Abstract
Context
Primary hyperparathyroidism is a common condition and results in hypercalcaemia, especially in older women. Thus, it is critical to obtain a robust estimate for the upper limit of the reference interval for albumin‐adjusted serum calcium in the general population. The current reference interval in use in the UK (Pathology Harmony range, 2.20 to 2.60 mmol/L) was based on a consensus.
Objectives
To establish a reference interval for albumin‐adjusted serum calcium in men and women.
Design
Cross‐sectional study of men and women who did not have chronic kidney disease or vitamin D deficiency; outliers were identified statistically and then rejected and then a 99% reference interval was calculated.
Patients
502 524 men and women aged 40 to 69 years from the UK Biobank Study.
Measurements
Serum total calcium, albumin, 25‐hydroxyvitamin D, estimated glomerular function (eGFR).
Results
We developed an equation for albumin‐adjusted serum calcium and applied it to 178 377 men and women who did not have chronic kidney disease or vitamin D deficiency. We identified 2962 (1.7%) as outliers, and when excluded, we report a 99% reference interval of 2.19 to 2.56 mmol/L (8.76 to 10.24 mg/dL). We found that for older (55‐69 years) and younger women (40‐55 years) the upper limits were 2.59 mmol/L and 2.57 mmol/L and that for all men, the upper limit was 2.55 mmol/L.
Conclusions
We have established an upper limit of the reference range for older women that would identify all high outliers (2.60 mmol/L and above). The upper limit for young women and for men is lower, at 2.57 and 2.55 mmol/L respectively. The current reference interval in use has to be updated and improved based on these findings. These upper limits may prove helpful for identifying hypercalcaemic disorders like primary hyperparathyroidism in clinical practice.
Keywords: adjusted calcium equation, calcium, reference interval, reference intervals, UK Biobank
1. INTRODUCTION
A reference interval is an important concept in everyday clinical practice, as it gives a range of values for which a measurement is considered physiologic in healthy subjects. Values above or below this reference interval suggest the existence of a disease. In the case of calcium metabolism, it is important to identify the upper and lower limits of the reference interval for serum calcium in order to diagnose patients with disorders of calcium homeostasis like primary hyperparathyroidism (PHPT). 1 This requires a very large population of people who have well‐characterized vitamin D and estimated glomerular filtration rate (eGFR) status. In the United Kingdom, the Pathology Harmony reference range has been in use for adjusted calcium since 2011; the range reported is 2.20 ‐ 2.60 mmol/L. Its establishment was based on a consensus. 2 , 3
Moreover, it is essential to consider that total calcium can vary significantly depending on protein concentration as approximately forty‐five per cent is protein bound, mainly to albumin. 4 NICE guidance advises the use of albumin‐adjusted calcium when assessing patients for calcium‐related disorders. 5
Adjusting total calcium for albumin concentration is a practical means of determining serum calcium. 6 There are several equations available in literature with the most commonly used being the following: adjusted calcium (mmol/L) = total calcium + [0.02 (40−albumin in g/L)]. 7 However, a derived regression equation would be better to use as it is based on the local patient population. 6
UK Biobank is a large health resource which has the aim of improving prevention, diagnosis and treatment of a wide range of serious illnesses. 8 It has data on half a million participants aged 40‐69 years, equally represented from both sexes. With information on a total of thirty‐four biomarkers (blood and urine), UK Biobank is an excellent resource for research.
The aims of this study were to calculate a reference interval for calcium based on the UK Biobank population cohort and to provide an equation that could be used by scientists studying calcium metabolism using UK Biobank data.
We considered two approaches. In the first, we have applied an outlier identification approach to a population‐based study to identify the range of abnormal results; the least abnormal result would provide us with our upper and lower limit estimate. We would expect to find a prevalence of hypercalcaemia of about 1% overall, with higher values in older women. 9 Primary hyperparathyroidism is a distinct abnormality of calcium homeostasis that is commonly due to a parathyroid adenoma. Hypoparathyroidism is much less common than primary hyperparathyroidism and so we would expect fewer cases. 10 In the second approach, we excluded all outliers identified using the first approach and then used a robust approach to calculate the 99% reference interval. We chose 99% as we would expect fewer than 1% diseased individuals in the tails of the distribution after excluding the outliers. This would also provide us with our upper and lower limit estimates.
2. MATERIALS AND METHODS
The participants of the UK Biobank (94% white) were recruited throughout the UK between 2006 and 2010. The information on the biochemical measurements was retrieved by Document Ref: BCM023, Version 1.0, 12 Aug 2015, the Companion Document to Accompany Serum Biomarker Data, Version 1.0, 11 March 2019 and the Biomarker assay quality procedures: approaches used to minimize systematic and random errors (and the wider epidemiological implications), Version 1.2, 02 April 2019. 8 The serum samples collected were not fasting and venepuncture was performed using a tourniquet (information obtained after communication with the UK Biobank). The participants could have their blood measurements at any point in the day. Calcium and albumin were measured using colorimetric assays in a Beckman Coulter AU5800 analyser. The coefficient of variation reported was between 1.29% and 1.61% for calcium and between 2.09% and 2.13% for albumin.
We had databases released in 2019, with data on a total of 502 524 participants (54% female, mean age 57 years).
In order to calculate the albumin‐adjusted calcium equation a previous protocol described by Barth et al was followed 6 ; this is the protocol also used in our laboratory at Sheffield Teaching Hospitals National Health Service Foundation Trust. According to this, patients with extreme levels of albumin (≥55 g/L and <20 g\L), substantial renal impairment (urea ≥15 mmol/L and/or creatinine ≥200 μmol/L) and/or deranged liver function tests [alanine transaminase (ALT) ≥41 U/L or >34 U/L for females and/or alkaline phosphatase (ALP) ≥130 U/L] were excluded. To comply to this protocol, the mean of the reference interval for total calcium has to be used. The mean of the Pathology Harmony reference range is 2.40 mmol/L, which was different from the mean calcium reported by the UK Biobank (2.38 mmol/L). Therefore, a new reference interval for total calcium had to be calculated first to allow the calculation of the adjusted calcium equation.
In order to establish the total calcium reference interval, all participants with a measurement of serum calcium at the UK Biobank baseline assessment were included. Exclusion criteria were low estimated glomerular filtration rate (eGFR < 60 mL/min/1.73 m2) and/or low vitamin D (<50 nmol/L). 11 As the UK Biobank did not give information on eGFR, the 2009 CKD‐EPI creatinine equation was used for its calculation. 12 When applying this equation, a different calculation is used for Black people, so these need to be identified. UK Biobank uses different codings for ethnic backgrounds and defines as ‘black’ the following categories: 4: Black or Black British; 4001: Caribbean; 4002: African; 4003: any other black background.
Using the derived adjusted calcium equation, adjusted calcium was calculated in order to find the reference interval. All participants with an estimate of adjusted calcium were included. Once again, exclusion criteria were low eGFR and/or low vitamin D as defined above.
A further analysis was conducted in order to check for age and gender differences in the adjusted calcium reference range. The same approach as described above was followed for each subgroup. Patients below 55 years were defined as young, while ≥55 years as old.
The analyses were performed using the RStudio statistical software version 1.1.442 (RStudio, Inc Boston). For the reference interval analysis, Horn's method of outlier detection using Tukey's interquartile fences was used. This method identifies an outlier if it lies more than 1.5 quartiles above or below the interquartile range (IQR). 13 The outliers were rejected and the refLimit function was applied. The robust method was used for the reference interval calculations adjusting the method to calculate a 99% reference interval, while the bootstrapping method was used for the confidence intervals adjusting it to 90% limits. 13
3. RESULTS
3.1. Reference interval for total calcium
In total, 178 442 participants had a total calcium result at baseline and had normal vitamin D and eGFR as defined above. Out of the women with data on menopause, 2873 were postmenopausal and 330 premenopausal. Calcium was higher in the menopausal group (2.39 vs 2.35 mmol/L, respectively, P < .0001).
The total calcium measurements of this population (n = 178 442) were used in RStudio, and the derived 99% reference interval was 2.16‐2.60 mmol/L after rejecting 2356 outliers (the majority being above of the reference interval). The mean calcium of the data was 2.38 mmol/L.
For patients with normal eGFR, independent of their vitamin D status, the respective reference interval was 2.15 το 2.60 mmol/L. The mean calcium was 2.38 mmol/L.
3.2. Albumin‐adjusted calcium equation
In total, 372 804 participants were selected based on the Barth et al protocol criteria and a plot was created of total calcium (y‐axis) against albumin (x‐axis). The y‐intercept was found to be 1.58 while the slope of the graph was 0.0177 (Figure 1).
FIGURE 1.
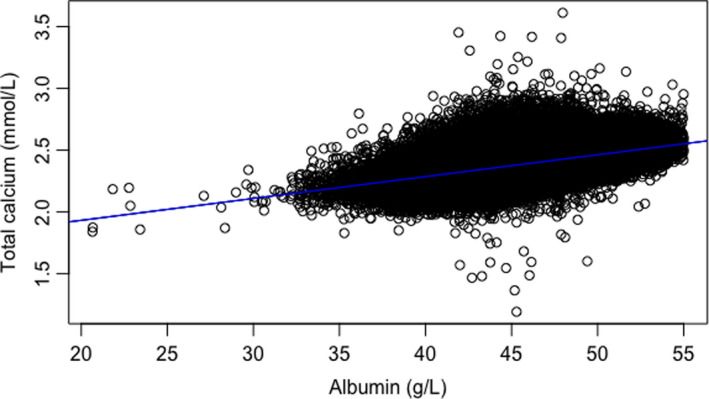
Plot of total calcium (y‐axis) against albumin (x‐axis) created to find the coefficients for the adjusted calcium equation. The y‐intercept was found to be 1.58 while the slope of the graph was 0.0177
Thus, the equation y = (slope)× + intercept, became y = 0.0177× + 1.58.
This was further processed:
Total calcium (TCa) = 0.0177 (albumin) + 1.58
According to Barth et al, albumin‐adjusted calcium = total calcium ‐ (slope * albumin) + (mean normal total calcium ‐ intercept calcium) 6
Albumin‐adjusted calcium (ACa) = TCa–0.0177 (albumin) + (mean total calcium–1.58)
ACa = TCa–0.0177 (albumin) + 0.80
ACa = TCa–(albumin–0.80/0.0177)
Albumin‐adjusted calcium = Total calcium + 0.0177 (45.2–albumin)
3.3. Reference interval for albumin‐adjusted calcium
Using the equation calculated above, the albumin‐adjusted calcium for the whole population was calculated. This included 178 377 adults with a total calcium and albumin result at baseline and normal vitamin D and eGFR as defined above. The outlier rejection approach identified 2961 outliers (1.66%) which were rejected. The reference interval for albumin‐adjusted calcium was found to be 2.19 to 2.56 mmol/L (Table 1). The mean albumin for this population was 45.1 g/L (SD 2.58).
TABLE 1.
Calculated reference intervals for total and albumin‐adjusted calcium
| Albumin‐adjusted calcium (mmol/L) | |
|---|---|
| Lower limit of 99% reference interval (mmol/L) | 2.194 |
| 90% confidence intervals (mmol/L) | 2.193‐2.194 |
| Higher limit of 99% reference interval (mmol/L) | 2.564 |
| 90% confidence intervals (mmol/L) | 2.563‐2.565 |
3.4. Age‐ and gender‐adjusted reference intervals for albumin‐adjusted calcium
Using equations derived for each subgroup, we calculated the adjusted calcium for each group and applied the outlier rejection approach as described above (Table 2). We found different reference intervals for albumin‐adjusted calcium (Table 3) with the highest levels in the older group of women and the lowest levels in the two groups of men.
TABLE 2.
Outlier rejection for the different groups. Young: < 55 y, old ≥55 y
| Population |
Older women N = 59 877 |
Younger women N = 35 497 |
Older men N = 55 879 |
Younger men N = 27 124 |
|---|---|---|---|---|
| Number of outliers (%) | 1249 (2.1) | 443 (1.2) | 667 (1.2) | 405 (1.5) |
| Number of outliers above the IQR (%) | 888 (1.5) | 285 (0.8) | 425 (0.8) | 220 (0.8) |
| Range of calcium for the outliers above the IQR (mmol/L) | 2.61‐3.43 | 2.60‐3.07 | 2.58‐3.51 | 2.57‐2.81 |
| Number of outliers below the IQR (%) | 361 (0.6) | 158 (0.4) | 242 (0.4) | 185 (0.7) |
| Range of calcium for the outliers below the IQR (mmol/L) | 1.12‐2.22 | 1.78‐2.20 | 1.51‐2.19 | 1.54‐2.22 |
IQR: interquartile range.
TABLE 3.
Reference intervals and 90% confidence intervals for the upper and lower limit for albumin‐adjusted calcium
| Population | Older women | Younger women | Older men | Younger men |
|---|---|---|---|---|
| Lower limit of 99% reference interval (mmol/L) | 2.210 | 2.195 | 2.192 | 2.211 |
| 90% confidence intervals (mmol/L) | 2.209‐2.211 | 2.194‐2.196 | 2.191‐2.193 | 2.210‐2.212 |
| Higher limit of 99% reference interval (mmol/L) | 2.586 | 2.565 | 2.549 | 2.552 |
| 90% confidence intervals (mmol/L) | 2.585‐2.587 | 2.563‐2.566 | 2.548‐2.550 | 2.550‐2.553 |
Young: <55 y, old ≥55 y.
4. DISCUSSION
This study has provided reference intervals for total and albumin‐adjusted calcium. An interesting observation was that the reference interval found for adjusted calcium was narrower than the Pathology Harmony range (2.2 to 2.6 mmol/L) which is the established range in the UK. This is expected, as the Pathology Harmony range was based on a consensus. The calculated reference interval for this population was 2.19‐2.56 mmol/L for albumin‐adjusted calcium. Interestingly, the confidence intervals of the lower and upper limit did not include the current limits of the Pathology Harmony interval. This observation has clinical consequences. Moving the reference interval to either direction would affect the number of individuals that are labelled as having a disorder of calcium metabolism and can change the reported prevalence of disorders like primary hyperparathyroidism and normocalcaemic hyperparathyroidism; a more narrow range would increase the prevalence of primary hyperparathyroidism (high calcium, high parathyroid hormone) and decrease the prevalence of normocalcaemic hyperparathyroidism (normal calcium, high parathyroid hormone).
We also found different age‐ and gender‐adjusted reference intervals for albumin‐adjusted calcium. Postmenopausal women had the highest upper limit (2.59 mmol/L). This estimate was supported by the range of outliers for older women of 2.61‐3.43 mmol/L as they were all above this threshold. Thus, an upper limit of 2.60 mmol/L may be suitable for older women. However, for younger women and for men the upper limit should be around 2.55‐2.57 mmol/L.
The higher limit found in older women could reflect the higher prevalence of primary hyperparathyroidism in this group 14 ; however, we rejected outliers before calculating the reference interval. There was also an increased number of outliers detected above the IQR in this group when compared to the other groups. Another explanation for this could be the increased resorption described after menopause 15 and the change in set point resulting from changes in oestrogen status. 16 We found increased levels of calcium in the postmenopausal group.
This study also provided an equation for the calculation of albumin‐adjusted calcium derived from UK Biobank data. This could be used when working using these data in future research projects.
There are several limitations to this study. Parathyroid hormone would be useful to have to allow exclusions of patients with parathyroid disorders. Moreover, we did not exclude people known to have diseases or taking medication affecting calcium metabolism; our only exclusions were vitamin D deficiency and CKD. As mentioned, patients were not fasting and this could have had an increasing effect on the measurements. 17 Total calcium seems to be affected by postural changes, with a peak late morning and a nadir starting in the evening. 18 , 19 Food intake can also affect the result slightly. 17 Thus, if the samples had been taking in the fasting state, the mean levels and reference interval may have been different, but it is difficult to predict in which direction. The oldest patients included were 70 years old; the results might be different in older people. We have used some non‐standard approaches to defining reference intervals. We chose outlier rejection as primary hyperparathyroidism is known to be a very common disorder; of course, we did not have PTH measurements so we cannot be sure all the outliers had this disorder. We also used the 99% rather than 95% CI to establish the reference interval. We did this because we had already excluded the outliers and did not want to have a high false‐positive rate of 5%.
There is still debate about whether it is best to measure albumin‐adjusted calcium or ionised calcium. Ionized (free) calcium is considered an alternative way of assessing the calcium status, and its importance in assessing PHPT has been previously emphasized. 20 However, measuring ionized calcium can be a challenge. This is due mainly to influences from the pH. There are also limitations due to binding of calcium to the common anticoagulants. The measurement is usually performed in blood gas analysers, and this is problematic because of frequent electrode dysfunction. The performance depends on staff training and quality control standards. The instrument must be perfectly calibrated, and the measurement has to be performed immediately after the collection. Finally, the lack of well‐established reference intervals for ionized calcium does limit its use. 21
Overall, this study provided a reference interval for total and albumin‐adjusted calcium which was based on a large population and has questioned whether the Pathology Harmony range is the appropriate reference interval to use, especially for younger women and men. It has also suggested that age‐ and gender‐adjusted reference intervals might be more appropriate to use. Finally, it provided an adjusted calcium equation to use when studying calcium metabolism using UK Biobank data.
CONFLICTS OF INTEREST
MS received funding for her fellowship from the Medical Research Council Centre of Excellence for Musculoskeletal Ageing and from Osteoporosis 2000 support group and grant funding from Roche Diagnostics. FMH has no conflicts of interest. JSW receives speaker's honoraria from Eli Lilly and Sandoz, grant funding from Alexion and Immunodiagnostic Systems, donation of drug from Eli Lilly, ProStrakan (Kyowa Kirin) and Consilient for clinical studies, donation of assay kits from Biomedica, consulting fees from Shire, Mereo BioPharma, Kyowa Kirin, UCB Pharma and Pharmacosmos. RE receives consultancy funding from IDS, Roche Diagnostics, GSK Nutrition, FNIH, Mereo, Lilly, Sandoz, Nittobo, AbbVie, Samsung and Haoma Medica and grant funding from Nittobo, IDS, Roche, Amgen and Alexion.
AUTHOR'S CONTRIBUTIONS
The authors confirm that the manuscript is original and has not been submitted elsewhere. Each author acknowledges that he/she has contributed in a substantial way to the work described in the manuscript and its preparation.
Schini M, Hannan FM, Susan Walsh J, Eastell R. Reference interval for albumin‐adjusted calcium based on a large UK population. Clin Endocrinol (Oxf). 2021;94:34–39. 10.1111/cen.14326
Funding information
MS received funding for her fellowship from the Medical Research Council Centre of Excellence for Musculoskeletal Ageing, from the Osteoporosis 2000 support group and from Roche Diagnostics.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Crowley R, Gittoes N. How to approach hypercalcaemia. Clin Med (London, England). 2013;13(3):287‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berg J. The approach to pathology harmony in the UK. Clin Biochem Rev. 2012;33(3):89‐93. [PMC free article] [PubMed] [Google Scholar]
- 3. Berg J, The UK. Pathology Harmony initiative; The foundation of a global model. Clin Chim Acta. 2014;432:22‐26. [DOI] [PubMed] [Google Scholar]
- 4. Favus MJ, Goltzman D. Regulation of calcium and magnesium. In: Rosen CJ, Bouillon R, Compston JE, Rosen V, eds. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. John Wiley & Sons, Inc.; 2013:171‐179. [Google Scholar]
- 5. NICE . Hyperparathyroidism (primary): diagnosis, assessment and initial management. In: National Institute for Health and Care Excellence (UK); 2019. https://www.ncbi.nlm.nih.gov/books/NBK542087/. Accessed September 14, 2020. [PubMed]
- 6. Barth JH, Fiddy JB, Payne RB. Adjustment of serum total calcium for albumin concentration: effects of non‐linearity and of regression differences between laboratories. Ann Clin Biochem. 1996;33(1):55‐58. [DOI] [PubMed] [Google Scholar]
- 7. James M, Zhang J, Lyon A, Hemmelgarn B. Derivation and internal validation of an equation for albumin‐ adjusted calcium. BMC Clin Pathol. 2008;8(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. UKBiobank . https://www.ukbiobank.ac.uk. Accessed September 14, 2020.
- 9. Khan AA, Hanley DA, Rizzoli R, et al. Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus; 2017. [DOI] [PMC free article] [PubMed]
- 10. Bollerslev J, Rejnmark L, Marcocci C, et al. European Society of Endocrinology Clinical Guideline: treatment of chronic hypoparathyroidism in adults. Eur J Endocrinol. 2015;173(2):G1‐G20. [DOI] [PubMed] [Google Scholar]
- 11. Aspray TJ, Bowring C, Fraser W, et al. National osteoporosis society vitamin D guideline summary. Age Ageing. 2014;43(5):592‐595. [DOI] [PubMed] [Google Scholar]
- 12. KDIGO . KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. In; 2012. [DOI] [PubMed]
- 13. Finnegan D.Package ‘referenceIntervals’ version 1.2.0; 2020. https://cran.r‐project.org/web/packages/referenceIntervals/referenceIntervals.pdf. Accessed September 14, 2020.
- 14. Silva BC, Cusano NE, Bilezikian JP. Primary hyperparathyroidism. Best Pract Res Clin Endocrinol Metab. 2018;32(5):593‐607. [DOI] [PubMed] [Google Scholar]
- 15. Khosla S, Atkinson EJ, Melton LJ, Riggs BL. Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: a population‐ based study1. J Clin Endocrinol Metab. 1997;82(5):1522‐1527. [DOI] [PubMed] [Google Scholar]
- 16. Boucher A, D'Amour P, Hamel L, et al. Estrogen replacement decreases the set point of parathyroid hormone stimulation by calcium in normal postmenopausal women. Maturitas. 1989;11(3):255. [DOI] [PubMed] [Google Scholar]
- 17. Goldstein D. Serum Calcium (Chapter 143) in Clinical Methods. In: Walker HKHW, Hurst JW, eds. The History, Physical, and Laboratory Examinations, 3rd edn. Boston: Butterworths; 1990:677–679. [PubMed] [Google Scholar]
- 18. Markowitz M, Rotkin L, Rosen JF. Circadian rhythms of blood minerals in humans. Science. 1981;213(4508):672‐674. [DOI] [PubMed] [Google Scholar]
- 19. Markowitz ME, Arnaud S, Rosen JF, Thorpy M, Laximinarayan S. Temporal interrelationships between the circadian rhythms of serum parathyroid hormone and calcium concentrations. J Clin Endocrinol Metab. 1988;67(5):1068‐1073. [DOI] [PubMed] [Google Scholar]
- 20. Ong GS, Walsh JP, Stuckey BG, et al. The importance of measuring ionized calcium in characterizing calcium status and diagnosing primary hyperparathyroidism. J Clin Endocrinol Metab. 2012;97(9):3138‐3145. [DOI] [PubMed] [Google Scholar]
- 21. Baird GS. Ionized calcium. Clin Chim Acta. 2011;412(9–10):696‐701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


