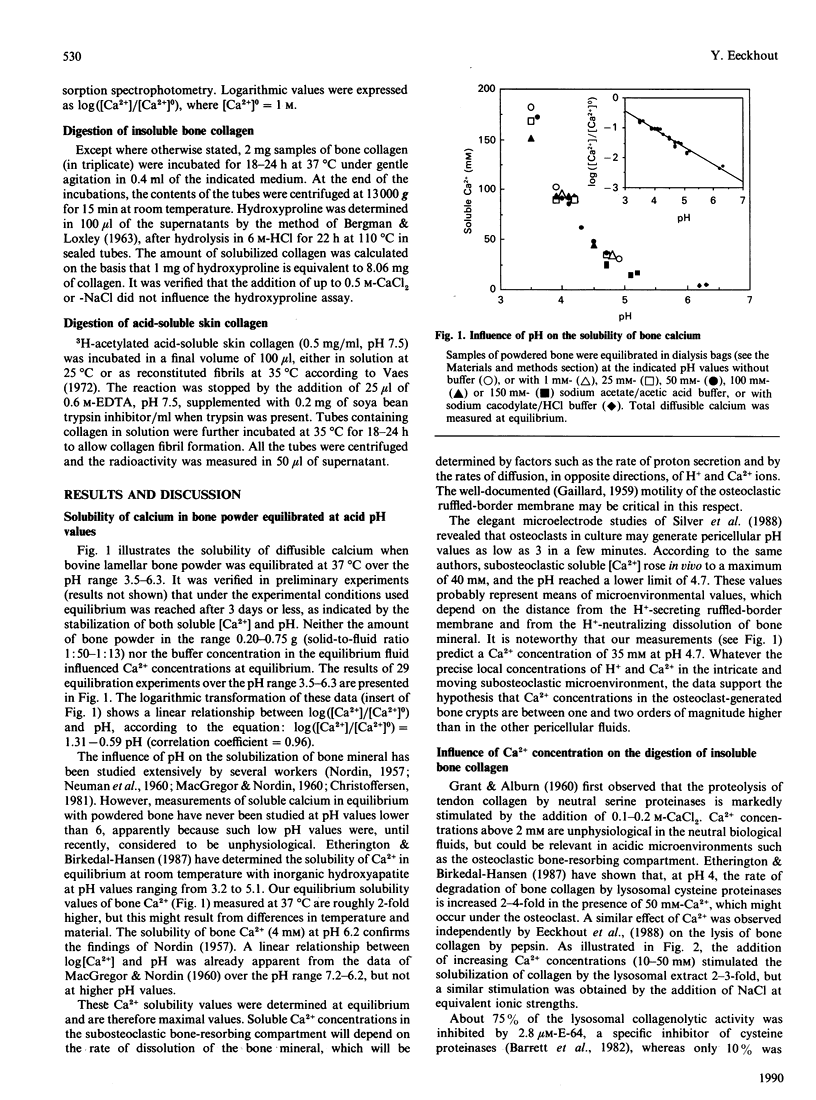
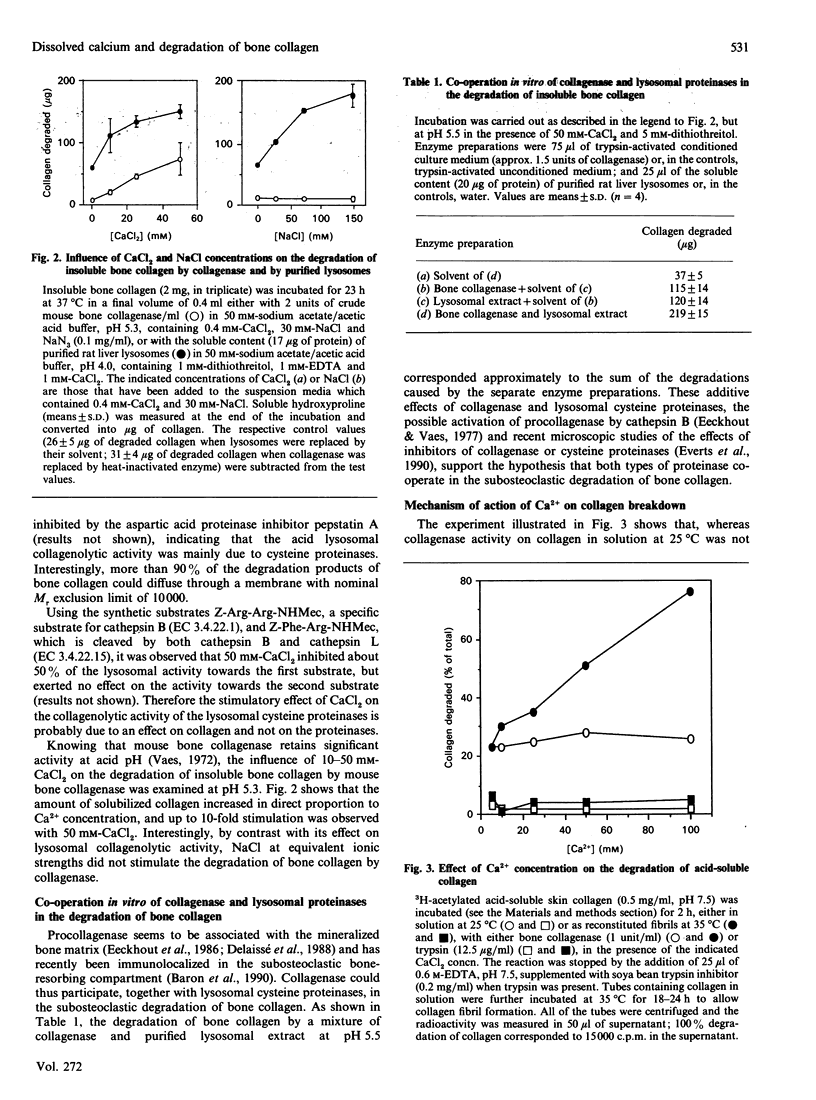
Equilibrium experiments with bone powder, at pH values ranging from 6.3 to 3.5, show a linear relation between log([Ca2+]/[Ca2+]0) (where [Ca2+]0 = 1 M-Ca2+) and pH, indicating that [Ca2+] could reach levels of 25 mM at pH 5 and 90 mM at pH 4. These elevated Ca2+ concentrations stimulated the lysis of insoluble bone collagen in vitro by purified lysosomes and by mouse bone collagenase, whose activities were additive at acid pH. At neutral pH, the addition of 10-100 mM-CaCl2 did not influence the susceptibility of acid-soluble skin collagen in solution towards bone collagenase, but increased it markedly towards collagen in the fibrillar form. Increasing the [Ca2+] did not influence the susceptibility of collagen to trypsin. Elevated [Ca2+] and a co-operation between lysosomal cysteine proteinases and matrix collagenase could thus participate in the osteoclastic breakdown of bone collagen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron R., Neff L., Louvard D., Courtoy P. J. Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J Cell Biol. 1985 Dec;101(6):2210–2222. doi: 10.1083/jcb.101.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair H. C., Kahn A. J., Crouch E. C., Jeffrey J. J., Teitelbaum S. L. Isolated osteoclasts resorb the organic and inorganic components of bone. J Cell Biol. 1986 Apr;102(4):1164–1172. doi: 10.1083/jcb.102.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston T. E., Barrett A. J. A rapid and reproducible assay for collagenase using [1-14C]acetylated collagen. Anal Biochem. 1979 Nov 1;99(2):340–345. doi: 10.1016/s0003-2697(79)80017-2. [DOI] [PubMed] [Google Scholar]
- Christoffersen J. Dissolution of calcium hydroxyapatite. Calcif Tissue Int. 1981;33(6):557–560. doi: 10.1007/BF02409491. [DOI] [PubMed] [Google Scholar]
- Delaisse J. M., Eeckhout Y., Vaes G. Bone-resorbing agents affect the production and distribution of procollagenase as well as the activity of collagenase in bone tissue. Endocrinology. 1988 Jul;123(1):264–276. doi: 10.1210/endo-123-1-264. [DOI] [PubMed] [Google Scholar]
- Delaissé J. M., Eeckhout Y., Vaes G. In vivo and in vitro evidence for the involvement of cysteine proteinases in bone resorption. Biochem Biophys Res Commun. 1984 Dec 14;125(2):441–447. doi: 10.1016/0006-291x(84)90560-6. [DOI] [PubMed] [Google Scholar]
- Eeckhout Y., Delaisse J. M. The role of collagenase in bone resorption. An overview. Pathol Biol (Paris) 1988 Nov;36(9):1139–1146. [PubMed] [Google Scholar]
- Eeckhout Y., Delaissé J. M., Vaes G. Direct extraction and assay of bone tissue collagenase and its relation to parathyroid-hormone-induced bone resorption. Biochem J. 1986 Nov 1;239(3):793–796. doi: 10.1042/bj2390793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherington D. J., Birkedahl-Hansen H. The influence of dissolved calcium salts on the degradation of hard-tissue collagens by lysosomal cathepsins. Coll Relat Res. 1987 Aug;7(3):185–199. doi: 10.1016/s0174-173x(87)80009-2. [DOI] [PubMed] [Google Scholar]
- GRANT N. H., ALBURN H. E. Collagen solubilization by mammalian proteinases. Arch Biochem Biophys. 1960 Aug;89:262–270. doi: 10.1016/0003-9861(60)90052-7. [DOI] [PubMed] [Google Scholar]
- MACGREGOR J., NORDIN B. E. Equilibration studies with human bone powder. J Biol Chem. 1960 Apr;235:1215–1218. [PubMed] [Google Scholar]
- NORDIN B. E. [The solubility of powdered bone]. J Biol Chem. 1957 Aug;227(2):551–564. [PubMed] [Google Scholar]
- Seltzer J. L., Welgus H. G., Jeffrey J. J., Eisen A. Z. The function of Ca+ in the action of mammalian collagenases. Arch Biochem Biophys. 1976 Mar;173(1):355–361. doi: 10.1016/0003-9861(76)90270-8. [DOI] [PubMed] [Google Scholar]
- Silver I. A., Murrills R. J., Etherington D. J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988 Apr;175(2):266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- Trouet A. Isolation of modified liver lysosomes. Methods Enzymol. 1974;31:323–329. doi: 10.1016/0076-6879(74)31034-8. [DOI] [PubMed] [Google Scholar]
- Vaes G. Cellular biology and biochemical mechanism of bone resorption. A review of recent developments on the formation, activation, and mode of action of osteoclasts. Clin Orthop Relat Res. 1988 Jun;(231):239–271. [PubMed] [Google Scholar]
- Vaes G. The release of collagenase as an inactive proenzyme by bone explants in culture. Biochem J. 1972 Jan;126(2):275–289. doi: 10.1042/bj1260275. [DOI] [PMC free article] [PubMed] [Google Scholar]


