Abstract
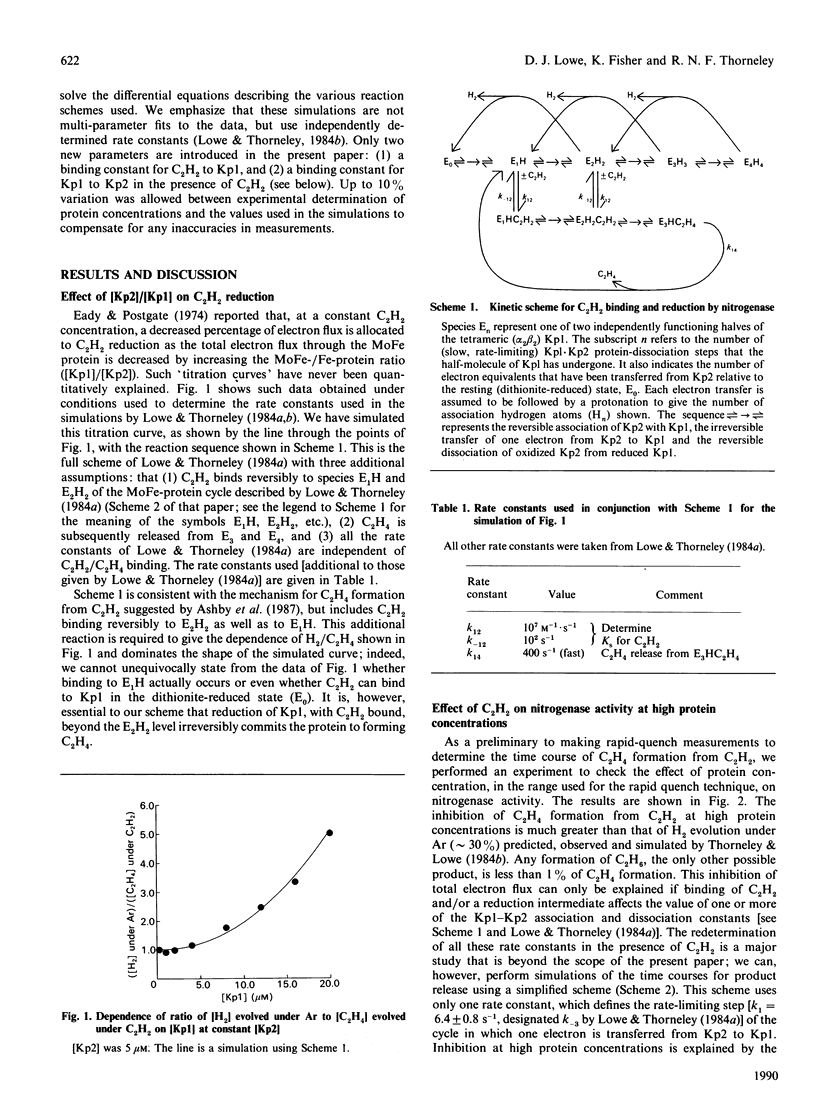
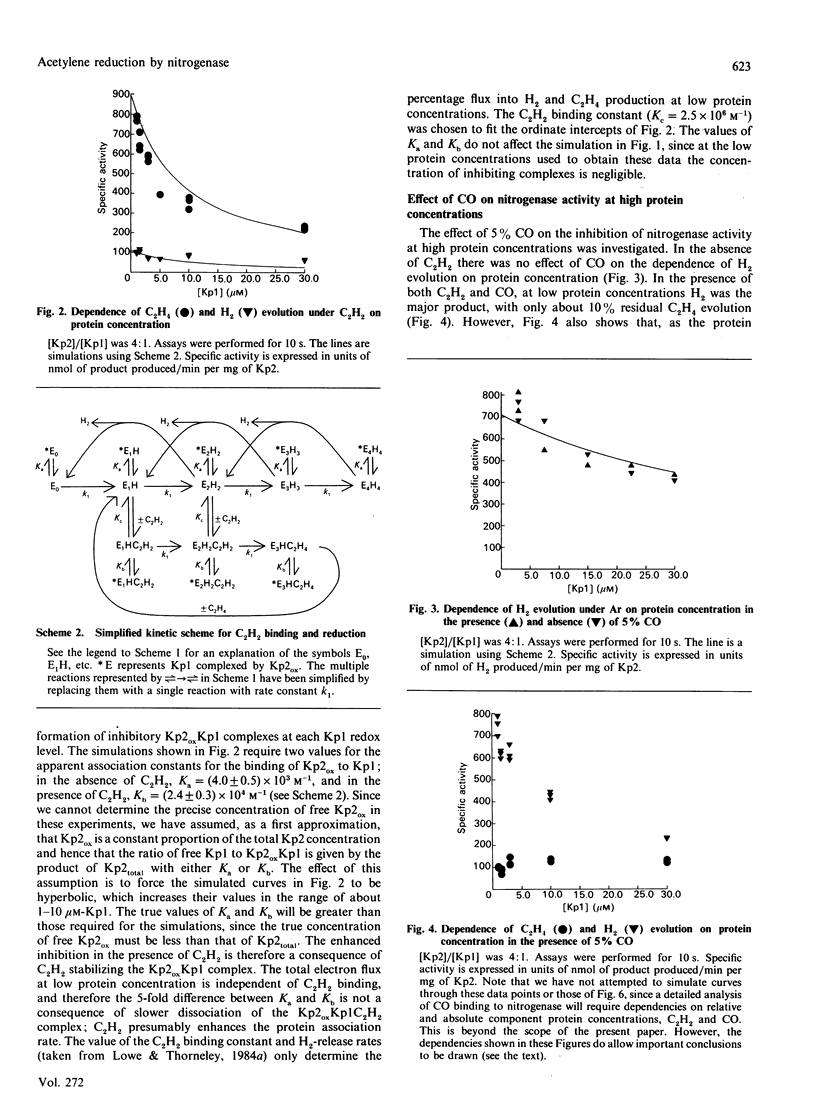
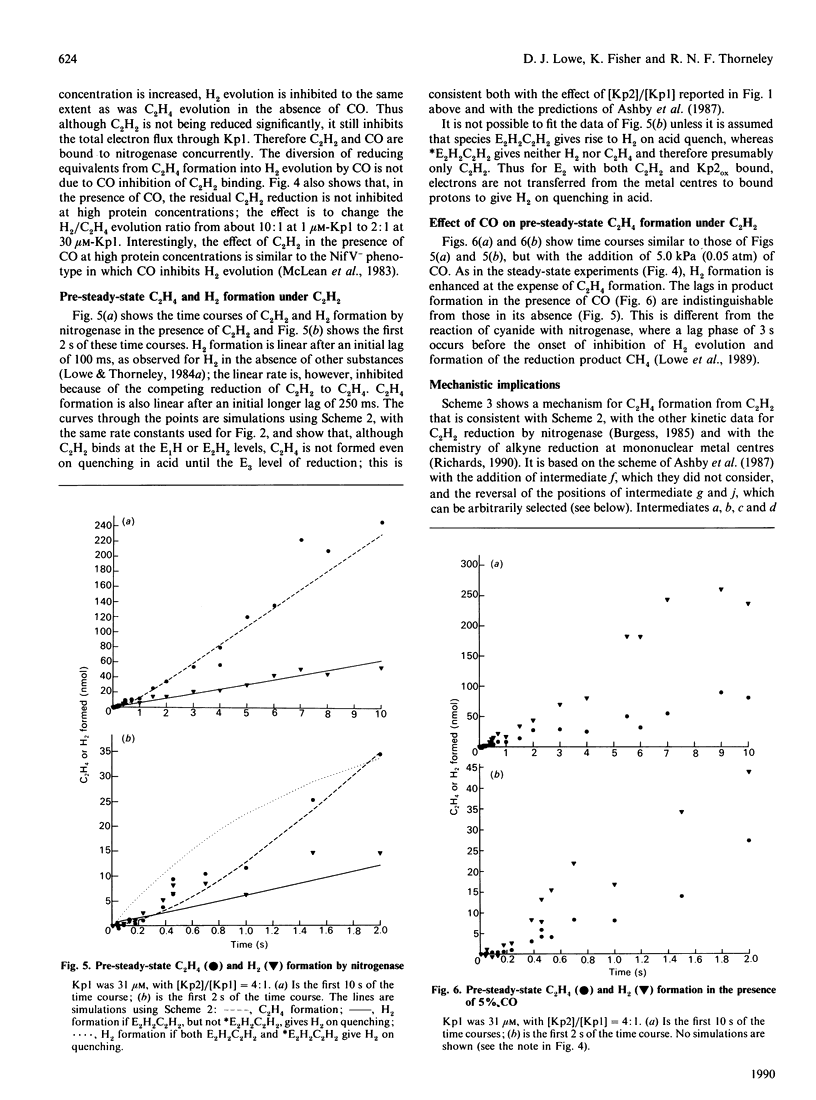
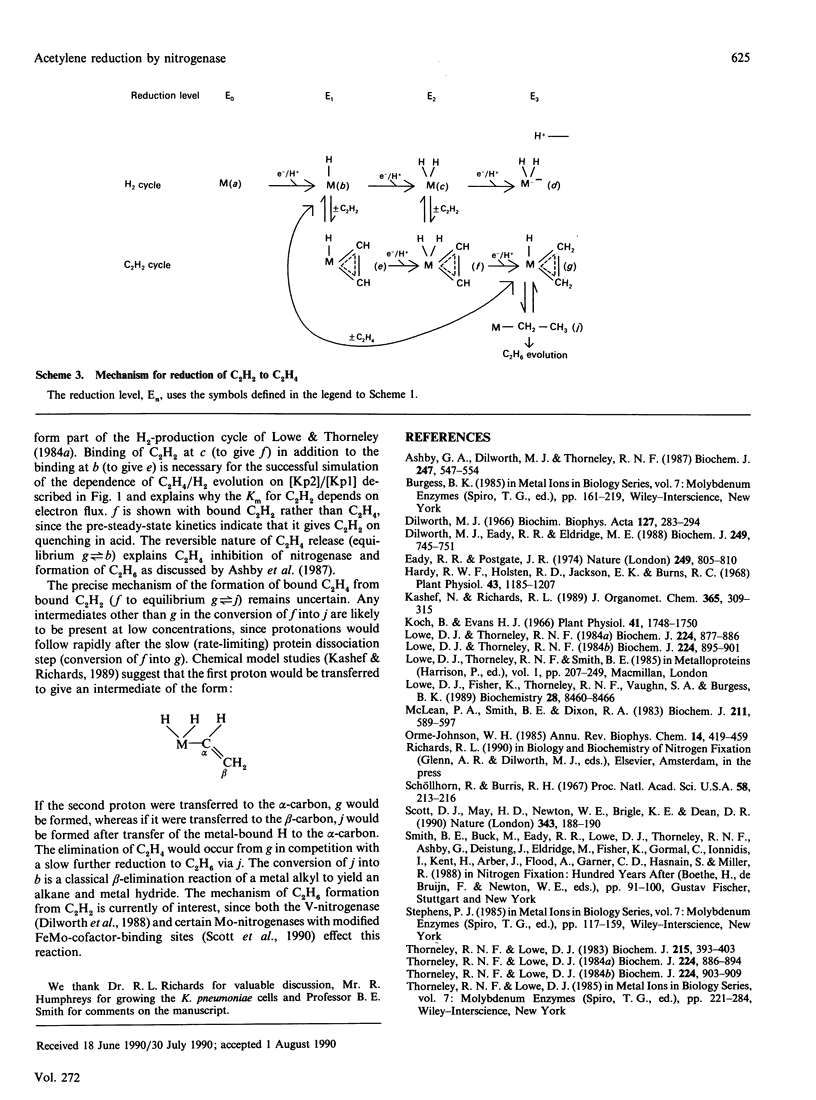
The electron flux through the MoFe-protein of nitrogenase from Klebsiella pneumoniae determines the absolute and relative rates of 2H+ reduction to H2 and acetylene (C2H2) reduction to ethylene (C2H4) at saturating levels of reductant (Na2S2O4) and MgATP. High electron flux, induced by a high Fe-protein (Kp2)/MoFe protein (Kp1) ratio, favours C2H2 reduction. These data can be explained if ethylene, the two-electron reduction product of C2H2, is not released until three electrons have been transferred from Kp2 to Kp1. This explanation is also consistent with a pre-steady-state lag phase for C2H4 formation of 250 ms observed when functioning enzyme is quenched with acid. Electron flux through nitrogenase is inhibited by C2H2 at high protein concentrations. This is because the association rate between Kp1 and oxidized Kp2 is enhanced by C2H2, leading to an increased steady-state concentration of the inhibitory complex Kp2oxKp1C2H2. This effect is not relieved by CO. Thus CO and C2H2 (or C2H4) must be bound at the same time to distinct sites, presumably at Mo or Fe centres, on the enzyme.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby G. A., Dilworth M. J., Thorneley R. N. Klebsiella pneumoniae nitrogenase. Inhibition of hydrogen evolution by ethylene and the reduction of ethylene to ethane. Biochem J. 1987 Nov 1;247(3):547–554. doi: 10.1042/bj2470547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth M. J. Acetylene reduction by nitrogen-fixing preparations from Clostridium pasteurianum. Biochim Biophys Acta. 1966 Oct 31;127(2):285–294. doi: 10.1016/0304-4165(66)90383-7. [DOI] [PubMed] [Google Scholar]
- Dilworth M. J., Eady R. R., Eldridge M. E. The vanadium nitrogenase of Azotobacter chroococcum. Reduction of acetylene and ethylene to ethane. Biochem J. 1988 Feb 1;249(3):745–751. doi: 10.1042/bj2490745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Postgate J. R. Nitrogenase. Nature. 1974 Jun 28;249(460):805–810. doi: 10.1038/249805a0. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch B., Evans H. J. Reduction of acetylene to ethylene by soybean root nodules. Plant Physiol. 1966 Dec;41(10):1748–1750. doi: 10.1104/pp.41.10.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J., Fisher K., Thorneley R. N., Vaughn S. A., Burgess B. K. Kinetics and mechanism of the reaction of cyanide with molybdenum nitrogenase from Azotobacter vinelandii. Biochemistry. 1989 Oct 17;28(21):8460–8466. doi: 10.1021/bi00447a028. [DOI] [PubMed] [Google Scholar]
- Lowe D. J., Thorneley R. N. The mechanism of Klebsiella pneumoniae nitrogenase action. Pre-steady-state kinetics of H2 formation. Biochem J. 1984 Dec 15;224(3):877–886. doi: 10.1042/bj2240877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J., Thorneley R. N. The mechanism of Klebsiella pneumoniae nitrogenase action. The determination of rate constants required for the simulation of the kinetics of N2 reduction and H2 evolution. Biochem J. 1984 Dec 15;224(3):895–901. doi: 10.1042/bj2240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean P. A., Smith B. E., Dixon R. A. Nitrogenase of Klebsiella pneumoniae nifV mutants. Biochem J. 1983 Jun 1;211(3):589–597. doi: 10.1042/bj2110589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme-Johnson W. H. Molecular basis of biological nitrogen fixation. Annu Rev Biophys Biophys Chem. 1985;14:419–459. doi: 10.1146/annurev.bb.14.060185.002223. [DOI] [PubMed] [Google Scholar]
- Schöllhorn R., Burris R. H. Acetylene as a competitive inhibitor of N-2 fixation. Proc Natl Acad Sci U S A. 1967 Jul;58(1):213–216. doi: 10.1073/pnas.58.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. J., May H. D., Newton W. E., Brigle K. E., Dean D. R. Role for the nitrogenase MoFe protein alpha-subunit in FeMo-cofactor binding and catalysis. Nature. 1990 Jan 11;343(6254):188–190. doi: 10.1038/343188a0. [DOI] [PubMed] [Google Scholar]
- Thorneley R. N., Lowe D. J. Nitrogenase of Klebsiella pneumoniae. Kinetics of the dissociation of oxidized iron protein from molybdenum-iron protein: identification of the rate-limiting step for substrate reduction. Biochem J. 1983 Nov 1;215(2):393–403. doi: 10.1042/bj2150393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Lowe D. J. The mechanism of Klebsiella pneumoniae nitrogenase action. Pre-steady-state kinetics of an enzyme-bound intermediate in N2 reduction and of NH3 formation. Biochem J. 1984 Dec 15;224(3):887–894. doi: 10.1042/bj2240887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Lowe D. J. The mechanism of Klebsiella pneumoniae nitrogenase action. Simulation of the dependences of H2-evolution rate on component-protein concentration and ratio and sodium dithionite concentration. Biochem J. 1984 Dec 15;224(3):903–909. doi: 10.1042/bj2240903. [DOI] [PMC free article] [PubMed] [Google Scholar]


