Abstract
Aim
This study aimed to contrast the associations of five common diet scores with severe non‐alcoholic fatty liver disease (NAFLD) incidence.
Materials and Methods
In total, 162 999 UK Biobank participants were included in this prospective population‐based study. Five international diet scores were included: the 14‐Item Mediterranean Diet Adherence Screener (MEDAS‐14), the Recommended Food Score (RFS), the Healthy Diet Indicator (HDI), the Mediterranean Diet Score and the Mediterranean‐DASH Intervention for Neurodegenerative Delay score. As each score has different measurements and scales, all scores were standardized and categorized into quartiles. Cox proportional hazard models adjusted for confounder factors investigated associations between the standardized quartiles and severe NAFLD incidence.
Results
Over a median follow‐up of 10.2 years, 1370 participants were diagnosed with severe NAFLD. When the analyses were fully adjusted, participants in quartile 4 using the MEDAS‐14 and RFS scores, as well as those in quartiles 2 and 3 using the HDI score, had a significantly lower risk of severe incident NAFLD compared with those in quartile 1. The lowest risk was observed in quartile 4 for the MEDAS‐14 score [hazard ratio (HR): 0.76 (95% confidence interval (CI): 0.62‐0.94)] and the RFS score [HR: 0.82 (95% CI: 0.69‐0.96)] and as well as in quartile 2 in the HDI score [HR: 0.80 (95% CI: 0.70‐0.91)].
Conclusion
MEDAS‐14, RFS and HDI scores were the strongest diet score predictors of severe NAFLD. A healthy diet might protect against NAFLD development irrespective of the specific approach used to assess diet. However, following these score recommendations could represent optimal dietary approaches to mitigate NAFLD risk.
Keywords: diet, non‐alcoholic fatty liver disease, incidence
1. INTRODUCTION
Non‐alcoholic fatty liver disease (NAFLD) is defined as excessive fat accumulation in the liver. 1 , 2 NAFLD, including its progressive form [non‐alcoholic steatohepatitis (NASH)], is the leading cause of global liver disease. 1 In 2022, a meta‐analysis highlighted that the incidence of NAFLD rose to almost 47 cases per 1000 person‐years. 2 Although this meta‐analysis included mainly data from Asian populations, 2 other global estimates are consistent with this figure. 3
Unhealthy diets, such as high‐calorie diets, diets high in refined carbohydrates, fructose, ultra‐processed foods or saturated fats, have been linked with obesity, an independently recognized risk factor in NAFLD development. 4 As a result, it is unsurprising that diet plays a crucial role in preventing or causing NAFLD. 5 , 6 , 7 , 8 As no specific pharmacotherapy treatment for NAFLD exists, 9 lifestyle modifications are still the first line recommendation for prevention and treatment. 10 , 11
Individual foods and nutrients are not consumed in isolation. 12 Therefore, studying one dietary risk factor without considering others could under‐ or overestimate overall associations between diet and adverse health outcomes such as NAFLD. Similarly, this approach fails to appreciate the complex synergistic/cumulative impact of different foods/nutrients on health. Using a cumulative combination of nutrients and foods as an overall dietary pattern may provide a more robust assessment of the association between diet and NAFLD. Previous prospective studies have investigated the association between individual diet scores, such as the Mediterranean Diet and NAFLD, separately. 6 , 13 , 14 , 15 , 16 , 17 , 18 , 19 However, to date, there have been no comparisons of the association between different scores of diet quality and severe NAFLD. The latter would be valuable to help identify if there is an optimal diet for NAFLD prevention. Therefore, considering that each score includes different food elements and methodologies and that there is no unique dietary intervention/recommendation for NAFLD, this study used the UK Biobank cohort study to contrast the associations of five common diet scores with severe NAFLD incidence. We decided to use NAFLD instead of the recent term, metabolic dysfunction‐associated steatotic liver disease (MASLD), as the cardiometabolic criteria requested for its definition were not available during the follow‐up. 20
2. METHODS
The UK Biobank is a prospective cohort that enrolled over 500 000 participants aged 37‐73 years from the general UK population at baseline (5.5% response rate). 21 From 2006 to 2010, these participants visited one of 22 assessment centres located throughout Scotland, England and Wales. During their initial visit, participants completed a questionnaire on a touch‐screen device, underwent physical measurements, and provided biological samples. 22 , 23 More information about the UK biobank protocol can be found online (https://www.ukbiobank.ac.uk).
2.1. Diet scores
Dietary intake was measured using the Oxford WebQ, a web‐based 24‐h dietary assessment tool that collects information on 206 foods and 32 beverages consumed during the previous 24 h. 24 , 25 Energy and nutrient intake were calculated using McCance and Widdowson's The Composition of Food, 5th edition. 26 Information from the dietary assessment tool was collected according to the previous day's intake using questions such as: ‘Did you have any of these yesterday?’ or ‘How much of the following did you drink yesterday?’ Determining daily nutrient intake involves multiplying the frequency of food or beverage consumption by standard portion size and the nutrient composition specific to each item. 27 For this study, the average of 24‐h recalls was used [the information was collected on up to five occasions (only one time per occasion) between April 2009 (first instance) and June 2012 (last instance) as described elsewhere 27 and on the UK biobank webpage: https://biobank.ndph.ox.ac.uk/showcase/field.cgi?id=26008].
From these data, we calculated five international diet scores that are aligned with dietary guidelines and represent widely used but different ways of assessing diet quality: the 14‐Item Mediterranean Diet Adherence Screener (MEDAS‐14), Recommended Food Score (RFS), Healthy Diet Indicator (HDI), Mediterranean Diet Score (MDS) and Mediterranean‐Dietary Approaches to Stop Hypertension (DASH) Intervention for Neurodegenerative Delay (MIND) score.
2.1.1. Mediterranean Diet Adherence Screener
The MEDAS‐14 questionnaire is a concise assessment tool used to measure adherence to the Mediterranean diet based on a previously validated food index. 28 This adaptation was previously validated for the UK Biobank population 29 as described elsewhere. 29 , 30 A score of 1 was given when consumption of certain food groups met or exceeded the recommended levels. These food groups included olive oil, white meats, legumes, fish, nuts, self‐reported intake of tomato‐based sauces (as a proxy of sofrito), nuts and vegetables. A score of 1 was also given when the consumption of specific food groups fell below the maximum accepted intake. These food groups included commercial pastries, red meats and derivatives, carbonated beverages, and butter, margarine, or cream. The final score, ranging from 0 to 14, reflected overall adherence to the Mediterranean diet, with a higher score representing a more Mediterranean dietary pattern.
2.1.2. Recommended Food Score
The RFS is a food‐based index intended to evaluate the consumption of food groups that align with dietary guidelines. Following the methodology previously described by Livingstone et al. in 2021, 31 a total of five food groups were established: fruits (consisting of seven items), vegetables (seven items), whole grains (two items), meat and alternatives (three items), and reduced‐fat dairy products (two items). In this scoring system, 1 point was allocated when the consumption of food items exceeded the minimum thresholds, which were set at 15 g/day for non‐beverages and 30 g/day for beverages. If the intake fell below these thresholds, a score of 0 was given. The scores ranged from 0 to 21, with higher scores indicating better quality diet and greater consumption of recommended foods.
2.1.3. Healthy Diet Indicator
The HDI is an index that accounts for food‐ and nutrient‐based factors to assess the consumption of foods recommended for a healthy diet by the World Health Organization. This study used an adapted version of the HDI proposed by Livingstone et al., 31 which consisted of an 11‐point scale. The index included the following groups of foods/nutrients: saturated fat, polyunsaturated fat, protein, total carbohydrates, dietary fibre, fruits and vegetables, pulses and nuts, total non‐milk extrinsic sugars, fish, red meat and meat products, and calcium. Because of the lack of available data in UK Biobank on the intake of non‐milk extrinsic sugars, we adjusted the HDI by scoring intakes of total sugars instead. Intakes within the specified cut‐offs were assigned a score of 1, while those outside received a score of 0. The overall score ranged from 0 to 11, with a higher score indicating a higher diet quality.
2.1.4. Mediterranean Diet Score
The MDS is a scoring system that combines both food‐based and nutrient‐based components to assess adherence to a Mediterranean‐style diet. In this study, we used a nine‐item index described and adapted by Livingstone et al., as detailed elsewhere. 31 Food and nutrient intakes were evaluated based on nine specific components, including vegetables, legumes, fruits and nuts, cereals, fish and seafood, the ratio of monounsaturated fats to saturated fats, dairy products, meat and meat products, and alcohol. Sex‐specific median intakes were used as cut‐off points to determine the score for each component. Participants who had an intake of vegetables, legumes, fruits and nuts, cereals, fish and seafood, and a monounsaturated to saturated fats ratio above the median were assigned a score of 1. On the other hand, participants with an intake of dairy products and meat and meat products below the median were also assigned a score of 1. As for alcohol, a score of 1 was given for low to moderate intake, which meant consuming alcohol once or twice per day. A score of 0 was assigned for no alcohol intake or an intake more frequent than twice per day. The total MDS score ranged from 0 to 9, with higher scores indicating greater adherence to the Mediterranean diet.
2.1.5. Mediterranean‐Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay
MIND, previously described by Morris et al., 32 is a hybrid score between the Mediterranean and DASH diets. This scoring system comprises 15 components, which include 10 categories of brain‐healthy foods (leafy green vegetables, other vegetables, nuts, berries, legumes, whole grains, fish, poultry, olive oil and wine) and five categories of unhealthy foods (red meats, stick butter and margarine, cheese, pastries and sweets, and fried/fast food). Although the MIND diet was initially derived to support healthy brain ageing, this dietary pattern has attracted recent attention as a potential strategy to improve other health outcomes. 33 The scoring assigns 1 point to each food group meeting the whole recommended intake, 0.5 when half of the recommendation was met and 0 points for not meeting the recommendations, producing an overall scale ranging from 0 to 15. Notably, for oil consumption, 1 point is given if olive oil is the primary oil used at home, whereas 0 points are awarded if other types of oil are used. 32
2.1.6. Contrasting the scores
As each score has different measurements and scales, making direct comparison problematic, all scores were standardized (z‐score). Then, all scores were split into quartiles according to their distribution.
2.2. Severe non‐alcoholic fatty liver disease
Severe NAFLD was defined as hospitalization or death because of NAFLD or NASH and was ascertained from the linked hospital and death databases during the follow‐up. The date and cause of death were obtained from death certificates held by the National Health Service (NHS) Information Centre (England and Wales) and the NHS Central Register Scotland (Scotland). Dates and causes of hospital admissions were identified via record linkage to Health Episode Statistics (England and Wales) and the Scottish Morbidity Records (SMR01) (Scotland). Details of the linkage procedure can be found at https://content.digital.nhs.uk/services. Hospital admissions data were available until the end of March 2021 in England and Scotland and the end of March 2018 in Wales. Mortality data were available until the end of February 2021. Therefore, follow‐up was censored on these dates.
Using the International Classification of Diseases 10th revision (ICD‐10) and an Expert Panel Consensus Statement, 34 NAFLD was defined as ICD‐10 K76.0 [fatty (change of) liver, not elsewhere classified] and K75.8 (NASH, other specified inflammatory liver diseases).
2.3. Covariates
Age at baseline was determined from date of birth and baseline assessment. Sex was self‐reported at baseline. Deprivation (area‐based socioeconomic status) was derived from the postcode of residence using the Townsend score. 35 Ethnicity was self‐reported and categorized as: white and others. Self‐reported smoking status was categorized as never, former or current smoker. The components of the metabolic syndrome (central obesity, hyperglycaemia/diabetes, high blood pressure/hypertension, low HDL and high triglyceride) were ascertained using baseline data. Central obesity was defined as a waist circumference >88 cm in women and >102 cm in men. Hyperglycemia/diabetes was defined as fasting glucose ≥5.6 mmol/L or self‐report of a physician diagnosis of diabetes. High blood pressure/hypertension was defined as a systolic blood pressure ≥130 mmHg and/or a diastolic blood pressure ≥85 mmHg or self‐report of a physician diagnosis of hypertension. High triglyceride was defined as ≥1.7 mmol/L and low HDL‐cholesterol as <1.3 mmol/L in women and <1.0 mmol/L in men. 36 , 37 , 38 Finally, the level of physical activity was self‐reported using the International Physical Activity Questionnaire short form. 39 Additional information on the measurements is available on the UK Biobank website (https://www.ukbiobank.ac.uk).
2.4. Ethical approval
The UK biobank was approved by the North West Multi‐Centre Research Ethics Committee (Ref: 11/NW/0382). The study protocol is available online (https://www.ukbiobank.ac.uk/). This work was conducted under the UK biobank application number 71392.
2.5. Statistical analyses
Descriptive baseline characteristics by quartiles of each standardized diet score are presented as means with SD for quantitative variables and as frequencies and percentages for categorical variables.
Associations between the diet scores and severe NAFLD incidence were investigated using Cox proportional hazard models using standardized quartiles of the included diet scores. Individuals in the lowest quartile (least healthy) were used as the referent in each model. The results are reported as hazard ratios (HR) and their 95% confidence intervals (95% CIs). The proportional hazard assumptions were checked using Schoenfeld residuals, and the duration of follow‐up was used as the time‐dependent variable.
Participants with missing data for any diet index/score (n = 301 889) or those with missing data for one or more covariates (n = 34 732) were excluded. Using the Expert Panel Consensus Statement, 34 participants with other liver disease or alcohol/drug use disorders at or before baseline (n = 2661) were also excluded. In addition, to minimize the effect of reverse causality, all analyses were conducted using 2‐year landmark analyses, excluding all participants who experienced events within the first 2 years of follow‐up (n = 84) (Figure 1).
FIGURE 1.
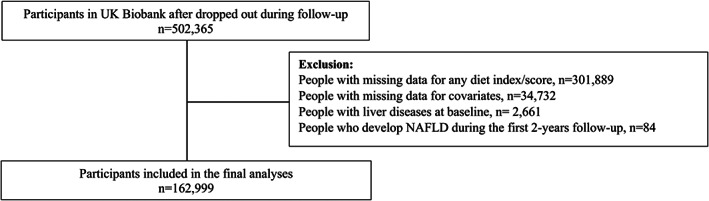
Diagram people included in the analyses. NAFLD, non‐alcoholic fatty liver disease.
Analyses were adjusted for confounding factors based on previous literature, 40 using the following three models: Model 1, was unadjusted. Model 2 was adjusted for sociodemographic factors (age, sex, deprivation and ethnicity). Model 3 was adjusted as per Model 2 but additionally included health‐related factors (the individual components of the metabolic syndrome (central obesity, hyperglycemia/diabetes, hypertension/high blood pressure, low HDL and high triglyceride) and lifestyle factors smoking and physical activity). As sensitivity analyses, (a) models were adjusted for total energy intake, which analysis was performed excluding people who reported unfeasible energy intake (i.e. <800 or >4000 kcal/day), and (b) non‐linear associations between the standardized continuous scores and severe NAFLD were explored using penalized cubic splines fitted in Cox proportional hazard models. 41 We decided to use this approach as the penalized cubic spline estimates the curvature based on the data and is more robust against human errors. 42
Finally, to investigate whether the association between the quartiles and severe NAFLD differed by population groups, we tested for interactions and stratified the analyses by age groups (≥ and <60 years), sex (men and women), alcohol intake (never/special occasion and regular drinkers) and central obesity (no and yes).
Stata 18 and R 4.3.0 were used to perform the analyses. A value of p < .05 was considered statistically significant.
3. RESULTS
After excluding participants with missing data for any diet scores included and covariates, and those with events over the first 2 years of follow‐up, 162 999 participants were included in the study (Figure 1). Over a median follow‐up of 10.2 years (interquartile range: 9.59‐10.9 years), 1370 individuals (0.8%) were diagnosed with severe NAFLD.
The baseline characteristics of participants by quartiles of the standardized diet scores are available in Table 1 (MEDAS‐14 score) and Tables S1–S4. Overall, participants would probably be women and from a white background. A high percentage of participants self‐reported never smoking (56.9%), walking for pleasure (75.0%) and almost 75% drank alcohol once or more per week. Compared with participants with the poorest quartile diets for the MEDAS‐14 score (quartile 1), participants with better diets (quartiles 3 and 4) would probably be women and to walk for pleasure as well as would probably be current smokers and had a higher prevalence of daily or almost daily alcohol intake. They also showed a lower prevalence of the individual components of the metabolic syndrome compared with those in quartile 1. In addition, participants in the lowest MEDAS‐14 score quartile had a mean score of 3.1, while those in the highest quartile of 8.5. These findings were similar regardless of the diet score used (Tables S1–S4).
TABLE 1.
Baseline characteristics of participants included by quartiles of the MEDAS‐14 score.
| Total | Quartile 1 (least healthy) | Quartile 2 | Quartile 3 | Quartile 4 (healthiest) | |
|---|---|---|---|---|---|
| n (%) | 162 999 (100) | 55 165 (33.8) | 31 401 (19.3) | 54 838 (33.6) | 21 595 (13.3) |
| Original score, mean (SD) | 5.3 (2.0) | 3.1 (1.0) | 5.0 (0) | 6.4 (0.5) | 8.5 (0.7) |
| Baseline age, years; mean (SD) | 56.2 (7.9) | 55.8 (8.1) | 56.2 (8.0) | 56.4 (7.8) | 56.4 (7.7) |
| Sex, n (%) | |||||
| Women | 89 555 (54.9) | 26 991 (48.9) | 17 034 (54.2) | 31 939 (58.2) | 13 591 (62.9) |
| Men | 73 444 (45.1) | 28 174 (51.1) | 14 367 (45.8) | 22 899 (41.8) | 8004 (37.1) |
| Deprivation index, mean (SD) | −1.6 (2.8) | −1.6 (2.9) | −1.7 (2.8) | −1.7 (2.8) | −1.5 (2.9) |
| Ethnicity, n (%) | |||||
| White | 156 532 (96.0) | 53 199 (96.4) | 30 129 (96.0) | 52 523 (95.8) | 20 681 (95.8) |
| Others | 6467 (4.0) | 1966 (3.6) | 1272 (4.0) | 2315 (4.2) | 914 (4.2) |
| Smoking status, n (%) | |||||
| Never | 92 738 (56.9) | 31 459 (57.0) | 18 122 (57.7) | 31 246 (57.0) | 11 911 (55.2) |
| Previous | 58 195 (35.7) | 18 753 (34.0) | 10 894 (34.7) | 20 130 (36.7) | 8418 (39.0) |
| Current | 12 066 (7.4) | 4953 (9.0) | 2385 (7.6) | 3462 (6.3) | 1266 (5.8) |
| Type of physical activity, n (%) | |||||
| Walking for pleasure (not as a means of transport) | 122 292 (75.0) | 38 844 (70.4) | 23 423 (74.6) | 42 631 (77.7) | 17 394 (80.6) |
| Other exercises (e.g. swimming, cycling, keep fit, bowling) | 20 231 (12.4) | 7236 (13.1) | 3927 (12.5) | 6552 (12.0) | 2516 (11.7) |
| Strenuous sports | 1288 (0.8) | 480 (0.9) | 240 (0.8) | 419 (0.7) | 149 (0.6) |
| Light DIY (e.g. pruning, watering the lawn) | 9476 (5.8) | 4117 (7.5) | 1892 (6.0) | 2671 (4.9) | 796 (3.7) |
| Heavy DIY (e.g. weeding, lawn mowing, carpentry, digging) | 3020 (1.9) | 1369 (2.5) | 604 (1.9) | 803 (1.5) | 244 (1.1) |
| None of the above or prefer not to answer | 6692 (4.1) | 3119 (5.6) | 1315 (4.2) | 1762 (3.2) | 496 (2.3) |
| Alcohol frequency intake, n (%) | |||||
| Daily or almost daily | 37 852 (23.2) | 9504 (17.2) | 6613 (21.1) | 14 690 (26.8) | 7045 (32.6) |
| 3‐4 times a week | 41 334 (25.4) | 11 913 (21.6) | 7751 (24.7) | 15 231 (27.8) | 6439 (29.8) |
| Once or twice a week | 40 435 (24.8) | 14 905 (27.0) | 8106 (25.8) | 12 853 (23.4) | 4571 (21.2) |
| 1‐3 times a month | 17 759 (10.9) | 7408 (13.4) | 3705 (11.8) | 5099 (9.3) | 1547 (7.2) |
| Special occasions only | 15 809 (9.7) | 6979 (12.7) | 3260 (10.4) | 4325 (7.9) | 1242 (5.8) |
| Never | 9810 (6.0) | 4456 (8.1) | 1966 (6.2) | 2637 (4.8) | 751 (3.4) |
| Hyperglycaemia/diabetes, yes; n (%) | 23 156 (14.2) | 8321 (15.1) | 4423 (14.1) | 7521 (13.7) | 2891 (13.4) |
| Low HDL, yes; n (%) | 28 566 (17.5) | 11 862 (21.5) | 5616 (17.9) | 8398 (15.3) | 2690 (12.5) |
| High triglycerides, yes; n (%) | 60 655 (37.2) | 23 300 (42.4) | 11 950 (38.1) | 18 912 (34.5) | 6413 (29.7) |
| Central obesity, yes; n (%) | 48 168 (29.6) | 19 188 (34.8) | 9547 (30.4) | 14 710 (26.8) | 4723 (21.9) |
| High blood pressure/hypertension, yes; n (%) | 110 657 (67.9) | 38 631 (70.0) | 21 443 (68.3) | 36 711 (66.9) | 13 872 (64.2) |
Note: Descriptive characteristics by quartiles of the score are presented as means (SD) for quantitative variables and as frequencies and percentages for categorical variables.
Abbreviations: HDL, high‐density lipoprotein; n, number; SD, standard deviation.
Associations between the quartiles of the standardized diet scores and severe NAFLD are available in Table 2. Overall, there was a trend whereby the risk of severe NAFLD became lower moving from the lowest to the highest quartile (p < .05) (Models 0 and 1). When the analyses were further adjusted for health‐related and lifestyle factors (Model 3), participants in quartiles 3 and 4 using the MEDAS‐14 and RFS scores, as well as those in quartiles 2 and 3 using the HDI score, showed a significantly lower risk of severe NAFLD incident compared with those in quartile 1 in their respective scores. Even if in all these quartiles the risk was lower, the lowest risk was observed in quartile 4 of the MEDAS‐14 and RFS score [HRMEDAS‐14: 0.76 (95% CI: 0.62‐0.94) and HRRFS: 0.82 (95% CI: 0.69‐0.96)] as well as in quartile 2 in the HDI score [HR: 0.80 (95% CI: 0.70‐0.91)]. After adjusting for energy intake in the sensitivity analyses, the associations remained but were slightly attenuated. Adjusting for energy intake also showed a significant risk reduction for individuals in quartile 4 using the HDI score (17% lower risk) (Table 2). There was no evidence of non‐linearity for any of the continuous scores analysed (Figure S1).
TABLE 2.
Associations between quartiles of five diet scores and incident severe NAFLD.
| Quartile 1 (least healthy) | Quartile 2 | Quartile 3 | Quartile 4 (healthiest) | Trend | |||||
|---|---|---|---|---|---|---|---|---|---|
| MEDAS‐14 | HR (95% CI) | HR (95% CI) | p‐Value | HR (95% CI) | p‐Value | HR (95% CI) | p‐Value | HR (95% CI) | p‐Value |
| Model 1 | 1.00 (Ref.) | 0.90 (0.78, 1.03) | .126 | 0.61 (0.53, 0.69) | <.001 | 0.49 (0.40, 0.60) | <.001 | 0.78 (0.75, 0.83) | <.001 |
| Model 2 | 1.00 (Ref.) | 0.91 (0.79, 1.05) | .199 | 0.62 (0.54, 0.71) | <.001 | 0.49 (0.40, 0.60) | <.001 | 0.79 (0.75, 0.83) | <.001 |
| Model 3 | 1.00 (Ref.) | 1.06 (0.92, 1.22) | .423 | 0.82 (0.71, 0.93) | .003 | 0.76 (0.62, 0.94) | .010 | 0.91 (0.86, 0.96) | <.001 |
| Sensitivity | 1.00 (Ref.) | 1.05 (0.91, 1.21) | .487 | 0.83 (0.72, 0.95) | .007 | 0.77 (0.62, 0.94) | .012 | 0.91 (0.86, 0.96) | .001 |
| RFS | |||||||||
| Model 1 | 1.00 (Ref.) | 0.86 (0.75, 1.00) | .055 | 0.73 (0.64, 0.84) | <.001 | 0.66 (0.57, 0.78) | <.001 | 0.87 (0.83, 0.91) | <.001 |
| Model 2 | 1.00 (Ref.) | 0.87 (0.75, 1.01) | .064 | 0.74 (0.65, 0.85) | <.001 | 0.67 (0.57, 0.79) | <.001 | 0.87 (0.83, 0.91) | <.001 |
| Model 3 | 1.00 (Ref.) | 0.91 (0.79, 1.06) | .228 | 0.84 (0.74, 0.96) | .012 | 0.82 (0.69, 0.96) | .014 | 0.93 (0.88, 0.98) | .003 |
| Sensitivity | 1.00 (Ref.) | 0.92 (0.79, 1.07) | .280 | 0.84 (0.74, 0.97) | .015 | 0.82 (0.69, 0.96) | .017 | 0.93 (0.88, 0.98) | .004 |
| HDI | |||||||||
| Model 1 | 1.00 (Ref.) | 0.78 (0.68, 0.89) | <.001 | 0.75 (0.65, 0.87) | <.001 | 0.74 (0.62, 0.88) | .001 | 0.90 (0.85, 0.94) | <.001 |
| Model 2 | 1.00 (Ref.) | 0.78 (0.68, 0.89) | <.001 | 0.75 (0.65, 0.87) | <.001 | 0.74 (0.62, 0.87) | .001 | 0.89 (0.84, 0.94) | <.001 |
| Model 3 | 1.00 (Ref.) | 0.80 (0.70, 0.91) | .001 | 0.81 (0.70, 0.94) | .006 | 0.84 (0.71, 1.01) | .059 | 0.93 (0.88, 0.98) | .005 |
| Sensitivity | 1.00 (Ref.) | 0.79 (0.69, 0.91) | .001 | 0.80 (0.68, 0.93) | .004 | 0.83 (0.69, 0.99) | .040 | 0.92 (0.87, 0.97) | .004 |
| MDS | |||||||||
| Model 1 | 1.00 (Ref.) | 0.88 (0.71, 1.09) | .234 | 0.68 (0.54, 0.86) | .001 | 0.67 (0.54, 0.84) | <.001 | 0.87 (0.82, 0.92) | <.001 |
| Model 2 | 1.00 (Ref.) | 0.89 (0.72, 1.10) | .296 | 0.69 (0.55, 0.87) | .002 | 0.68 (0.55, 0.85) | .001 | 0.87 (0.82, 0.92) | <0.001 |
| Model 3 | 1.00 (Ref.) | 0.95 (0.77, 1.17) | .627 | 0.80 (0.64, 1.01) | .058 | 0.87 (0.69, 1.08) | .202 | 0.95 (0.90, 1.00) | .062 |
| Sensitivity | 1.00 (Ref.) | 0.93 (0.75, 1.16) | .539 | 0.80 (0.63, 1.01) | .055 | 0.86 (0.68, 1.07) | .182 | 0.95 (0.90, 1.01) | .072 |
| MIND | |||||||||
| Model 1 | 1.00 (Ref.) | 0.80 (0.71, 0.91) | .001 | 0.64 (0.54, 0.77) | <.001 | 0.66 (0.56, 0.77) | <.001 | 0.86 (0.82, 0.90) | <.001 |
| Model 2 | 1.00 (Ref.) | 0.82 (0.72, 0.93) | .002 | 0.66 (0.55, 0.79) | <.001 | 0.67 (0.57, 0.78) | <.001 | 0.86 (0.82, 0.91) | <.001 |
| Model 3 | 1.00 (Ref.) | 0.95 (0.83, 1.08) | .401 | 0.86 (0.72, 1.03) | .107 | 0.94 (0.80, 1.11) | .471 | 0.97 (0.92, 1.02) | .270 |
| Sensitivity | 1.00 (Ref.) | 0.96 (0.84, 1.09) | .491 | 0.88 (0.73, 1.05) | .149 | 0.94 (0.80, 1.11) | .474 | 0.97 (0.92, 1.02) | .292 |
Note: Associations between severe NAFLD and standardized quartiles of the scores were investigated using Cox proportional hazard models. Individuals in the least healthy quartile for each score/index were used as the referent. All analyses were performed using a 2‐year landmark analysis, excluding participants who experienced events within the first 2 years of follow‐up and those with liver disease or alcohol/drug use disorder at baseline. Model 1 was unadjusted. Model 2 was adjusted for age, sex, deprivation and ethnicity. Model 3, as per Model 2, but additionally for the components of the metabolic syndrome (central obesity, high glycaemia/diabetes, high blood pressure/hypertension, low HDL and high triglyceride), smoking and physical activity. As a sensitivity analysis, Model 3 was adjusted by total energy intake removing people who reported unfeasible energy intake (i.e. <800 or >4000 kcal/day). A value of p < .05 was considered statistically significant.
Abbreviations: HDI, healthy diet indicator; MEDAS‐14, Mediterranean Diet Adherence; MDS, Mediterranean diet score; MIND, Mediterranean‐DASH Intervention for Neurodegenerative Delay; NAFLD, non‐alcoholic fatty liver disease; RFS, recommended food score.
Finally, analyses were re‐run and stratified by age categories, sex, alcohol intake and central obesity [Table 3 (MEDAS‐14) and Tables S5–S8 (other scores)]. While similar associations were observed across all studied subgroups using the MEDAS‐14 score, the magnitude of associations was stronger in participants <60 years, men, regular drinkers and individuals with central obesity (Table 3). The subgroup analyses of the other diet quality scores are available in Tables S5‐S8.
TABLE 3.
Associations between quartiles of MEDAS‐14 index and incident severe non‐alcoholic fatty liver disease.
| Total n | Events | Quartile 1 (lest healthy) | Quartile 2 | Quartile 3 | Quartile 4 (healthiest) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI | p‐Value | 95% CI | p‐Value | 95% CI | p‐Value | ||||
| Age, years | |||||||||
| <60 | 95 918 | 768 | 1.00 (Ref.) | 1.03 (0.86, 1.24) | .732 | 0.79 (0.66, 0.95) | .012 | 0.69 (0.52, 0.92) | .012 |
| ≥60 | 67 081 | 602 | 1.00 (Ref.) | 1.10 (0.89, 1.36) | .374 | 0.84 (0.69, 1.03) | .103 | 0.85 (0.64, 1.15) | .292 |
| p interaction | .479 | .315 | .130 | ||||||
| Sex | |||||||||
| Women | 89 555 | 709 | 1.00 (Ref.) | 1.14 (0.94, 1.39) | .188 | 0.94 (0.78, 1.13) | .533 | 0.86 (0.66, 1.14) | .304 |
| Men | 73 444 | 661 | 1.00 (Ref.) | 0.98 (0.80, 1.19) | .806 | 0.69 (0.57, 0.84) | <.001 | 0.68 (0.50, 0.93) | .015 |
| p interaction | .398 | .104 | .625 | ||||||
| Alcohol | |||||||||
| Never/special occ | 25 619 | 368 | 1.00 (Ref.) | 0.98 (0.75, 1.27) | .857 | 0.77 (0.59, 1.01) | .064 | 1.02 (0.66, 1.56) | .941 |
| Regular drinking | 137 380 | 1002 | 1.00 (Ref.) | 1.08 (0.92, 1.28) | .330 | 0.82 (0.70, 0.96) | .012 | 0.72 (0.56, 0.90) | .004 |
| p interaction | .653 | .998 | .070 | ||||||
| Central obesity | |||||||||
| No | 114 831 | 484 | 1.00 (Ref.) | 0.99 (0.77, 1.26) | .914 | 0.80 (0.64, 1.00) | .050 | 0.84 (0.62, 1.14) | .263 |
| Yes | 48 168 | 886 | 1.00 (Ref.) | 1.10 (0.92, 1.30) | .293 | 0.83 (0.70, 0.98) | .027 | 0.71 (0.54, 0.94) | .018 |
| p interaction | .297 | .321 | .970 | ||||||
Note: Associations between severe non‐alcoholic fatty liver disease and standardized quartiles of the scores were investigated using Cox proportional hazard models. Individuals in the least healthy quartile for each score/index were used as the referent. All analyses were performed using a 2‐year landmark analysis, excluding participants who experienced events within the first 2 years of follow‐up and those with liver disease or alcohol/drug use disorder at baseline. Analyses were adjusted for age, sex, deprivation, ethnicity, the components of the metabolic syndrome (central obesity, high glycaemia/diabetes, high blood pressure/hypertension, low HDL and high triglyceride), smoking and physical activity when these were not included as a subgroup. A value of p < .05 was considered statistically significant.
Abbreviations: HDI, healthy diet indicator; MEDAS‐14, Mediterranean Diet Adherence; MDS, Mediterranean diet score; MIND, Mediterranean‐DASH Intervention for Neurodegenerative Delay; Occ, occasions; RFS, recommended food score.
4. DISCUSSION
Using data from five standardized diet scores, we identified a trend whereby the risk of severe NAFLD incidence reduced from the least to most healthy quartile in the least adjusted models. Yet, there were varied strengths of associations where the MEDAS‐14, the RFS and HDI scores were the strongest predictors. This may be because of the components of these scores, which are largely measures of anti‐inflammatory effects. Even if the lowest risk was observed in quartile 4 for the MEDAS‐14 and RFS score, the identified association in quartile 2 for the HDI might be associated with power as in this quartile was classified 26.3% of the included participants, while in quartiles 3 and 4 only 19.8% and 13.5%, respectively. However, irrespective of the quartile, following the HDI, RFS or MEDAS‐14 recommendations could represent optimal dietary approaches to mitigate NAFLD risk.
The magnitude of associations was stronger among participants ≤60 years, men, regular drinkers and people with central obesity. Previous studies have shown that women may have a lower susceptibility to NAFLD until menopause, which is probably because of the protective role of estrogens. 43 , 44 Unfortunately, women with altered estrogen levels, such as post‐menopausal women and women with polycystic ovary syndrome, are more susceptible to NAFLD. 43 , 44 In terms of alcohol, a healthier diet (i.e. those in the highest quartiles in our study) may have decreased the detrimental impact of regular alcohol intake on the liver among those who did not have alcoholic liver disease at baseline in our study. Studies have shown that Western diets combined with alcohol are associated with a higher risk of NAFLD. 45 Yet, further research is needed to identify if a healthier diet may protect the liver against the effect of alcohol in people without liver damage. Regarding central obesity, obesity is a known metabolic risk factor associated with NAFLD. 7 , 9 Therefore, similar to participants with higher alcohol intake levels, those with central obesity may benefit most from a healthier diet.
Different mechanisms have been proposed to explain the known association between an unhealthy diet and NAFLD progression. Among them, diets high in fructose and/or fat have been most frequently investigated. 46 Fat was one of the first macronutrients associated with obesity and metabolic complications. In a recent meta‐analysis, Western diets, characterized by high‐fat dairy and red meat, were associated with a 56% (95% CI: 1.27‐1.92) higher NAFLD risk. 47 Yet, in spite of recommendations to reduce dietary fat intake, the incidence of both obesity and NAFLD continues to rise. Sugars, particularly fructose through sugar‐sweetened consumption, have been particularly important considering their effect on metabolic complications. 46 Excessive sugar intake stimulates de novo lipogenesis, which is later converted into fat, 45 stored as triglycerides and deposited in the liver, potentially resulting in steatosis. 45 Fructose is the monosaccharide that stimulates more hepatic de novo lipogenesis than other monosaccharides such as glucose 46 , 48 or polysaccharides such as starch. 46 , 49 It also induces liver inflammation, promoting insulin resistance, dyslipidemia and steatosis. 50 In addition, unhealthy diets or excessive energy intake may produce gut dysbiosis, leading to higher permeability. This has been linked to more proinflammatory cytokines and reactive oxygen species, increasing NAFLD susceptivity. 44 Although these macronutrients are receiving special attention, the European, 10 American 51 and Asian 52 societies recommend adopting a healthy dietary pattern overall, as this would probably capture the overall impact of diet on health.
Previous prospective studies have investigated the associations between diet and NAFLD. 13 , 14 , 15 , 16 , 17 , 18 , 19 However, to our knowledge, no study has compared which diet score might best differentiate diets associated with high and low risk of NAFLD. Previous studies have tended to focus on adherence to a Mediterranean diet, probably because of the high concentration of polyunsaturated fatty acids, which decreases oxidative stress markers and transaminases. 15 For instance, adherence to this type of diet has been shown to be associated with reduced risk of NAFLD [odds ratio: 0.64 (0.52‐0.78)], 17 less fatty liver accumulation [odds ratio: 0.74 (95% CI: 0.61‐0.90)] 14 and lower risk of hepatic steatosis [risk ratio: 0.85 (95% CI: 0.73‐0.99)]. 16 In Korea, women and men who were in the highest quartile of flour‐based food and meat consumption (high intake of noodles and dumplings, wheat flour and bread, red meat and its products, white meat and its products, eggs, dairy products and beverages) had 55% (95% CI: 1.22‐1.97) and 29% (95% CI: 1.00‐1.67), respectively, higher NAFLD risk than their counterparts in the lowest quartile. 18 In contrast, men and women who followed a prudent pattern (high consumption of potatoes, soybean pastes, beans, tofu, soymilk, green and yellow vegetables, light‐coloured vegetables, kimchi, mushrooms, fruits, fish, shellfish and seaweed) showed a 22% and 36% lower risk. 18 Similar patterns were observed among Chinese adults, where diets rich in sugar [HR: 1.11 (95% CI: 1.01‐1.23)] and an animal diet pattern [HR: 1.22 (95% CI: 1.10‐1.36)] were associated with higher NAFLD risk. 19
Using the UK Biobank study, we investigated the research question in a single, large and well‐characterized general population cohort of middle‐aged and older adults. Analyses were adjusted for a comprehensive set of covariates, including the common drivers of NAFLD. In addition, we could assess whether the associations were consistent across population subgroups. A major driver of potential information bias, knowledge of disease status, was obviated entirely by ascertaining outcomes from routine administrative databases. Finally, estimates of habitual diet were derived from the mean of up to five dietary calls.
This study also has limitations. First, although we included those confounding factors that were considered relevant and for which we had data, residual confounding because of unknown or unmeasured confounders is possible. Second, the diet scores were created from self‐reported data, which may result in some inaccuracies. Diet is subject to recall and misclassification bias and may change over time. This is one of the main limitations of the 24‐h recall method as its accuracy relies on participants' memory and ability to retain information for an extended period. 53 Moreover, we did not have data to create the original diet scores (proxies were used) and the number of participants who had repeated dietary data was <20% of the original cohort. However, there is no reason to suspect a systematic error in relation to associations between dietary quality and future NAFLD risk and, therefore, concern about the disease‐differential recall or misclassification bias. We minimized potential reverse causation by using a 2‐year landmark analysis. Third, ascertainment of NAFLD was based on hospital admission and death records and was, therefore, restricted to more advanced or severe cases of the disease. Biomarkers of disease severity, such as FIB‐4 (Fibrosis‐4 index for liver fibrosis), NFS (NAFLD Fibrosis Score), or APRI (aspartate aminotransferase‐to‐platelet ration index), were unavailable in the UK Biobank study, or the information used to estimate them was only available at baseline assessment. Fifth, we could not estimate MASLD during the follow‐up period as the cardiometabolic criteria requested for its classification were available only during the baseline assessment. 20 Using baseline data, a recent Letter to the Editor from Schneider and Schneider showed that from 10 656 participants with magnetic resonance imaging‐measured steatosis and at least one cardiovascular risk factor, 98.2% were classified as MASLD and the remaining as MetALD (those MASLD who consumed a greater amount of alcohol per week). 54 However, we tried to mitigate the role of metabolic syndrome including its components and alcohol as confounders in our models. Sixth, associations observed in an observational study cannot be assumed to infer causality. Finally, the UK Biobank does not represent the UK population regarding lifestyle and prevalent diseases. Therefore, while risk estimates can be generalized, 55 summary statistics such as prevalence and incidence should not. 56
In conclusion, participants with healthier diets had a lower risk of severe NAFLD. Our study showed that the MEDAS‐14, RFS and HDI scores were the strongest predictors. Moreover, the associations between diet and NAFLD were stronger in people ≤60 years of age, men, regular drinkers and people with central obesity. A healthy diet might protect against NAFLD development irrespective of the specific approach used to assess the healthiness of the diet. However, following these scores, recommendations could represent optimal dietary approaches to mitigate NAFLD risk, which requires substantiation in future randomized controlled trials.
AUTHOR CONTRIBUTIONS
FPR and CCM contributed to the conception and design of the study, advised on all statistical aspects, and interpreted the data. FCM and SPS created the codes for the scores with the support of CCM and KML. FPR performed the literature search and the analyses. All authors critically reviewed this and previous drafts. All authors approved the final draft for submission, with final responsibility for publication. CCM is the guarantor.
FUNDING INFORMATION
The UK Biobank study was established by the Wellcome Trust medical charity, Medical Research Council, Department of Health, Scottish Government and the Northwest Regional Development Agency. It has also had funding from the Welsh Assembly Government and the British Heart Foundation. Katherine M. Livingstone is supported by a National Health and Medical Research Council Emerging Leadership Fellowship (APP1173803). JB receives financial support from the Royal Thai Government Scholarship for her PhD. Solange Parra‐Soto receives financial support from the Chilean Government for doing their PhD (ANID‐Becas Chile).
CONFLICT OF INTEREST STATEMENT
All authors have no conflicts of interest to declare.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1111/dom.15378.
Supporting information
Data S1. Supporting information
ACKNOWLEDGMENTS
This research has been conducted using the UK biobank resource. We are grateful to UK Biobank participants.
Petermann‐Rocha F, Carrasco‐Marin F, Boonpor J, et al. Association of five diet scores with severe NAFLD incidence: A prospective study from UK Biobank. Diabetes Obes Metab. 2024;26(3):860‐870. doi: 10.1111/dom.15378
DATA AVAILABILITY STATEMENT
All UK Biobank information is available online on the webpage https://www.ukbiobank. Data access are available through applications. This research was conducted using the application number 71392.
REFERENCES
- 1. Teng ML, Ng CH, Huang DQ, et al. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2023;29:S32‐s42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2022;7:851‐861. [DOI] [PubMed] [Google Scholar]
- 3. Le MH, Yeo YH, Li X, et al. 2019 global NAFLD prevalence: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2022;20:2809‐2817. [DOI] [PubMed] [Google Scholar]
- 4. Fabbrini E, Klein SS. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ullah R, Rauf N, Nabi G, et al. Role of nutrition in the pathogenesis and prevention of non‐alcoholic fatty liver disease: recent updates. Int J Biol Sci. 2019;15:265‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol. 2018;24:2083‐2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. George ES, Forsyth A, Itsiopoulos C, et al. Practical dietary recommendations for the prevention and management of nonalcoholic fatty liver disease in adults. Adv Nutr. 2018;9:30‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zelber‐Sagi S, Ivancovsky‐Wajcman D, Fliss Isakov N, et al. High red and processed meat consumption is associated with non‐alcoholic fatty liver disease and insulin resistance. J Hepatol. 2018;68:1239‐1246. [DOI] [PubMed] [Google Scholar]
- 9. Rong L, Zou J, Ran W, et al. Advancements in the treatment of non‐alcoholic fatty liver disease (NAFLD). Front Endocrinol (Lausanne). 2022;13:1087260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. EASL‐EASD‐EASO . Clinical practice guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64:1388‐1402. [DOI] [PubMed] [Google Scholar]
- 11. Zelber‐Sagi S, Oren RV. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol. 2011;17:3377‐3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3‐9. [DOI] [PubMed] [Google Scholar]
- 13. Haigh L, Kirk C, El Gendy K, et al. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non‐alcoholic fatty liver disease (NAFLD): a systematic review and meta‐analysis. Clin Nutr. 2022;41:1913‐1931. [DOI] [PubMed] [Google Scholar]
- 14. Ma J, Hennein R, Liu C, et al. Improved diet quality associates with reduction in liver fat, particularly in individuals with high genetic risk scores for nonalcoholic fatty liver disease. Gastroenterology. 2018;155:107‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Videla LA, Hernandez‐Rodas MC, Metherel AH, Valenzuela R. Influence of the nutritional status and oxidative stress in the desaturation and elongation of n‐3 and n‐6 polyunsaturated fatty acids: impact on non‐alcoholic fatty liver disease. Prostaglandins Leukot Essent Fatty Acids. 2022;181:102441. [DOI] [PubMed] [Google Scholar]
- 16. Khalatbari‐Soltani S, Marques‐Vidal P, Imamura F, Forouhi NG. Prospective association between adherence to the Mediterranean diet and hepatic steatosis: the swiss CoLaus cohort study. BMJ Open. 2020;10:e040959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doustmohammadian A, Clark CCT, Maadi M, et al. Favorable association between Mediterranean diet (MeD) and DASH with NAFLD among Iranian adults of the Amol cohort study (AmolCS). Sci Rep. 2022;12:2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fu J, Shin S. Dietary patterns and risk of non‐alcoholic fatty liver disease in Korean adults: a prospective cohort study. BMJ Open. 2023;13:e065198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang S, Gu Y, Bian S, et al. Dietary patterns and risk of non‐alcoholic fatty liver disease in adults: a prospective cohort study. Clin Nutr. 2021;40:5373‐5382. [DOI] [PubMed] [Google Scholar]
- 20. Rinella ME, Lazarus JV, Ratziu V, et al. A multi‐society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;29:101133. [DOI] [PubMed] [Google Scholar]
- 21. Collins R. What makes UK biobank special? Lancet. 2012;379:1173‐1174. [DOI] [PubMed] [Google Scholar]
- 22. Palmer LJ. UK biobank: bank on it. Lancet. 2007;369:1980‐1982. [DOI] [PubMed] [Google Scholar]
- 23. Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS med. 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu B, Young H, Crowe FL, et al. Development and evaluation of the Oxford WebQ, a low‐cost, web‐based method for assessment of previous 24 h dietary intakes in large‐scale prospective studies. Public Health Nutr. 2011;14:1998‐2005. [DOI] [PubMed] [Google Scholar]
- 25. Greenwood DC, Hardie LJ, Frost GS, et al. Validation of the Oxford WebQ online 24‐hour dietary questionnaire using biomarkers. Am J Epidemiol. 2019;188:1858‐1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCance RA, Widdowson EM. McCance and Widdowson's the Composition of Foods. Royal Society of Chemistry; 2014. [Google Scholar]
- 27. Bradbury KE, Young HJ, Guo W, Key TJ. Dietary assessment in UK biobank: an evaluation of the performance of the touchscreen dietary questionnaire. J Nutr Sci. 2018;7:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martínez‐González MA, Fernández‐Jarne E, Serrano‐Martínez M, Wright M, Gomez‐Gracia E. Development of a short dietary intake questionnaire for the quantitative estimation of adherence to a cardioprotective Mediterranean diet. Eur J Clin Nutr. 2004;58:1550‐1552. [DOI] [PubMed] [Google Scholar]
- 29. Papadaki A, Johnson L, Toumpakari Z, et al. Validation of the English version of the 14‐item Mediterranean diet adherence screener of the PREDIMED study, in people at high cardiovascular risk in the UK. Nutrients. 2018;10. https://www.mdpi.com/2072-6643/10/2/138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shannon OM, Ranson JM, Gregory S, et al. Mediterranean diet adherence is associated with lower dementia risk, independent of genetic predisposition: findings from the UK biobank prospective cohort study. BMC med. 2023;21:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Livingstone KM, Abbott G, Bowe SJ, Ward J, Milte C, McNaughton SA. Diet quality indices, genetic risk and risk of cardiovascular disease and mortality: a longitudinal analysis of 77 004 UK biobank participants. BMJ Open. 2021;11:e045362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimers Dement. 2015;11:1007‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Golzarand M, Azizi MP. Adherence to the MIND diet and the risk of cardiovascular disease in adults: a cohort study. Food Funct. 2022;13:1651‐1658. [DOI] [PubMed] [Google Scholar]
- 34. Hagström H, Adams LA, Allen AM, et al. Administrative coding in electronic health care record‐based research of NAFLD: an expert panel consensus statement. Hepatology. 2021;74:474‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Townsend PPM, Beattie A. Health and Deprivation. Inequality and the North. Health Policy; 1988:10. [Google Scholar]
- 36. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC med. 2011;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo W, Bradbury KE, Reeves GK, Key TJ. Physical activity in relation to body size and composition in women in UK biobank. Ann Epidemiol. 2015;25:406‐413.e406. [DOI] [PubMed] [Google Scholar]
- 40. Petermann‐Rocha F, Gray SR, Forrest E, et al. Associations of muscle mass and grip strength with severe NAFLD: a prospective study of 333,295 UK biobank participants. J Hepatol. 2022;76:1021‐1029. [DOI] [PubMed] [Google Scholar]
- 41. Govindarajulu US, Malloy EJ, Ganguli B, Spiegelman D, Eisen EA. The comparison of alternative smoothing methods for fitting non‐linear exposure‐response relationships with cox models in a simulation study. Int J Biostat. 2009;5. https://www.degruyter.com/document/doi/10.2202/1557-4679.1104/html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ho FK, Cole TJ. Non‐linear predictor outcome associations. BMJ Med. 2023;2:e000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Della Torre S. Beyond the X factor: relevance of sex hormones in NAFLD pathophysiology. Cell. 2021;10:2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dolce A, Della Torre S. Sex, nutrition, and NAFLD: relevance of environmental pollution. Nutrients. 2023;15:2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Skinner RC, Hagaman JA. The interplay of Western diet and binge drinking on the onset, progression, and outlook of liver disease. Nutr Rev. 2021;80:503‐512. [DOI] [PubMed] [Google Scholar]
- 46. Inci MK, Park SH, Helsley RN, Attia SL, Softic S. Fructose impairs fat oxidation: implications for the mechanism of western diet‐induced NAFLD. J Nutr Biochem. 2023;114:109224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hassani Zadeh S, Hosseinzadeh Mansoori A (2021) Relationship between dietary patterns and non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. J Gastroenterol Hepatol 36, 1470–1478. [DOI] [PubMed] [Google Scholar]
- 48. Lecoultre V, Egli L, Carrel G, et al. Effects of fructose and glucose overfeeding on hepatic insulin sensitivity and intrahepatic lipids in healthy humans. Obesity. 2013;21:782‐785. [DOI] [PubMed] [Google Scholar]
- 49. Laube H, Klör H, Fussgänger R, et al. The effect of starch, sucrose, glucose and fructose on lipid metabolism in rats. Ann Nutr Metab. 1973;15:273‐280. [DOI] [PubMed] [Google Scholar]
- 50. Coronati M, Baratta F, Pastori D, Ferro D, Angelico F, del Ben M. Added fructose in non‐alcoholic fatty liver disease and in metabolic syndrome: a narrative review. Nutrients. 2022;14(6):1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. The diagnosis and management of nonalcoholic fatty liver disease . Practice guidance from the American Association for the Study of Liver Diseases. Clin Liver Dis (Hoboken). 2018;11(4):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eslam M, Sarin SK, Wong VW, et al. The Asian Pacific Association for the Study of the liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889‐919. [DOI] [PubMed] [Google Scholar]
- 53. Osadchiy T, Poliakov I, Olivier P, Rowland M, Foster E. Progressive 24‐hour recall: usability study of short retention intervals in web‐based dietary assessment surveys. J med Internet Res. 2020;22:e13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schneider KM, Schneider CVA. New era for steatotic liver disease: evaluating the novel nomenclature in the UK biobank. J Hepatol. 2023;30:876‐881. [DOI] [PubMed] [Google Scholar]
- 55. Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health‐related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Batty GD, Gale CR, Kivimäki M, Deary IJ, Bell S. Comparison of risk factor associations in UK biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta‐analysis. BMJ. 2020;368:m131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information
Data Availability Statement
All UK Biobank information is available online on the webpage https://www.ukbiobank. Data access are available through applications. This research was conducted using the application number 71392.


