Abstract
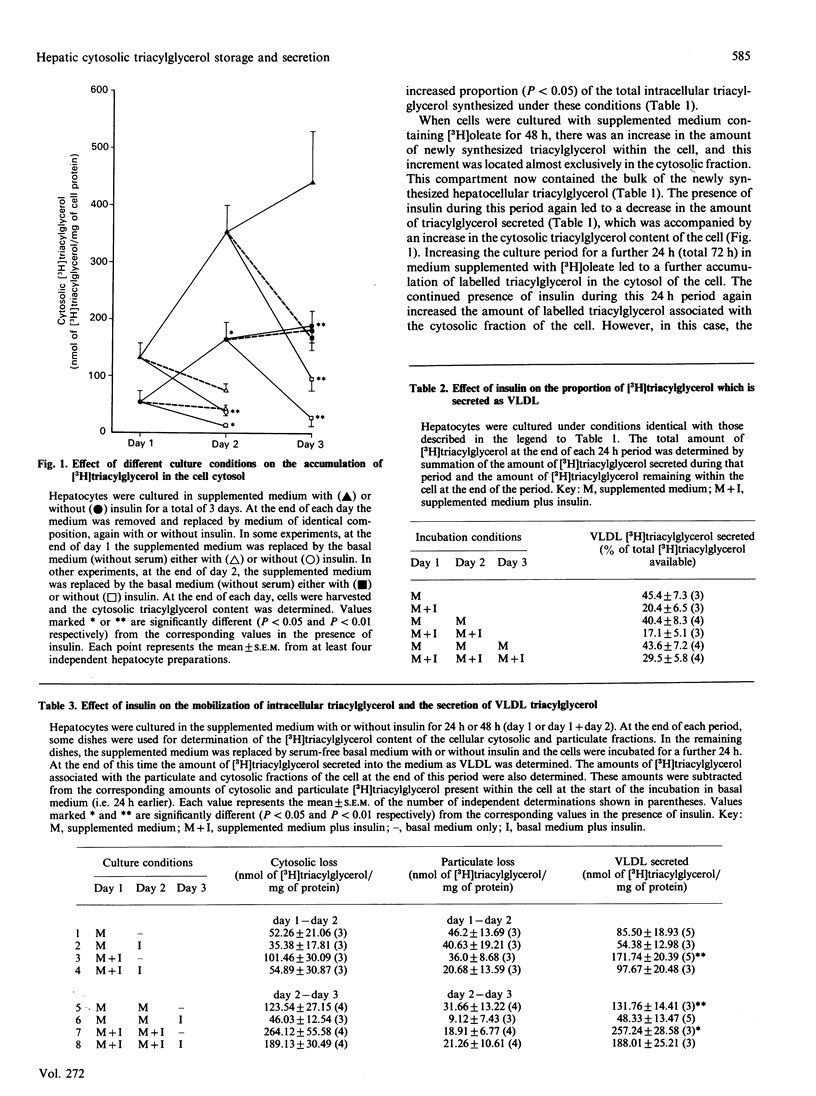
Cytosolic triacylglycerol labelled from [3H]oleate accounted for almost 50% (57 +/- 22 nmol/mg of protein) of the total cellular triacylglycerol which was newly synthesized by cultured hepatocytes during a 24 h incubation. Insulin decreased the export of triacylglycerol as very-low-density lipoprotein (VLDL) during this period. This resulted in a sequestration of newly synthesized triacylglycerol in the cytosol, rather than in the particulate fraction of the cell. Longer periods of incubation with [3H]oleate resulted in increased concentrations of newly synthesized triacylglycerol within the cell, most of which (78 +/- 3% after 48 h; 80 +/- 3% after 72 h) was located within the cytosolic fraction. The quantity of newly synthesized triacylglycerol in the cell cytosol was further increased by insulin. During these periods there were decreases in the amounts of triacylglycerol associated with the particulate fraction of the cell, irrespective of the presence or absence of insulin. In no case was a decrease in VLDL triacylglycerol secretion in response to insulin accompanied by an increased triacylglycerol content in the particulate fraction of the cell. In some experiments, the fate of the cytosolic triacylglycerol was studied by pulse labelling with [3H]oleate. In these cases, when insulin was removed from the medium of cells to which they had previously been exposed, more newly synthesized triacylglycerol was secreted compared with cells which had not been exposed to insulin. This extra triacylglycerol was mobilized from the cytosolic rather than from the particulate fraction of the cell. Subsequent addition of insulin to the medium prevented the mobilization of cytosolic triacylglycerol. These results suggest that insulin enhances the storage of hepatocellular triacylglycerol in a cytosolic pool. Deficiency of insulin in the medium stimulates the mobilization of this pool which is channelled into the secretory pathway, entering the extracellular medium as VLDL.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberger M. J., Lane M. D. Assembly of very low density lipoprotein in the hepatocyte. Differential transport of apoproteins through the secretory pathway. J Biol Chem. 1988 Aug 25;263(24):11868–11878. [PubMed] [Google Scholar]
- Bar-On H., Roheim P. S., Stein O., Stein Y. Contribution of floating fat triglyceride and of lecithin towards formation of secretory triglyceride in perfused rat liver. Biochim Biophys Acta. 1971 Oct 5;248(1):1–11. doi: 10.1016/0005-2760(71)90068-3. [DOI] [PubMed] [Google Scholar]
- Bartlett S. M., Gibbons G. F. Short- and longer-term regulation of very-low-density lipoprotein secretion by insulin, dexamethasone and lipogenic substrates in cultured hepatocytes. A biphasic effect of insulin. Biochem J. 1988 Jan 1;249(1):37–43. doi: 10.1042/bj2490037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt R. A., Davis R. A. Intrahepatic assembly of very low density lipoproteins. Rate of transport out of the endoplasmic reticulum determines rate of secretion. J Biol Chem. 1987 Dec 5;262(34):16394–16402. [PubMed] [Google Scholar]
- Boström K., Borén J., Wettesten M., Sjöberg A., Bondjers G., Wiklund O., Carlsson P., Olofsson S. O. Studies on the assembly of apo B-100-containing lipoproteins in HepG2 cells. J Biol Chem. 1988 Mar 25;263(9):4434–4442. [PubMed] [Google Scholar]
- Coleman R., Bell R. M. Evidence that biosynthesis of phosphatidylethanolamine, phosphatidylcholine, and triacylglycerol occurs on the cytoplasmic side of microsomal vesicles. J Cell Biol. 1978 Jan;76(1):245–253. doi: 10.1083/jcb.76.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti N., Williams D. L., Alaupovic P. Effects of oleate and insulin on the production rates and cellular mRNA concentrations of apolipoproteins in HepG2 cells. J Lipid Res. 1989 Sep;30(9):1365–1373. [PubMed] [Google Scholar]
- Debeer L. J., Beynen A. C., Mannaerts G. P., Geelen M. J. Lipolysis of hepatic triacylglycerol stores. FEBS Lett. 1982 Apr 19;140(2):159–164. doi: 10.1016/0014-5793(82)80884-3. [DOI] [PubMed] [Google Scholar]
- Duerden J. M., Bartlett S. M., Gibbons G. F. Long-term maintenance of high rates of very-low-density-lipoprotein secretion in hepatocyte cultures. A model for studying the direct effects of insulin and insulin deficiency in vitro. Biochem J. 1989 Nov 1;263(3):937–943. doi: 10.1042/bj2630937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden J. M., Gibbons G. F. Secretion and storage of newly synthesized hepatic triacylglycerol fatty acids in vivo in different nutritional states and in diabetes. Biochem J. 1988 Nov 1;255(3):929–935. doi: 10.1042/bj2550929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrington P. N., Newton R. S., Weinstein D. B., Steinberg D. Effects of insulin and glucose on very low density lipoprotein triglyceride secretion by cultured rat hepatocytes. J Clin Invest. 1982 Jul;70(1):63–73. doi: 10.1172/JCI110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Francone O. L., Kalopissis A. D., Griffaton G. Contribution of cytoplasmic storage triacylglycerol to VLDL-triacylglycerol in isolated rat hepatocytes. Biochim Biophys Acta. 1989 Mar 14;1002(1):28–36. doi: 10.1016/0005-2760(89)90060-x. [DOI] [PubMed] [Google Scholar]
- Gibbons G. F. Assembly and secretion of hepatic very-low-density lipoprotein. Biochem J. 1990 May 15;268(1):1–13. doi: 10.1042/bj2680001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R., Fleming R., O'Connell W., Kirschner J., Oppliger W. Apolipoproteins of the orotic acid fatty liver: implications for the biogenesis of plasma lipoproteins. J Lipid Res. 1988 Aug;29(8):981–995. [PubMed] [Google Scholar]
- Higgins J. A. Evidence that during very low density lipoprotein assembly in rat hepatocytes most of the triacylglycerol and phospholipid are packaged with apolipoprotein B in the Golgi complex. FEBS Lett. 1988 May 23;232(2):405–408. doi: 10.1016/0014-5793(88)80780-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mangiapane E. H., Brindley D. N. Effects of dexamethasone and insulin on the synthesis of triacylglycerols and phosphatidylcholine and the secretion of very-low-density lipoproteins and lysophosphatidylcholine by monolayer cultures of rat hepatocytes. Biochem J. 1986 Jan 1;233(1):151–160. doi: 10.1042/bj2330151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson S. O., Bjursell G., Boström K., Carlsson P., Elovson J., Protter A. A., Reuben M. A., Bondjers G. Apolipoprotein B: structure, biosynthesis and role in the lipoprotein assembly process. Atherosclerosis. 1987 Nov;68(1-2):1–17. doi: 10.1016/0021-9150(87)90088-8. [DOI] [PubMed] [Google Scholar]
- Palmer J. F., Cooper C., Shipley R. A. Rate of release of hepatic triacylglycerol into serum in the starved rat. Biochem J. 1978 May 15;172(2):219–226. doi: 10.1042/bj1720219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch W., Franz S., Schonfeld G. Role of insulin in lipoprotein secretion by cultured rat hepatocytes. J Clin Invest. 1983 May;71(5):1161–1174. doi: 10.1172/JCI110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch W., Gotto A. M., Jr, Patsch J. R. Effects of insulin on lipoprotein secretion in rat hepatocyte cultures. The role of the insulin receptor. J Biol Chem. 1986 Jul 25;261(21):9603–9606. [PubMed] [Google Scholar]
- Pullinger C. R., Gibbons G. F. Effects of hormones and pyruvate on the rates of secretion of very-low-density lipoprotein triacylglycerol and cholesterol by rat hepatocytes. Biochim Biophys Acta. 1985 Jan 9;833(1):44–51. doi: 10.1016/0005-2760(85)90251-6. [DOI] [PubMed] [Google Scholar]
- Pullinger C. R., North J. D., Teng B. B., Rifici V. A., Ronhild de Brito A. E., Scott J. The apolipoprotein B gene is constitutively expressed in HepG2 cells: regulation of secretion by oleic acid, albumin, and insulin, and measurement of the mRNA half-life. J Lipid Res. 1989 Jul;30(7):1065–1077. [PubMed] [Google Scholar]
- Sparks C. E., Sparks J. D., Bolognino M., Salhanick A., Strumph P. S., Amatruda J. M. Insulin effects on apolipoprotein B lipoprotein synthesis and secretion by primary cultures of rat hepatocytes. Metabolism. 1986 Dec;35(12):1128–1136. doi: 10.1016/0026-0495(86)90026-0. [DOI] [PubMed] [Google Scholar]
- Van Harken D. R., Dixon C. W., Heimberg M. Hepatic lipid metabolism in experimental diabetes. V. The effect of concentration of oleate on metabolism of triglycerides and on ketogenesis. J Biol Chem. 1969 May 10;244(9):2278–2285. [PubMed] [Google Scholar]


