Abstract
Background
Clinically relevant‐postoperative pancreatic fistula (CR‐POPF) is one of the dreaded complications of pancreatoduodenectomy. Vascularity of the stump of the pancreas during pancreatico‐enteric anastomosis is considered one of the major determinants of POPF. Indocyanine green (ICG) is one of the modality for vascular assessment; hence, we aimed to evaluate the role of ICG fluorescence imaging to assess the vascularity of the pancreatic stump during pancreatoduodenectomy.
Methodology
The study was conducted at Kathmandu Medical College, Kathmandu, Nepal, during the period of 1 year (June 01, 2022–July 31, 2023). All of the patients who were undergoing pancreatoduodenectomy were included in the study. In all cases, intraoperatively, ICG fluorescence detection at the pancreatic stump margin was evaluated using near‐infrared light.
Results
A total of 28 patients underwent PD during this period. The ICG fluorescence imaging was positive at the pancreatic stump margin in 25 out of the 28 patients (89%), and in three cases, the florescence was negative. In patients who had negative fluorescence, revision of the stump margin was performed. Clinically relevant POPF was noted in 2 out of 28 cases, which had a soft pancreas with a small duct, although the pancreatic stump margin had good ICG florescence per‐operatively.
Conclusion
ICG is inexpensive and a safe dye to use in clinical practice. We can objectively assess the pancreatic stump vascularity using intraoperative ICG fluorescence imaging, thereby potentially decreasing postoperative pancreatic fistula.
Keywords: fluorescence, indocyanine green, pancreatic stump, pancreatoduodenectomy, perfusion
1. INTRODUCTION
Pancreatoduodenectomy (PD) is a major abdominal surgery with a mortality rate of less than 5% but morbidity of 30%–65%. 1 Among surgical complications, clinically relevant postoperative pancreatic fistula (CR‐POPF) is the most dreaded complication encountered after PD, which affects mortality rate, length of hospital stay, and costs. The incidence of POPF is around 6%–14%. 2 , 3 , 4 Various factors play a role in the development of pancreatic fistulas, which include patients general condition factors, disease‐related risk factors, including pancreatic texture and duct size, procedure‐related factors, and surgeons experience. 3 , 5 , 6 , 7 Among the various factors, good vascularity of the pancreatic stump has a role in reducing the incidence of CR‐POPF. 8
ICG florescence is widely used to detect the vascularity status of the bowel margin, mainly in colorectal cases, 9 which can be implemented in pancreatic surgery as well. Hence, we aimed to evaluate the role of ICG fluorescence imaging for detection of perfusion at the pancreatic stump margin in patients undergoing pancreatoduodenectomy.
2. METHODOLOGY
All patients who underwent pancreatoduodenectomy from June 1, 2022, to July 31, 2023 (1 year) were enrolled in the study. The patients undergoing laparoscopic surgeries were excluded from the study. Written consent was obtained from all patients included in the study and ethical clearance was obtained from the Institutional Review Board (IRB number: 05042024/01) of Kathmandu Medical College Teaching Hospital.
2.1. Study design and end points
This was a hospital‐based descriptive study. In adherence to the principles of rigorous reporting, this study conformed to the revised Strengthening the Reporting of Observational Studies in Epidemiology statement. 10 The objectives of the study were to assess the pancreatic stump margin vascularity using ICG fluorescence imaging under near‐infrared (NIR) light and to correlate the findings with the incidence of CR‐POPF. The definition and classification of POPF were as per the ISGPS postoperative pancreatic fistula classification. 11 The same group of surgeons performed all surgeries.
2.2. Surgical technique
A standard pancreatoduodenectomy was performed, during which the pancreatic neck transection line was located directly over the superior mesenteric/portal vein axis.
ICG dosing: 25 milligrams (mg) of powdered ICG were mixed in 10 milliliters (mL) of normal saline (2.5 mg in 1 mL solution), and 3 mL of the solution was administered intravenously (IV), followed by a 10 mL saline flush. Under NIR light, ICG fluorescence was assessed at the pancreatic stump margin after 15–45 s of IV administration of ICG. Stump perfusion adequacy was assessed; homogenous enhancement indicated adequate perfusion, and heterogenous or absent enhancement indicated hypoperfusion. If classified as hypoperfusion, the pancreatic margin was revised by 1.5–2.0 cm to improve perfusion and re‐assessed using ICG fluorescence imaging.
Pancreatic anastomosis was performed using either pancreaticojejunostomy or pancreaticogastrostomy techniques, based on the surgeon's choice. Postoperatively, the CR‐POPF rate was recorded.
2.3. Data analysis and statistical analysis
The collected data was stored in an electronic database (MS Excel sheet). The incidence of revision of stump margins based on ICG fluorescence and the incidence of CR‐POPF were recorded. The results were analyzed using appropriate statistical methods.
3. RESULTS
A total of 28 patients underwent standard pancreatoduodenectomy during the study period. Out of the total, 17 patients were male, and the mean age was 57 ± 3 years. Details on patient inclusion are provided in Table 1.
Table 1.
Indications of patients undergoing pancreatoduodenectomy.
| S. No. | Indications | Number (n = 28) |
|---|---|---|
| 1. | Carcinoma head pancreas | 10 |
| 2. | Ampullary mass | 9 |
| 3. | Distal cholangiocarcinoma | 6 |
| 4. | Duodenal carcinoma | 1 |
| 5. | Neuroendocrine tumors | 1 |
| 6. | Chronic pancreatitis | 1 |
The median postoperative hospital stay was 8 days and ranged from five to eleven days. The morbidity of the surgical procedures was graded based on Clavien‐Dindo grading (Table 2).
Table 2.
Categorization of patients based on the Clavien‐Dindo grading.
| Morbidity (Clavien‐Dindo grading) | Number |
|---|---|
| Grade 1 and 2 | 17 |
| Grade 3 and 4 | 3 |
| Grade 5 | 0 |
The ICG fluorescence detection at the pancreatic stump margin was noted in 25 out of the 28 patients intra‐operatively (89.2%). Three cases required revision of the stump margin due to hypoperfusion, for which the pancreas was cut back 1.5–2.0 cm to improve the vascularity and thus the ICG florescence. Clinically relevant POPF was noted in 2 out of 28 (7.14%) cases, which had soft pancreas with small duct and a diagnosis of distal cholangiocarcinoma. Both of these cases had good ICG fluorescence peroperatively. The intraoperative assessment of pancreatic stump perfusion is shown in Figures 1 and 2, where Figure 1 depicts an inadequately perfused pancreatic stump margin and Figure 2 an adequately perfused pancreatic stump margin. Following a pancreaticogastrostomy, Figure 3 illustrates a viable pancreatic stump.
Figure 1.
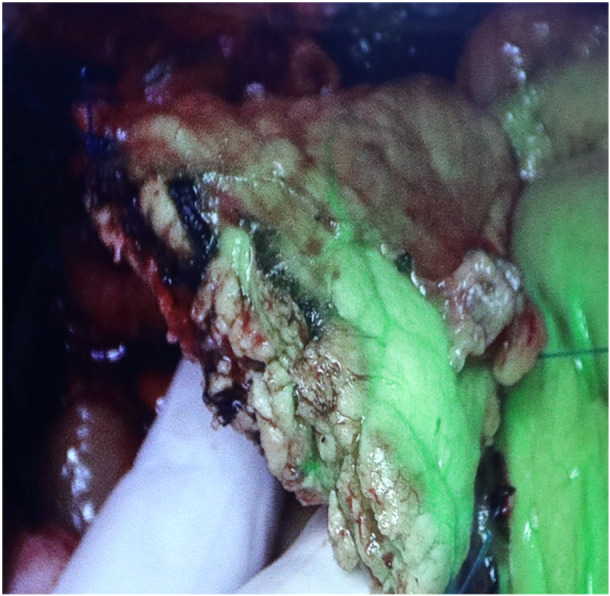
Pancreatic stump margin showing inadequate perfusion.
Figure 2.
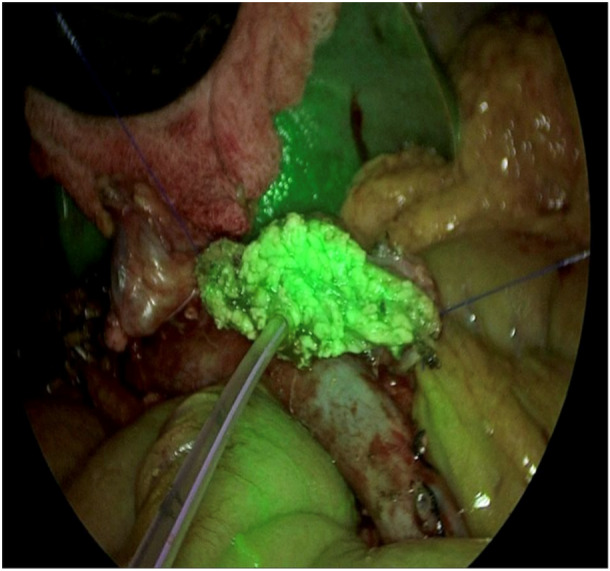
Pancreatic stump margin showing adequate perfusion.
Figure 3.
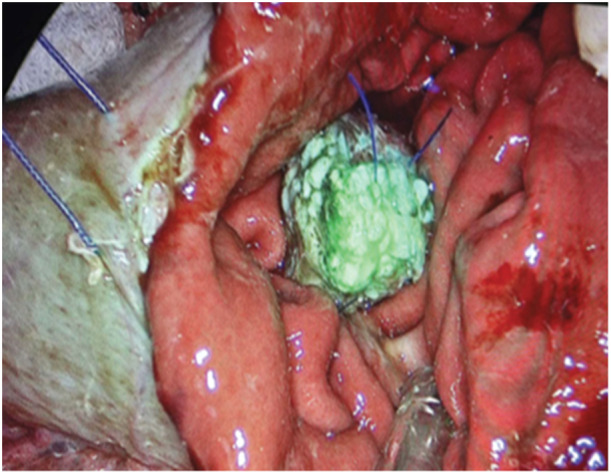
Pancreatic viable stump after pancreaticogastrostomy.
4. DISCUSSION
In our series, 25 out of 28 (89.2%) had adequate perfusion, and the remaining three required revision of the stump. All pancreatico‐enteric anastomosis was performed following the confirmation of adequate perfusion at the pancreatic stump margin based on ICG fluorescence imaging.
The importance of adequate vascularity in the pancreatic margin during PD has been underscored by the study done by Strasberg et al. 8 , 12 In this study, the initial line of transection of the pancreatic neck was directly over the superior mesenteric/portal vein axis, and Strasberg et al. noted that 62% of the patients had good vascularity at the margin based on a naked eye examination. For the rest of the patient, the pancreatic margin was revised toward the left side to achieve good vascularity before performing the pancreatico‐enteric anastomosis, which leads to a decrease in the incidence of CR‐POPF.
There are several factors that contribute to POPF. One of the main limitations is the stump's perfusion status. Busnardo et al. 13 identified a hypo‐vascular area, located between the isthmus and the body of the pancreas, along the border of these two portions. Based on this theory, pancreatic stumps should not include watershed areas. 14 Adequate vascularity in this area can be assessed with the naked eye; however, ICG fluorescence imaging enables us to identify and document microvascular perfusion. In our study, due to poor micro‐vascular perfusion status on ICG imaging in three cases, the pancreatic margin was cut back to the left side before pancreatico‐enteric anastomosis. 8 The ability to detect micro‐vascular perfusion using ICG therefore ensures good vascularity peroperatively at the pancreatic stump margin, potentially decreasing the incidence of CR‐POPF, which is one of the most dreaded complications following PD. Even computed tomography perfusion data (arterial flow and mean transit time) show a correlation with the occurrence of POPF in PD. 15
However, there were some limitations to the present study. This study included data from a single center, and other determinants of CR‐POPF were not assessed adequately, which limited the validity of the study. Further multicenter, prospective, and randomized controlled trials should be performed to overcome these limitations.
5. CONCLUSION
ICG can be a useful tool to assess and document the adequacy of the vascularity of the pancreatic stump intra‐operatively, which can potentially help to decrease the rate of clinically significant POPF.
AUTHOR CONTRIBUTIONS
Roshan Ghimire: Conceptualization; methodology; investigation; data curation; formal analysis; writing—original draft; writing—review and editing. Yugal Limbu: Writing—original draft; visualization; formal analysis; data curation. Sujan Regmee: Data curation; visualization; formal analysis. Dhiresh Kumar Maharjan: Data curation; formal analysis; visualization; investigation. Aakash Mishra: Writing—review and editing. Rabin Pahari: Data curation. Prabin Bikram Thapa: Supervision; writing—review and editing; conceptualization.
CONFLICT OF INTEREST STATEMENT
The authors do not have any conflicts of interest to disclose. This study was presented at the 34th World Congress of the International Association of Surgeons, Gastroenterologists, and Oncologists (IASGO) in Verona, Italy, in September 2023.
ETHICS STATEMENT
Institutional review committee‐Kathmandu Medical College (IRC‐KMC) approved this study.
TRANSPARENCY STATEMENT
The lead author Roshan Ghimire affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
The authors state that they did not receive any financial assistance or funding for this study.
Ghimire R, Limbu Y, Regmee S, et al. Indocyanine green fluorescence imaging: assessment of perfusion at pancreatic resection margin during pancreatoduodenectomy: a cross sectional study. Health Sci Rep. 2024;7:e70153. 10.1002/hsr2.70153
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single‐institution experience. J Gastrointest Surg. 2006;10(9):1199‐1211. 10.1016/j.gassur.2006.08.018 [DOI] [PubMed] [Google Scholar]
- 2. Mathur A, Pitt HA, Marine M, et al. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg. 2007;246(6):1058‐1064. 10.1097/SLA.0b013e31814a6906 [DOI] [PubMed] [Google Scholar]
- 3. Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244(1):10‐15. 10.1097/01.sla.0000217673.04165.ea [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lai ECH. Measures to prevent pancreatic fistula after pancreatoduodenectomy: a comprehensive review. AArch Surg. 2009;144(11):1074. 10.1001/archsurg.2009.193 [DOI] [PubMed] [Google Scholar]
- 5. Oneil Machado N. Pancreatic fistula after pancreatectomy: definitions, risk factors, preventive measures, and management—review. Int J Surg Oncol. 2012;2012:1‐10. 10.1155/2012/602478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Figueras J, Sabater L, Planellas P, et al. Randomized clinical trial of pancreaticogastrostomy versus pancreaticojejunostomy on the rate and severity of pancreatic fistula after pancreaticoduodenectomy. Br J Surg. 2013;100(12):1597‐1605. 10.1002/bjs.9252 [DOI] [PubMed] [Google Scholar]
- 7. Romano G, Agrusa A, Galia M, et al. Whipple's pancreaticoduodenectomy: surgical technique and perioperative clinical outcomes in a single center. International Journal of Surgery. 2015;21:S68‐S71. 10.1016/j.ijsu.2015.06.062 [DOI] [PubMed] [Google Scholar]
- 8. Strasberg SM, Drebin JA, Mokadam NA, et al. Prospective trial of a blood supply‐based technique of pancreaticojejunostomy: effect on anastomotic failure in the whipple procedure. J Am Coll Surg. 2002;194(6):746‐758. 10.1016/S1072-7515(02)01202-4 [DOI] [PubMed] [Google Scholar]
- 9. Wojcik M, Doussot A, Manfredelli S, et al. Intra‐operative fluorescence angiography is reproducible and reduces the rate of anastomotic leak after colorectal resection for cancer: a prospective case‐matched study. Colorectal Dis. 2020;22(10):1263‐1270. 10.1111/codi.15076 [DOI] [PubMed] [Google Scholar]
- 10. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453‐1457. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 11. Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584‐591. 10.1016/j.surg.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 12. Strasberg SM, McNevin MS. Results of a technique of pancreaticojejunostomy that optimizes blood supply to the pancreas. J Am Coll Surg. 1998;187(6):591‐596. 10.1016/S1072-7515(98)00243-9 [DOI] [PubMed] [Google Scholar]
- 13. Busnardo AC, DiDio LJA, Thomford NR. Anatomicosurgical segments of the human pancreas. Surg Radiol Anat. 1988;10(1):77‐82. 10.1007/BF02094076 [DOI] [PubMed] [Google Scholar]
- 14. Renard Y, De Mestier L, Perez M, Avisse C, Lévy P, Kianmanesh R. Unraveling pancreatic segmentation. World J Surg. 2018;42(4):1147‐1153. 10.1007/s00268-017-4263-5 [DOI] [PubMed] [Google Scholar]
- 15. Sugimoto M, Takahashi S, Kobayashi T, et al. Pancreatic perfusion data and post‐pancreaticoduodenectomy outcomes. J Surg Res. 2015;194(2):441‐449. 10.1016/j.jss.2014.11.046 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


