Abstract
Background
The natural polyphenolic compound known as Rosmarinic acid (RosA) can be found in various plants. Although its potential health benefits have been extensively studied, its effect on osteoarthritis (OA) progression and cartilage regeneration function still needs to be fully elucidated in OA animal models. This study elucidated the effect of RosA on OA progression and cartilage regeneration.
Methods
In vitro assessments were conducted using RT-PCR, qRT-PCR, Western blotting, and ELISA to measure the effects of RosA. The molecular mechanisms of RosA were determined by analyzing the translocation of p65 into the nucleus using immunocytochemistry (ICC). Histological analysis of cartilage explant was performed using alcian blue staining and immunohistochemistry (IHC). For in vivo analysis, the destabilization of the medial meniscus (DMM)-induced OA mouse model was utilized to evaluate cartilage destruction through Safranin-O staining. The expression of catabolic and anabolic factors in mice knee joints was quantified by immunohistochemistry.
Results
The expression of catabolic factors in chondrocytes was significantly impeded by RosA. It also suppressed the NF-κB signaling pathway by decreasing phosphorylation of p65 and reducing degradation of IκB protein. In ex vivo experiments, RosA protected sulfated proteoglycan erosion triggered by IL-1β and suppressed the catabolic factors in cartilage explant. RosA treatment in animal models resulted in preventing cartilage destruction and reducing catabolic factors in the cartilage. RosA was also found to promote the expression of Sox9, Col2a1, and Acan in vitro, ex vivo, and in vivo analyses.
Conclusions
RosA attenuated the OA progression by suppressing the catabolic factors expression. These effects were facilitated through the suppression of the NF-κB signaling pathway. Additionally, it promotes cartilage regeneration by inducing anabolic factors. Therefore, RosA shows potential as an effective therapeutic agent for treating OA.
Keywords: Osteoarthritis, Rosmarinic acid, Extracellular matrix, NF-κB, Sox9
Abbreviations
- Cox2
Cyclooxygenase2
- DMM
Destabilization of the medial meniscus
- i.a:
Intra-articular
- IκB
I kappa B
- IL-1β
Interleukin-1beta
- MMPs
Matrix metalloproteinases
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- OA
Osteoarthritis
- OARSI
Osteoarthritis Research Society International
- PGE2
Prostaglandin E2
- RosA
Rosmarinic acid
- Sox9
SRY-Box transcription factor 9
1. Introduction
OA is a complex joint disease that progressively degrades articular cartilage, resulting in pain, stiffness, and limited joint function [1]. Chondrocytes are specialized cells within the cartilage that are essential for preserving the equilibrium of catabolic and anabolic processes that regulate the turnover of Extracellular matrix (ECM) components in the cartilage, notably Type II collagen and aggrecan [2]. However, various factors, such as mechanical stress, inflammation, imbalanced signaling pathways, and cellular changes, collectively disrupt the equilibrium of anabolic and catabolic activities within chondrocytes. This disruption leads to an imbalance in which the breakdown of matrix components outweighs their synthesis, resulting in gradual cartilage tissue erosion over time [3,4].
The major pathological mechanism underlying cartilage degradation involves activating catabolic factors, specifically matrix metalloproteinases (MMPs) [5]. Within the MMPs, MMP3 and MMP13 are particularly important in the pathogenesis of OA. These enzymes can remodel the ECM, involving the degradation of type II collagen and aggrecan in cartilage [6,7]. Another critical player in OA is Cyclooxygenase2 (Cox2), an enzyme responsible for prostaglandin E2 synthesis [8]. Prostaglandins E2 serve as potent mediators of inflammation and pain. Increased Cox2 expression in osteoarthritic joints leads to enhanced production of inflammatory prostaglandins E2, perpetuating the inflammatory cascade and contributing to joint deterioration [[8], [9], [10]]. The upregulation of Mmps and Cox2 is often induced by pro-inflammatory cytokines, including Interleukin-1beta (IL-1β). The pro-inflammatory mediator IL-1β has been identified as a key factor in the process of cartilage degradation [10,11]. Within chondrocytes, it triggers several signaling pathways, notably the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway [7,12,13].
NF-κB is a transcription factor that regulates inflammatory responses, cell survival, and differentiation. NF-κB comprises p65 and p50 subunits and remains inactive in the cytoplasm, bound in a complex with IκB protein [14,15]. However, in OA, IL-1β leads to the breakdown of the IκB protein and the p65 phosphorylation. Subsequently, p65 migrates into the nucleus, where it promotes the catabolic factors expression and suppresses the SRY-Box Transcription Factor 9 (Sox9) expression, further promoting cartilage degradation [16,17]. The transcription factor Sox9 is essential for both the development and maintenance of cartilage [18]. It is pivotal in the differentiation of mesenchymal stem cells into chondrocytes, the cells responsible for cartilage formation. Sox9 is involved in promoting the production of ECM components, which includes type II collagen and proteoglycans, like aggrecan. Impaired Sox9 function can accelerate the progression of degenerative arthritis by disrupting cartilage formation and repair [19,20].
Currently, the primary treatment option for OA is the use of nonsteroidal anti-inflammatory drugs (NSAIDs) [21]. However, these treatments primarily focus on relieving symptoms and can cause a variety of side effects, including gastrointestinal issues, cardiovascular risks, and kidney function impairment [22,23]. These side effects arise because NSAIDs can damage the stomach lining, increase blood pressure, and reduce blood flow to the kidneys. Furthermore, since cartilage does not regenerate once damaged, effective treatment for OA requires drugs that can relieve symptoms and promote cartilage regeneration [[23], [24], [25]].
Rosmarinic acid (RosA), a natural polyphenolic compound, is prevalent in various plants, such as oregano (Origanum vulgare), basil (Ocimum basilicum), and rosemary (Rosmarinus officinalis) [26]. It is chemically defined as α-o-caffeoyl-3, 4-dihydroxyphenyllactic acid, featuring a structure that combines caffeic acid and 3, 4-dihydroxyphenyllactic acid (Fig. S1A). This configuration enables it to scavenge free radicals effectively and enhances its solubility and stability, making RosA a valuable compound for various health-related applications [26,27]. The potential health benefits of RosA have been extensively studied. It is recognized for its potent antioxidant, anti-inflammatory, neuroprotective effects, and antimicrobial activity [[28], [29], [30], [31]]. Recent studies have demonstrated the effectiveness of RosA in treating degenerative joint diseases in chondrocytes of rats and rabbits [32,33]. However, the role of RosA in most degenerative conditions, including OA and cartilage regeneration, remains unclear. Additionally, the efficacy of RosA in treating osteoarthritis has not been completely revealed in pre-clinical animal models.
Here, we demonstrated through a pre-clinical animal model that RosA treatment, through the induction of Sox9, restored the expression of Type II collagen and Aggrecan that had been reduced by IL-1β. Additionally, our findings provided evidence that the suppression of the NF-κB pathway led to a reduction in the expression of Mmp3, Mmp13, and Cox2. Furthermore, we sought to determine whether RosA has therapeutic potential in preventing OA development and promoting cartilage regeneration.
2. Material and method
2.1. Reagents and treatment
RosA was obtained from Sigma-Aldrich (St. Louis, MO, USA) and reconstituted in 100 % ethanol. Recombinant IL-1β proteins used in this study were obtained from Gen Script (Piscataway, NJ, USA) and dissolved in sterile double-distilled water for treatment. In our experimental setup, primary chondrocytes in culture were simultaneously treated with IL-1β (1 ng/ml) and RosA at indicated concentrations (10, 50, and 100 μM) for 24 h [34,35].
2.2. Animal
The Institutional Animal Care and Use Committee (IACUC) of Ajou University reviewed and authorized our animal study protocol (protocol number 2023-0016). Male C57BL/6 mice weighing 18–20 g were obtained from DBL (Chungcheongbuk-do, South Korea). These mice were maintained in a controlled environment with a temperature of 25 °C, humidity of approximately 50 %, and a 12 h light-dark cycle. Throughout the study, mice had unrestricted access to both chow and water, allowing them to consume food and water freely according to their requirements.
2.3. Primary mouse articular chondrocyte culture
Articular chondrocytes were acquired from 5-day-old ICR mice purchased from DBL. The chondrocytes were harvested from each mouse's tibial plateaus and femoral condyles. Cartilage tissue underwent enzymatic digestion employing 0.2 % type II collagenase, following a previously established procedure [7,35]. Subsequently, the isolated cells were maintained in a culture medium consisting of Dulbecco's Modified Eagle's Medium (DMEM), enriched with 10 % fetal bovine serum (FBS) and 1 % antibiotic solution.
2.4. Cytotoxicity analysis
Following the manufacturer's guidelines, cytotoxicity was determined through the EZ-Cytox Cell Viability Assay Kit (DoGenBio, Seoul, Korea). Briefly, primary cultured chondrocytes were plated in a 96-well plate at a density of 9 × 103 cells per well and incubated for 48 h before treatment. RosA was applied at the specified concentrations (50, 100, and 200 μM), after which the cells were incubated for 24 h in serum-free DMEM. For the WST-1 assay, a solution of 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2, 4-disulfophenyl)-2H-tetrazolium (WST-1) mixed with DMEM (without FBS) at a ratio of 1:100 (v/v) was added to the chondrocytes and incubated for 3 h in the dark. Subsequently, a microplate reader (BioTek Instruments, Winooski, VT, USA) was used to measure the absorbance at 450 nm.
2.5. Cartilage explant culture
Femur and tibia cartilage explants were obtained from 5-day-old ICR mice. These isolated explants were cultured in DMEM supplemented with 10 % FBS, IL-1β, and RosA for 72 h. The culture medium, IL-1β, and RosA were renewed every 24 h during the incubation. After a 72 h incubation period, all cartilage explants underwent fixation in 4 % paraformaldehyde solution for 30 min. Subsequently, the fixed explants were subjected to dehydration, embedding, and sectioning, where the sections were sliced to a thickness of 5 μm. Proteoglycan restoration was assessed using Alcian blue staining [36].
2.6. Real-time PCR and quantitative (q)RT-PCR analysis
Total mRNA was isolated from primary chondrocytes utilizing TRIzol reagent (Molecular Research Center Inc., Cincinnati, OH, USA), followed by reverse transcription to generate cDNA for PCR amplification. The specific primers used for PCR amplification are listed in Table A.1. To measure the expression levels of each target gene, we conducted a qRT-PCR using SYBR® Premix Ex Taq (TaKaRa Bio, Shiga, Japan). Gapdh served as the housekeeping gene to normalize the expression levels of each target gene.
2.7. Collagenase and prostaglandin E2 (PGE2) assay
To assess PGE2 production, primary cultured chondrocytes were plated in 96-well plates at a density of 9 × 103 cells per well and maintained for 48 h before treatment. Subsequently, RosA was administered at indicated concentrations of 10, 50, and 100 μM. PGE2 production was analyzed in the supernatant of chondrocytes using a Prostaglandin E2 ELISA kit (Abcam, Cambridge, UK) per the manufacturer's instructions. The level of collagenase activity present in the chondrocyte culture supernatant was quantified using the EnzCheck Gelatinase/Collagenase Assay Kit (Thermo Scientific, Waltham, MA, USA) per the manufacturer's guidelines. The measurements were performed at 490/530 nm wavelengths using a Microplate Reader [7].
2.8. Western blotting
To analyze protein expression in mouse articular chondrocytes, total cell lysates were extracted using a RIPA buffer containing 100 mM NaCl, 1 % NP-40, 10 mM Tris, 2 mM EDTA, and 50 mM NaF supplemented with an additional 1 mM phosphatase and protease inhibitors. For western blot analysis, we used the antibodies: anti-Mmp3, anti-Mmp13, anti-Cox2 (Abcam), anti-p65, anti-Phospho p65, and anti-IκB (Cell Signaling Technology, Beverly, MA, USA). To determine the Mmp3 and Mmp13 protein levels, we isolated secreted proteins from the cell culture supernatant using the trichloroacetic acid precipitation method. Densitometry analyses were performed to quantify band intensities using the Image J software. GAPDH served as the loading control for the protein analysis.
2.9. Experimental arthritis mouse model and intra-articular (i.a) injection
A mouse model of arthritis was established by subjecting 10-week-old male C57BL/6 mice to DMM surgery, following the standard protocol (N = 5). For histological analysis, we extracted the mice's knee joints 10 weeks post-surgery and immersed them in a 4 % paraformaldehyde solution for fixation. The joints then underwent a decalcification procedure lasting two weeks, after which they were embedded in paraffin. To evaluate the degree of cartilage degradation, we applied Safranin O staining to the tissue sections. The severity of the observed damage was then quantified using the scoring method established by the Osteoarthritis Research Society International (OARSI) [7,35]. For the intra-articular (i.a) injection of RosA, mice received injections twice a week for 5 weeks, after which the mice were euthanized for further analysis. Immunohistochemical analysis was conducted on sections of mouse knee joints and cartilage explant using antibodies for anti-Mmp3, anti-Mmp13, anti-Cox2 (abcam), anti-Type II collagen (Thermo Scientific), anti-Aggrecan (Abclonal, Woburn, MA, USA), anti-Sox9, anti-IκB and anti - phospho-p65 (Cell Signaling Technology).
2.10. Immunofluorescence staining
Chondrocyte cells were cultured on coverslips and treated with 50 and 100 μM concentrations of RosA for 24 h. After incubation, the cells were fixed through a 20-min treatment with a 4 % paraformaldehyde solution. Subsequently, the cells underwent permeabilization with 0.1 % Triton X-100 (VWR Life Science, Radnor, Pennsylvania, USA) for 5 min. Subsequently, cells were blocked using 10 % FBS (Gibco, Montana, USA) in 1х PBS and then exposed to primary antibodies targeting NF-κB (Cell Signaling Technology) in blocking buffer at 4 °C overnight. Cells were rinsed with 1 × PBS, then exposed to secondary antibodies conjugated to Alexa Fluor (Sigma-Aldrich) for 1.5 h. For nuclear staining, the cells were counterstained with 4,6-diamidino-2-phenylindole (Tocris, Bristol, UK) for 1 min. The coverslips with the cells were mounted using an amino gel/mount solution (Biomeda, San Jose, USA), and images were acquired utilizing a Nikon A1R microscope at the Three-Dimensional Immune System Imaging Core Facility of Ajou University. Using the NIS software, data analysis was performed.
2.11. Statistical analysis
Data are presented as the mean ± standard error of the mean. The histological samples were independently prepared by two researchers to ensure accuracy and reliability. To validate the results and increase statistical power, each experiment was replicated a minimum of three times independently. One-way ANOVA using Dunnett's multiple post-analysis comparison test or one-way ANOVA followed by Bonferroni post-test was used for data analysis. Significance was determined at a p-value of ≤0.05, indicating that differences observed in the data were unlikely due to random chance and were likely to result from the experimental treatments or conditions being compared.
3. Results
3.1. RosA suppresses catabolic factor expression induced by IL-1β in chondrocytes
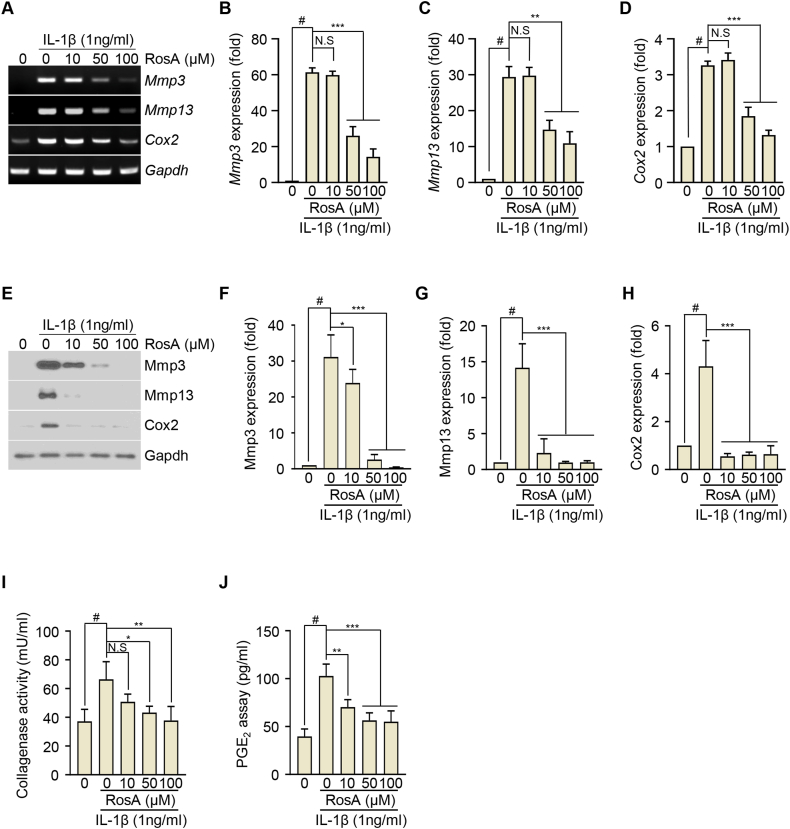
We initially investigated the potential cytotoxic effects of RosA on chondrocytes using a WST-1 assay. Chondrocytes were exposed to indicated concentrations of RosA (50, 100, and 200 μM) for 24h. Fig. S1B demonstrates that chondrocyte viability remained comparable to the control group. This suggests that RosA did not exhibit cytotoxicity at the concentrations used in the experiment. Based on these non-toxic results, we selected 10, 50, and 100 μM concentrations for further experimental treatments lasting 24 h. The presence of catabolic factors including Mmp3, Mmp13, and Cox2 regulates cartilage degradation in OA. Therefore, we aimed to investigate whether RosA could modulate the expression of these factors. Chondrocytes were exposed to IL-1β, a widely recognized pro-inflammatory cytokine that stimulates the expression of catabolic factors. RT-PCR analysis revealed that IL-1β stimulation of chondrocytes resulted in a time-dependent upregulation of Mmp3, Mmp13, and Cox2 expression levels (Fig. S2A). This was further confirmed by densitometry (Figs. S2B–D) and qRT-PCR (Figs. S2E–G). Next, we exposed the chondrocytes to a combined treatment of IL-1β and RosA at specific concentrations (10, 50, and 100 μM) for 24 h. The results demonstrated that exposure to IL-1β led to a notable upregulation in the expression of Mmp3, Mmp13, and Cox2, indicating cartilage degradation. However, co-treatment of RosA resulted in a reduction of IL-1β-mediated upregulation of Mmp3, Mmp13, and Cox2, as confirmed by both RT-PCR (Fig. 1A), densitometry (Fig. 1B–D) and qRT-PCR (Figs. S3A–C) analyses. To validate these observations at the protein level, western blot assays were conducted (Fig. 1E), followed by densitometry analysis (Fig. 1F–H). These experiments further confirmed RosA's suppressive effect on the expression of Mmp3, Mmp13, and Cox2. We also assessed collagenase activity and PGE2 production. Treatment with RosA decreased the IL-1β-induced collagenase activity (Fig. 1I) and PGE2 (Fig. 1J) production. These results suggest that RosA protects against OA progression by inhibiting Mmp3, Mmp13, and Cox2.
Fig. 1.
RosA inhibits Mmp3, Mmp13, and Cox2 expression induced by IL-1β in articular chondrocytes.
(A, B, C, D) Chondrocytes were co-treated with IL-1β and RosA for 24 h Mmp3, Mmp13, and Cox2 expression were evaluated by RT-PCR (A), densitometry (B; Mmp3, C; Mmp13, and D; Cox2), western blot (E) and densitometry (F; Mmp3, G; Mmp13, H; Cox2) analysis. Collagenase activity (I) and PGE2 production (J) were detected in chondrocytes cultured medium. Values are presented as the mean ± standard deviation. One-way analysis of variance followed by Bonferroni's test was used to determine significant differences. None significant (N.S), ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, #P < 0.05 compared to the control group. Original gel (Fig. S6) and blots (Fig. S7) can be seen in supplementary figures file.
3.2. RosA restores the IL-1β induced sulfated proteoglycan erosion in cartilage explant
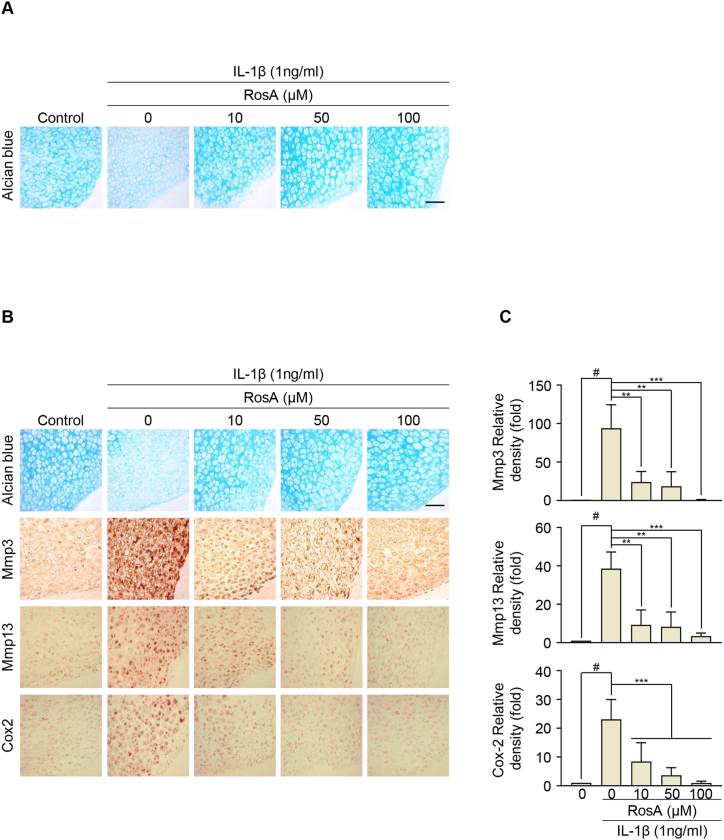
We evaluated the potential protective effects of RosA against cartilage destruction by conducting ex vivo experiments using cartilage explants. These explants were cultured for 72 h in the presence and absence of IL-1β and RosA. We analyzed the erosion of extracellular sulfate proteoglycans using Alcian blue staining. Exposure of the cartilage explants to IL-1β resulted in a reduction of sulfated proteoglycan content. However, co-treatment with RosA and IL-1β demonstrated protective effects against the reduction of sulfated proteoglycan in the cartilage explants (Fig. 2A). Immunohistochemical staining revealed that Mmp3, Mmp13, and Cox2 expression in cartilage explants was decreased by RosA treatment (Fig. 2B and C; upper; Mmp3, middle; Mmp13, lower; Cox2). This result is consistent with our in vitro experiments, suggesting that RosA effectively countered the reduction of extracellular sulfate proteoglycans.
Fig. 2.
RosA protects against extracellular matrix (ECM) degradation in cartilage explants.
(A) Mouse knee cartilage explants were co-treated with IL-1β and RosA at the indicated concentration. The accumulation of sulfated-proteoglycans was evaluated by Alcian blue staining. (B) Expression of Mmp3, Mmp13, and Cox2 in cartilage explant after co-treated with IL-1β and RosA assessed by Immunohistochemical staining and (C) quantification (Mmp3; upper panel, Mmp13; middle panel, Cox2; lower panel). Scale bars, 100 μm. Values are presented as the mean ± standard deviation. One-way analysis of variance followed by Bonferroni's test was used to determine significant differences ∗∗P < 0.01, ∗∗∗P < 0.001, #P < 0.05 compared to the control group.
3.3. RosA inhibits cartilage destruction in the DMM-induced OA model
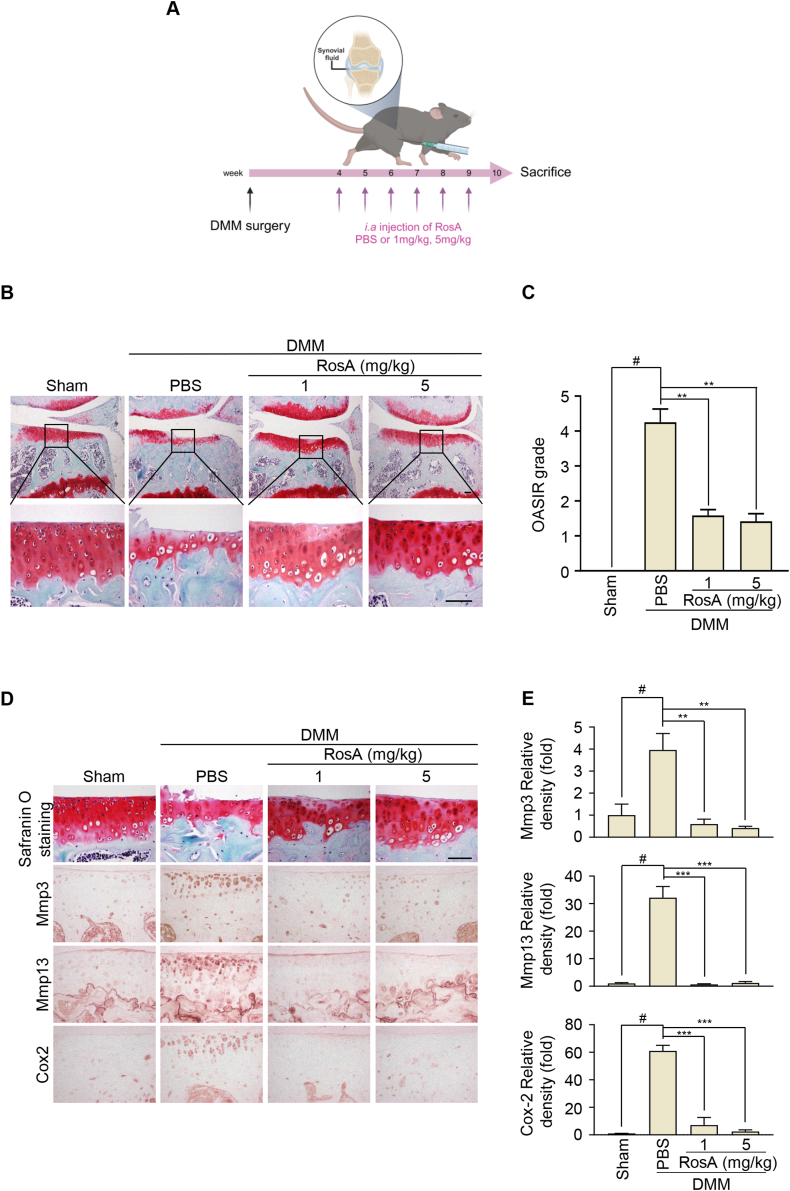
To investigate the potential protective effects of RosA against cartilage destruction, we conducted an in vivo study using a DMM-induced OA model. Mice were (i.a) - injected with PBS or RosA once a week for a total of 6 weeks, starting at 4 weeks after DMM (Fig. 3A). Compared to the control group, (i.a) - injection of RosA effectively prevented cartilage destruction (Fig. 3B). Furthermore, the RosA-treated group exhibited significantly reduced OARSI scores compared to the DMM group (Fig. 3C). These results indicated that RosA prevents cartilage destruction. Furthermore, immunohistochemistry and densitometry analyses indicated that DMM-induced OA mice treated with RosA injections exhibited a notable reduction in Mmp3, Mmp13, and Cox2 protein expression (Fig. 3D and E; upper: Mmp3, middle: Mmp13, lower: Cox2). Our findings indicate that RosA exhibits a protective effect against cartilage degradation in the DMM-induced OA model, through the suppression of Mmp3, Mmp13, and Cox2 expression.
Fig. 3.
RosA suppresses cartilage degradation in the DMM-induced OA mice model.
(A) Experimental framework outlining the establishment of the DMM-induced OA model. (B) Following DMM induction, mice received intra-articular (i.a) injections of RosA for 6 weeks. The degree of cartilage degradation was assessed using Safranin-O staining. (C) OA severity was quantified utilizing the OARSI scoring system at 10 weeks post-DMM induction. (D) Immunohistochemical staining was performed to assess the Mmp3, Mmp13, and Cox2 protein levels in experimental OA mice after i,a injection of RosA and (E) quantification. Scale bars, 100 μm. Values are presented as the mean ± standard deviation. One-way analysis of variance followed by Bonferroni's test was used to determine significant differences ∗∗P < 0.01, ∗∗∗P < 0.001, #P < 0.05 compared to the control group.
3.4. RosA inhibits the NF-κB signaling pathway in the articular chondrocyte
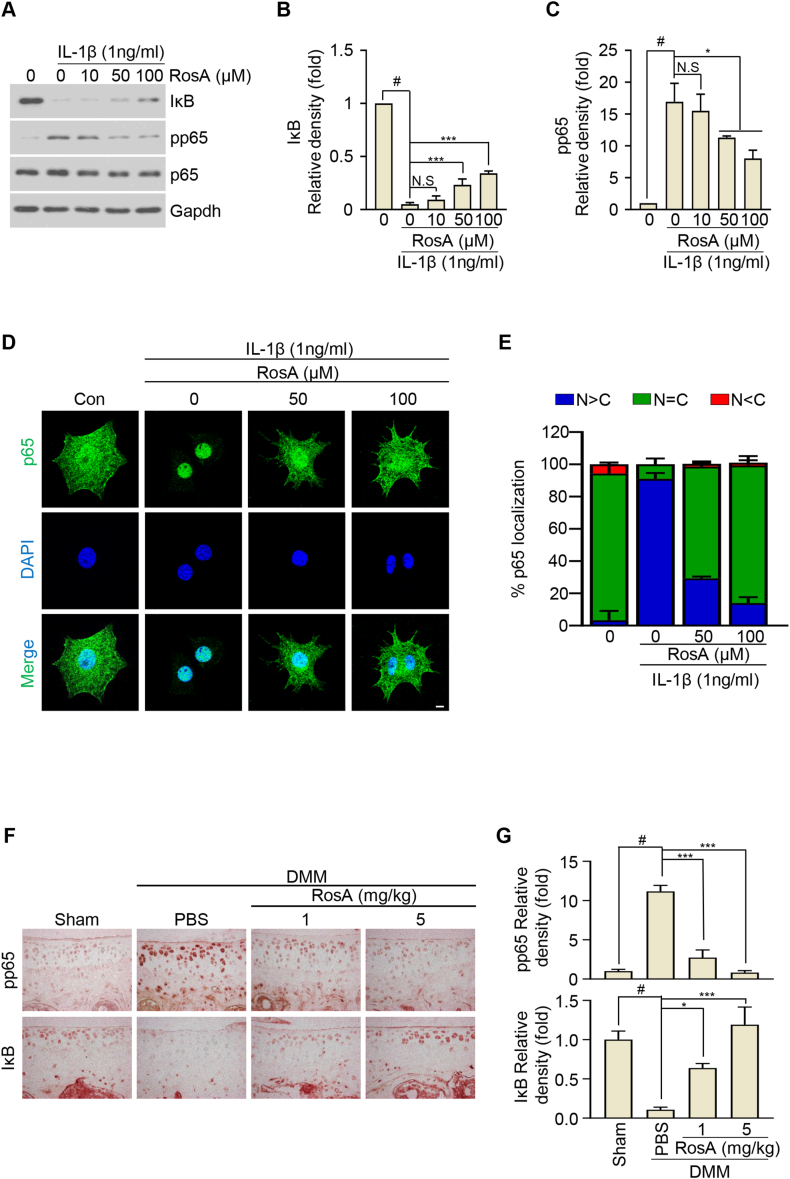
Next, to examine RosA's potential to inhibit the NF-κB signaling cascade, we cultured articular chondrocytes for 24 h in the absence or presence of indicated concentrations of RosA. After incubation for 24 h, we treated with IL-1β in chondrocytes for 15 min. RosA markedly inhibited the phosphorylated p65 by IL-1β compared with the control. Further, we also confirmed that IL-1β induced IκB degradation was inhibited by RosA treatment, as determined by western blot (Fig. 4A) and analyzed densitometry (Fig. 4B and C). Additionally, immunocytochemistry and densitometry analysis reveal that RosA treatment inhibited the IL-1β-induced nuclear translocation of p65 (Fig. 4D and E). Furthermore, the effects of RosA were also investigated in a DMM-induced OA mouse model to extend our findings to in vivo conditions. Notably, phosphorylated p65 and IκB degradation were reduced in the cartilage of mice treated with RosA (Fig. 4F and G). These data suggest that RosA inhibits the NF-κB signaling pathway by preventing p65 phosphorylation and IκB protein degradation. These findings indicate that RosA effectively protects against cartilage degradation through suppressing NF-κB signaling pathway.
Fig. 4.
RosA suppresses the expression of catabolic factors via the NF-κB signaling pathway.
(A, B, C) Chondrocytes were exposed to RosA at the indicated concentration for 24 h before being treated to IL-1β (1 ng/ml) for 15 min. Western blot analysis was performed to evaluate the Phosphorylation of p65 and the degradation of IκB (A) and densitometry analysis (B; IκB, C; pp65). (D) Chondrocytes were subjected to immunofluorescence staining for p65 (green) and 4′, 6-diamidino-2-phenylindole (DAPI) (blue) was used for nuclear staining. (E) The nuclear (N)-cytoplasmic (C) ratio of p65 was analyzed in three randomly chosen regions from three separate experiments (n = 100 cells per field). Scale bars, 10 μm. (F) The pp65 and IκB protein levels in OA mice cartilage after i,a-injection of RosA assessed by Immunohistochemical staining and (G) quantification. Scale bars, 100 μm. Values are presented as the mean ± standard deviation. One-way analysis of variance followed by Bonferroni's test was used to determine significant differences. None significant (N.S), ∗P < 0.05, ∗∗∗P < 0.001, #P < 0.05 compared to the control group. Original blots (Fig. S8) can be seen in supplementary figures file.
3.5. RosA mediates cartilage regeneration via Sox9 induction
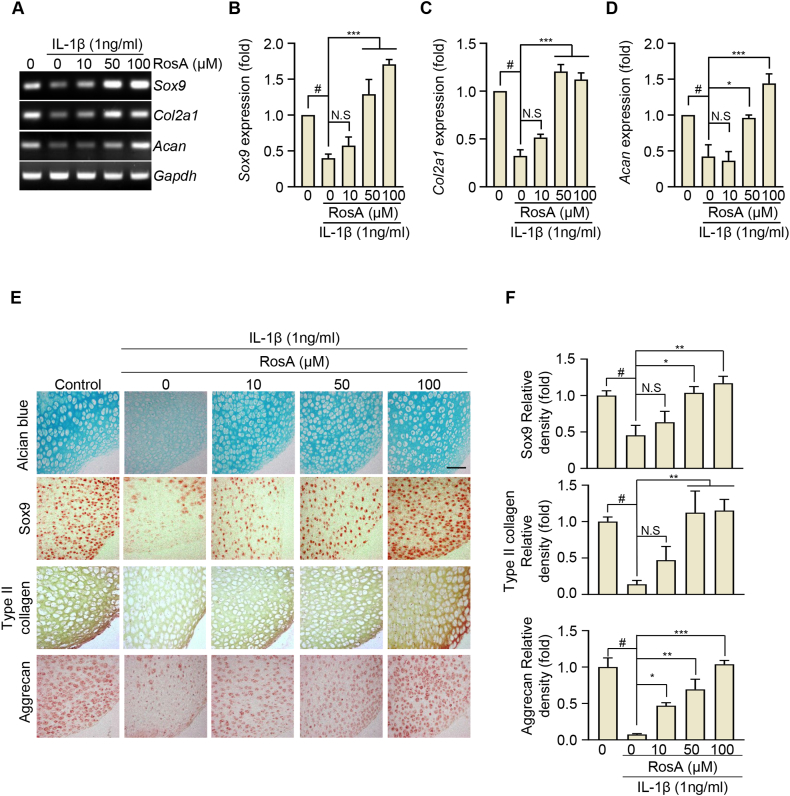
We also investigated whether RosA promoted the synthesis of Col2a1 and Acan through Sox9 expression. Initially, IL-1β treatment of chondrocytes resulted in a time-dependent reduction of Sox9, Col2a1, and Acan expression, as determined by RT-PCR (Fig. S4A) followed by densitometry (Figs. S4B–D) and qRT-PCR (Figs. S4E–G). To examine whether RosA could enhance the expression of Sox9, Col2a1, and Acan which IL-1β reduced in chondrocytes, we co-administered IL-1β and RosA to chondrocytes for 24 h. We observed that RosA dose-dependently upregulated the expression of Sox9, Col2a1, and Acan effectively countering the reduction caused by IL-1β, as confirmed by both RT-PCR (Fig. 5A), densitometry (Fig. 5B–D) and qRT-PCR (Figs. S5A–C) analyses. This phenomenon was also evident in ex vivo cartilage explants, where RosA treatment consistently recovered the expression of Sox9, Type II collagen, and Aggrecan that was diminished by IL-1β (Fig. 5E and F; upper: Sox9, middle; Type II collagen, lower; Aggrecan). Furthermore, in vivo analyses consistently revealed a decrease in the protein levels of Sox9, Type II collagen, and Aggrecan in the DMM-induced OA group. However, these levels were substantially restored upon (i.a) - injection of RosA (Fig. 6A and B; upper: Sox9, middle; Type II collagen, lower; Aggrecan). These data suggest that RosA effectively restores the expression of anabolic factors.
Fig. 5.
RosA restores the expression of Type II collagen, Aggrecan and Sox9 reduced by OA progression.
(A, B, C, D) Chondrocytes were co-treated with IL-β and RosA for 24 h Sox9, Col2a1, and Acan expressions were evaluated by RT-PCR (A) and densitometry (B; Sox9, C; Col2a1, and D; Acan) analysis. (E) Sox9, Type II collagen and Aggrecan expressions in cartilage explant after being co-treated with IL-1β and RosA measured by Immunohistochemical staining and (F) quantification (Sox9; upper panel, Type II collagen; middle panel, Aggrecan; lower panel). Values are presented as the mean ± standard deviation. One-way analysis of variance followed by Bonferroni's test was used to determine significant differences. None significant (N.S), ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and #P < 0.05 compared to the control group. Original gel (Fig. S9) can be seen in supplementary figures file.
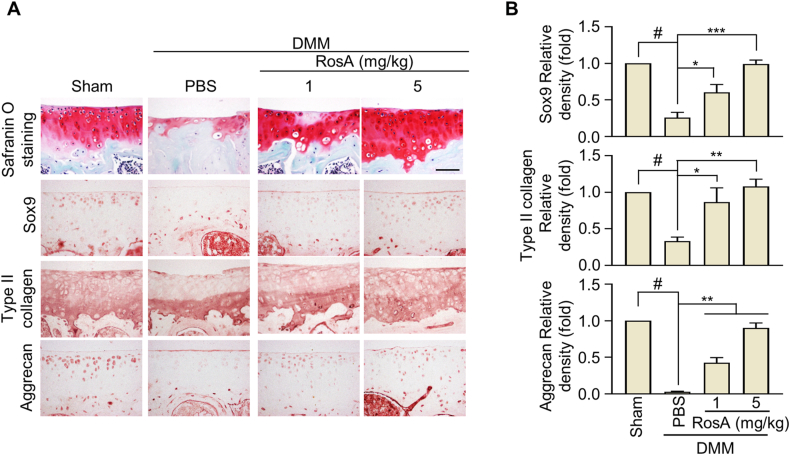
Fig. 6.
RosA induced the expression of Type II collagen, Aggrecan and Sox9 protein in DMM-induced OA mice model.
(A) The Sox9, Type II collagen, and Aggrecan, protein levels were assessed through Immunohistochemical staining in the cartilage of OA mice following i,a - injection of RosA, and (B) quantification was performed. Scale bars, 100 μm. Values are presented as the mean ± standard deviation. One-way analysis of variance followed by Bonferroni's test was used to determine significant differences. None significant (N.S), ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, #P < 0.05 compared to the Sham group.
4. Discussions
OA is characterized by the destruction of ECM components in cartilage, which are crucial for smooth joint function and shock absorption [37,38]. Current therapeutic options for OA, such as non-steroidal anti-inflammatory drugs (NSAIDs), primarily aim to relieve symptoms by reducing pain and inflammation. However, these medications do not facilitate cartilage regeneration and are often associated with side effects that can exacerbate joint damage over time [[23], [24], [25]]. In this context, RosA, a naturally occurring polyphenol, emerges as a promising therapeutic alternative with the potential to alleviate symptoms and promote cartilage regeneration.
RosA exhibits a broad spectrum of beneficial biological activities in managing OA. Its potent antioxidant properties help neutralize oxidative stress, a known contributor to cellular damage in chondrocytes. Furthermore, RosA exerts significant anti-inflammatory effects by modulating inflammatory pathways, which may alleviate the chronic inflammation characteristic of OA [25,39,40]. Significantly, our study has explored the molecular mechanisms underlying RosA's beneficial effects in OA. We discovered that RosA treatment effectively inhibited proteoglycan loss and attenuated the expression of critical catabolic enzymes, including MMPs and Cox2 in chondrocytes and cartilage explant tissues. Additionally, results from the DMM-induced OA animal model confirmed that RosA prevents cartilage breakdown and suppresses the expression of catabolic factors. These enzymes are critical in breaking ECM proteins, suggesting that RosA helps maintain cartilage integrity. Furthermore, RosA modulates the IL-1β-mediated signaling pathway, a major contributor to the progression of OA. Our findings confirmed that RosA attenuated NF-κB activity, thereby reducing the expressions of catabolic factors. Many studies indicate that suppression of the NF-κB pathway leads to a decrease in catabolic factors production in chondrocytes, which reduces cartilage destruction and inhibits the progression of OA. Moreover, studies indicated that various molecules targeting the NF-κB pathway can effectively block the progression of OA [10,35,41]. Therefore, RosA suppresses the NF-κB signaling pathway and diminishes the production of catabolic factors, suggesting its potential crucial role in mitigating OA progression.
Our research further demonstrates that RosA significantly promotes the production of essential ECM components, including type II collagen and aggrecan. These components play a crucial role in preserving the structural stability and functional characteristics of cartilage tissue. Enhanced expression of Sox9, a transcription factor crucial for chondrogenesis, was observed in both in vitro and in vivo OA models treated with RosA. The activation of these anabolic factors by RosA enables chondrocytes to produce matrix components more effectively, supporting the repair process of cartilage damage. Several studies have indicated that the expression of Sox9 during OA treatment can lead to the expression of type II collagen and aggrecan and that these effects can be beneficial for cartilage regeneration and OA treatment [[42], [43], [44]]. This suggests that RosA prevents degradation and actively contributes to the anabolic processes of cartilage repair and regeneration.
Despite these promising findings, current research has limitations, primarily relating to extrapolating animal model results to human clinical cases. While animal models help understand disease mechanisms and test initial hypotheses, they often do not fully reproduce the complex pathology and heterogeneity of humans. Further clinical trials are required to validate the therapeutic benefits and safety of RosA in osteoarthritis patients, focusing mainly on long-term treatment outcomes. These studies will help determine the appropriate dose, duration of treatment, and potential side effects to effectively and safely translate the promising effects observed in animal studies into the clinic.
Collectively, our findings suggest that RosA has the potential not only for symptomatic relief in OA but also for addressing the underlying biological causes of cartilage degradation. By promoting cartilage regeneration and inhibiting inflammatory pathways, RosA may offer a more sustainable and effective treatment strategy for OA, highlighting its potential as a novel therapeutic agent in the ongoing fight against this debilitating disease. Further research is needed to confirm these benefits in a clinical setting and fully understand the scope of RosA's therapeutic effects.
5. Conclusions
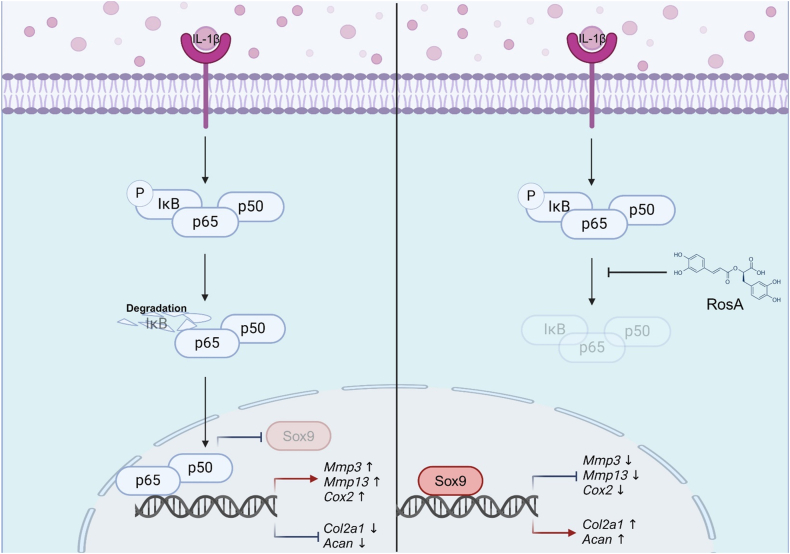
In conclusion, this study highlights the therapeutic potential of RosA in osteoarthritis. We have demonstrated that RosA effectively suppresses the expression of catabolic factors, preserves cartilage integrity, and inhibits critical inflammatory mediators within a DMM-induced OA model. Additionally, RosA modulates the NF-κB signaling pathway and enhances the expression of essential cartilage maintenance genes, including Sox9, Col2a1, and Acan, countering their reduction induced by IL-1β, as depicted in Fig. 7. Collectively, these results indicated that RosA shows promise as a potential therapeutic option for OA, with dual actions on inflammation suppression and molecular pathway modulation crucial for cartilage health. These findings position RosA as a promising candidate for OA treatment, addressing inflammation and influencing crucial molecular pathways. Further research and clinical trials are necessary to substantiate these findings and establish the efficacy and safety of RosA in managing OA.
Fig. 7.
A schematic abstract illustrating RosA's effects on both inhibiting catabolic factors and promoting ECM synthesis through the inhibition of the NF-κB pathway.
Data availability statement
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.
Funding
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (2021R1I1A1A01051938, NRF-2020R1A2C2011016). This research was supported by a Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education. (grant no. 2019R1A6C1010003). This research was supported by the Korea Initiative for Fostering the University of Research and Innovation Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. NRF2021M3H1A104892211). This work was supported by research fund of Ajou University Medical Center (2023)
Ethics approval
The entire animal experimental protocol was conducted with approval from the Ajou University Animal Care and Use Committee (protocol number: 2023-0016).
CRediT authorship contribution statement
Ye Eun Sim: Methodology, Formal analysis, Data curation. Cho-Long Kim: Writing – review & editing, Methodology, Formal analysis, Data curation. Dong Hyun Kim: Formal analysis, Data curation. Ji-Ae Hong: Methodology, Formal analysis, Data curation. In-Jeong Lee: Methodology, Formal analysis, Data curation. Jong-Young Kwak: Writing – review & editing, Validation, Methodology. Li-Jung Kang: Writing – original draft, Supervision, Funding acquisition, Conceptualization. Jung-Soon Mo: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by research fund of Ajou University Medical Center (2023)
Footnotes
Table A.1.
| Gene | Origin | Strand | Sequence | Size (bp) |
ATa (°C) |
|---|---|---|---|---|---|
| Mmp3 | Mouse |
bS cAs |
5'-CTGTGTGTGGTTGTGTGCTCATCCTAC-3' 5'-GGCAAATCCGGTGTATAATTCACAATC-3' |
350 | 60 |
| Mmp13 | Mouse | S As |
5'-TGATGGACCTTCTGGTCTTCTGGC-3' 5'-CATCCACATGGTTGGGAAGTTCTG-3' |
473 | 60 |
| Cox-2 | Mouse | S As |
5'-GGTCTGGTGCCTGGTCTGATGAT-3' 5'-GTCCTTTCAAGGAGAATGGTGC-3' |
724 | 63 |
| Col2a1 | Mouse | S As |
5'-CACACTGGTAAGTGGGGCAAGA-3' 5'-GGATTGTGTTGTTTCAGGGTTCG-3' |
173 | 58 |
| Acan | Mouse | S As |
5'-CCTGCTACTTCATCGACCC-3' 5'-AGATGCTGTTGACTCGAACCT-3' |
150 | 60 |
| Sox9 | Mouse | S As |
5'-GAGCCGGATCTGAAGAGGGA-3' 5'- GCTTGACGTGTGGCTTGTTC-3' |
151 | 58 |
| Gapdh | Mouse | S As |
5'-TCACTGCCACCCAGAAGAC-3' 5'-TGTAGGCCATGAGGTCCAC-3' |
450 | 60 |
Contributor Information
Li-Jung Kang, Email: rkdflwnd@aumc.ac.kr.
Jung-Soon Mo, Email: j5mo@ajou.ac.kr.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Yao Q., Wu X., Tao C., Gong W., Chen M., Qu M., Zhong Y., He T., Chen S., Xiao G. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct. Targeted Ther. 2023;8(1):56. doi: 10.1038/s41392-023-01330-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rapp A.E., Zaucke F. Cartilage extracellular matrix-derived matrikines in osteoarthritis. Am J Physiol Cell Physiol. 2023;324(2):C377–c394. doi: 10.1152/ajpcell.00464.2022. [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum F., Griffin T.M., Liu-Bryan R. Review: metabolic regulation of inflammation in osteoarthritis. Arthritis Rheumatol. 2017;69(1):9–21. doi: 10.1002/art.39842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujii Y., Liu L., Yagasaki L., Inotsume M., Chiba T., Asahara H. Cartilage homeostasis and osteoarthritis. Int. J. Mol. Sci. 2022;23(11) doi: 10.3390/ijms23116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehana E.E., Khafaga A.F., El-Blehi S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: an updated review. Life Sci. 2019;234 doi: 10.1016/j.lfs.2019.116786. [DOI] [PubMed] [Google Scholar]
- 6.Dong Y., Lin L., Ji Y., Cheng X., Zhang Z. Cabozantinib prevents AGEs-induced degradation of type 2 collagen and aggrecan in human chondrocytes. Aging (Albany NY) 2023;15(23):13646–13654. doi: 10.18632/aging.205186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang L.J., Yoon J., Rho J.G., Han H.S., Lee S., Oh Y.S., Kim H., Kim E., Kim S.J., Lim Y.T., et al. Self-assembled hyaluronic acid nanoparticles for osteoarthritis treatment. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120967. [DOI] [PubMed] [Google Scholar]
- 8.Jiang W., Jin Y., Zhang S., Ding Y., Huo K., Yang J., Zhao L., Nian B., Zhong T.P., Lu W., et al. PGE2 activates EP4 in subchondral bone osteoclasts to regulate osteoarthritis. Bone Research. 2022;10(1):27. doi: 10.1038/s41413-022-00201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jo H.G., Baek C.Y., Kim D., Kim S., Han Y., Park C., Song H.S., Lee D. Network analysis, in vivo, and in vitro experiments identified the mechanisms by which Piper longum L. [Piperaceae] alleviates cartilage destruction, joint inflammation, and arthritic pain. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1282943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang G., Lee S.A., Hong J.H., Park B.R., Kim D.K., Kim C.S. Chondroprotective effects of 4,5-dicaffeoylquinic acid in osteoarthritis through NF-κB signaling inhibition. Antioxidants. 2022;11(3) doi: 10.3390/antiox11030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miao Z., Dong M., Wang Z., Ma J., Lin Y., Wu Y. Linalool inhibits the progression of osteoarthritis via the Nrf2/HO-1 signal pathway both in vitro and in vivo. Int Immunopharmacol. 2022;113(Pt A) doi: 10.1016/j.intimp.2022.109338. [DOI] [PubMed] [Google Scholar]
- 12.Avenoso A., D'Ascola A., Scuruchi M., Mandraffino G., Calatroni A., Saitta A., Campo S., Campo G.M. Hyaluronan in the experimental injury of the cartilage: biochemical action and protective effects. Inflamm. Res. 2018;67(1):5–20. doi: 10.1007/s00011-017-1084-9. [DOI] [PubMed] [Google Scholar]
- 13.Xu Z., Ke T., Zhang Y., Guo L., Chen F., He W. Danshensu inhibits the IL-1β-induced inflammatory response in chondrocytes and osteoarthritis possibly via suppressing NF-κB signaling pathway. Mol Med. 2021;27(1):80. doi: 10.1186/s10020-021-00329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X., Qian F., Fan Q., Lin L., He M., Li P., Cai H., Ma L., Cheng X., Yang X. NF-κB activation impedes the transdifferentiation of hypertrophic chondrocytes at the growth plate of mouse embryos in diabetic pregnancy. J Orthop Translat. 2021;31:52–61. doi: 10.1016/j.jot.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fei J., Liang B., Jiang C., Ni H., Wang L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed. Pharmacother. 2019;109:1586–1592. doi: 10.1016/j.biopha.2018.09.161. [DOI] [PubMed] [Google Scholar]
- 16.Kang D.G., Lee H.J., Lee C.J., Park J.S. Inhibition of the expression of matrix metalloproteinases in articular chondrocytes by resveratrol through affecting nuclear factor-kappa B signaling pathway. Biomol Ther (Seoul) 2018;26(6):560–567. doi: 10.4062/biomolther.2018.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng D., Kang X., Wang R., Chen H., Zhang K., Feng W., Li H., Zhu Y., Wu S. Progranulin modulates cartilage-specific gene expression via sirtuin 1-mediated deacetylation of the transcription factors SOX9 and P65. J. Biol. Chem. 2020;295(39):13640–13650. doi: 10.1074/jbc.RA119.011164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horváth E., Sólyom Á., Székely J., Nagy E.E., Popoviciu H. Inflammatory and metabolic signaling interfaces of the hypertrophic and senescent chondrocyte phenotypes associated with osteoarthritis. Int. J. Mol. Sci. 2023;24(22) doi: 10.3390/ijms242216468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haseeb A., Kc R., Angelozzi M., de Charleroy C., Rux D., Tower R.J., Yao L., Pellegrino da Silva R., Pacifici M., Qin L., et al. SOX9 keeps growth plates and articular cartilage healthy by inhibiting chondrocyte dedifferentiation/osteoblastic redifferentiation. Proc Natl Acad Sci U S A. 2021;118(8) doi: 10.1073/pnas.2019152118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q., Ji Q., Wang X., Kang L., Fu Y., Yin Y., Li Z., Liu Y., Xu X., Wang Y. SOX9 is a regulator of ADAMTSs-induced cartilage degeneration at the early stage of human osteoarthritis. Osteoarthritis Cartilage. 2015;23(12):2259–2268. doi: 10.1016/j.joca.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Katz J.N., Arant K.R., Loeser R.F. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325(6):568–578. doi: 10.1001/jama.2020.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magni A., Agostoni P., Bonezzi C., Massazza G., Menè P., Savarino V., Fornasari D. Management of osteoarthritis: expert opinion on NSAIDs. Pain Ther. 2021;10(2):783–808. doi: 10.1007/s40122-021-00260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richard M.J., Driban J.B., McAlindon T.E. Pharmaceutical treatment of osteoarthritis. Osteoarthritis Cartilage. 2023;31(4):458–466. doi: 10.1016/j.joca.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Cooke M.E., Lawless B.M., Jones S.W., Grover L.M. Matrix degradation in osteoarthritis primes the superficial region of cartilage for mechanical damage. Acta Biomater. 2018;78:320–328. doi: 10.1016/j.actbio.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 25.Armiento A.R., Alini M., Stoddart M.J. Articular fibrocartilage - why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev. 2019;146:289–305. doi: 10.1016/j.addr.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Sik B., Kapcsándi V., Székelyhidi R., Hanczné E.L., Ajtony Z. Recent advances in the analysis of rosmarinic acid from herbs in the lamiaceae family. Nat. Prod. Commun. 2019;14(7) doi: 10.1177/1934578X19864216. 1934578X19864216. [DOI] [Google Scholar]
- 27.Bardakcı H., Akaydin G., Kırmızıbekmez H., Yesilada E. Validated HPTLC method for quantification of rosmarinic acid in seven Salvia species. Planta Med. 2011;77 doi: 10.1055/s-0031-1282229. [DOI] [Google Scholar]
- 28.Fachel F.N.S., Schuh R.S., Veras K.S., Bassani V.L., Koester L.S., Henriques A.T., Braganhol E., Teixeira H.F. An overview of the neuroprotective potential of rosmarinic acid and its association with nanotechnology-based delivery systems: a novel approach to treating neurodegenerative disorders. Neurochem. Int. 2019;122:47–58. doi: 10.1016/j.neuint.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Hahn H.J., Kim K.B., An I.S., Ahn K.J., Han H.J. Protective effects of rosmarinic acid against hydrogen peroxide-induced cellular senescence and the inflammatory response in normal human dermal fibroblasts. Mol. Med. Rep. 2017;16(6):9763–9769. doi: 10.3892/mmr.2017.7804. [DOI] [PubMed] [Google Scholar]
- 30.Ivanov M., Kostić M., Stojković D., Soković M. Rosmarinic acid–Modes of antimicrobial and antibiofilm activities of a common plant polyphenol. South Afr. J. Bot. 2022;146:521–527. doi: 10.1016/j.sajb.2021.11.050. [DOI] [Google Scholar]
- 31.Rocha J., Eduardo-Figueira M., Barateiro A., Fernandes A., Brites D., Bronze R., Duarte C.M., Serra A.T., Pinto R., Freitas M., et al. Anti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin. Pharmacol. Toxicol. 2015;116(5):398–413. doi: 10.1111/bcpt.12335. [DOI] [PubMed] [Google Scholar]
- 32.Chen W.P., Jin G.J., Xiong Y., Hu P.F., Bao J.P., Wu L.D. Rosmarinic acid down-regulates NO and PGE(2) expression via MAPK pathway in rat chondrocytes. J. Cell Mol. Med. 2018;22(1):346–353. doi: 10.1111/jcmm.13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eo S.H., Kim S.J. Rosmarinic acid induces rabbit articular chondrocyte differentiation by decreases matrix metalloproteinase-13 and inflammation by upregulating cyclooxygenase-2 expression. J. Biomed. Sci. 2017;24(1):75. doi: 10.1186/s12929-017-0381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim C.L., Shin Y.S., Choi S.H., Oh S., Kim K., Jeong H.S., Mo J.S. Extracts of perilla frutescens var. Acuta (odash.) kudo leaves have antitumor effects on breast cancer cells by suppressing YAP activity. Evid Based Complement Alternat Med. 2021;2021 doi: 10.1155/2021/5619761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han S.J., Lim M.J., Lee K.M., Oh E., Shin Y.S., Kim S., Kim J.S., Yun S.P., Kang L.J. Safflower seed extract attenuates the development of osteoarthritis by blocking NF-κB signaling. Pharmaceuticals. 2021;14(3) doi: 10.3390/ph14030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee H., Jang D., Jeon J., Cho C., Choi S., Han S.J., Oh E., Nam J., Park C.H., Shin Y.S., et al. Seomae mugwort and jaceosidin attenuate osteoarthritic cartilage damage by blocking IκB degradation in mice. J. Cell Mol. Med. 2020;24(14):8126–8137. doi: 10.1111/jcmm.15471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorvina A., Antoniou M., Esmaeili Z., Kochetkova M. Unusual suspects: bone and cartilage ECM proteins as carcinoma facilitators. Cancers. 2023;15(3) doi: 10.3390/cancers15030791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urlić I., Ivković A. Cell sources for cartilage repair-biological and clinical perspective. Cells. 2021;10(9) doi: 10.3390/cells10092496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dahchour A. Anxiolytic and antidepressive potentials of rosmarinic acid: a review with a focus on antioxidant and anti-inflammatory effects. Pharmacol. Res. 2022;184 doi: 10.1016/j.phrs.2022.106421. [DOI] [PubMed] [Google Scholar]
- 40.Kernou O.-N., Azzouz Z., Madani K., Rijo P. Application of rosmarinic acid with its derivatives in the treatment of microbial pathogens. Molecules. 2023;28(10):4243. doi: 10.3390/molecules28104243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi M.C., Jo J., Park J., Kang H.K., Park Y. NF-κB signaling pathways in osteoarthritic cartilage destruction. Cells. 2019;8(7) doi: 10.3390/cells8070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagata K., Hojo H., Chang S.H., Okada H., Yano F., Chijimatsu R., Omata Y., Mori D., Makii Y., Kawata M., et al. Runx2 and Runx3 differentially regulate articular chondrocytes during surgically induced osteoarthritis development. Nat. Commun. 2022;13(1):6187. doi: 10.1038/s41467-022-33744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song H., Park K.H. Regulation and function of SOX9 during cartilage development and regeneration. Semin. Cancer Biol. 2020;67(Pt 1):12–23. doi: 10.1016/j.semcancer.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Jeon J., Kang L.J., Lee K.M., Cho C., Song E.K., Kim W., Park T.J., Yang S. 3'-Sialyllactose protects against osteoarthritic development by facilitating cartilage homeostasis. J. Cell Mol. Med. 2018;22(1):57–66. doi: 10.1111/jcmm.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.