Abstract
Background:
The predictors of intracranial haemorrhagic transformation (HT) in acute ischaemic stroke (AIS) patients undergoing dual antiplatelet therapy (DAPT) are not well known.
Objectives:
The aim of this study is to identify the possible clinical and radiological predictors of HT in patients, irrespective of clinical indication for this treatment.
Design:
This study is a monocentric cohort retrospective study.
Methods:
We enrolled consecutive AIS patients, from our prospective register, admitted to Stroke Unit between June 2021 and June 2023 undergoing DAPT with Acetylsalicylic Acid and Clopidogrel within 72 h from symptoms onset. According to current guidelines, DAPT indication was for patients with a minor stroke, symptomatic intracranial artery stenosis and carotid angioplasty stenting. We collected clinical, demographical and radiological data. We used ABC/2 method to measure stroke volume in magnetic resonance imaging (MRI)/Diffusion-weighted imaging (DWI) sequences performed within 48 h. The primary outcome was the presence of HT at non-contrast brain computed tomography, performed 7 days after commencing DAPT.
Results:
One hundred ninety-four patients were included. Twenty-eight (14.4%) presented HT. Higher NIH Stroke Scale (NIHSS) and MRI/DWI lesion volume related to increased risk of HT (p < 0.001). Reperfusion therapy and mechanical thrombectomy (MT), stent placement and a loading dose (LD) of dual antiplatelet or Clopidogrel were associated with a higher occurrence of HT (p < 0.05). Furthermore, we individuated an NIHSS cut-off value >4 (area under the curve (AUC) 0.80, sensitivity 0.82, specificity 0.65) and a volume cut-off value >8.2 ml (AUC 0.82, sensitivity 0.79, specificity 0.80) associated with an increased risk of HT (respectively, adjusted odds ratio (adj. OR) 6.5, confidence interval (CI) 1.3–32.7, p = 0.024 and adj. OR 11.0, CI 3.1–39.2, p < 0.001).
Conclusion:
In clinical practice, MT treatment, antiplatelet LD administration, stent placement and clinical severity may relate to a higher risk of HT in patients with AIS and DAPT in the acute phase. In particular, we found that lesion volume cut-off could help to identify patients at greater risk of HT, regardless of the indication for DAPT.
Keywords: acute ischaemic stroke, biomarkers, dual antiplatelet therapy, haemorrhagic transformation, MRI
Plain language summary
Possible clinical and radiological features able to predict the risk of haemorrhagic transformation in patients affected by acute cerebral ischemic stroke undergoing treatment with dual antiplatelet, Acid Acetylsalicylic and Clopidogrel
This monocentric cohort retrospective study aims to identify predictive factors for haemorrhagic transformation (HT) in patients with acute ischaemic stroke (AIS) and dual antiplatelet therapy (DAPT). DAPT is indicated for minor strokes, symptomatic intracranial artery stenosis and carotid stent placement. Although there are guidelines on this subject, there are some grey areas due to the emergence of new possible uses of DAPT and to a lack of studies addressing some issues (e.g. patients with moderate to severe AIS undergoing DAPT). We selected patients >18 years old from our prospective registry, who were admitted for AIS and started DAPT within 72 hours from the event. We collected clinical and radiological data. All patients underwent brain magnetic resonance imaging (MRI). We calculated the volume of the AIS using an easily reproducible methodology (ABC/2). We then identified which patients developed HT after one week of therapy and examined the factors potentially associated with an increased risk of HT. Our study provided useful insights for clinical practice. We observed an increased risk of HT in patients with higher scores on the stroke clinical severity scale (NIHSS), larger infarcts, treatment with mechanical thrombectomy, administration of antiplatelet loading doses and stent placement. Furthermore, we identified a 11-fold increased risk of HT in patients with acute ischaemic lesion volumes on MRI >8.2 ml, and a 6-fold increased risk for patients with NIHSS >4. This study is easily reproducible in clinical practice, as it utilizes readily available clinical and radiological parameters. It highlights how the integration of clinical and radiological data can assist neurologists in navigating grey areas of treatment. In this way, it might be possible to identify patients at risk of haemorrhage, who should be monitored more closely to prevent adverse effects that could lead to the interruption of DAPT, thereby reducing the risk of a new ischemic stroke.
Introduction
Dual antiplatelet therapy (DAPT) is a stroke secondary prevention therapy currently administered in the early stage following a carotid stenting, high-risk transitory ischaemic attack (TIA), minor stroke and symptomatic intracranial stenosis, in accordance with current guidelines. 1
In recent years, four randomised controlled trials (the Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT), the Fast Assessment of Stroke and Transient Ischaemic Attack to Prevent Early Recurrence (FASTER), the Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) and lastly the Acute Stroke or Transient Ischaemic Attack Treated With Ticagrelor and Aspirin for Prevention of Stroke and Death (THALES) trials) have demonstrated a reduced risk of ischaemic recurrence in patients with high-risk TIA and minor stroke undergoing DAPT within 24 h from symptoms’ onset, for 21–30 days after the index event.2–5 Recently, the results from the Intensive Statin and Antiplatelet Therapy for Acute High-Risk Intracranial or Extracranial Atherosclerosis – INSPIRES trial, confirmed the efficacy of DAPT even when started later (i.e. within 72 h from symptoms’ onset) and in patients with NIHSS < 6 and extracranial or intracranial atherosclerosis. 6
However, due to strict selection criteria, the study population included in these trials did not fully reflect the clinical complexity observed in the real-life setting (i.e. patients undergoing intravenous thrombolysis and previous statin treatment). Indeed, a recent multicentre Italian real-life study showed that almost half of the cases of DAPT prescription did not adhere to trial inclusion and exclusion criteria. 7 Recent data from the INSPIRES trial suggests that the clinical severity of stroke and the timing of DAPT introduction may be potential indicators of HT risk, 6 due to a slightly higher frequency of HT than observed in previous trials.3,5
It should be noted that the timing of introduction and duration of treatment might vary in clinical trials based on the specific scenario. Neurologists dealing with acute stroke settings should be cautious about the risk of haemorrhagic complications that can occur with the early use of DAPT. 8 Currently, data on the predictors of the risk of HT in acute ischaemic stroke (AIS) patients on DAPT for other indications (i.e. symptomatic intracranial stenosis, early carotid stenting) are poorly available.
Therefore, our study aimed to identify possible clinical and radiological predictors of cerebral haemorrhagic complications in consecutive AIS patients receiving DAPT for different clinical indications within 72 h from symptoms’ onset in a real-life population.
Methods
Patient selection
We retrospectively collected data from our prospective stroke registry concerning all consecutive AIS patients aged ⩾18 years old, hospitalised in the Stroke Unit of the University Hospital of Rome Tor Vergata from June 2021 to June 2023. In this register, clinical, demographical and radiological features are collected regularly by trained members of our Stroke Team. Inclusion criteria were: (i) patients with AIS treated with DAPT (Clopidogrel combined with Aspirin) within 72 h from the symptoms’ onset (indications for DAPT treatment included: mild or moderate AIS, symptomatic intracranial artery stenosis and symptomatic carotid angioplasty stenting) and (ii) patients who presented a recent ischaemic lesion in DWI brain MRI sequences performed at DAPT initiation time. Exclusion criteria were: (i) ongoing DAPT or anticoagulant treatment at admission, (ii) unavailable MRI imaging information and (iii) periprocedural complications.
Clinical, biological, neuroradiological data and procedural information from the stroke registry were analysed for patients receiving reperfusion treatment. Patients were followed up for 3 months after the index event to assess efficacy and safety outcomes.
Clinical data
We collected: (i) demographic data; (ii) vascular risk factors and related treatments; (iii) medical history; (iv) baseline vital signs; (v) baseline laboratory tests; (vi) severity of stroke determined by NIHSS score assessed on arrival, at 24 h from symptoms onset and on discharge; (vii) pre-stroke disability and functional outcome at 3 months assessed by modified Rankin Scale (mRS) score and (viii) characteristics of the stroke.
Neuroimaging data
All patients underwent a standardised stroke imaging protocol at admission, which included brain non-contrast-computed tomography (NCCT), CT-angiography and CT-perfusion imaging when indicated. A first follow-up NCCT was repeated at 24 h in patients who underwent reperfusion therapies (i.e. intravenous thrombolysis and mechanical thrombectomy). Brain 1.5 Tesla magnetic resonance imaging (MRI) (including axial DWI, 3D time-of-flight MR-angiography of the intracranial vessels, axial FLAIR and axial gradient echo imaging) was performed at the time of DAPT introduction in all patients. Patients with early HT that contraindicated the start of DAPT, detected at 24-h brain NCCT or at 48-h brain MRI were excluded. According to the internal guidelines of common clinical practice, a second follow-up NCCT was performed 7 days (±2) after DAPT introduction or at discharge. Two blinded neurologists (I.M. and M.D.), expert in neurovascular disease, independently assessed all the scans.
Haemorrhagic transformation (HT) was categorised according to ECASS III classification based on radiological appearance on follow-up NCCT. Symptomatic HT was defined as any extravascular blood in the brain or within the cranium associated with early clinical deterioration (defined by an increase of 4 points or more in the NIHSS score). 9
Pure subarachnoid haemorrhage was defined as subarachnoid hyperdense lesions caused by vessel rupture and was similarly assessed on follow-up NCCT. Concerning lesion characteristics, we evaluated the number and volume of lesions by the ABC/2 method in the DWI images. 10 In the case of multiple lesions, we considered the most extensive lesion.
Small vessel disease (SVD) was evaluated by radiological signs including the presence of lacunae and areas of hyperintensity on T2 FLAIR images, the presence of perivascular spaces, the presence and number of microbleeds and the presence of cortical superficial siderosis on T2 Gradient Echo images. 11 The Fazekas scale was used to calculate scores for periventricular and subcortical leukoaraiosis, ranging from 0 for the minimum to 3 for the maximum score. 12
We summed up radiological signs of SVD by adapting the Cerebral Small Vessel Disease Score (CSVD), as follows: 1 point for ⩾11 periventricular spaces in the basal ganglia; 1 point in presence of a microbleed; 1 in presence of a lacuna and 1 point for patients with a Fazekas score ⩾2. 13
DAPT administration
All patients in this study started DAPT within 72 h from symptoms’ onset. According to treatment indications, we have categorised the timing of initiation into four-time intervals (<12, 12–24, 24–48 and ⩾48 h). We recorded the use of either a single loading dose (LD) (300 mg for Clopidogrel or 250 mg for Aspirin) when the patient was already on single antiplatelet treatment (SAPT) or a dual LD of Aspirin and Clopidogrel. Following current guidelines and indications, the DAPT assumption continued for 21 days for minor stroke, 90 days for symptomatic intracranial stenosis and up to 180 days for stent placement.
Outcome evaluation
The primary outcome of the study was to evaluate the safety of the treatment, particularly regarding the risk of HT of the ischaemic lesion. Safety was determined based on the presence of any symptomatic or asymptomatic HT at follow-up NCCT images. Secondary outcomes included (i) other intracranial or systemic haemorrhagic complications, and (ii) the occurrence of ischaemic stroke, transient ischaemic attack or acute coronary syndrome (ACS) in a composite ischaemic outcome. At 90 days after the event, we conducted an in-person visit during which we collected medical reports on any secondary outcomes. The diagnosis of ACS was made according to the definition given in the guidelines of the European Society of Cardiology, 14 while the definition of TIA was made following the American Heart Association statement of 2023. 15
Finally, we assessed the possible association between several risk factors (clinical, neuroimaging, biological) and the incidence of HT.
Statistical analysis
Quantitative variables are expressed as the mean (standard deviation) in case of a normal distribution or median (interquartile range) otherwise. Categorical variables are expressed as percentages. We compared the groups for the categorical variables with the χ2 test with Yates correction or Fisher’s exact test when appropriate and for the continuous variables with the Mann–Whitney U test. The normality of the distributions was assessed using histograms and the Shapiro–Wilk test. We used the receiver operating characteristic (ROC) curve to determine the predictive values of the area under the curve (AUC) and the 95% confidence interval (CI) for ischaemic lesion volume. For the value that best predicts the outcome, we also determine the cut-off point that best distinguishes the presence and absence of the primary endpoint. We determined the cut-off point as the maximum Youden’s index (sum of sensitivity and specificity). We considered an AUC value of 0.70 or higher as indicative of moderately accurate discrimination. We conducted logistic regression analysis with the primary endpoint as the dependent variable. The independent variables included in the analysis were selected from a bivariate analysis, using a level of 0.1 as a screening criterion to increase association detection. The lesion volume variable was forced into the model as a dichotomous variable according to the cut-off established with the ROC curve. Correlations between independent variables were checked for possible collinearity between the variables (defined as rho >0.6). The adjusted odds ratios (adj. OR) and the 95% CI were calculated from logistic regression analyses. A two-tailed p-value < 0.05 was considered significant. The data were analysed using Statistical Package for the Social Sciences 26 for Windows (IBM Corp., Armonk, NY, USA). A significance level of p < 0.05 was considered for all results.
Results
Study population
A total of 706 patients with AIS were collected. Out of these, 194 (66.5% male, median age 70 years) fulfilled the inclusion criteria and were eligible for analysis. The baseline characteristics of the entire cohort are shown in Table 1.
Table 1.
Baseline and DAPT characteristics of the study population and bivariate comparison between patients with and without haemorrhagic transformation.
| Variable | Population (n = 194) | Haemorrhagic transformation | p-Value | |
|---|---|---|---|---|
| Yes (n = 28) | No (n = 166) | |||
| Demographic features | ||||
| Gender, M | 129 (66.5) | 22 (79) | 107 (64.5) | 0.2 |
| Age a | 70 (61–78) | 65 (64–71) | 71 (61–79) | 0.1 |
| Medical history | ||||
| Arterial hypertension | 141 (73) | 18 (643) | 123 (743) | 0.3 |
| Diabetes mellitus | 45 (23) | 9 (32) | 36 (22) | 0.2 |
| Hypercholesterolaemia | 62 (32) | 8 (29) | 54 (32.5) | 0.6 |
| Hypertriglyceridaemia | 64 (33) | 9 (32) | 55 (33.1) | 0.9 |
| Smoking habit | 91 (47) | 11 (39) | 80 (48) | 0.3 |
| Previous TIA/stroke | 27 (14) | 4 (14) | 23 (14) | 0.9 |
| History of neoplasm | 14 (7) | 2 (7) | 12 (7) | 0.9 |
| Previous MI | 20 (10) | 3 (11) | 17 (10) | 0.9 |
| Heart failure | 7 (4) | 1 (4) | 6 (4) | 0.9 |
| Peripheral artery disease | 7 (4) | 2 (7) | 5 (3) | 0.2 |
| Ongoing therapies | ||||
| Aspirin | 63 (33) | 6 (22) | 57 (34.5) | 0.2 |
| Clopidogrel | 9 (5) | 1 (4) | 8 (5) | 0.7 |
| Statins | 62 (32) | 8 (28) | 54 (33) | 0.7 |
| Antihypertensives | 132 (69) | 20 (74) | 112 (68) | 0.5 |
| Hypoglycaemics | 35 (18) | 5 (18.5) | 30 (18) | 0.9 |
| Baseline clinical and biological characteristics | ||||
| SAP, a mmHg | 160 (140–175) | 155 (145–180) | 160 (140–175) | 0.9 |
| DAP, a mmHg | 80 (72–90) | 90 (75–100) | 80 (70–90) | 0.1 |
| Total cholesterol, a mg/dl | 177 (148–208) | 168 (148–191) | 178 (148–209) | 0.2 |
| HDL cholesterol, a mg/dl | 42 (33–50) | 37 (30–48) | 43 (34–50) | 0.1 |
| LDL cholesterol, a mg/dl | 110 (81–141) | 109 (80–123) | 111 (81–143) | 0.6 |
| Triglycerides, a mg/dl | 114 (87–144) | 112 (87–134) | 114 (88–149) | 0.4 |
| Blood glucose concentration, a mg/dl | 104 (90–127) | 107 (100–133) | 102 (90–125) | 0.1 |
| CRP concentration, a mg/dl | 3.9 (1.3–9.3) | 5.2 (1.9–20.9) | 3.8 (1.3–9.1) | 0.1 |
| Stroke characteristics and procedures | ||||
| Baseline NIHSS score a | 4 (2–8) | 11 (5–19) | 3 (2–7) | <0.01 |
| 24-h NIHSS score a | 3 (1–5) | 5 (3–10) | 2 (1–5) | <0.01 |
| 7-day NIHSS score a | 2 (0–3) | 3 (2–5) | 1 (0–3) | <0.01 |
| Intravenous thrombolysis | 51 (26.3) | 9 (32.1) | 42 (25.3) | 0.4 |
| Mechanical thrombectomy | 43 (22.2) | 15 (53.6) | 28 (16.9) | <0.01 |
| Reperfusion therapies | 75 (38.6) | 17 (60.7) | 58 (34.9) | <0.01 |
| TOAST classification | 0.09 | |||
| Large vessel | 105 (54.5) | 21 (75) | 84 (51) | |
| Lacunar | 35 (18) | 2 (7) | 33 (20) | |
| Cardioembolic | 2 (1) | 0 (0) | 2 (1) | |
| Undetermined | 41 (21) | 4 (14) | 37 (22) | |
| Other | 10 (5) | 1 (4) | 9 (5.5) | |
| DAPT indication | 0.01 | |||
| Stent placement | 87 (45) | 20 (71.5) | 68 (41) | |
| Intracranial stenosis | 46 (24) | 4 (14) | 42 (25) | |
| Minor stroke | 61 (31) | 4 (14) | 57 (34) | |
| Timing DAPT | 0.09 | |||
| <12 h | 49 (25.5) | 10 (37) | 39 (24) | |
| 12–24 h | 54 (28) | 5 (18.5) | 49 (30) | |
| 24–48 h | 38 (20) | 2 (7) | 36 (22) | |
| >48 h | 51 (27) | 10 (37) | 41 (25) | |
| DAPT administration | ||||
| Aspirin LD | 97 (50) | 17 (61) | 80 (48) | 0.2 |
| Clopidogrel LD | 53 (27) | 12 (43) | 41 (25) | 0.04 |
| Dual LD | 34 (17.5) | 9 (32) | 25 (15) | 0.03 |
Unless specified values are number of patients (%).
median value (interquartile range).
Bold values denote statistical significance at the p < 0.05 level.
CRP, C-reactive protein; DAP, diastolic blood pressure; DAPT, dual antiplatelet therapy; LD, loading dose; MI, myocardial infarction; NIHSS, NIH Stroke Scale; SAP, systolic blood pressure; DAP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CRP, C-reactive protein; TIA, transitory ischaemic attack.
Twenty-eight patients (14.4%) reported an HT after DAPT administration; of these, four patients had a parenchymal hematoma (PH) subtype (two patients with PH1 and two patients with PH2). Two patients had asymptomatic intracranial haemorrhage.
A higher incidence of HT was observed in patients who underwent carotid stenting compared to those who were on DAPT either following a minor stroke or for a stroke due to intracranial stenosis (71.5% in the stenting vs 14.2% in the minor stroke vs 14.2% in intracranial stenosis group, p < 0.01) (Table 1). Reperfusion therapy was correlated with a higher occurrence of HT (p < 0.01), particularly in the case of MT (p < 0.01), while intravenous thrombolysis alone did not affect it (p = 0.4) (Table 1).
Patients with HT presented a more severe stroke at baseline (NIHSS score median values 11 vs 3, p < 0.01) and at 24-h (NIHSS score median values 5 vs 2, p < 0.01) (Table 1).
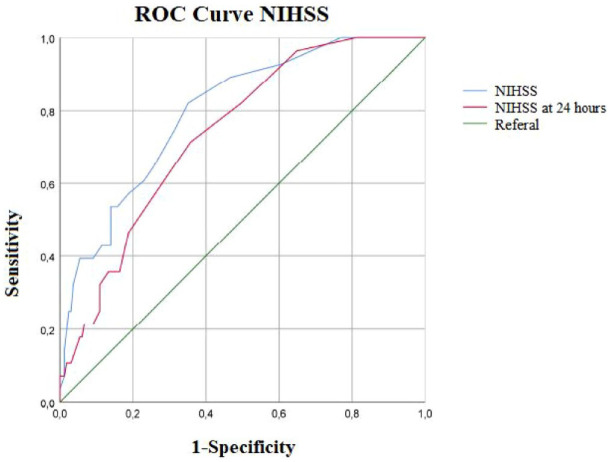
By ROC curve, we determined a cut-off value of baseline NIHSS > 4 that correlated with a 5.86-fold higher risk of HT, AUC 0.80 (95% CI 0.75–0.88), sensitivity 0.82, specificity 0.65, p < 0.001 (Figure 1).
Figure 1.
Receiver operating characteristic curves for the HT. (Blue line) AUC for HT for NIHSS, AUC 0.80 (95% CI 0.75–0.88), sensitivity 0.82, specificity 0.65, p < 0.001. (Red line) AUC for HT NIHSS at 24 h. AUC 0.74 (95% CI 0.72–0.82), sensitivity 0.71, specificity 0.65.
AUC, area under the curve; CI, confidence interval; HT, haemorrhagic transformation; NIHSS, NIH Stroke Scale.
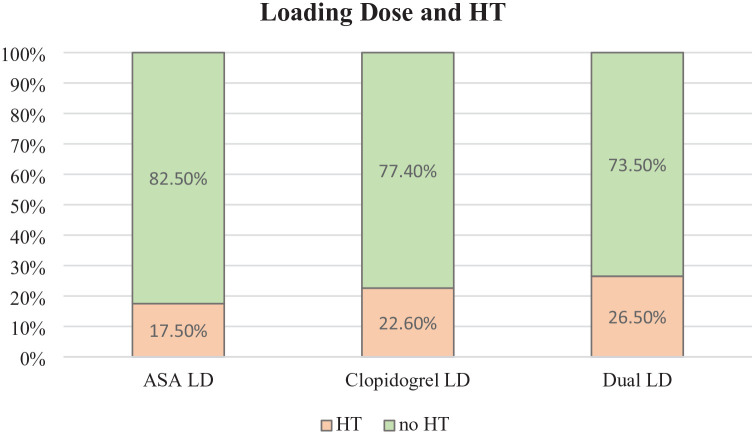
Patients treated with Clopidogrel LD and those treated with Clopidogrel + Aspirin LD were more likely to experience HT (respectively, 22.6% and 26.5% with p = 0.04 and p = 0.03 vs 17.5% for Aspirin LD) (Figure 2). No differences were observed in the case of Aspirin LD, p = 0.2. Different timing of DAPT initiation within 72 h, treatment seems not to influence HT occurrence (p = 0.09) (Table 1).
Figure 2.
Comparison of the percentage of HT among patients who received Aspirin loading dose (n = 97), Clopidogrel loading dose (n = 53) and Dual loading dose (n = 34).
HT, haemorrhagic transformation.
Neuroimaging
A larger lesion volume calculated with the ABC/2 method on MRI DWI sequences was related to higher HT occurrence (p < 0.001) (Table 2).
Table 2.
Neuroradiological characteristics of the study population and bivariate comparison between patients with and without haemorrhagic transformation.
| Variable | Population (n = 194) | Haemorrhagic transformation | p-Value | |
|---|---|---|---|---|
| Yes (n = 28) | No (n = 166) | |||
| Microbleeds | 30 (15.5) | 6 (21) | 24 (12) | 0.2 |
| Cortical superficial siderosis | 1 (1) | 0 (0) | 1 (1) | 0.6 |
| Atrophy | 53 (31) | 7 (28) | 46 (31.5) | 0.7 |
| Perivascular spaces | 51 (27) | 6 (21) | 45 (27) | 0.5 |
| Lacunae | 104 (54) | 10 (36) | 94 (57) | 0.03 |
| White matter hyperintensity | 159 (83) | 21 (75) | 138 (84) | 0.3 |
| Number of acute lesions (>2) | 127 (66) | 21 (75) | 106 (65) | 0.4 |
| CT lesion volume, a cm3 | 0 (0–0.1) | 0.5 (0–2.9) | 0 (0–0.8) | <0.01 |
| MRI lesion volume, a cm3 | 2 (0.3–10) | 23.1 (8.5–58.1) | 1.3 (0.2–5.3) | <0.01 |
| Fazekas scale (deep white matter) | 0.2 | |||
| 0 | 40 (21) | 9 (32) | 31 (19) | |
| 1 | 109 (56.5) | 14 (50) | 95 (58) | |
| 2 | 35 (18) | 5 (18) | 30 (18) | |
| 3 | 9 (5) | 0 (0) | 9 (5.5) | |
| Fazekas scale (periventricular white matter) | 0.5 | |||
| 0 | 42 (22) | 8 (29) | 34 (21) | |
| 1 | 89 (46) | 14 (50) | 75 (45.5) | |
| 2 | 50 (26) | 5 (18) | 45 (27) | |
| 3 | 12 (6) | 1 (4) | 11 (7) | |
| CSVD score | 0.1 | |||
| 0 | 57 (29.5) | 13 (46) | 44 (27) | |
| 1 | 65 (34) | 8 (29) | 57 (34.5) | |
| 2 | 52 (27) | 5 (18) | 47 (28.5) | |
| 3 | 12 (6) | 0 (0) | 12 (7) | |
| 4 | 7 (4) | 2 (7) | 5 (3) | |
Unless specified, values are number of patients (%).
= median value (interquartile range).
Bold values denote statistical significance at the p < 0.05 level.
CSVD, cerebral small vessel disease; CT, computed tomography; MRI, magnetic resonance imaging.
We obtained a cut-off volume value maximising sensitivity and specificity in discriminating the probability of HT at follow-up NCCT. Good reliability was associated with a lesion volume ⩾8.2 ml (AUC 0.82, 95% CI 0.74–0.90, p < 0.001, sensitivity 0.79, specificity 0.80), with a risk of HT 10-fold more frequent (Figure 3).
Figure 3.
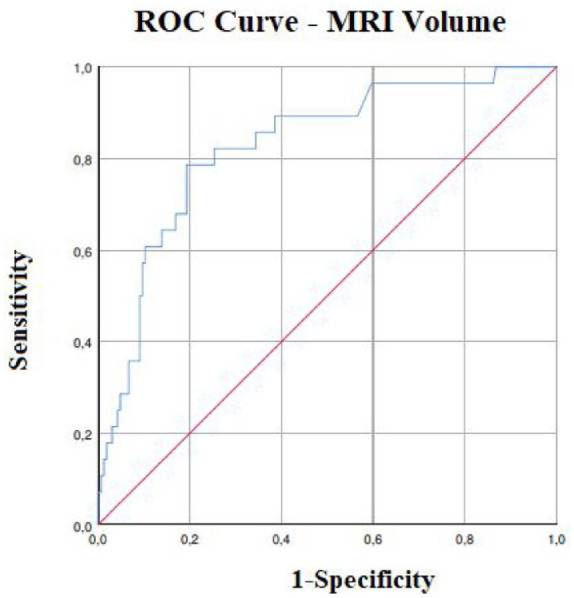
Predictive values of volumes defined at DWI MRI for HT.
Receiver operating characteristic curves for HT. AUC for HT (AUC 0.82, 95% CI 0.74–0.90, p < 0.001, sensitivity 0.79, specificity 0.80).
AUC, area under the curve; CI, confidence interval; HT, haemorrhagic transformation; MRI, magnetic resonance imaging.
The logistic regression model demonstrated a 6-fold increased risk of developing HT with a NIHSS > 4 (adj. OR 6.5, CI 1.3–32.7, p = 0.024). Additionally, patients with a lesion volume of 8.2 ml or greater on MRI were found to have over 10 times the risk of developing HT (adj. OR 11, CI 3.1–39.2, p < 0.001). This statistical model included different DAPT indications, reperfusion therapy, dual LD and Clopidogrel LD, confirming that the results could be applicable in our heterogeneous population (Table 3).
Table 3.
Results of the logistic regression analysis with HT as dependent variable.
| Dependent variables | Logistic regression | Independent variables | Adj. OR (95% CI) | p-value | Variables entered in the model but not selected |
|---|---|---|---|---|---|
| HT | p < 0.001 | MRI LV ⩾8.2 cm3 | 11.0 (3.1–39.2) | <0.001 | Reperfusion therapy, DAPT indication, Clopidogrel LD, Dual LD |
|
R2 = 0.403 Classified = 85.6% |
NIHSS > 4 | 6.5 (1.3–32.7) | 0.024 |
Adj. OR, adjusted odds ratio; CI, confidence interval; DAPT, dual antiplatelet therapy; HT, haemorrhagic transformation; LD, loading dose; LV, lesion volume; MRI, magnetic resonance imaging; NIHSS, NIH Stroke Scale.
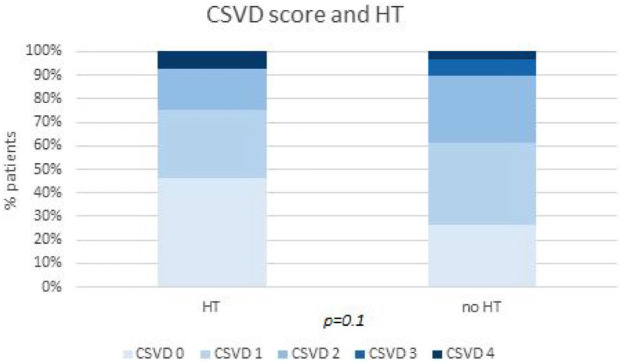
The markers of cerebral SVD, both individually and cumulatively in the CSVD score (Figure 4), as well as the Fazekas score, did not differ between patients with or without HT. However, the presence of lacunae was found to be less associated with the risk of HT (p = 0.03) (Table 2). The interrater reliability for neuroradiological assessment was strong (k = 0.91).
Figure 4.
Comparison of the percentage of patients scoring from 0 to 4 in CSVD score between those with or without HT. This figure emphasises the controversial relationships between SVD, in particular CSVD score and HT. CSVD scores of 0 and 1 are more expressed in the HT population, although not statistically significant (p = 0.1).
CSVD, cerebral small vessel disease; HT, haemorrhagic transformation; SVD, small vessel disease.
Two patients presented other systemic haemorrhagic complications, one patient experienced melena 20 days after DAPT initiation, and one had a delayed nucleocapsular haemorrhage 2 months later.
Secondary outcomes
Three patients reported TIA/stroke at 3-month follow-up: one reported TIA at 2 months, and two had ischaemic stroke 45 and 30 days after the initial event, respectively. In addition, we registered one case of ACS 6 weeks after the event.
Discussion
The increasing use of DAPT as an early treatment in patients with AIS calls for the identification of predictive factors for possible haemorrhagic complications. Within the heterogeneous population of ischaemic stroke patients, it is crucial for clinicians to have markers that guide treatment choices in areas not clearly delineated by guidelines.
The study results indicate that a NIHSS cut-off value greater than 4 was associated with a 6-fold increased risk of HT. Furthermore, a volume cut-off value greater than 8.2 ml was associated with an 11-fold increased risk of HT in AIS patients receiving DAPT.
A previous single-centre cohort study on DAPT-treated stroke patients with non-minor stroke (defined as NIHSS ⩾4) reported no significant increase in the rate of major bleeding during hospitalisation when compared to those treated with SAPT, even after NIHSS stratification (NIHSS 4–7 vs NIHSS ⩾8) and considering thrombolysis or thrombectomy pre-treatment. 16
In our study, 28 patients (14.4%) presented HT at follow-up NCCT after DAPT initiation. Only four patients (2%) had a symptomatic HT, while two presented with other haemorrhagic complications (1%). Our larger population confirms the safety of DAPT in AIS, even for patients with NIHSS scores > 4, compared to Khazaal et al. 16 observations. In patients with HTs, the haemorrhagic complications were not life threatening. Therefore, our results confirm that DAPT is a safe therapy for current indications, even in cases of moderate stroke early after the ischaemic event. Nevertheless, the presence of any HT leads the clinician to face the dilemma of DAPT discontinuation, balancing individual risk/benefit ratio and possibly exposing the patient to a higher risk of early ischaemic recurrence or stent occlusion.
Stroke severity has been reported to be closely related to infarct volume, 17 and direct volume measurements have a strong correlation with the risk of bleeding. However, it should be noted that the NIHSS score, a commonly used measure of stroke severity, might have a bias towards one hemisphere. This is because the severity of stroke on one side of the brain may be underestimated, leading to a difference in the clinical assessment of patients depending on the affected hemisphere. 18 Therefore, evaluating the baseline NIHSS score together with the lesion volume, may offer a more accurate assessment of the tissue at risk for haemorrhagic reperfusion damage, compared to NIHSS score alone.
In a review concerning the risk of HT after stroke, it was found that lesions with diameters <1.5 cm were at lower risk of HT with an increasing risk for larger sizes, without a specific cut-off and regardless of treatment. 19
Beyond the volume of the acute lesion, MRI provides more accurate information on the presence and extent of SVD radiological features. While individual markers of SVD or their combined score (CSVD) were not found to be predictors of a higher risk of HT, the presence of lacunae was associated with a lower frequency of HT. This result confirms the controversial findings in the literature about the relationship between SVD and HT, where an increased risk of HT for intermediate burdens of SVD and a decreased risk for high SVD burdens are reported. 20
Thus, an MRI picture of diffuse white matter damage and lacunae should not exclude a priori an AIS patient candidate for DAPT.
The previous thrombolytic reperfusion treatment did not appear to influence the risk of bleeding complications in our population. Two Asian retrospective studies have already shown a substantially equal haemorrhagic risk between patients with minor stroke undergoing thrombolysis followed by DAPT compared to SAPT after thrombolysis.21,22 It must be mentioned that none of these studies included patients with NIHSS > 4, differently from our more heterogeneous population with NIHSS even greater than 4.
Instead, patients who underwent reperfusion therapy, in particular MT or stent placement, had a higher incidence of HT (p < 0.001), in line with the findings of studies on the safety of TANDEM occlusion recanalisation.23,24 This observation highlights the need for clinicians to carefully consider the timing and modality of DAPT administration, particularly in patients undergoing stenting in the acute stage.
Regarding the impact of antiplatelet therapy, both the Clopidogrel LD and the dual LD resulted in a higher frequency of HT. This evidence, together with the higher frequency of HT after stenting, emphasises the need for a careful risk assessment in this subgroup of patients and closer clinical and radiological monitoring.
Concerning the efficacy of DAPT at 3 months, out of the observed study population, there were five ischaemic events, including three strokes, one TIA and one myocardial infarction.
The main strength of the study relies on its possible implications in clinical practice.
Our study aimed to provide valuable prognostic criteria, easily obtainable and reproducible in everyday practice, for risk stratification of HT occurrence to support clinicians in decision-making. Apart from cases where the inclusion of DAPT is considered mandatory (i.e. acute carotid stenting), the very early introduction of DAPT treatment could increase the risk of HT and lead to poor long-term outcomes. Thus, a better selection with a combined clinical–radiological approach of patients could be desirable to optimise secondary prevention strategies. In particular, in MT treatment, the need for single or dual LD, higher baseline and 24-h NIHSS, larger brain MRI volume may help identify a subset of patients at higher risk of HT when undergoing DAPT therapy soon after AIS.
This study has some limitations. First, it has a retrospective and monocentric design even though patients were enrolled from a consecutive and prospective stroke registry, which limits the risk of biases. Secondly, the study population is heterogeneous, due to the inclusion of patients with different indications for DAPT and stroke aetiologies. However, the study was precisely designed to mirror the complexity of real-world clinical practice in which clinicians must face different scenarios and customise therapies on a patient-by-patient basis. A further limitation is the different patterns of DAPT administration, influenced by different indications and attitudes of neurologists in the absence of established protocols for timing and dose loading in all scenarios. Furthermore, HT was assessed with a brain NCCT scan 1 week after the start of DAPT according to the ECASS III criteria for symptomatic intracerebral hemorrahge, 9 so it cannot be excluded that other asymptomatic events may have occurred after this period. The number of patients enrolled did not allow us to employ matching methods in the statistical analysis, particularly when comparing the populations undergoing intravenous thrombolysis and MT; however, we included reperfusion treatment in our logistical regression model (Table 3). In conclusion, no power analysis was performed to calculate the sample size.
Conclusion
In the acute phase of moderate strokes, although DAPT seems to be a safe therapeutic option, certain clinical and radiological predictors were associated with a higher occurrence of HT: higher NIHSS at baseline and after 24 h, reperfusion treatment with MT, stent placement, dual antiplatelet/Clopidogrel LD and larger ischaemic stroke in MRI DWI/ADC sequences. Furthermore, considering the inherent differences in such a heterogeneous real-life population, we identified a brain MRI cut-off for ischaemic lesions which is highly predictive of HT. This cut-off, along with a thorough individual clinical evaluation, may help detect warning markers to avoid early HT, which could lead to treatment discontinuation and potentially expose the patient to a higher risk of early ischaemic recurrence.
Acknowledgments
None.
Footnotes
ORCID iD: Ilaria Maestrini  https://orcid.org/0000-0003-4996-7506
https://orcid.org/0000-0003-4996-7506
Contributor Information
Maria Rosaria Bagnato, Stroke Center, Department of Systems Medicine, University Hospital of Rome ‘Tor Vergata’, Rome, Italy.
Ilaria Maestrini, Stroke Center, Department of Systems Medicine, University Hospital of Rome ‘Tor Vergata’, Viale Oxford 81, Rome 00133, Italy.
Leonardo Bruno, Stroke Center, Department of Systems Medicine, University Hospital of Rome ‘Tor Vergata’, Rome, Italy.
Ilaria Ciullo, Stroke Center, Department of Systems Medicine, University Hospital of Rome ‘Tor Vergata’, Rome, Italy.
Federica D’Agostino, Stroke Center, Department of Systems Medicine, University Hospital of Rome ‘Tor Vergata’, Rome, Italy.
Giordano Lacidogna, Stroke Center, Department of Systems Medicine, University Hospital of Rome ‘Tor Vergata’, Rome, Italy.
Federico Marrama, Stroke Center, Department of Systems Medicine, University Hospital of Rome ‘Tor Vergata’, Rome, Italy.
Alfredo Paolo Mascolo, Stroke Center, Department of Systems Medicine, University Hospital of Rome ‘Tor Vergata’, Rome, Italy.
Alessandro Rocco, Stroke Center, Department of Systems Medicine, University Hospital of Rome ‘Tor Vergata’, Rome, Italy.
Marina Diomedi, Stroke Center, Department of Systems Medicine, University Hospital of Rome ‘Tor Vergata’, Rome, Italy.
Declarations
Ethics approval and consent to participate: All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments. All the procedures performed were part of the routine care. The local ethics committee of the University Hospital of Rome ‘Tor Vergata’ approved prospective data collection on patients undergoing EVT (R.S. 25/18) and their use for retrospective studies and analysis. The patients who were enrolled signed a written informed consent for study participation, collection and use of data.
Consent for publication: Patients gave their written consent to anonymous scientific publication of their data.
Author contributions: Maria Rosaria Bagnato: Conceptualisation; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Ilaria Maestrini: Data curation; Conceptualisation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Leonardo Bruno: Data curation; Writing – review & editing.
Ilaria Ciullo: Data curation; Writing – review & editing.
Federica D’Agostino: Data curation; Writing – review & editing.
Giordano Lacidogna: Data curation; Writing – review & editing.
Federico Marrama: Data curation; Writing – review & editing.
Alfredo Paolo Mascolo: Data curation; Writing – review & editing.
Alessandro Rocco: Data curation; Writing – review & editing.
Marina Diomedi: Conceptualisation; Investigation; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable requests.
References
- 1. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke 2021; 52: e364–e467. [DOI] [PubMed] [Google Scholar]
- 2. Johnston SC, Easton JD, Farrant M, et al. Clinical Research Collaboration, Neurological Emergencies Treatment Trials Network, and the POINT Investigators. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018; 379(3): 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kennedy J, Hill MD, Ryckborst KJ, et al. Fast Assessment of Stroke and Transient Ischaemic Attack to Prevent Early Recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol 2007; 6: 961–969. [DOI] [PubMed] [Google Scholar]
- 4. Wang Y, Wang Y, Zhao X, et al. CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013; 369(1): 11–19. [DOI] [PubMed] [Google Scholar]
- 5. Johnston SC, Amarenco P, Denison H, et al. THALES Investigators. The acute stroke or transient ischemic attack treated with Ticagrelor and Aspirin for Prevention of Stroke and Death (THALES) trial: rationale and design. Int J Stroke 2019; 14(7): 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao Y, Chen W, Pan Y, et al. INSPIRES Investigators. Dual antiplatelet treatment up to 72 hours after ischemic stroke. N Engl J Med 2023; 389(26): 2413–2424. [DOI] [PubMed] [Google Scholar]
- 7. De Matteis E, De Santis F, Ornello R, et al. READAPT Study Group. Divergence between clinical trial evidence and actual practice in use of dual antiplatelet therapy after transient ischemic attack and minor stroke. Stroke 2023; 54(5): 1172–1181. [DOI] [PubMed] [Google Scholar]
- 8. Shah J, Liu S, Yu W. Contemporary antiplatelet therapy for secondary stroke prevention: a narrative review of current literature and guidelines. Stroke Vasc Neurol 2022; 7(5): 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hacke W, Kaste M, Bluhmki E, et al. ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359(13): 1317–1329. [DOI] [PubMed] [Google Scholar]
- 10. Sananmuang T, Dejsiripongsa T, Keandoungchun J, et al. Reliability of ABC/2 method in measuring of infarct volume in magnetic resonance diffusion-weighted image. Asian J Neurosurg 2019; 14(3): 801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Lancet Neurol 2013; 12(8): 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valdés Hernández MdC, Morris Z, Dickie DA, et al. Close correlation between quantitative and qualitative assessments of white matter lesions. Neuroepidemiology 2013; 40(1): 13–22. [DOI] [PubMed] [Google Scholar]
- 13. Yilmaz P, Ikram MK, Niessen WJ, et al. Practical small vessel disease score relates to stroke, dementia, and death. Stroke 2018; 49(12): 2857–2865. [DOI] [PubMed] [Google Scholar]
- 14. Byrne RA, Rossello X, Coughlan JJ, et al. ESC Scientific Document Group. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J 2023; 44(38): 3720–3826. [DOI] [PubMed] [Google Scholar]
- 15. Amin HP, Madsen TE, Bravata DM, et al. AHA Emergency Neurovascular Care Committee of the Stroke Council and Council on Peripheral Vascular Disease. Diagnosis, workup, risk reduction of transient ischemic attack in the emergency department setting: a scientific statement from the American Heart Association. Stroke 2023; 54(3): e109–e121. [DOI] [PubMed] [Google Scholar]
- 16. Khazaal O, Rothstein A, Husain MR, et al. Dual-antiplatelet therapy may not be associated with an increased risk of in-hospital bleeding in patients with moderate or severe ischemic stroke. Front Neurol 2021; 12: 728111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Furlanis G, Ajcˇević M, Stragapede L, et al. Ischemic volume and neurological deficit: correlation of computed tomography perfusion with the National Institutes of Health Stroke Scale score in acute ischemic stroke. J Stroke Cerebrovasc Dis 2018; 27(8): 2200–2207. [DOI] [PubMed] [Google Scholar]
- 18. Vitti E, Kim G, Stockbridge MD, et al. Left hemisphere bias of NIH stroke scale is most severe for middle cerebral artery strokes. Front Neurol 2022; 13: 912782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grifoni E, Bini C, Signorini I, et al. Predictive factors for hemorrhagic transformation in acute ischemic stroke in the REAL-World Clinical Practice. Neurologist 2023; 28(3): 150–156. [DOI] [PubMed] [Google Scholar]
- 20. Wei C, Liu J, Li J, et al. A non-linear association between total small vessel disease score and hemorrhagic transformation after ischemic stroke with atrial fibrillation and/or rheumatic heart disease. Front Neurol 2019; 10: 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao G, Lin F, Wang Z, et al. Dual antiplatelet therapy after intravenous thrombolysis for acute minor ischemic stroke. Eur Neurol 2019; 82: 93–98. [DOI] [PubMed] [Google Scholar]
- 22. Xu Z, Chen N, Sun H, et al. Dual antiplatelet therapy in patients with minor stroke receiving intravenous thrombolysis. Front Neurol 2022; 13: 819896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buckel P, Perez MA, Almatter M, et al. Functional outcome and safety of intracranial thrombectomy after emergent extracranial stenting in acute ischemic stroke due to tandem occlusions. Front Neurol 2018; 9: 940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pop R, Burel J, Finitsis SN, et al. Comparison of three antithrombotic strategies for emergent carotid stenting during stroke thrombectomy: a multicenter study. J Neurointerv Surg 2023; 15(e3): e388–e395. [DOI] [PubMed] [Google Scholar]