Abstract
Background:
Animal research suggests that repeated heat exposures may stimulate skeletal muscle protein synthesis and downregulate protein degradation.
Hypothesis:
Repeated heat exposures during ankle immobilization and rehabilitation would preserve human muscle strength and mass.
Study Design:
Controlled laboratory study.
Methods:
A total of 20 male participants (age, 33.6 ± 2.8 years; weight, 83.8 ± 9.2 kg; height, 182 ± 6 cm) underwent 4 weeks of supervised training, 2 weeks of single-lower leg immobilization, and 2 weeks of supervised rehabilitation before return to sports (RTS). Participants were split into 2 groups: (1) whole-body heat therapy (HEAT) and (2) sham treatment (SHAM) throughout the immobilization and rehabilitation periods. Measures of muscle strength (isometric and isokinetic), volume (magnetic resonance imaging and ultrasound), and muscle biopsies were obtained preimmobilization, postimmobilization, and at RTS.
Results:
Maximal isometric strength of the plantarflexors was lower at RTS compared with preimmobilization in SHAM (P = .027) but not HEAT (P = .301). Isokinetic strength during a fatigue test was higher at RTS compared with preimmobilization in HEAT (P = .039) but not SHAM (P = .245). Pennation angle and muscle thickness were lower at postimmobilization compared with preimmobilization only in SHAM (P≤ .027). Muscle cross-sectional area decreased in soleus and both gastrocnemius medialis and lateralis (all P≤ .035) in SHAM, but only in gastrocnemius medialis in HEAT. There was a large (d = 0.91) but not significant (P = .054) decrease in the ratio of phosphorylated/total nuclear factor-kappa B (NFκB) from preimmobilization to postimmobilization in HEAT only. There was an increase in phosphorylated fork head box O proteins (FoxO) only in HEAT (P = .034), suggesting a decrease in FoxO activity. Caspase 3 expression increased from preimmobilization to postimmobilization in SHAM only (P = .004).
Conclusion:
These results indicate that using heat therapy throughout immobilization and rehabilitation reduces skeletal muscle atrophy and maintains plantarflexor strength in healthy humans. Moreover, heat therapy may lead to the inactivation of the FoxO and NFκB signaling pathways involved in atrophy.
Clinical Relevance:
Repeated heat exposures should be considered a novel therapeutic intervention to counteract muscle atrophy during immobilization.
Keywords: ankle, athletic training, biologic healing enhancement, muscle injuries, muscle physiology, physical therapy modalities, physical therapy/rehabilitation
Injury, illness, or surgery may be associated with whole-body or single-limb immobilization,17,29 leading to skeletal muscle atrophy and strength loss. 9 For example, it was described nearly 50 years ago that the gastrocnemii showed rapid atrophy during the first 2 weeks of immobilization in rats. 4 A decrease in muscle cross-sectional area (CSA) and strength may be visible even within 3 days of immobilization in healthy humans, 8 jeopardizing return to sports (RTS) in athletes.1,27 Conversely, repeated heat exposures have been reported to increase cell proliferation and muscle protein content in vitro and in rodents,14,45 thus reducing muscle atrophy during immobilization in rodents.28,36 Although the effects of heat exposures on the signaling cascade affecting muscle atrophy remain to be characterized in humans via muscle biopsies, a few studies have suggested that repeated local and whole-body passive heat exposures may improve skeletal muscle contractility and strength in active healthy humans.13,23,34 However, this result is not universal as heat exposure may not have an additional effect on skeletal muscle mass in an active participant with a mechanical stimulus.25,39 To date, the only study applying heat therapy in immobilized humans showed that repeated heat exposure partly reduced vastus lateralis atrophy during knee immobilization. 15 However, the results of this study remain to be confirmed, 20 especially in other muscles such as postural muscles more affected by disuse. 8 Moreover, the functional outcome of such intervention on patient muscle strength remains to be investigated. Finally, to match the clinical reality of an immobilized athlete, heat therapy interventions should also include the rehabilitation phase as animal studies have suggested that heat exposures after immobilization may facilitate the recovery of atrophied muscles.12,37
Thus, the current study aimed to verify the hypothesis that applying heat therapy throughout immobilization and rehabilitation can limit (1) skeletal muscle atrophy and (2) reductions in muscle strength in an ankle immobilization model in healthy humans. Moreover, we investigated the molecular pathways underpinning the effects of heat therapy on skeletal muscle.
Methods
Ethical Approval
The project received approval from the hospital scientific committee (reference No. ASC/0000205/ak) and an external institutional review board (reference No. F202007002). All procedures followed ethical principles for medical research of the Declaration of Helsinki, and written consent was obtained from participants. The study ran between November 26, 2020, and July 12, 2021.
Participants
From an initial sample of 22 healthy adults, 2 participants did not complete the protocol for personal reasons, thus 20 participants completed the entire study (age, 33.6 ± 2.8 years; weight, 83.8 ± 9.2 kg; height, 182 ± 6 cm). They were separated into 2 groups, 1 exposed to heat (HEAT, n = 10) and the other to sham treatment (SHAM, n = 10). Participants were healthy and had a sports-specific background, no neuromuscular disorders, and no ankle injuries. All were former competitive athletes, primarily in athletics, now working as fitness coaches. Groups were counterbalanced by maximal isometric force, the study's primary metric. A noninferiority sample size calculation (G*Power, software Version 3.1.9.6; Heinrich Heine Universität Düsseldorf) based on maximal isometric force from a previous study showed that a minimum of 9 participants was required per group with significance and power set at .05 and 80%, respectively. 34 Of note, the project was designed and ethically approved for male participants only, given the higher thrombosis risk with ankle immobilization compared with female participants.
General Procedure
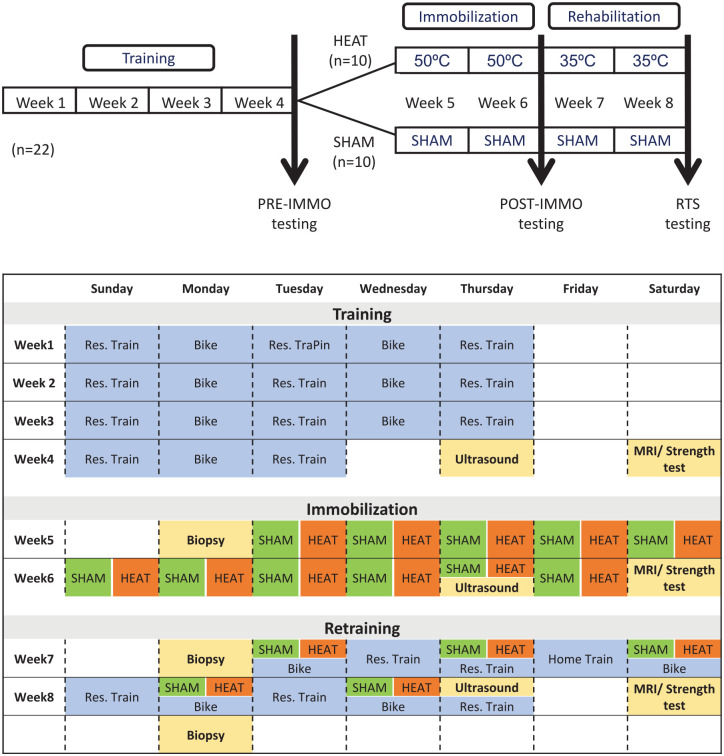
One week after a comprehensive familiarization session where participants practiced all the neuromuscular tests, all participants completed an 8-week intervention program including (1) 4 weeks of supervised training, (2) 2 weeks of single-leg immobilization with an orthopaedic walking boot, and (3) 2 weeks of supervised rehabilitation postimmobilization (Figure 1). Participants were tested (ie, neuromuscular function, imaging and muscle biopsy; see the following) before the immobilization (preimmobilization), after the immobilization (postimmobilization), and at RTS. All tests were performed at the same time of day for a given participant.
Figure 1.
General procedure for the 8-week protocol. The training phase lasted for 4 weeks, the immobilization for 2 weeks, and the rehabilitation postimmobilization for 2 weeks. The HEAT group was in an environmental chamber set at 50ºC for the passive heat exposures (ie, immobilization) and 35ºC for the active heat exposures (ie, rehabilitation). The SHAM group was in an altitude chamber set to a simulated altitude of 200 m. MRI, magnetic resonance imaging; POST-IMMO, postimmobilization; PRE-IMMO, preimmobilization; Res., resistance; RTS, return to sports.
Standardization
First, participants underwent 4 weeks of a standardized training program to ensure a common training base before starting the immobilization procedure. Each week included 5 sessions (3 resistance training sessions and 2 endurance training sessions) (Figure 1). Moreover, all participants received 4 nutritional interventions, including face-to-face consultations with a nutritionist and educational videos to standardize their daily energy and protein intake throughout training, immobilization, and rehabilitation phases. Participants maintained a minimum of 3 daily doses of 20 g each of animal protein throughout this study. In addition, participants were fasted for the muscle biopsies and were provided with standardized meals and snacks for 48 hours preceding each biopsy.
Immobilization
The left ankle of each participant was immobilized at an angle of 90° for 14 consecutive days using a therapeutic boot (Airselect Elite, Aircast, DJO). The participants were given crutches and instructed not to engage in weightbearing activity. Before starting the immobilization protocol, participants were educated on using the medical arm crutches and therapeutic boots. Regular check-in during the immobilization period ensured proper boot use and adherence to the nonweightbearing requirement.
Rehabilitation
After the immobilization period, participants followed a structured rehabilitation program for 2 weeks before RTS, including 3 sessions of general conditioning and 2 sessions of neuromuscular training per week (Figure 1).
Intervention
After the 4-week training period, participants were separated into 2 groups, 1 exposed to heat (HEAT) and the other to sham treatment (SHAM) during the immobilization and rehabilitation periods. Participants were not informed of the hypothesis until the end of the study and both groups received interventions.
During the immobilization period, participants received 11 passive interventions of 60 minutes. The HEAT group sat in a heat chamber at 48°C to 50°C and 50% relative humidity (0 m altitude). 33 The SHAM group sat in an altitude chamber, but it was set at 200 m only to create a placebo effect while avoiding any effect of altitude (24°C and 40% relative humidity).
During the rehabilitation period, participants received 5 active interventions of 60 minutes (ie, conditioning sessions, Figure 1). The HEAT group performed the sessions in the heat chamber at 35°C and 60% relative humidity (0 m altitude), 32 while the SHAM group performed the sessions in the altitude room at 200 m altitude and in a sham condition (24°C and 40% relative humidity). The rehabilitation neuromuscular training (ie, resistance training and proprioception) sessions were performed in standard conditions for both groups (temperate environment, sea level).
Neuromuscular Testing
Isometric Maximal Voluntary Contraction
After a standardized warm-up (ie, progressive isometric contractions), participants performed 3 isometric maximal voluntary contractions (iMVCs) of the plantarflexors lasting 5 seconds, interspersed by 60 seconds of rest. Participants were instructed to push as hard as possible against a dynamometric pedal (Captels). Torque was recorded at 2000 Hz (Biopac MP 35). Peak torque within a 650-millisecond window was identified as the iMVC torque, with visual feedback and verbal encouragement provided throughout.
Dynamic Fatigue Test
Participants performed 30 concentric plantarflexions at 90 deg/s (return at 60 deg/s) using an isokinetic dynamometer (Biodex System 3), 5 between 25° of dorsiflexion and 45° of plantarflexion, with the participants seated with the hip joint at about 110° (full hip extension, 180°) and the knee at 100° (full knee extension, 180°). The mean peak torque was derived from the 30 contractions.
Imaging
Magnetic Resonance Imaging
Participants underwent magnetic resonance imaging (MRI) scanning (1.5-T, MR B19) in a supine position with the foot around 20° of plantarflexion. Two coils extended the field of view (FOV), accommodating parallel imaging techniques; 90 slices (3 sets of 30 transverse planes) from the femoral condyle to the Achilles tendon covered the calf muscles. Parameters for T1 spin echo axial images included a repetition time of 730 milliseconds, echo time of 14 milliseconds, and others. A custom MATLAB script (R2017a, The MathWorks Inc) analyzed images to track each muscle's CSA. All CSA contours underwent manual validation by the same medical doctor (M.A.), blinded to the participant intervention. Maximal CSA for the soleus (SOL) and gastrocnemius medialis (GM) and lateralis (GL) were extracted, and total muscle volume was determined.
Ultrasound
Muscle thickness, pennation angle, and fascicle length of the GM were determined from images taken along the longitudinal axis of the muscle belly utilizing a 2-dimensional, B-mode ultrasound (12-MHz probe; depth, 8 cm; FOV, 14 × 47 mm; Logiq E, GE Healthcare).2,22 Measurements were standardized at 30% of shank length distal from the medial tibial condyle along the muscle belly. 24 Participants were assessed lying prone after 5 minutes of inactivity, with hips and knees in a neutral position. All analyses were done by the same medical doctor (M.A.), blinded to the participant intervention.
Muscle Biopsy
Muscle Sampling
GM muscle samples were obtained using a microbiopsy system (MAX-CORE, Bard Biopsy Systems). After 1% xylocaine anesthetic was injected and a 5-mm skin incision was made, 4 samples (60-80 mg) were extracted with a 14-gauge needle. Samples were frozen in liquid nitrogen and stored at -80°C for subsequent analysis. Biopsies were sampled early morning, in a fasted state.
Protein Extraction
Approximately 20 mg of muscle biopsy tissue were homogenized by TissueLyser II (Qiagen) using stainless steel beads 2 × 2 minutes at 25 Hz in ice-cold lysis buffer (20 mM Tris-HCl at pH 7.0, 270 mM sucrose, 5 mM EGTA, 1 mM EDTA, 1 mM sodium orthovanadate, 50 mM β-glycerophosphate, 5 mM sodium pyrophosphate, 50 mM sodium fluoride, 1 mM dithiothreitol, and 1% Triton-X 100) supplemented with cOmplete Protease Inhibitor Cocktail (Roche). After removing the beads, lysates were vortexed for 5 minutes and centrifuged at 10,000g for 10 minutes at 4°C. Supernatants were carefully separated and stored at -80°C. Protein concentration was determined by the DC Protein Assay kit (Bio-Rad), and bovine serum albumin was used as a protein concentration standard.
Western Blotting
Samples were prepared for SDS-polyacrylamide gel electrophoresis (PAGE) with Laemmli buffer (60 mM Tris at pH 6.8, 2% [wt/vol] SDS, 10% [vol/vol] glycerol, 0.01% [wt/vol] bromophenol blue, and 1.25% [vol/vol] β-mercaptoethanol). Equal amounts of protein were loaded and separated using 10% to 15% SDS-PAGE and transferred to PVDF membranes (Immun-Blot PVDF Membrane, Bio-Rad). Ponceau S staining of the membrane was used to check the quality of protein loading and transfer; 5% nonfat milk in Tris-buffered saline tween (TBST) (20 mM Tris-HCl at pH 7.6, 137 mM NaCl, and 0.02% Tween-20) was added for 1 hour to block membranes. Membranes were incubated overnight at 4°C with primary antibodies diluted in TBST with 0.1% (wt/vol) bovine serum albumin. The following antibodies were used from Cell Signaling: phospho-Akt Ser473 (catalog no. 4060), total Akt (catalog no. 9272), phospho-NF-kB Ser536 (cat. no. 3033), total NF-kB (catalog no. 8242), phospho-FoxO1 (Thr24) (catalog no. 9464), total FoxO1 (catalog no. 2880), caspase 3 (catalog no. 14220). Membranes were washed with TBST and incubated with species appropriate secondary antibody (1:10,000 in TBST with 5% nonfat milk) to which horseradish conjugated peroxidase was added. Proteins were then visualized by enhanced chemiluminescence (Immobilon Forte Western HRP substrate, Sigma-Aldrich). Images were captured with GeneSnap from Syngene. Band density was quantified using a free online gel analyzer (www.gelanalyzer.com).
Real-Time Polymerase Chain Reaction Analysis
Approximately 15 mg of muscle biopsy samples were homogenized in 1 mL Trizol reagent (Invitrogen) by TissueLyser II (Qiagen) using stainless steel beads 2 × 2 min at 25 to 30 Hz. RNA was isolated according to the manufacturer's instructions. Nanodrop spectrophotometer (Thermo Fisher Scientific) was used to assess RNA quality and quantity. According to the manufacturer's instructions, 1 µg RNA was used for reverse transcription using the iScript cDNA Synthesis Kit from Bio-Rad Laboratories. The following polymerase chain reaction (PCR) thermal profile was used: 3 minutes at 95°C, followed by 40 cycles of 15 seconds at 95°C and 30 seconds at 60°C. PCRs were carried out in a final volume of 10 µL containing 5 µL of appropriately diluted cDNA samples, 4.8 µL SsoAdvanced Universal SYBR Green SuperMix (Bio-Rad Laboratories), and 0.1 µL of each primer (100 nM final concentration). The primers used are listed in Table 1. To compensate for variations in RNA amounts input and efficiency of reverse transcription, the expression of the following housekeeping genes was quantified: ribosomal protein L19, beta2-microglobulin, and ribosomal protein L4. Gene expression of each gene of interest was normalized to the geometric mean of the 3 housekeeping genes.
Table 1.
Primer Sequences a
| Forward | Reverse | |
|---|---|---|
| B2M | ATGAGTATGCCTGCCGTGTGA | GGCATCTTCAAACCTCCATG |
| HSP70 | GGTGCTGACCAAGATGAAG | CTGCGAGTCGTTGAAGTAG |
| HSP90 | ATCAAACTTGGTCTGGGTATT | GATGTGTCGTCATCTCCTTC |
| MAFbx | GTGGTACTGAAAGTCCTTGAA | CTCTTTGGACCAGTGTACATAA |
| MuRF-1 | CTATCTGCCTGGAGATGTTTAC | TTTGCAGCCTGGAAGATG |
| RPL4 | ATACGCCATCTGTTCTGCCCT | GCTTCCTTGGTCTTCTTGTAGCCT |
| RPL19 | CGCTGTGGCAAGAAGAAGGTC | GGAATGGACCGTCACAGGC |
B2M, beta2-microglobulin; HSP, heat shock protein; MAFbx, muscle atrophy F-box protein; MuRF-1, muscle RING-finger protein-1; RPL4, ribosomal protein L4; RPL19, ribosomal protein L19.
Statistical Analysis
Although the number of participants was larger than in previous immobilization studies, 8 not all parameters showed normal distribution, necessitating nonparametric statistics. Immobilization and rehabilitation effects were analyzed using repeated measures (ie, Friedman test) for both the SHAM and HEAT groups. Effect sizes for the Friedman test were assessed by Kendall W with a W in the range of 0.00 to 0.30 representing a weak agreement, 0.30 to 0.50 a moderate agreement, 0.50 to 0.70 a good agreement, and 0.70 to 1.00 a strong agreement.6,21 A Wilcoxon signed-rank test with a Sidàk correction was used for pairwise post hoc comparison. Effect sizes for the pairwise comparisons were assessed by Cohen d with d in the range of 0.2 to 0.5 representing a small effect size, 0.5 to 0.8 a medium effect size, and values >0.8 a large effect size. Significance was declared at P≤ .05. Data were analyzed in Wizard (Version 1.9.41, Evan Miller). Data are presented as means and standard deviations.
Results
Torque
The iMVC did not change in HEAT (P = .247; W = 0.16) but tended to decrease in SHAM (P = .052; W = 0.28), with a lower torque at RTS than preimmobilization in SHAM (P = .027; d = 0.95) but not HEAT (P = .301; d = 0.54) (Figure 2A). The mean dynamic torque did not change significantly in SHAM (P = .150; W = 0.19) but increased in HEAT (P = .039; W = 0.33), with an increase from preimmobilization to RTS in HEAT (P = .039; d = 0.97) but not SHAM (P = .245; d = 0.47) (Figure 2B).
Figure 2.
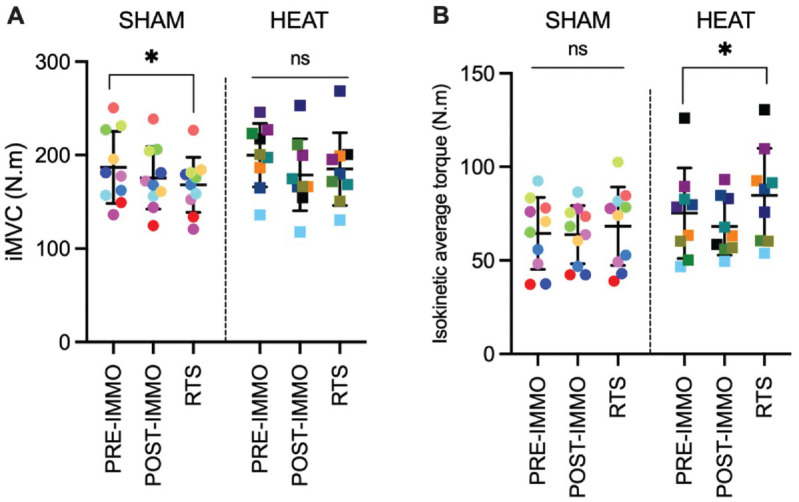
Individual and mean maximal torque during (A) iMVC and (B) isokinetic fatigue tests of the HEAT and control (SHAM) groups preimmobilization (PRE-IMMO), postimmobilization (POST-IMMO), and after rehabilitation (RTS). *P < .05. iMVC, isometric maximal voluntary contraction; ns, nonsignificant; RTS, return to sport.
Ultrasound
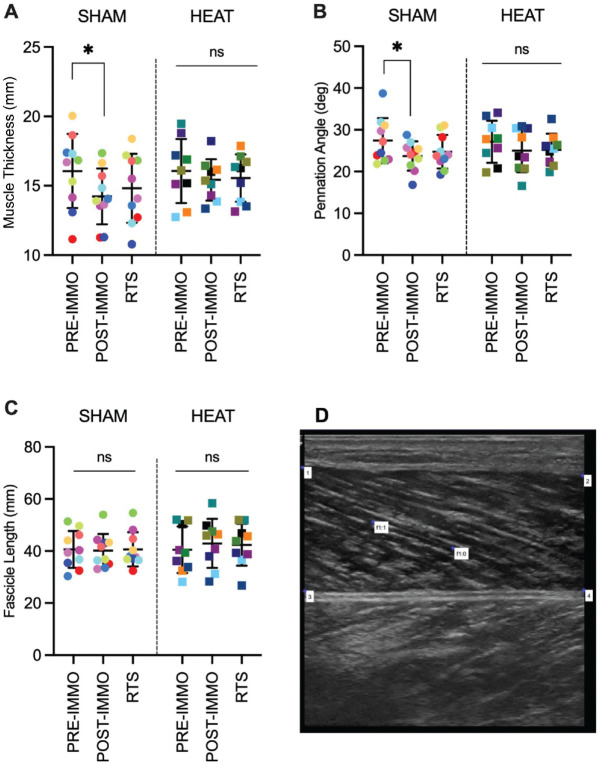
Ultrasound measures showed trends for changes in SHAM for pennation angle (P = .059; W = 0.27) and muscle thickness (P = .120; W = 0.21) but not in HEAT (P = .485; W = 0.09). The changes in SHAM were due to a lower pennation angle (P = .027; d = 0.40) and muscle thickness (P = .019; d = 0.53) in postimmobilization than in preimmobilization (Figure 3, A and B). There was no change in fascicle length in either SHAM (P = .947) or HEAT (P = .419) (Figure 3C).
Figure 3.
Ultrasound of the GM. Individual and mean (±SD) of (A) muscle thickness, (B) pennation angle, and (C) fascicle length of the SHAM (control) and HEAT groups preimmobilization (PRE-IMMO), postimmobilization (PRE-IMMO), and after rehabilitation (RTS). *P < .05. (D) Representative ultrasound image; muscle thickness was calculated as the mean of the vertical distances between points 1 and 3, and 2 and 4; pennation angle and fascicle length were calculated using the f1:0 and f1:1 markers. 2 GM, gastrocnemius medialis; ns, nonsignificant; RTS, return to sport.
Muscle Volume
MRI measurements showed moderate to strong (0.31 ≤W≤ 0.79) and significant (P≤ .035) decrease in CSA in the SHAM group for all muscles (GL, GM, and SOL; Figure 4), with post hoc analyses showing a decrease from preimmobilization to postimmobilization in GM (P = .004; d = 2.11) and SOL (P = .039; d = 0.91) and from preimmobilization to RTS in GM (P = .004; d = 1.27) and GL (P = .012; d = 1.17). In HEAT, CSA decreased for GM (P < .001; W = 0.61) with decreases from preimmobilization to postimmobilization (P = .039; d = 1.18) and RTS (P = .008; d = 1.05) but did not change for GL (P = .905; W = 0.01) or SOL (P = .739; W = 0.04). Total muscle volume decreased in both SHAM and HEAT (P≤ .002; W≥ 0.49) due to a decrease from preimmobilization to postimmobilization in both groups (P≤ .012; d≥ 1.21).
Figure 4.
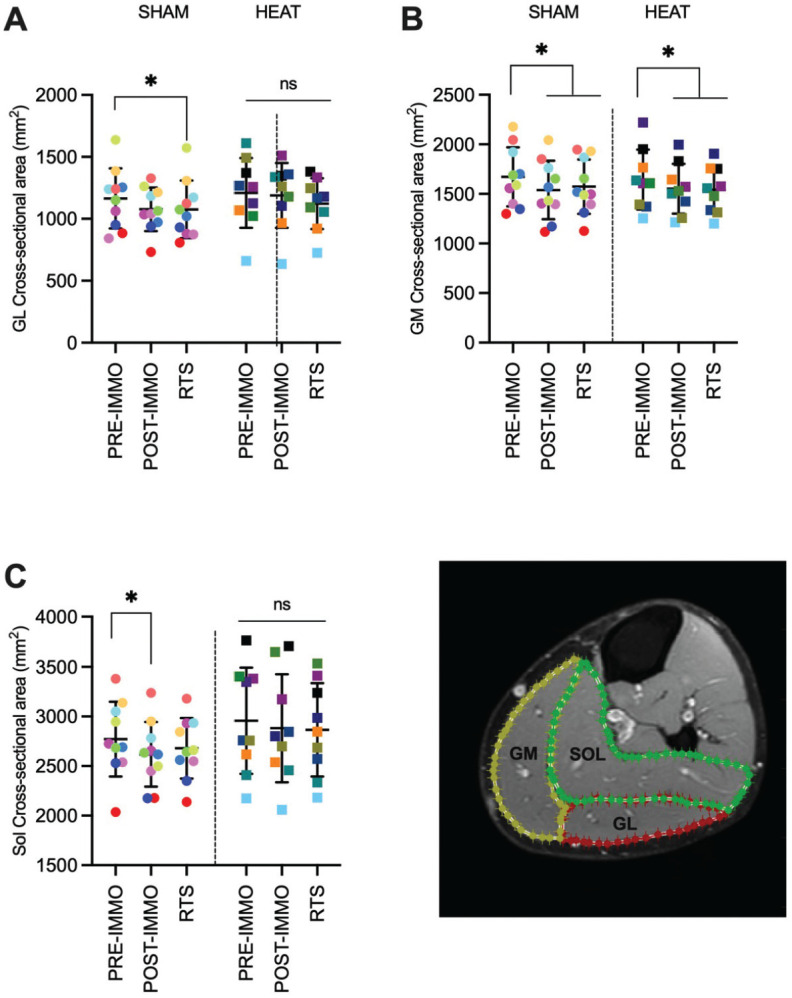
Individual and mean (±SD) cross-sectional areas were obtained from MRI scans for the (A) GL, (B) GM, and (C) SOL muscles. Data are shown for the SHAM and HEAT groups preimmobilization (PRE-IMMO), postimmobilization (PRE-IMMO), and after rehabilitation (RTS). (D) Representative image. *P < .05. GL, gastrocnemius lateralis; GM, gastrocnemius medialis; MRI, magnetic resonance imaging; ns, nonsignificant; RTS, return to sport; SOL, soleus.
Intramuscular Molecular Regulation
Protein kinase B (AKT) phosphorylated, total, and the phosphorylated/total ratio did not change over time in either SHAM or HEAT groups (all P≥ .120; W≤ 0.21) (Figure 5A, Table 2). Fork head box O proteins (FoxO) total protein expression and the phosphorylated/total ratio did not change over time in either SHAM or HEAT (all P≥ .390; W≤ 0.11). The phosphorylation level of FoxO1Thr24 did not change in SHAM (P = .693; W = 0.04) but significantly increased in HEAT (P = .034; W = 0.035). Post hoc pairwise comparisons revealed moderate (d≥ 0.62) but nonsignificant increases from preimmobilization and postimmobilization to RTS (P = .143) (Figure 5B, Table 2).
Figure 5.
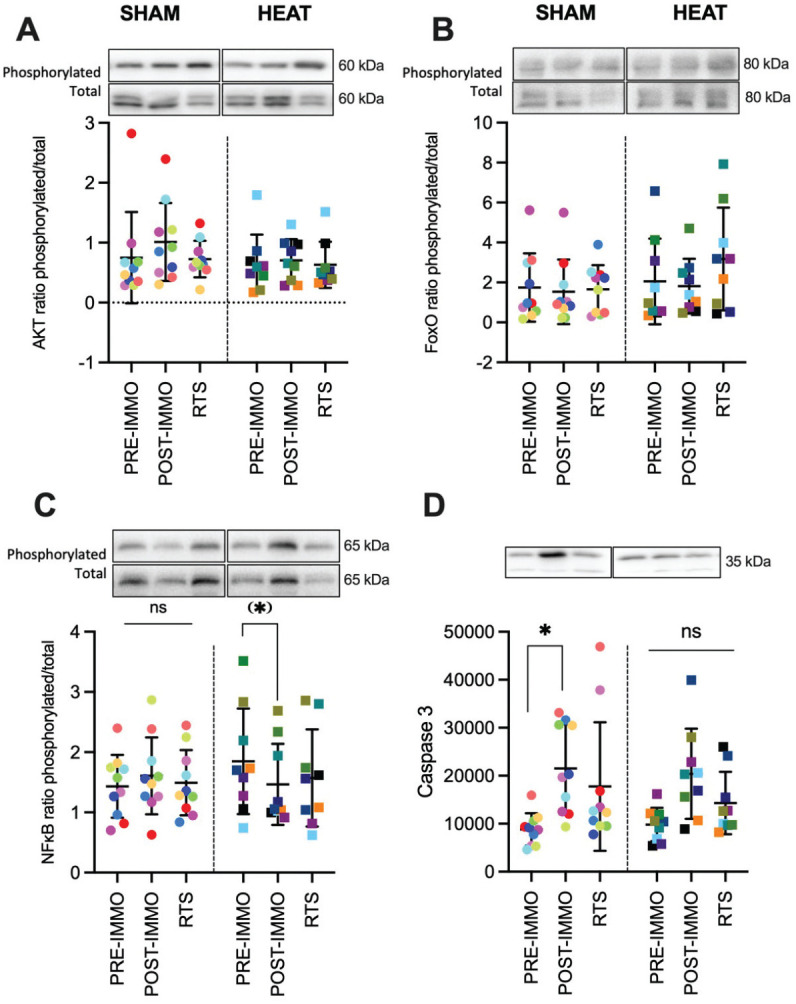
Results of muscle biopsies. Representative protein blot (phosphorylated and total when applicable) and analyses for (A) protein kinase B (AKT), (B) fork head box O proteins (FoxO), (C) nuclear factor-kappa B (NFκB), and (D) caspase 3. Data are shown for the SHAM group and HEAT groups preimmobilization (PRE-IMMO), postimmobilization (PRE-IMMO), and after rehabilitation (RTS). Differences with PRE-IMMO: *P < .05, (*)P = .054. ns, nonsignificant; RTS, return to sport.
Table 2.
mRNA and Protein Expression a
| HEAT | SHAM | |||||
|---|---|---|---|---|---|---|
| PRE-IMMO | POST-IMMO | RTS | PRE-IMMO | POST-IMMO | RTS | |
| mRNA expression (A.U.) | ||||||
| HSP70 | 27.2 ± 1.1 | 27.4 ± 1.2 | 27.6 ± 1.1 | 27.0 ± 1.4 | 28.0 ± 2.1 | 26.7 ± 1.5 |
| HSP90 | 23.0 ± 0.6 | 23.2 ± 0.6 | 23.3 ± 0.6 | 23.1 ± 0.9 | 23.1 ± 0.8 | 22.6 ± 0.7 |
| MuRF-1 | 27.5 ± 2 | 28.1 ± 1.8 | 28.4 ± 1.6 | 27.8 ± 1.9 | 28.4 ± 1.6 | 27.1 ± 1.5 |
| MAFbx/atrogin-1 | 0.9 ± 0.6 | 0.5 ± 0.2 | 0.6 ± 0.3 | 1.2 ± 0.4 | 0.6 ± 0.4 | 0.8 ± 0.5 |
| Protein expression (A.U.) | ||||||
| p-AKT | 14.9 ± 7.8 | 18.7 ± 5.6 | 16.3 ± 5.5 | 12.2 ± 5.1 | 23.1 ± 10.5 | 18.6 ± 8.5 |
| AKT total | 28.6 ± 13.8 | 31.7 ± 15.9 | 29.3 ± 9.4 | 24.2 ± 13.4 | 29.9 ± 14.6 | 28.5 ± 14.8 |
| p-NFκB | 12.1 ± 5.3 | 14.6 ± 5.2 | 12.2 ± 4.5 | 12.0 ± 4.9 | 20.2 ± 9.0 | 15.3 ± 8.8 |
| NFκB total | 7.4 ± 3.7 | 11.5 ± 6.0 | 9.6 ± 4.7 | 9.1 ± 3.8 | 12.9 ± 5.1 | 10.3 ± 3.9 |
| p-FoxO | 46.7 ± 27.9 b | 49.4 ± 25.4 b | 63.9 ± 27.3 b | 48.4 ± 35. 1 | 55.8 ± 35.6 | 64.0 ± 43.7 |
| FoxO1 total | 39.3 ± 23.1 | 36.4 ± 20.0 | 36.4 ± 32.4 | 41.4 ± 28.0 | 51.8 ± 32.5 | 55.6 ± 51.8 |
The caspase 3 and phosphorylated/total ratios are presented in Figure 5. AKT, protein kinase B; A.U., arbitrary units; FoxO, fork head box O proteins; HEAT, heat therapy group; HSP, heat shock protein; MAFbx/atrogin-1, muscle atrophy F-box; MuRF-1, muscle RING-finger protein-1; NFκB, nuclear factor-kappa B; p-, phosphorylated; POST-IMMO, postimmobilization; PRE-IMMO, preimmobilization; RTS, Return To Sport (i.e. test after rehabilitation); SHAM, control group. Data in mean ±SD.
P < .05, effect of time in HEAT.
The changes in nuclear factor-kappa B (NFκB) phosphorylation level, total protein expression, and the phosphorylated/total ratio did not reach significance in either SHAM (P≥ .120; 0.07 ≤W≤ 0.21) or HEAT (P≥ .091; 0.09 ≤W≤ 0.26). However, the trend for change in the ratio of phosphorylated/total for NFκB in HEAT (P = .091; W = 0.26) was due to a large (d = 0.91) decrease from preimmobilization to postimmobilization (P = .054) (Figure 5C).
Caspase 3 protein expression increased in both SHAM (P = .002; W = 0.49) and HEAT (P = .005; W = 0.48) due to significant increases from preimmobilization to postimmobilization in both SHAM (P = .004; d = 1.84) and HEAT (P = .016; d = 1.49); however the values at RTS were still higher than preimmobilization in SHAM (P = .027; d = 0.92) but not HEAT (P = .590; d = 0.86) (Figure 5D).
Table 2 demonstrates detailed phosphorylated and total protein results when ratios are presented. There were no changes in heat shock protein (HSP) 70 and 90 mRNA expression over time in SHAM and HEAT conditions (all P≥ .242; W≤ 0.21; Table 2). The changes in muscle RING-finger protein-1 (MuRF-1) and muscle atrophy F-box protein (MAFbx)/atrogin-1 mRNA expression did not reach significance in SHAM (.059 ≤P≤ .099; 0.21 ≤W≤ 0.24). Neither MuRF-1 nor MAFbx/atrogin-1 mRNA expression changed in HEAT (P = .715; W < 0.09).
Discussion
This study examined the potential of heat therapy to counteract muscle atrophy and strength loss during lower leg immobilization in healthy trained males. Our primary findings showed that using heat therapy during 2 weeks of immobilization and 2 weeks of rehabilitation (1) lowered muscle atrophy as evidenced by both MRI and ultrasound methods and (2) maintained and/or improved plantarflexor strength at RTS. Moreover, these effects were accompanied by inactivation of the FoxO and NFκB signaling pathways.
Muscle Volume and Architecture
Muscle atrophy is one of the most commonly reported effects of immobilization. 35 In the current study, MRI analyses confirmed a decrease in CSA in all plantarflexors (GM, GL, and SOL) in the SHAM group. However, this decrease was limited to the GM in the HEAT group (Figure 4). Similarly, ultrasound measurements showed decreased muscle thickness in the SHAM but not in the HEAT group (Figure 3). This suggests repeated heat exposure can partially avert muscle atrophy during ankle immobilization in humans.
It has been reported previously that heat stress may facilitate hypertrophy in cultured cells and rodents.14,45 Heat therapy was also shown to be partly effective in reducing muscle atrophy during rodent immobilization. 36 So far, only 3 studies have investigated the effect of repeated heat exposure on human muscle CSA. Two of these studies used prolonged intervention (ie, 6 to 10 weeks) in healthy/active participants and reported conflicting results, with either an increase in quadriceps CSA or no changes in calf CSA.13,25 The third study showed that heat therapy reduced quadriceps atrophy during 10 days of knee immobilization. 15 The current data extend these findings by showing that heat therapy reduced calf atrophy after ankle immobilization and revealed the benefit of pursuing heat therapy during rehabilitation. This aligns with previous animal studies showing that heat therapy could improve muscle regrowth during postimmobilization reloading.12,37
As previously reported in the literature, 40 data showed a decrease in muscle thickness with immobilization in the SHAM group, accompanied by a decrease in pennation angle without changes in fascicle length (Figure 3). Conversely, the HEAT group did not show such changes, further confirming that repeated heat exposures partially blunt the effect of immobilization. Of note, it remains unclear as to why immobilization affected muscle thickness and CSA when architectural adaptations precede gains in muscle CSA in response to training38,43; it also remained unclear as to why immobilization decreased pennation angle when athletes with a unilateral history of muscle strain showed greater pennation on their previously injured limb compared with the uninjured limb. 44
Muscle Force
Immobilization has been reported to impair iMVC,8,17 with a persisting decrease of ~10% after 2 weeks of recovery from a 3-week single leg immobilization period. 17 Similarly, the current data showed an 11% deficit in iMVC (P = .027) at RTS in the SHAM group. However, this decrease was not significant in the HEAT group (-5%; P = .301) (Figure 2), suggesting that heat therapy may have preserved muscle function at RTS. Although this effect may not be cumulative with the mechanical stimulus of the resistance training,25,39 the current data suggest that heat therapy might improve the muscle force of patients with limited training ability, such as during and after immobilization.
Moreover, while there was no changes in mean torque during a dynamic fatigue test in the SHAM group, repeated heat exposure increased this parameter from preimmobilization to RTS (+14%; Figure 2). This increase confirms a previous study reporting increased plantarflexor torque after repeated passive heat exposures without immobilization 34 and further confirms a potential benefit of heat therapy on muscle strength.
Molecular Regulation
The mechanisms by which heat therapy regulates muscle mass remain unclear, particularly in humans. For instance, HSP expression is enhanced after an acute heat exposure,19, 26 and some have concluded to a HSP-mitochondrial-centered mechanism regulating muscle mass after heat therapy.15,16 However, another study reported heat-induced regulation of muscle mass with negligible changes in HSP70 and HSP90 mRNA. 23 Importantly, the measurement technique in the current study (ie, PCR at rest, >48 hours after the last heat exposure) cannot rule out acute changes during the intervention that would normalize afterward. Similarly, whereas a previous study showed acute increases in AKT after a single heat exposure, 19 we did not observe chronic changes in AKT. Thus, our data agree with human immobilization studies reporting decreased protein synthesis rate, without concomitant changes in AKT-mTOR signaling,7,11 and showed no effect of heat therapy in this context. 18 This suggests that muscle atrophy in immobilized humans may be regulated through other signaling pathways, such as mechanosensory pathways via altered focal adhesion kinase signaling. 7 By extension, the attenuated decline in muscle mass and strength conferred by heat therapy during immobilization is likely due to attenuated protein degradation. In the current study, this is evidenced by the increased phosphorylation (and inactivation) of FOXO and a tendency toward decreased NFκB phosphorylation after heat therapy (Table 2). Both transcription factors have been shown to be pivotal regulators of muscle protein degradation. Moreover, the findings here corroborate with our previous work in humans demonstrating increased phosphorylation (ie, inactivation) of FOXO after acute heat treatment, 19 as well as with others demonstrating decreased NFκB activation after heat treatment in cultured cells and rodents.30,31 Key atrogenes downstream of FOXO and NFκB include MuRF-1 and MAFbx/atrogin-1,3,10 which purportedly account for 80% of proteolysis during muscle atrophy. 42 However, limited changes were observed in these biomarkers following immobilization, heat therapy, or recovery (Table 2), probably because those atrogenes returned near baseline by the time of PCR measurement. Indeed, human immobilization studies have reported increases in the mRNA of MuRF-1 and/or MAFbx in the initial periods of immobilization (ie, 2 to 10 days), that thereafter reverted toward baseline or subbaseline levels after 4 to 10 days.7,40
The caspase-3 is a crucial proteolytic enzyme mediating immobilization-induced muscle atrophy. 41 In rodents, repeated heat stress has been shown to protect against diaphragmatic atrophy and contractile dysfunction, in part due to reduced caspase-3 activation. 46 In the current study, marked increases in caspase-3 expression were evident after immobilization, which was averted with the inclusion of heat therapy (Figure 5D). Collectively, our data showed that heat therapy prevented muscle atrophy and strength loss, primarily through attenuating muscle degrading pathways.
Limitations
The current data complement the existing literature by investigating a human model of ankle immobilization. However, plantarflexors have different fiber typologies, and the biopsy from the GL and GM may not reflect changes in the SOL. Importantly, it should be acknowledged that immobilization is generally used after an injury with a specific cascade of physiological responses that may influence the transfer of the current data to injured patients. Likewise, it is necessary to consider that these findings could be specific to the model investigated, and more constringent immobilization modalities, such as bed rest, might yield more pronounced disparities. Potential positive and negative effects of heat therapy after an injury remain unknown. Finally, training the participants for 4 weeks before initiating immobilization allowed to standardize the overall study but could have minimized the differences between groups as it could have upregulated some protective pathways in all participants.
Conclusion
Our findings showed that heat therapy during immobilization and rehabilitation could prevent muscle atrophy and strength deficits at RTS in healthy humans. In addition, the current data suggest that repeated heat exposures may inhibit the atrophy signaling response to immobilization. These results could offer a therapeutic solution to minimize muscle deconditioning in immobilized patients such as injured athletes.
Acknowledgments
The authors thank the participants of this study for their dedication and commitment during the research process.
Footnotes
Final revision submitted March 22, 2024; accepted April 5, 2024.
The authors declare that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the hospital scientific committee (reference No. ASC/0000205/ak) and Aspire Zone Foundation (reference No. F202007002).
Mariem Labidi, PhD, Aspetar Orthopaedic and Sports Medicine Hospital, Research and Scientific Support Department, Doha, Qatar, Aspetar Orthopaedic and Sports Medicine Hospital, Education Department, Doha, Qatar, and Faculty of Sport Sciences and Physical Education, CETAPS, University of Rouen, France; Marine Alhammoud, MD, PhD, Aspetar Orthopaedic and Sports Medicine Hospital, Surgery Department, Doha, Qatar; Inter-University Laboratory of Human Movement Biology, Universite Claude Bernard Lyon 1, Villeurbanne, France. Khouloud Mtibaa, PhD, College of Health and Life Sciences, Hamad Bin Khalifa University, Doha, Qatar; Mohammed Ihsan, PhD, Scientific Conditioning Centre, Elite Training Science and Technology Division, Hong Kong Sports Institute, Hong Kong, Rabi Elhusseiny, Institute of Neuroscience, Université Catholique de Louvain, Louvain-la-Neuve, Belgium; Louise Deldicque, PhD, Institute of Neuroscience, Université Catholique de Louvain, Louvain-la-Neuve, Belgium; Nelda Nader, Aspetar Orthopaedic and Sports Medicine Hospital, Research and Scientific Support Department, Doha, Qatar; Nada Nasir, Aspetar Orthopaedic and Sports Medicine Hospital, Research and Scientific Support Department, Doha, Qatar and College of Health and Life Sciences, Hamad Bin Khalifa University, Doha, Qatar; Emmanouil Papakostas, MD, Aspetar Orthopaedic and Sports Medicine Hospital, Surgery Department, Doha, Qatar; Bruno Olory, MD, Aspetar Orthopaedic and Sports Medicine Hospital, Surgery Department, Doha, Qatar; Flavio Cruz, MD, Aspetar Orthopaedic and Sports Medicine Hospital, Surgery Department, Doha, Qatar; Mohammed Farooq, PhD, Aspetar Orthopaedic and Sports Medicine Hospital, Research and Scientific Support Department, Doha, Qatar; Antony M.J. Sanchez, PhD, University of Perpignan Via Domitia, Laboratoire Performance Santé Altitude, EA 4604, Font-Romeu, France; Pieter d’Hooghe, MD, PhD, Aspetar Orthopaedic and Sports Medicine Hospital, Surgery Department, Doha, Qatar; Claire Tourny, PhD,Faculty of Sport Sciences and Physical Education, CETAPS, University of Rouen, France
ORCID iD: Louise Deldicque  https://orcid.org/0000-0003-3393-5278
https://orcid.org/0000-0003-3393-5278
Contributor Information
Mariem Labidi, Aspetar Orthopaedic and Sports Medicine Hospital, Research and Scientific Support Department, Doha, Qatar, Aspetar Orthopaedic and Sports Medicine Hospital, Education Department, Doha, Qatar, and Faculty of Sport Sciences and Physical Education, CETAPS, University of Rouen, France.
Marine Alhammoud, Aspetar Orthopaedic and Sports Medicine Hospital, Surgery Department, Doha, Qatar; Inter-University Laboratory of Human Movement Biology, Universite Claude Bernard Lyon 1, Villeurbanne, France.
Khouloud Mtibaa, College of Health and Life Sciences, Hamad Bin Khalifa University, Doha, Qatar.
Mohammed Ihsan, Scientific Conditioning Centre, Elite Training Science and Technology Division, Hong Kong Sports Institute, Hong Kong, Rabi Elhusseiny, Institute of Neuroscience, Université Catholique de Louvain, Louvain-la-Neuve, Belgium.
Louise Deldicque, Institute of Neuroscience, Université Catholique de Louvain, Louvain-la-Neuve, Belgium; Nelda Nader, Aspetar Orthopaedic and Sports Medicine Hospital, Research and Scientific Support Department, Doha, Qatar.
Nada Nasir, Aspetar Orthopaedic and Sports Medicine Hospital, Research and Scientific Support Department, Doha, Qatar and College of Health and Life Sciences, Hamad Bin Khalifa University, Doha, Qatar.
Emmanouil Papakostas, Aspetar Orthopaedic and Sports Medicine Hospital, Surgery Department, Doha, Qatar.
Bruno Olory, Aspetar Orthopaedic and Sports Medicine Hospital, Surgery Department, Doha, Qatar.
Flavio Cruz, Aspetar Orthopaedic and Sports Medicine Hospital, Surgery Department, Doha, Qatar.
Mohammed Farooq, Aspetar Orthopaedic and Sports Medicine Hospital, Research and Scientific Support Department, Doha, Qatar.
Antony M.J. Sanchez, University of Perpignan Via Domitia, Laboratoire Performance Santé Altitude, EA 4604, Font-Romeu, France.
Pieter d’Hooghe, Aspetar Orthopaedic and Sports Medicine Hospital, Surgery Department, Doha, Qatar.
Claire Tourny, Faculty of Sport Sciences and Physical Education, CETAPS, University of Rouen, France.
Sebastien Racinais, Universite de Montpellier.
References
- 1. Anderson L, Close GL, Konopinski M, et al. Case study: muscle atrophy, hypertrophy, and energy expenditure of a premier league soccer player during rehabilitation from anterior cruciate ligament injury. Int J Sport Nutr Exerc Metab. 2019;29(5):559-566. doi: 10.1123/ijsnem.2018-0391 [DOI] [PubMed] [Google Scholar]
- 2. Blazevich AJ, Gill ND, Zhou S. Intra- and intermuscular variation in human quadriceps femoris architecture assessed in vivo. J Anat. 2006;209(3):289-310. doi: 10.1111/j.1469-7580.2006.00619.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2014;307(6):E469-E484. doi: 10.1152/ajpendo.00204.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Booth FW. Time course of muscular atrophy during immobilization of hindlimbs in rats. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(4):656-661. doi: 10.1152/jappl.1977.43.4.656 [DOI] [PubMed] [Google Scholar]
- 5. Bosquet L, Maquet D, Forthomme B, Nowak N, Lehance C, Croisier JL. Effect of the lengthening of the protocol on the reliability of muscle fatigue indicators. Int J Sports Med. 2010;31(2):82-88. doi: 10.1055/s-0029-1243168 [DOI] [PubMed] [Google Scholar]
- 6. Cafiso S, Di Graziano A, Pappalardo G. Using the Delphi method to evaluate opinions of public transport managers on bus safety. Saf Sci. 2013;57:254-263. doi:10.1016/j.ssci.2013.03.001 [Google Scholar]
- 7. de Boer MD, Selby A, Atherton P, et al. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol. 2007;585(pt 1):241-251. doi: 10.1113/jphysiol.2007.142828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Demangel R, Treffel L, Py G, et al. Early structural and functional signature of 3-day human skeletal muscle disuse using the dry immersion model. J Physiol. 2017;595(13):4301-4315. doi: 10.1113/jp273895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fitts RH, Trappe SW, Costill DL, et al. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J Physiol. 2010;588(18):3567-3592. doi: 10.1113/jphysiol.2010.188508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37(10):1974-1984. doi: 10.1016/j.biocel.2005.04.018 [DOI] [PubMed] [Google Scholar]
- 11. Glover EI, Phillips SM, Oates BR, et al. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008;586(24):6049-6061. doi: 10.1113/jphysiol.2008.160333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goto K, Honda M, Kobayashi T, et al. Heat stress facilitates the recovery of atrophied soleus muscle in rat. Jpn J Physiol. 2004;54(3):285-293. doi: 10.2170/jjphysiol.54.285 [DOI] [PubMed] [Google Scholar]
- 13. Goto K, Oda H, Kondo H, et al. Responses of muscle mass, strength and gene transcripts to long-term heat stress in healthy human subjects. Eur J Appl Physiol. 2011;111(1):17-27. doi: 10.1007/s00421-010-1617-1 [DOI] [PubMed] [Google Scholar]
- 14. Goto K, Okuyama R, Sugiyama H, et al. Effects of heat stress and mechanical stretch on protein expression in cultured skeletal muscle cells. Pflugers Arch. 2003;447(2):247-253. doi: 10.1007/s00424-003-1177-x [DOI] [PubMed] [Google Scholar]
- 15. Hafen PS, Abbott K, Bowden J, Lopiano R, Hancock CR, Hyldahl RD. Daily heat treatment maintains mitochondrial function and attenuates atrophy in human skeletal muscle subjected to immobilization. J Appl Physiol (1985). 2019;127(1):47-57. doi: 10.1152/japplphysiol.01098.2018 [DOI] [PubMed] [Google Scholar]
- 16. Hafen PS, Preece CN, Sorensen JR, Hancock CR, Hyldahl RD. Repeated exposure to heat stress induces mitochondrial adaptation in human skeletal muscle. J Appl Physiol (1985). 2018;125(5):1447-1455. doi: 10.1152/japplphysiol.00383.2018 [DOI] [PubMed] [Google Scholar]
- 17. Hortobágyi T, Dempsey L, Fraser D, et al. Changes in muscle strength, muscle fibre size and myofibrillar gene expression after immobilization and retraining in humans. J Physiol. 2000;524(1):293-304. doi: 10.1111/j.1469-7793.2000.00293.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hyldahl R, Hafen P, Nelson W, et al. Passive muscle heating attenuates the decline in vascular function caused by limb disuse. J Physiol. 2021;599(20):4581-4596. doi: 10.1113/JP281900 [DOI] [PubMed] [Google Scholar]
- 19. Ihsan M, Deldicque L, Molphy J, Britto F, Cherif A, Racinais S. Skeletal muscle signaling following whole-body and localized heat exposure in humans. Front Physiol. 2020;11:839. doi: 10.3389/fphys.2020.00839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ihsan M, Périard JD, Racinais S. Integrating heat training in the rehabilitation toolbox for the injured athlete. Front Physiol. 2019;10:1488. doi: 10.3389/fphys.2019.01488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kendall MG, Smith BB. The problem of m rankings. Ann Math Stat. 1939;10(3):275-287. [Google Scholar]
- 22. Khamwong P, Nosaka K, Pirunsan U, Paungmali A. Prophylactic effect of hot pack on symptoms of eccentric exercise-induced muscle damage of the wrist extensors. Eur J Sport Sci. 2012;12(5):443-453. doi: 10.1080/17461391.2011.566359 [DOI] [Google Scholar]
- 23. Kim K, Reid BA, Casey CA, et al. Effects of repeated local heat therapy on skeletal muscle structure and function in humans. J Appl Physiol (1985). 2020;128(3):483-492. doi: 10.1152/japplphysiol.00701.2019 [DOI] [PubMed] [Google Scholar]
- 24. Kumagai K, Abe T, Brechue WF, Ryushi T, Takano S, Mizuno M. Sprint performance is related to muscle fascicle length in male 100-m sprinters. J Appl Physiol (1985). 2000;88(3):811-816. doi: 10.1152/jappl.2000.88.3.811 [DOI] [PubMed] [Google Scholar]
- 25. Labidi M, Ihsan M, Behan FP, et al. Six weeks of localized heat therapy does not affect muscle mass, strength and contractile properties in healthy active humans. Eur J Appl Physiol. 2021;121(2):573-582. doi: 10.1007/s00421-020-04545-9 [DOI] [PubMed] [Google Scholar]
- 26. McClung JP, Hasday JD, He JR, et al. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol. 2008;294(1):R185-R191. doi: 10.1152/ajpregu.00532.2007 [DOI] [PubMed] [Google Scholar]
- 27. Milsom J, Barreira P, Burgess DJ, Iqbal Z, Morton JP. Case study: muscle atrophy and hypertrophy in a premier league soccer player during rehabilitation from ACL injury. Int J Sport Nutr Exerc Metab. 2014;24(5):543-552. doi: 10.1123/ijsnem.2013-0209 [DOI] [PubMed] [Google Scholar]
- 28. Naito H, Powers SK, Demirel HA, Sugiura T, Dodd SL, Aoki J. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J Appl Physiol (1985). 2000;88(1):359-363. doi:10.1152/jappl .2000.88.1.359 [DOI] [PubMed] [Google Scholar]
- 29. Nash CE, Mickan SM, Del Mar CB, Glasziou PP. Resting injured limbs delays recovery: a systematic review. J Fam Pract. 2004;53(9):706-712. [PubMed] [Google Scholar]
- 30. Ohno Y, Yamada S, Sugiura T, Ohira Y, Yoshioka T, Goto K. A possible role of NF-kappaB and HSP72 in skeletal muscle hypertrophy induced by heat stress in rats. Gen Physiol Biophys. 2010;29(3):234-242. doi: 10.4149/gpb_2010_03_234 [DOI] [PubMed] [Google Scholar]
- 31. Ohno Y, Yamada S, Sugiura T, Ohira Y, Yoshioka T, Goto K. Possible role of NF-kB signals in heat stress-associated increase in protein content of cultured C2C12 cells. Cells Tissues Organs. 2011;194(5):363-370. doi: 10.1159/000323324 [DOI] [PubMed] [Google Scholar]
- 32. Périard JD, Racinais S. Self-paced exercise in hot and cool conditions is associated with the maintenance of %VO2peak within a narrow range. J Appl Physiol (1985). 2015;118(10):1258-1265. doi: 10.1152/japplphysiol.00084.2015 [DOI] [PubMed] [Google Scholar]
- 33. Racinais S, Wilson MG, Gaoua N, Periard JD. Heat acclimation has a protective effect on the central but not peripheral nervous system. J Appl Physiol (1985). 2017;123(4):816-824. doi:10.1152/japplph ysiol.00430.2017 [DOI] [PubMed] [Google Scholar]
- 34. Racinais S, Wilson MG, Periard JD. Passive heat acclimation improves skeletal muscle contractility in humans. Am J Physiol Regul Integr Comp Physiol. 2017;312(1):R101-R107. doi: 10.1152/ajpregu.00431.2016 [DOI] [PubMed] [Google Scholar]
- 35. Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280(17):4294-4314. doi: 10.1111/febs.12253 [DOI] [PubMed] [Google Scholar]
- 36. Selsby JT, Dodd SL. Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am J Physiol Regul Integr Comp Physiol. 2005;289(1):R134-R139. doi: 10.1152/ajpregu.00497.2004 [DOI] [PubMed] [Google Scholar]
- 37. Selsby JT, Rother S, Tsuda S, Pracash O, Quindry J, Dodd SL. Intermittent hyperthermia enhances skeletal muscle regrowth and attenuates oxidative damage following reloading. J Appl Physiol (1985). 2007;102(4):1702-1707. doi: 10.1152/japplphysiol.00722.2006 [DOI] [PubMed] [Google Scholar]
- 38. Seynnes OR, Boer Md, Narici MV. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J Appl Physiol. 2007;102(1):368-373. doi: 10.1152/japplphysiol.00789.2006 [DOI] [PubMed] [Google Scholar]
- 39. Stadnyk AMJ, Rehrer NJ, Handcock PJ, Meredith-Jones KA, Cotter JD. No clear benefit of muscle heating on hypertrophy and strength with resistance training. Temperature (Austin). 2018;5(2):175-183. doi: 10.1080/23328940.2017.1391366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suetta C, Frandsen U, Jensen L, et al. Aging affects the transcriptional regulation of human skeletal muscle disuse atrophy. PLoS One. 2012;7(12):e51238. doi: 10.1371/journal.pone.0051238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Talbert EE, Smuder AJ, Min K, Kwon OS, Powers SK. Calpain and caspase-3 play required roles in immobilization-induced limb muscle atrophy. J Appl Physiol (1985). 2013;114(10):1482-1489. doi: 10.1152/japplphysiol.00925.2012 [DOI] [PubMed] [Google Scholar]
- 42. Tawa NE, Jr, Odessey R, Goldberg AL. Inhibitors of the proteasome reduce the accelerated proteolysis in atrophying rat skeletal muscles. J Clin Invest. 1997;100(1):197-203. doi: 10.1172/jci119513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Timmins RG, Ruddy JD, Presland J, Maniar N, Williams M. Architectural changes of the biceps femoris long head after concentric or eccentric training. Med Sci Sports Exerc. 2016;48(3):499-508. doi: 10.1249/mss.0000000000000795 [DOI] [PubMed] [Google Scholar]
- 44. Timmins RG, Shield AJ, Williams MD, Lorenzen C, Opar DA. Biceps femoris long head architecture: a reliability and retrospective injury study. Med Sci Sports Exerc. 2015;47(5):905-913. doi: 10.1249/mss.0000000000000507 [DOI] [PubMed] [Google Scholar]
- 45. Uehara K, Goto K, Kobayashi T, et al. Heat-stress enhances proliferative potential in rat soleus muscle. Jpn J Physiol. 2004;54(3):263-271. doi: 10.2170/jjphysiol.54.263 [DOI] [PubMed] [Google Scholar]
- 46. Yoshihara T, Ichinoseki-Sekine N, Kakigi R, et al. Repeated exposure to heat stress results in a diaphragm phenotype that resists ventilator-induced diaphragm dysfunction. J Appl Physiol (1985). 2015;119(9):1023-1031. doi: 10.1152/japplphysiol.00438.2015 [DOI] [PubMed] [Google Scholar]