Abstract
The envelope protein E of the flavivirus tick-borne encephalitis (TBE) virus is, like the alphavirus E1 protein, a class II viral fusion protein that differs structurally and probably mechanistically from class I viral fusion proteins. The surface of the native TBE virion is covered by an icosahedrally symmetrical network of E homodimers, which mediate low-pH-induced fusion in endosomes. At the pH of fusion, the E homodimers are irreversibly converted to a homotrimeric form, which we have found by intrinsic fluorescence measurements to be more stable than the native dimers. Thus, the TBE virus E protein is analogous to the prototypical class I fusion protein, the influenza virus hemagglutinin (HA), in that it is initially synthesized in a metastable state that is energetically poised to be converted to the fusogenic state by exposure to low pH. However, in contrast to what has been observed with influenza virus HA, this transition could not be triggered by input of heat energy alone and membrane fusion could be induced only when the virus was exposed to an acidic pH. In a previous study we showed that the dimer-to-trimer transition appears to be a two-step process involving a reversible dissociation of the dimer followed by an irreversible trimerization of the dissociated monomeric subunits. Because the dimer-monomer equilibrium in the first step apparently depends on the protonation state of E, the lack of availability of monomers for the trimerization step at neutral pH could explain why low pH is essential for fusion in spite of the metastability of the native E dimer.
The capacity to undergo triggered conformational changes is a crucial property of viral surface glycoproteins that mediate the fusion of the viral membrane with a cellular membrane during entry. Not only do structural changes in these fusion proteins lead to the exposure of a previously buried functional element (fusion peptide), but the energy released by these conformational changes is also believed to be necessary for the fusion process (16, 30).
It is a characteristic feature of many viral fusion proteins that they are synthesized in an inactive precursor form that either must itself be proteolytically cleaved for activation or requires the cleavage of an accessory protein. This generates a metastable state that is potentially fusion active and can be irreversibly converted to a more stable conformation by the fusion trigger (receptor binding or low pH) (16). The fusion protein of rhabdoviruses is an exception because the low-pH-induced conformational change to the fusion-active state is reversible (for a review, see reference 9).
The fusion proteins that exhibit irreversible conformational changes can be divided into at least two general classes. Class I includes the fusion proteins of orthomyxo-, retro-, paramyxo- and filoviruses, all of which are activated by proteolytic cleavage (20). They are synthesized as trimers, their fusion peptides are at or near the amino terminus (7, 17, 23, 30), and their final (postfusion) state contains a characteristic α-helical coiled-coil core structure (28, 30). Class II includes the fusion proteins of flaviviruses and alphaviruses, both of which form part of an icosahedral envelope structure that rearranges upon exposure to low pH, the physiological trigger of fusion. The class II fusion proteins are not proteolytically cleaved during maturation but are associated with a second protein whose cleavage is essential for activation of fusion activity (12, 19). They possess internal fusion peptides (12, 19) and, unlike the class I proteins, are not predicted to form coiled coils (27). The fusion protein E of the flavivirus tick-borne encephalitis (TBE) virus was the first class II fusion protein for which the structure was determined to atomic resolution (25), but very recently, the X-ray structure of the E1 protein of the alphavirus Semliki Forest virus (SFV) was solved to 3.5 Å and shown to have very similar structural features (21).
Flaviviruses enter cells via the endocytic pathway, and fusion of the viral membrane with the endosomal membrane is induced by the acidic pH in the endosome (12). The native E protein is a membrane-anchored homodimer that lies flat on the surface of the virion and forms a regular icosahedral lattice through specific lateral interactions (8, 25). Exposure to low pH triggers a concerted rearrangement of the lattice structure, resulting in an irreversible and quantitative conversion of the E dimers to a homotrimeric state of yet unknown structure (2). This rearrangement is believed to initiate the fusion process by causing an internal fusion peptide to become exposed and attach to the target membrane (1), and it might also be involved in subsequent membrane merger steps. The fusion process itself is triggered by low pH and does not require the presence of specific proteins and—in contrast to alphaviruses—does not have an absolute requirement for specific lipids in the target membrane (6).
Earlier studies with the class I fusion protein of influenza virus (hemagglutinin, HA) showed that fusion activity as well as the associated conformational changes that are normally triggered by acidic pH could also be induced by elevated temperatures and other protein-destabilizing conditions such as urea treatment (5, 11, 26). Since the final form is more stable, it was concluded that native HA is kinetically trapped in a metastable state (4). The induction of fusion at elevated temperatures has also been observed with other class I fusion proteins, namely, the F proteins of the paramyxoviruses Sendai virus (31) and simian virus 5 (24). In contrast, with SFV, whose fusion protein belongs to class II, it was not possible to induce fusion by heat or urea treatment (10).
In this study we investigated the relative stability of native and low-pH forms of the TBE virus E protein and the ability of low pH and elevated temperatures to induce the dimer-trimer transition and membrane fusion. We show that the trimeric form of the E protein is more stable than the native dimeric form. However, in contrast to what occurs with the class I fusion proteins of orthomyxoviruses and paramyxoviruses, and similarly to what occurs with the E1 fusion protein of SFV, elevated temperatures cannot substitute for low pH as a trigger.
MATERIALS AND METHODS
Virus growth and purification.
The TBE virus prototype strain Neudoerfl (22) was grown in primary chicken embryo cells, harvested 48 h after infection, and purified by two cycles of sucrose density gradient centrifugation (14). For membrane fusion assays the virions were metabolically labeled with 1-pyrenehexadecanoic acid (Molecular Probes, Leiden, The Netherlands) as described previously (6).
Preparation of E rosettes.
E rosettes were obtained by centrifugation of Triton X-100-solubilized untreated or low-pH pretreated virions in an SW 40 rotor (Beckman) at 38,000 rpm and 4°C for 24 h in 10 to 40% (wt/wt) detergent-free sucrose density gradients in TAN buffer (0.05 M triethanolamine [pH 8.0], 0.1 M NaCl) with a top layer of 5% (wt/wt) sucrose containing 0.25% Triton X-100 (13). Fractions were collected by upward displacement, and E protein was quantitated by four-layer enzyme-linked immunosorbent assay after denaturation with 0.5% sodium dodecyl sulfate at 65°C (15).
Tryptophan fluorescence.
Thermal denaturation was monitored by measuring intrinsic tryptophan fluorescence. Fluorescence spectra of E rosettes (40 μg/ml in TAN buffer [pH 8.0]) or 1 μM l-tryptophan (Sigma) as a control were recorded using a Perkin-Elmer LS 50B fluorescence spectrophotometer with an excitation wavelength of 285 nm (slit width, 10 nm) and an emission scan of 300 to 500 nm (slit width, 10 nm) using a continuously stirred fluorimeter cuvette. The measurements were made in increments of 5°C from 25 to 75°C. At each time point the sample was incubated for 60 s before the emission spectrum was recorded. The signal at the fluorescence maximum at 25°C was defined as 100%, and the change in fluorescence intensity at this wavelength was calculated.
Fusion assay.
Fusion of virions with liposomes was measured by monitoring the decrease in pyrene excimer fluorescence caused by the dilution of pyrene-labeled viral phospholipids into unlabeled liposomes (6). Virions were mixed with liposomes consisting of phosphatidylcholine, phosphatidylethanolamine, sphingomyelin, and cholesterol in a 1:1:1:1.5 molar ratio as described by Corver et al. (6). Fluorescence was recorded continuously for 60 s at 480 nm using a Perkin-Elmer LS 50B fluorescence spectrophotometer and an excitation wavelength of 343 nm.
The liposomes (0.2 mM phospholipid) were preincubated in 10 mM triethanolamine (pH 8.0)–140 mM NaCl–0.1% bovine serum albumin (BSA) in a continuously stirred fluorimeter cuvette at the appropriate temperature for 60 s. Pyrene-labeled virions (1 μM phospholipid) were added in a small volume and, for controls, immediately acidified to pH 5.5 by the addition of preheated 300 mM morpholinoethanesulfonic acid with 0.1% BSA. For the measurement at pH 8.0, the same amount of preheated TAN buffer (pH 8.0; containing 0.1% BSA) was added. The initial excimer fluorescence after mixing was defined as 100%. To determine the residual excimer fluorescence after fusion, the detergent n-octa(ethylene glycol) n-dodecyl monoether was added to a final concentration of 10 mM to disperse the viral and liposomal membranes.
Sedimentation analysis.
The conversion of E dimers into trimers was measured by sedimentation analysis as described previously (2). Purified virions (3 μg) in TAN buffer containing 0.1% BSA were incubated for 10 min at various temperatures either at pH 8.0 or, after acidification with 150 mM morpholinoethanesulfonic acid (containing 0.1% BSA), at pH 5.5. After cooling in an ice bath, the samples were back-neutralized, solubilized with 1% Triton X-100, and applied to 7 to 20% sucrose gradients containing 0.1% Triton X-100. Samples were centrifuged for 20 h in an SW 40 rotor (Beckman) at 38,000 rpm and 15°C. Fractions were collected by upward displacement, and E protein was quantitated by four-layer enzyme-linked immunosorbent assay after denaturation with 0.5% sodium dodecyl sulfate at 65°C (15).
RESULTS
Thermostability of E dimers and trimers.
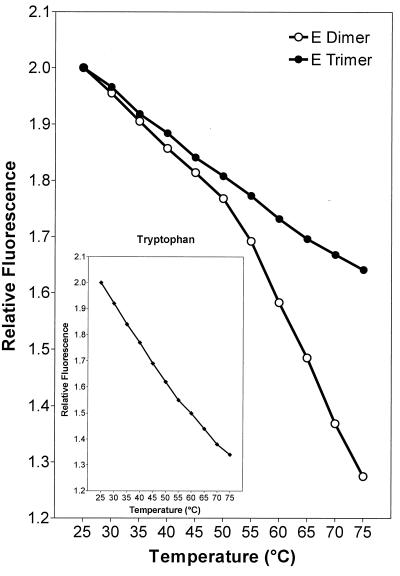
To examine the thermostability of native E dimers and E trimers formed by low-pH treatment, we measured the change in intrinsic tryptophan fluorescence accompanying thermally induced protein unfolding. E dimers and E trimers were isolated by solubilization of native or low-pH-treated virions with Triton X-100. It was then necessary to remove Triton X-100 from the preparations to avoid interference from the detergent at the wavelengths used. This resulted in the formation of E protein rosettes (13) in which the original oligomeric state (i.e., dimer or trimer) was retained, as confirmed by resolubilization with Triton X-100 (data not shown).
In the case of the native E dimers, a characteristic inflection in the fluorescence curve was observed at about 50°C, suggesting that a change in conformation had begun to occur at this threshold temperature (Fig. 1). With the trimeric, low-pH form of E, an increase in the temperature from 25 to 75°C resulted in a continuous decrease in fluorescence (Fig. 1) that was similar to the normal temperature-dependent fluorescence decrease exhibited by free l-tryptophan at the same pH (Fig. 1, inset), indicating that, unlike the dimer, the trimer is stable throughout this temperature range. These results are consistent with the idea that acidic pH induces a conversion from a metastable state to a more stable one, as has been described for influenza virus HA (5, 26).
FIG. 1.
Thermal stability of E rosettes containing native E dimers (open symbols) or E trimers obtained by low-pH-treatment (closed symbols) monitored by measuring intrinsic tryptophan fluorescence. The inset shows the fluorescence curve of free l-tryptophan as a control. At each temperature, the fluorescence intensity relative to the signal at 25°C is indicated. The fluorescence curves are representative of three experiments.
Temperature and pH dependence of fusion.
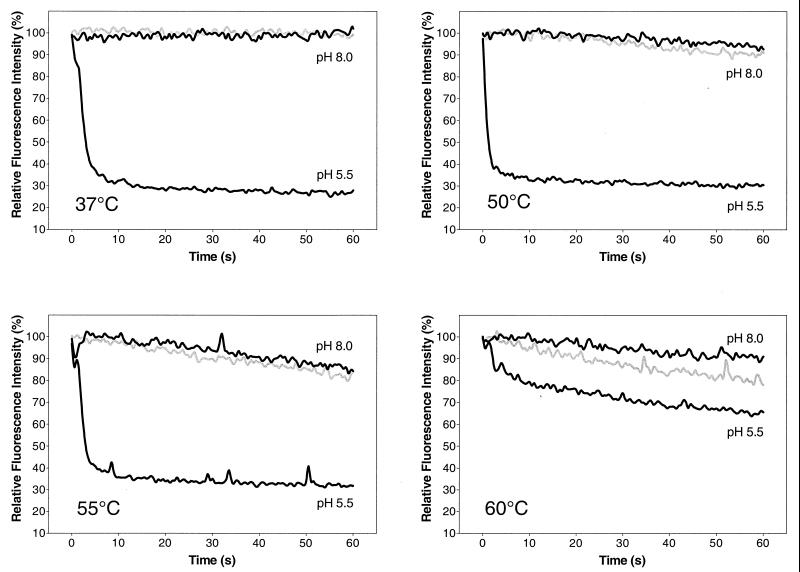
To investigate whether heating alone, in the absence of the low pH trigger, can also induce fusion of TBE virus with membranes, as was the case with influenza virus HA (5, 11, 26), we used an in vitro fusion assay based on pyrene-excimer fluorescence (6).
As shown in Fig. 2, heating the virus to 50, 55, or 60°C in the presence of liposomes at pH 8.0 did not lead to fusion. A slight gradual decrease in fluorescence was observed, but this change did not exceed that of controls treated at the corresponding temperature in the absence of a target membrane. This slow loss of fluorescence at pH 8.0 was therefore most likely due to a gradual degradation of the viral membrane at elevated temperatures rather than actual fusion with the target membrane. The same results were obtained by heating the virus at pH 7.4 or 7.0 or in the presence of urea (data not shown). In contrast, acidification to pH 5.5 immediately induced rapid fusion of the virus with liposomes at temperatures ranging from 37 to 55°C, as indicated by the rapid decrease of fluorescence (Fig. 2). At 60°C and pH 5.5 the observed fluorescence decrease was considerably smaller. This was probably due to heat inactivation of the virus in the few seconds between the addition of virus to the preheated buffer-liposome mixture and acidification.
FIG. 2.
Temperature dependence of fluorescence change in a pyrene excimer fusion assay. Virions whose membranes had been metabolically labeled with pyrene-conjugated lipids were incubated with liposomes at various temperatures either at pH 5.5 or at pH 8.0, and the change in pyrene excimer fluorescence was monitored continuously. Black curves represent the pyrene-labeled virions incubated with liposomes at the indicated pH. Gray curves show the rate of fluorescence loss with pyrene-labeled virions that were incubated at pH 8.0 without liposomes as background controls. The data shown are representative of three experiments.
Conversion of dimers to trimers.
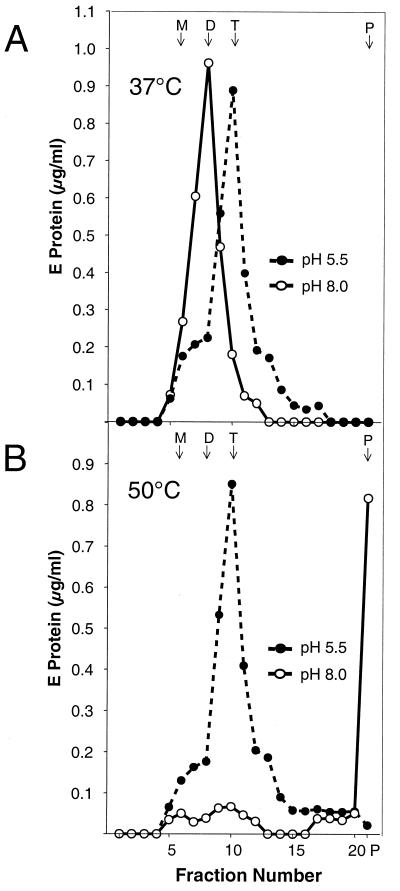
Although heat treatment did not result in functional activity, we were interested in finding out whether heat treatment can induce the characteristic dimer-trimer transition normally associated with low-pH-triggered fusion. We therefore incubated native and low-pH-pretreated virions for 10 min at 37 or 50°C, either at pH 8.0 or at pH 5.5, and analyzed the oligomeric state of the E protein in these samples by rate zonal sucrose density gradient centrifugation after solubilization with Triton X-100. This technique allows the monomeric, dimeric, and trimeric forms of the E protein to be distinguished by their sedimentation behaviors (2).
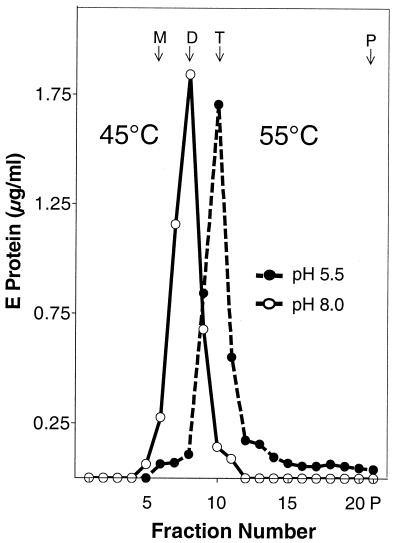
As expected, exposure of the virions to pH 5.5 at 37 or 50°C caused a quantitative, irreversible conversion from the dimeric to the trimeric form (Fig. 3). At pH 8.0, however, incubation at 50°C resulted in the loss of the dimer peak without the appearance of a peak corresponding to a defined oligomeric state of the E protein, indicating that the same specific dimer-trimer transition that is induced by acidic pH could not be triggered by simple heating of the virions (Fig. 3B). Instead, most of the material was found as a pellet at the bottom of the gradient, suggesting that heating had caused the E protein to aggregate (Fig. 3B). We were not able to solubilize these aggregates with Triton X-100 or define a specific oligomeric state by sedimentation analysis or cross-linking (data not shown). The fact that low-pH-treated virions yielded detergent-soluble trimers even at 50°C without significant aggregation (Fig. 3B) further supports the idea that the trimeric form of the E protein is more stable than the dimeric form.
FIG. 3.
Effects of elevated temperature on the oligomeric state of native and low-pH-treated E proteins. Virions that had been pretreated at pH 8.0 (solid line and open symbols) or pH 5.5 (dashed line and closed symbols) were incubated for 10 min either at 37°C (A) or at 50°C (B), solubilized with Triton X-100, and analyzed by sedimentation in 7 to 20% sucrose gradients containing 0.1% Triton X-100. The sedimentation direction is from left to right, and the positions of E monomer (M), dimer (D), trimer (T), and the pellet (P) are indicated. The data shown are representative of five experiments.
Temperature dependence of denaturation and aggregation.
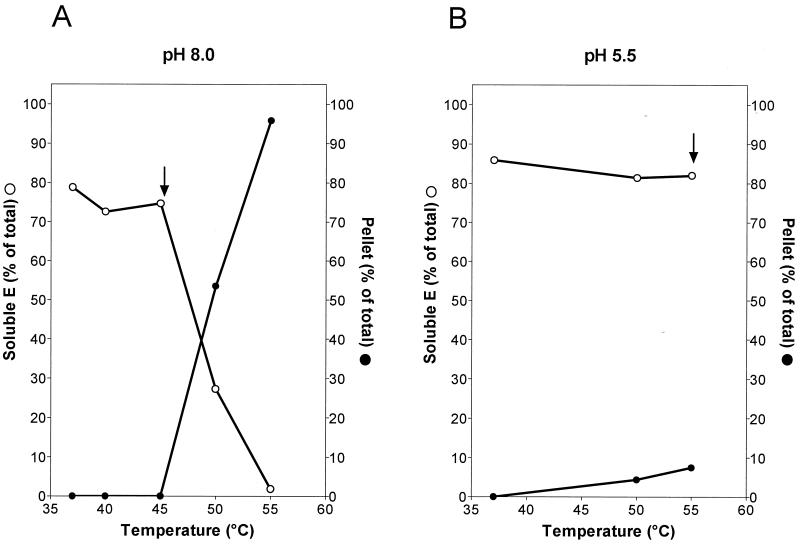
To investigate the temperature lability of the E protein more precisely, we incubated native and low-pH-treated virions at various temperatures and analyzed the state of the E protein in these samples by sucrose density centrifugation after Triton X-100 solubilization as described above.
With native virions, no change in the sedimentation behavior was observed up to a temperature of 45°C and the E protein remained Triton X-100 soluble (Fig. 4A). Significant aggregation was observed, however, at 50°C and above, leading to the formation of a Triton X-100-insoluble pellet (Fig. 4A). The temperature threshold of aggregation corresponded to the inflection point in the tryptophan fluorescence curve of E dimers shown in Fig. 1, suggesting that the same structural transition is being detected in each case. In contrast to what was shown with native virions, no significant aggregation was observed with low-pH-pretreated virions up to 55°C (Fig. 4B). Even at 65°C about 50% of the trimers remained soluble (data not shown), again confirming that the trimeric form of the E protein is more resistant to heating than the dimeric form. We did not find any evidence for a heat-induced dissociation of the E dimer at temperatures below the aggregation threshold. As shown in Fig. 5, the 45°C sample marked by an arrow in Fig. 4A still sedimented as a dimer. Likewise, the low-pH form remained trimeric up to at least 55°C (Fig. 5).
FIG. 4.
Temperature dependence of aggregation. Virions that had been pretreated at pH 8.0 (A) or pH 5.5 (B) were incubated at various temperatures, solubilized with Triton X-100, and analyzed by sedimentation in sucrose gradients as described in the legend for Fig. 3. The symbols indicate the percentages of soluble (open circles) or pelleted (closed circles) E protein. Each curve is the average of at least three experiments. Arrows indicate the samples that were used for the sedimentation analysis shown in Fig. 5.
FIG. 5.
Oligomeric state of the E protein of the samples indicated by arrows in Fig. 4. Virions that had been pretreated at pH 8.0 (solid line and open symbols) or pH 5.5 (dashed line and closed symbols) were incubated for 10 min at either 45°C (solid line and open symbols) or 55°C (dashed line and closed symbols), solubilized with Triton X-100, and analyzed by sedimentation in 7 to 20% sucrose gradients containing 0.1% Triton X-100. The sedimentation direction is from left to right, and the positions of E monomer (M), dimer (D), trimer (T), and the pellet (P) are indicated. The data shown are representative of three experiments.
DISCUSSION
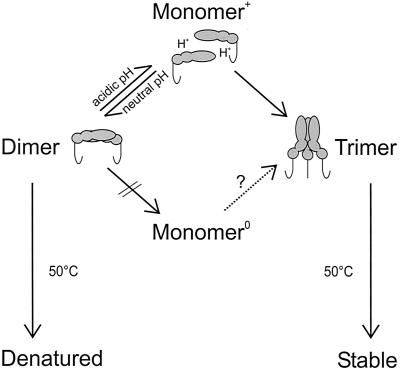
The data presented here provide evidence that the native E dimer of TBE virus—a representative of the class II viral fusion proteins—is metastable and is irreversibly converted to a more stable state by the fusion trigger, consistent with what has been observed so far with almost all enveloped viruses. The only exception are the rhabdoviruses, which control fusion by a series of reversible conformational changes (9). In contrast to the class I viral fusion protein of influenza virus, it was not possible to induce fusion or the required structural transition by the simple input of heat energy. The combined information derived from this and previous studies (2, 29) is summarized schematically in Fig. 6.
FIG. 6.
Schematic model of low-pH- and heat-induced structural alterations of the E protein of TBE virus. Upon exposure to low pH, native E dimers dissociate reversibly into monomers, which are subsequently converted in an irreversible step into homotrimers. Heating alone does not induce this transition but instead causes the E dimers to denature and aggregate. In contrast, E trimers obtained after incubation at an acidic pH are more thermostable and retain their oligomeric state at 50°C. We propose that protonation of the native E dimer, caused by exposure to low pH, is essential for shifting the equilibrium toward the monomeric form (Monomer+), thereby providing the intermediate required for the trimerization step. The question mark indicates that it is still not known whether a hypothetical unprotonated monomer (Monomer0) would be able to trimerize at a neutral pH.
We have previously shown that the dimer-trimer transition is likely to be at least a two-step process (29). Step 1 consists of a low-pH-induced dissociation of the dimer, which was shown with a soluble form of the E protein lacking the stem-anchor region to be reversible when the pH is returned to neutral. This suggests that the dimeric state is thermodynamically favored at neutral pH and the monomeric state is favored at an acidic pH (Fig. 6). Step 2 is an irreversible conversion of monomers to trimers, which occurs rapidly with full-length E containing an intact C-terminal stem-anchor, but does not occur with isolated ectodomains lacking this region (3, 29).
It is probable that the first dissociation step makes TBE virus fusion absolutely dependent on an acidic pH. Without the protonation of specific amino acid residues the equilibrium would be shifted away from the monomeric intermediate, making the subsequent formation of trimers impossible (Fig. 6). In contrast to the situation with influenza virus HA, an input of energy effected by raising the temperature at a pH above 7.0 was not sufficient to induce the change to the fusogenic state, possibly because for TBE virus the energy barrier for denaturation and aggregation of the native dimer is lower than that for dissociation to the monomeric intermediate at a neutral pH. This idea is consistent with the observation that the E protein was still completely dimeric at 45°C, just below the aggregation threshold. Alternatively, an acidic pH might induce not only the dissociation of the dimer but also a reversible conformational change that is essential for the subsequent trimerization step. We currently do not have any evidence as to whether the conformation of the monomer at low pH is different from what it is in the native dimer or if the conversion of a monomer to a trimer would be possible at a neutral pH.
In contrast, influenza virus HA, which normally uses low pH as a trigger, can be induced to undergo fusion-specific structural alterations and fusion itself by heat and other destabilizing conditions (5, 11, 26), indicating that its energy barrier for fusion at a neutral pH is lower than the one for denaturation. Fusion at elevated temperatures has also been described for the paramyxoviruses Sendai virus and simian virus 5 (24, 31). It is therefore possible that this is a general property of class I viral fusion proteins, but more viruses of this class will have to be investigated before such a generalization can be made.
Very recently, results that were similar to those reported here for TBE virus were obtained with the low-pH-dependent fusion protein E1 of the alphavirus SFV (10). Like the TBE virus E protein, the E1 protein of SFV forms at low pH a homotrimer which is more stable than the metastable native form, a heteromeric complex consisting of the E1 and E2 envelope proteins. In the case of SFV also it was not possible to trigger the oligomeric rearrangements needed for fusion by heat or urea treatment alone (10). This property is one of many that are shared by alphaviruses and flaviviruses but are different from those of viruses with class I fusion proteins. Other distinguishing features include an icosahedral envelope structure, the lack of proteolytic cleavage of the fusion protein, the association of the fusion protein in a complex with a second glycoprotein, the activation of the fusion potential by cleavage of this accessory protein, the presence of an internal fusion peptide, and low-pH-triggered oligomeric rearrangements leading to the formation of homotrimers of the fusion protein (reviewed in references 12 and 19). In addition, the structural similarity between the fusion proteins of flaviviruses and alphaviruses has recently been revealed by the determination of a 3.5-Å crystal structure of E1 isolated from SFV (21). The overall shape and structural organization are strikingly similar to those of the flavivirus E protein, but the fusion machineries of flaviviruses and alphaviruses also have distinctive features that relate to the details of oligomerization, receptor binding, and lipid requirements for fusion. It will be interesting to see whether these class II fusion proteins have other viral or even cellular homologues, as has been found with class I viral fusion proteins (18, 28).
ACKNOWLEDGMENTS
We thank Angela Dohnal and Walter Holzer for excellent technical assistance.
REFERENCES
- 1.Allison S L, Schalich J, Stiasny K, Mandl C W, Heinz F X. Mutational evidence for an internal fusion peptide in the flavivirus envelope protein E. J Virol. 2001;75:4268–4275. doi: 10.1128/JVI.75.9.4268-4275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison S L, Schalich J, Stiasny K, Mandl C W, Kunz C, Heinz F X. Oligomeric rearrangement of tick-borne encephalitis virus envelope proteins induced by an acidic pH. J Virol. 1995;69:695–700. doi: 10.1128/jvi.69.2.695-700.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison S L, Stiasny K, Stadler K, Mandl C W, Heinz F X. Mapping of functional elements in the stem-anchor region of tick-borne encephalitis virus envelope protein E. J Virol. 1999;73:5605–5612. doi: 10.1128/jvi.73.7.5605-5612.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker D, Agard D A. Influenza hemagglutinin: kinetic control of protein function. Structure. 1994;2:907–910. doi: 10.1016/s0969-2126(94)00091-3. [DOI] [PubMed] [Google Scholar]
- 5.Carr C M, Chaudhry C, Kim P S. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc Natl Acad Sci USA. 1997;94:14306–14313. doi: 10.1073/pnas.94.26.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corver J, Ortiz A, Allison S L, Schalich J, Heinz F X, Wilschut J. Membrane fusion activity of tick-borne encephalitis virus and recombinant subviral particles in a liposomal model system. Virology. 2000;269:37–46. doi: 10.1006/viro.1999.0172. [DOI] [PubMed] [Google Scholar]
- 7.Delos S E, Gilbert J M, White J M. The central proline of an internal viral fusion peptide serves two important roles. J Virol. 2000;74:1686–1693. doi: 10.1128/jvi.74.4.1686-1693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferlenghi I, Clarke M, Rutten T, Allison S L, Schalich J, Heinz F X, Harrison S C, Rey F A, Fuller S D. Molecular organization of a recombinant subviral particle from tick-borne encephalitis virus. Mol Cell. 2001;7:593–602. doi: 10.1016/s1097-2765(01)00206-4. [DOI] [PubMed] [Google Scholar]
- 9.Gaudin Y. Reversibility in fusion protein conformational changes. The intriguing case of rhabdovirus-induced membrane fusion. Subcell Biochem. 2000;34:379–408. doi: 10.1007/0-306-46824-7_10. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons D L, Ahn A, Chatterjee P K, Kielian M. Formation and characterization of the trimeric form of the fusion protein of Semliki Forest virus. J Virol. 2000;74:7772–7780. doi: 10.1128/jvi.74.17.7772-7780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haywood A M, Boyer B P. Time and temperature dependence of influenza virus membrane fusion at neutral pH. J Gen Virol. 1986;67:2813–2817. doi: 10.1099/0022-1317-67-12-2813. [DOI] [PubMed] [Google Scholar]
- 12.Heinz F X, Allison S L. Structures and mechanisms in flavivirus fusion. Adv Virus Res. 2000;55:231–269. doi: 10.1016/S0065-3527(00)55005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinz F X, Kunz C. Formation of polymeric glycoprotein complexes from a flavivirus: tick-borne encephalitis virus. J Gen Virol. 1980;49:125–132. doi: 10.1099/0022-1317-49-1-125. [DOI] [PubMed] [Google Scholar]
- 14.Heinz F X, Kunz C. Homogeneity of the structural glycoprotein from European isolates of tick-borne encephalitis virus: comparison with other flaviviruses. J Gen Virol. 1981;57:263–274. doi: 10.1099/0022-1317-57-2-263. [DOI] [PubMed] [Google Scholar]
- 15.Heinz F X, Stiasny K, Püschner-Auer G, Holzmann H, Allison S L, Mandl C W, Kunz C. Structural changes and functional control of the tick-borne encephalitis virus glycoprotein E by the heterodimeric association with protein prM. Virology. 1994;198:109–117. doi: 10.1006/viro.1994.1013. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez L D, Hoffman L R, Wolfsberg T G, White J M. Virus-cell and cell-cell fusion. Annu Rev Cell Dev Biol. 1996;12:627–661. doi: 10.1146/annurev.cellbio.12.1.627. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez L D, White J M. Mutational analysis of the candidate internal fusion peptide of the avian leukosis and sarcoma virus subgroup A envelope glycoprotein. J Virol. 1998;72:3259–3267. doi: 10.1128/jvi.72.4.3259-3267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jahn R, Sudhof T C. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- 19.Kielian M, Chatterjee P K, Gibbons D L, Lu Y E. Specific roles for lipids in virus fusion and exit. Examples from the alphaviruses. Subcell Biochem. 2000;34:409–455. doi: 10.1007/0-306-46824-7_11. [DOI] [PubMed] [Google Scholar]
- 20.Klenk H D, Garten W. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 1994;2:39–43. doi: 10.1016/0966-842x(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 21.Lescar J, Roussel A, Wien M W, Navaza J, Fuller S D, Wengler G, Wengler G, Rey F A. The fusion glycoprotein shell of Semliki Forest virus. Cell. 2001;105:137–148. doi: 10.1016/s0092-8674(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 22.Mandl C W, Heinz F X, Kunz C. Sequence of the structural proteins of tick-borne encephalitis virus (western subtype) and comparative analysis with other flaviviruses. Virology. 1988;166:197–205. doi: 10.1016/0042-6822(88)90161-4. [DOI] [PubMed] [Google Scholar]
- 23.Martin I, Ruysschaert J, Epand R M. Role of the N-terminal peptides of viral envelope proteins in membrane fusion. Adv Drug Deliv Rev. 1999;38:233–255. doi: 10.1016/s0169-409x(99)00031-9. [DOI] [PubMed] [Google Scholar]
- 24.Paterson R G, Russell C J, Lamb R A. Fusion protein of the paramyxovirus SV5: destabilizing and stabilizing mutants of fusion activation. Virology. 2000;270:17–30. doi: 10.1006/viro.2000.0267. [DOI] [PubMed] [Google Scholar]
- 25.Rey F A, Heinz F X, Mandl C, Kunz C, Harrison S C. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature. 1995;375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 26.Ruigrok R W, Martin S R, Wharton S A, Skehel J J, Bayley P M, Wiley D C. Conformational changes in the hemagglutinin of influenza virus which accompany heat-induced fusion of virus with liposomes. Virology. 1986;155:484–497. doi: 10.1016/0042-6822(86)90210-2. [DOI] [PubMed] [Google Scholar]
- 27.Singh M, Berger B, Kim P S. LearnCoil-VMF: computational evidence for coiled-coil-like motifs in many viral membrane-fusion proteins. J Mol Biol. 1999;290:1031–1041. doi: 10.1006/jmbi.1999.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skehel J J, Wiley D C. Coiled coils in both intracellular vesicle and viral membrane fusion. Cell. 1998;95:871–874. doi: 10.1016/s0092-8674(00)81710-9. [DOI] [PubMed] [Google Scholar]
- 29.Stiasny K, Allison S L, Marchler-Bauer A, Kunz C, Heinz F X. Structural requirements for low-pH-induced rearrangements in the envelope glycoprotein of tick-borne encephalitis virus. J Virol. 1996;70:8142–8147. doi: 10.1128/jvi.70.11.8142-8147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weissenhorn W, Dessen A, Calder L J, Harrison S C, Skehel J J, Wiley D C. Structural basis for membrane fusion by enveloped viruses. Mol Membr Biol. 1999;16:3–9. doi: 10.1080/096876899294706. [DOI] [PubMed] [Google Scholar]
- 31.Wharton S A, Skehel J J, Wiley D C. Temperature dependence of fusion by Sendai virus. Virology. 2000;271:71–78. doi: 10.1006/viro.2000.0280. [DOI] [PubMed] [Google Scholar]