Abstract
Xuefu zhuyu Tang (XFZYT) is a classic formula used for promoting blood circulation and resolving blood stasis in Traditional Chinese Medicine. Clinical data have indicated that XFZYT plays a significant therapeutic role in diabetes-induced erectile dysfunction (DIED) disease, but the underlying mechanism remains elusive. Male Sprague–Dawley (SD) rats were randomly categorized into normal, model, and treatment groups. The diabetic rat model was established via intraperitoneal injection of streptozotocin. DIED rats were screened using apomorphine, and the number of erections was measured after 8 weeks of XFZYT treatment. Serum nitric oxide (NO) and endothelin-1 levels as well as penile tissue structure alterations were assessed by hematoxylin–eosin staining and electron microscopy. CaSR/PLC/PKC and MEK/ERK/RSK pathway-related proteins in the penile tissue were detected by western blotting (WB) analysis and polymerase chain reaction (PCR). Compared with the blank group, the model group rats showed a significant decrease in weight and erectile function. The pathological damage in the penile tissues of the model rats was indicated by a significantly decreased serum NO level and an increased endothelin-1 content. After treatment with XFZYT, the protein expression of CaSR, PLCβ1, PKCβ, MEK1, ERK1, and RSK1 in the penile tissue was significantly increased. Overall, the treatment group showed significant improvements in the evaluated indexes. In conclusion, this study revealed that XFZYT improves erectile function in diabetic rats, and the underlying mechanism might be linked with the regulation of CaSR/PLC/PKC and related molecules of the MEK/ERK/RSK pathway, which promotes the vascular endothelial diastolic effect.
Keywords: herbal, sexual dysfunction, protein pathway, vascular endothelial function
Introduction
Diabetes mellitus (DM) is one of the three major chronic diseases recognized by the World Health Organization. About 415 million people globally suffer from or are at risk of early-stage DM (Ogurtsova et al., 2017). Literature data published in JAMA in 2017 showed that China ranked first with the most number of DM cases and the prevalence of diabetes was as high as 10.9% (L. Wang et al., 2017). Diabetes-induced erectile dysfunction (DIED) is one of the common complications of DM, has a high prevalence rate, and is closely associated with cardiovascular lesions and other conditions (Bergman et al., 2014). The National Institutes of Health (NIH) expert meeting indicated that DM is one of the key etiological factors responsible for erectile dysfunction (ED) in men. The prevalence of ED in DM patients was found to be 1.9 to 5 times higher than in non-DM patients, and the global prevalence of DIED was reported as 20% to 75% (Bivalacqua et al., 2004). With the increase in age, metabolic diseases, including DM, greatly impact men, especially those who are sexually active.
In recent years, the over-activation of the protein kinase C (PKC) pathway is a hotspot in the study of DM hyperglycemic environment that induces vascular endothelial dysfunction and leads to the formation of vascular complications (Xiao et al., 2023). Studies have shown that the extracellular hyperglycemic environment can activate the PKC pathway from multiple pathways. The CaSR/PLC/PKC pathway activation is a hallmark of vascular atherosclerosis with decreased compliance and is essentially involved in platelet aggregation and thrombosis (Breitwieser, 2008). The ERK signaling pathway has also been observed to be activated during endothelial differentiation and angiogenesis (Shi et al., 2022; Zachary & Gliki, 2001). The 90 ribosomal S6 kinase (RSK) family is downstream of ERK. In addition, the ERK pathway is an important component of the mitogen-activated protein kinase signaling pathway, which is crucially linked with the regulation of cell proliferation, survival, and apoptosis (Cui et al., 2016). The aforementioned studies suggest that CaSR/PLC/PKC and ERK/RSK pathways are essential for regulating angiogenesis and endothelial cell function. Clinically, the first-line drugs for ED treatment are phosphodiesterase-5 (PDE-5) inhibitors, but they have poor efficacy against diabetic vascular endothelial disorders, and their therapeutic effects on DM are undetermined (Anita et al., 2022). The therapeutic effect of traditional Chinese medicine (TCM) on DIED has been confirmed in recent years (Li et al., 2022; J. S. Wang et al., 2021). TCM is a safer and more effective treatment option for ED and the prolonged control of diabetes progression. According to Chinese medicine scholars, “blood stasis and obstruction of collaterals” is a key pathogenesis of DIED. Xuefu zhuyu Tang (XFZYT) is a representative formula of clinically effective blood-activating and collaterals-opening methods used in TCM. Whether XFZYT can boost the erectile function of DIED rats via the CaSR/PLC/PKC and MEK/ERK/RSK pathways modulation remains unaddressed.
This study used XFZYT, the representative formula of the blood activation and circulation method, as a DIED intervention to observe its effects on CaSR/PLC/PKC and MEK/ERK/RSK pathways in rats. The study aimed to explore the possible XFZYT mechanisms, that improve erectile function in DIED rats and to comprehensively understand the underlying mechanisms of DIED occurrence. The research idea is presented in Figure 1.
Figure 1.
Overall Process Based on Biological Network Research and Animal Experiments
Materials and Methods
Experimental Animals
Twenty-five healthy SPF (Sun Protection Factor)-grade Sprague–Dawley (SD) white male rats (weight = 180–220 g, age = 8 weeks) were purchased from Zhengzhou Huiji Huaxing Laboratory Animal Breeding Farm Co, Ltd. (Animal Production License No.: SCXK [Yu] 2019-0002). The mating experiments were carried out to confirm the normal sexual function of all the rats. The animals were kept in independent ventilated cage boxes in the animal experiment room of the Central Laboratory of the First Affiliated Hospital of Henan University of Chinese Medicine (HUCM), with 12-h day/night alternation, 23 ± 2°C; room temperature (RT), 60% ± 10% relative humidity, and ad libitum food and water. All the animal experiments were approved by the Animal Ethics Committee of the First Affiliated Hospital of HUCM (YFYDW2021023).
Drugs and Reagents
The formula granules of XFZYT were provided by the formula granule room of the First Affiliated Hospital of HUCM and were uniformly prepared by New Green Pharmaceuticals with the same batch number and sealed and stored for subsequent use. Streptozotocin (STZ; Beijing Solepol Technology Co., Ltd, batch number: 421D022), apomorphine (APO, APExBO, batch number: B6936113377BD, USA), nitric oxide (NO) kit (Shanghai Biyuntian Company, batch number S0021S) were used. Endothelin-1 was purchased from Shanghai Biyuntian Company, whereas Endothelin-1 (ET-1) kit (batch no. E-EL-R1458c) was purchased from Wuhan Elabscience Company. Rabbit-derived anti-rat CaSR (item no. ab259846), PLCβ1 antibody (item no. ab107455), PKCβ (item no. ab181558), ERK1 (item no. ab109282), RSK1 (item no. ab32413), MEK1 (item no. ab307509) antibodies were purchased from Abcam, USA. Strong RIPA Lysate (item no. P0013B) and BCA Protein assay kit (P0011) were purchased from Shanghai Biyuntian Company.
Construction of Diabetic Erectile Dysfunction Rat Model
All the SD rats were acclimatized with ad libitum chow for 1 week, and then, analyzed for any abnormality. Normal healthy rats were then numbered, and their body weights were recorded. According to the random number table method, six rats were included in the blank control group, and the remaining 19 rats were added to the DIED pre-built model group.
DM Model Preparation
For the model establishment, 19 SD rats were randomly selected, fasted for 12 h, and then injected with STZ solution (55 mg/kg) intraperitoneally into the right lower abdomen. The blood was collected from the tail vein at 72 h, 1 week, and 2 weeks after the drug administration to measure their blood glucose using a glucose meter. Rats with random blood glucose > 16.7 mmol/L accompanied by increased urine and diet were initially regarded as the DM rat model.
Diabetes-Induced Erectile Dysfunction Model Screening
According to Heaton’s method (Heaton et al., 1991), the body weight of each rat was measured. Then, the experimental rats were administered apomorphine (APO; 100 μg/kg) in the neck to induce a penile erection. A video camera was used to record the state of the rats in a normal dimly lit and quiet environment for 30 min to record the presence or absence of penile erection. The erection was confirmed if the penis increased and grew, and the end of the penis was exposed. Rats without an erection within 30 min were included in the model group. Finally, 13 rats without erections were identified as DIED model rats.
Experimental Drug Injections and Subgroups
Blank control group (Grp C, n = 6) received routine feed and from the fourth day were intraperitoneally injected with a buffer of 0.1 mmol/L citric acid–sodium citrate to mimic the injury in the model group; 10 mL/kg of deionized water was given daily at 10 a.m. via gavage.
Model group (Grp M, n = 6), after successful modeling, received acclimatization feeding for 3 days, and from the 4th day received 10 mL/kg of deionized water daily at 10 a.m. via gavage.
The treatment group (Grp T, n = 6), after successful modeling, received acclimatization feeding for 3 days, and from the fourth day received deionized water configured with XFZYT granule suspension (7.8 g/kg/d) daily at 10 a.m. via gavage (six times the human dosage).
Preparation and Collection of Tissue Samples
After 8 weeks of treatment, rats were fasted for 12 h and anesthetized by intraperitoneal injection of 1% sodium pentobarbital (30 mg/kg), 1 h after the last drug administration. Blood was collected from the abdominal aorta in ethylenediaminetetraacetic acid (EDTA) anticoagulation and serum tubes, respectively. The samples were centrifuged at a low temperature and 4,000 rpm for 20 min to separate plasma and serum for NO and ET-1 tests. The rat penile corpus cavernosum tissue was used for hematoxylin–eosin (HE) staining and related molecular assays.
Assessment of Erectile Function
After 8 weeks of treatment, the body weight of all the rats from each group was measured, and apomorphine (APO; 100 μg/kg) was administered in the neck of the experimental rats to induce a penile erection. The presence or absence of penile erection and the number of times of erection were recorded within 30 min.
Nitrate Reductase Assay for the Detection of NO Level
The cavernous tissue of the rat penis was homogenized in a homogenizer, and centrifuged at 4,000 rpm (centrifugation radius = 10 cm) for 10 min. The supernatant was collected for the detection of the NO level of rats by using an NO assay kit (Nitrate reductase assay), per the manufacturer’s guide.
Enzyme-Linked Immunosorbent Assay Detection of ET-1 Level in Rat Penis Tissue
Each group rat’s serum and the standards were added to the microtiter enzyme labeling plate for assessing ET-1 level using the ELISA (enzyme-linked immunosorbent assay) kit, per the kit’s instructions. Immediately after the experiment completion, the enzyme labeling instrument was used to determine the absorbance of each well, the standard curve was plotted, and the ET-1 level was calculated.
Morphological Detection of Penile Tissue
The middle region of the penile tissue was fixed with 4% paraformaldehyde before incubation in a dehydration box. Next, the alcoholic series-based dehydration was performed, followed by wax block embedding, slicing, xylene dewaxing, hematoxylin staining, alcohol differentiation, rinsing, anhydrous ethanol-based dehydration, eosin staining, xylene clearing, neutral resin fixation, and photography under a microscope.
For the electron microscopy, the penile tissue was dissected from the root, the residual fascia and skin were removed, and the middle part of the penis was retained. The tissue was initially fixed in 2.5% and then in 3% glutaraldehyde solution; the tissue was cut into pieces (about 1 × 1 × 1 mm), double-fixed in 2.5% glutaraldehyde and 1% osmic acid, dehydrated in gradient ethanol and acetone, embedded in epoxy resin, and ultrathin sectioned (about 100 nm in thickness), stained with uranium acetate and observed under transmission electron microscopy.
Protein Blot Analysis
Western blotting analysis determined the expression levels of CaSR, PLCβ1, PKCβ, MEK1, ERK1, and RSK1 proteins in the penis tissue. Following the collection and weighing of the penile tissue, it was homogenized in RIPA buffer on an ice bath and then centrifuged for supernatant collection. The total protein was quantified via the BCA kit. The protein samples in the homogenate were separated by gel electrophoresis at 80 V before transfer to a PVDF film. The film was then incubated with 5% skim milk for 1 h at RT. Based on the molecular weight of the target proteins, the film was sectioned and incubated overnight (ON) with primary antibody (diluted at 1:1,000) at 4°C, rinsed with Tris-buffered saline with Tween 20 (TBST) thrice, treated with secondary antibody at RT for 4 h (1:1,000 dilution), washed against with TBST three times, and developed with chemiluminescent via an automatic chemiluminescence imaging system to visualize protein bands.
Real-Time Reverse Transcription-Quantitative Polymerase Chain Reaction Assay
Total RNA was extracted from rat penis tissue using Trizol reagent and cDNA was synthesized via a reverse transcription kit. GAPDH mRNA expression was set as an internal reference for standardization. Real-time reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was performed and the 2-ΔΔCt method was used to calculate the relative expressions. Table 1 summarizes the primers used.
Table 1.
The Primer Sequences for RT-PCR
| Primer | Forward primer | Reverse primer |
|---|---|---|
| CASR | 5’-GCAGACCTGGCATTGAGAAG-3’ | 5’-ACAATGACCTTGGCCGTAGA-3’ |
| PLCβ1 | 5’-CTTCTGGAATGCTGGCTGTC-3’ | 5’-GTCTGGCCTCCTCATGAACT-3’ |
| PKCβ | 5’-GCGGCAGAAGTTTGAGAGAG-3’ | 5’-GTCCCTGTTGCCATTGTTGT-3’ |
| MEK1 | 5’-AGTGGTGTGTTCAGCTCAGA-3’ | 5’-CAGAGCGTTTGATGAAGGCA-3’ |
| ERK1 | 5’-GAGCCAGCAGCTGAGCAATG-3’ | 5’-AAGGTCGCAGGTGGTGTTGA-3’ |
| RSK1 | 5’-AGAAGCTGGACTTCAGCCAT-3’ | 5’-GGGATGGATCAGCCTTCTCA-3’ |
| GAPDH | 5’-CAACTCCCTCAAGATTGTCAGCAA-3’ | 5’-GGCATGGACTGTGGTCATGA-3’ |
Statistical Processing
All data were analyzed using SPSS 23.0 software. Data with normal distribution are presented as mean ± standard deviation (x ± s). For homogeneous variance data, one-way analysis of variance (ANOVA), and intergroup pairwise comparison the least significant difference test was performed. Nonparametric rank-sum tests were employed for data with non-normal distribution. Unordered count data were assessed via the chi-square test. p < .05 indicated statistical significance.
Results
Comparison of Body Weight and Blood Glucose in Rats
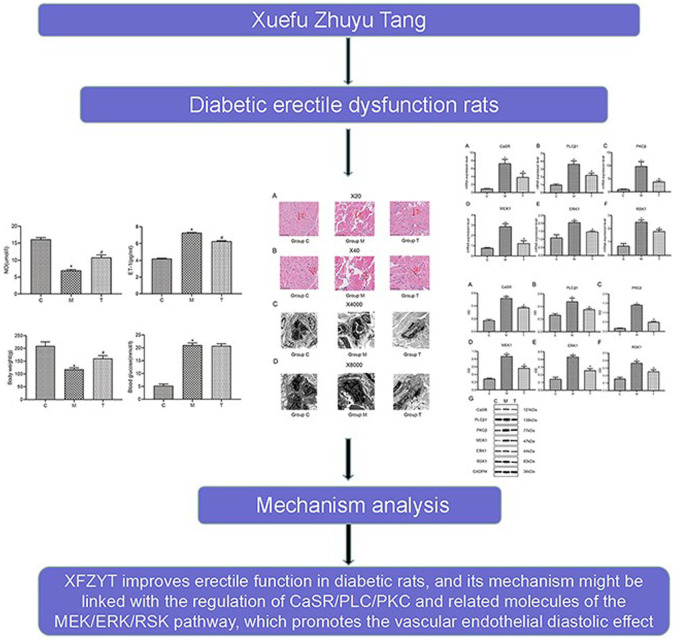
Compared with Grp C, the body weight of rats in Grp M decreased significantly (p < .05), whereas their blood glucose increased significantly (p < .05). After drug intervention, Grp T rats indicated significantly increased body weight (p < .05) relative to Grp M, but there was no significant difference in the blood glucose levels (p > .05; Figure 2).
Figure 2.
The Body Weight and Blood Glucose in Rats From the C, M, and T Groups
Data are expressed as the mean ± SEM. Independent sample t-tests were employed for comparison between the two groups.
Differences with p < .05 were considered statistically significant. *p< .05,the M group versus the C group; #p< .05, the T group versus the M group.
Comparison of Erectile Function in Rats
Following APO stimulation, the Grp M rat’s erection frequency was markedly reduced than Grp C. However, drug treatment markedly elevated the erection frequency in Grp T compared with Grp M (p < .05; Table 2).
Table 2.
Erectile Times of Rats in Each Group After Drug Intervention
Note. Differences with p < .05 were considered statistically significant *p < .05, the M group versus the C group; # p < .05, the T group versus the M group.
NO and ET-1 Levels in Rats Following Drug Intervention
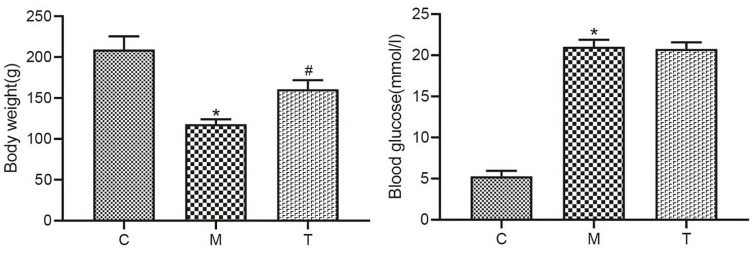
The ET-1 expression of rats in Grp M was significantly higher, and the NO expression was significantly lower compared with Grp C. Following drug treatment, the ET-1 contents in Grp T were reduced, and the NO expression was markedly elevated relative to Grp M (p < .05; Figure 3).
Figure 3.
The NO and ET-1 Levels in Rats From the C, M, and T Groups
Data are expressed as the mean ± SEM. Independent sample t-tests were employed for comparison between the two groups.
Differences with p < .05 were considered statistically significant. *p < .05, the M group versus the C group; #p < .05, the T group versus the M group.
Comparison of Pathological Changes in Rats
The HE staining indicated that in Grp C, muscle and collagen fibers were neatly arranged, and the blood sinusoids were structurally intact, containing a small number of erythrocytes. The model group indicated broken muscle fibers, collagen fibers were twisted into strips, and the blood sinusoids were reduced, whereas in Grp T there were still breaks in the smooth muscle fibers, but the collagen fibers and blood sinusoids were restored (Figure 4A and 4B).
Figure 4.
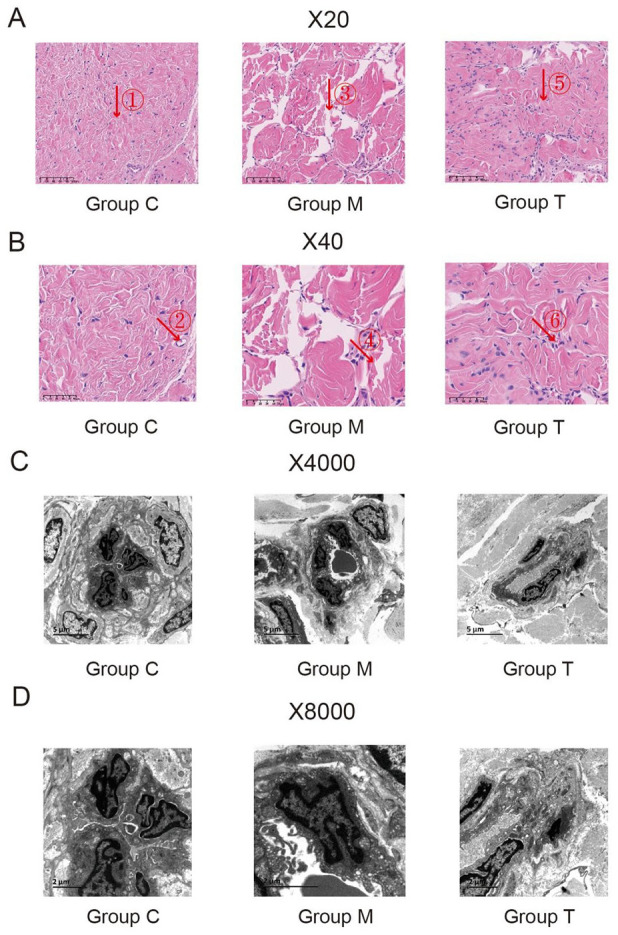
(A) and (B) HE Staining of Penile Tissue of Rats From the C, M, and T Groups (×20 and ×40). (a) In the C group: the muscle and collagen fibers were neatly arranged, and the blood sinusoids were structurally intact, containing a small number of erythrocytes (Arrow ①②);(b) In the M group: the broken muscle fibers, collagen fibers were twisted into strips, and the blood sinusoids were reduced (Arrow ③④); (c) In the T group: there were still breaks in the smooth muscle fibers, but the collagen fibers and blood sinusoids were restored (Arrow ⑤⑥). Figure (C) and (D) Ultrastructure of penile tissue of rats from the C, M, and T Groups (×4,000 and ×8,000)
Under the electron microscope, the vascular structure of rats in Grp C was normal with good vascular endothelial cell morphology, regular arrangement, intact cytoplasmic membrane, and normal organelles, such as mitochondria, endoplasmic reticulum, and Golgi apparatus. The model group indicated fractured endothelium of blood sinusoids, narrowed lumen, swollen endothelial cells, broken cytoplasmic membrane, reduced organelles, and condensed nuclear chromatin. Compared with the model group, the endothelium of the treatment group showed different degrees of hyperplasia, and organelles, such as mitochondria, endoplasmic reticulum, and Golgi apparatus increased significantly, and the morphology and structure of the endothelial cells were markedly improved (Figure 4C and 4D).
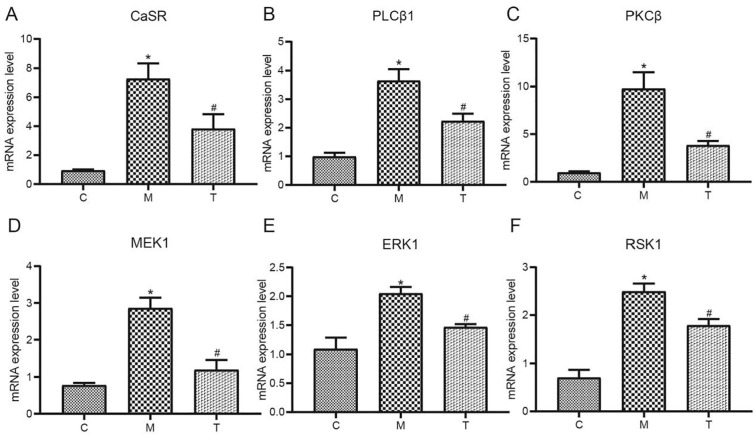
Effects of XFZYT on the Expression Levels of CaSR, PLCβ1, PKCβ, MEK1, ERK1, and RSK1 Proteins in the Penile Tissues of Rats in Each Group
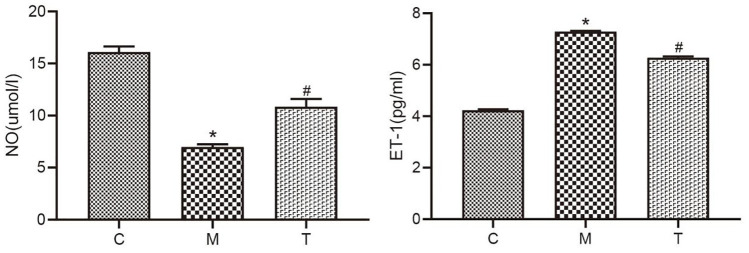
The results of the study showed that the protein expression of CaSR, PLCβ1, PKCβ, MEK1, ERK1, and RSK1 in the penile tissue of rats in Grp M was significantly increased compared with Grp C (p < .05). After drug treatment, the protein expression of CaSR, PLCβ1, PKCβ, MEK1, ERK1, and RSK1 in Grp T was significantly reduced compared with Grp M (p < .05; Figure 5).
Figure 5.
(A) to (F) The Expression Levels of Six Proteins (CaSR, PLCβ1, PKCβ, MEK1, ERK1, and RSK1) in Rats From the C, M, and T Groups Determined Using Western Blotting. (G) Electrophoretogram of Six Proteins (CaSR, PLCβ1, PKCβ, MEK1, ERK1, and RSK1) in Rats From the C, M, and T Groups
Data are expressed as the mean ± SEM. Independent sample t-tests were employed for comparison between the two groups. differences with p < .05 were considered statistically significant. *p < .05, the M group versus the C group; #p < .05, the T group versus the M group.
Effects of XFZYT on the Expression Levels of CaSR, PLCβ1, PKCβ, MEK1, ERK1, and RSK1 mRNA in the Penile Tissues of Rats in All Groups
It was revealed that the mRNA expression of CaSR, PLCβ1, PKCβ, MEK1, ERK1, and RSK1 in the Grp M rat’s penile tissue was significantly higher than Grp C (p < .05). Following drug treatment, the mRNA expression of CaSR, PLCβ1, PKCβ, MEK1, ERK1, and RSK1 in Grp T was significantly reduced significantly compared with Grp M (p < .05; Figure 6).
Figure 6.
(A) to (F) The mRNA Expression Levels of Six Proteins (CaSR, PLCβ1, PKCβ, MEK1, ERK1, and RSK1) in Rats From the C, M, and T Groups Determined Using RT-qPCR
Data are expressed as the mean ± SEM. Independent sample t-tests were employed for comparison between the two groups. differences with p < .05 were considered statistically significant. *p < .05, the M group versus the C group; #p < .05, the T group versus the M group.
Discussion
The pathogenesis of DIED is complex and is associated with penile vascular endothelial and smooth muscle lesions and sensory nerve dysfunction caused by high circulating glucose toxicity because of DM, which involves multiple genes and targets (Cayetano-Alcaraz et al., 2023). This study revealed that XFZYT significantly improved the erectile function of DIED rats, and the mechanism of action may be closely related to the regulation of CaSR/PLC/PKC and MEK/ERK/RSK pathways. This investigation combined the animal experimental results to introduce possible mechanism hypotheses for further studies.
This study found that compared with Grp C, the number of erections of rats in Grp M decreased significantly. The HE and electron microscopy revealed that the myofibrils in Grp M were broken, collagen fibers were twisted into strips, blood sinusoids were markedly reduced, the blood sinusoidal lumen was narrowed, the endothelial cells were swollen, the cytoplasmic membrane was broken, and there was a substantial decrease in the cellular organelles, which was a direct cause of the decreased erectile function of the DIED rats. After XFZYT treatment, the number of erections increased, the collagen fibers and blood sinusoids of the penile tissue of rats in the treatment group were restored, the organelles, such as mitochondria, endoplasmic reticulum, and Golgi apparatus were increased, and the morphology and structure of endothelial cells were notably improved. These results suggested that XFZYT could significantly improve the pathological penile histological structure of rats with DIED.
NO is an important vasodilatory substance for vascular endothelial cells (Wu et al., 2022), and excessive generation of oxygen-free radicals in patients with DM can decrease vascular NO levels (Tu et al., 2022). During high glucose conditions, the vascular wall is damaged, the vascular endothelial cells destruct, and NO synthase production decreases, which reduces NO synthesis (Liu et al., 2018). The decrease in NO leads to decreased vasodilatory capacity of vessels and cavernous blood flow to the penis, leading to decreased erection (Yadav & Mishra, 2023). The decrease in NO reduces vasodilatory capacity and blood flow to the penile corpus cavernosum, which in turn decreases erectile function (Yadav & Mishra, 2023). This study indicated alleviated NO in the model group rats, which significantly increased after XFZYT treatment, confirming that XFZYT can improve the erectile function of DIED rats by increasing NO content.
The ET-1 is a mitogenic peptide produced by endothelial cell, vascular smooth muscle cell (VSMC), and inflammatory cell, which is involved in the regulation of vascular tone, and has a strong and long-lasting vasoconstrictor effect. Furthermore, ET-1 can regulate an organism’s function as a potentially vasoactive substance, which can mediate the contraction of a variety of muscles, including cavernous and urethral cavernous smooth muscle, and so on (Chang et al., 2018; Corder et al., 2001). Glucose is an ET-releasing agent, and long-term glycation of erythrocytes and hemoglobin in DM patients reduces their deformation and oxygen-carrying capacity, causing ischemic and hypoxic damage to the vascular endothelium and cells, which can activate the endogenous coagulation system and elevate ET levels (Ali et al., 2019; Jain et al., 2019). ET-1 has been observed to increase Ca2+ influx by binding to specific penile tissue receptors and affecting NO production, causing persistent contraction of the penile corpus cavernosum, simultaneously inhibiting endothelial NO bioavailability, promoting constriction of penile arteries, and consequently promoting ED (SDomoti et al., 2014). This study showed that ET-1 was significantly increased in the DIED model group, and decreased significantly after treatment with XFZYT, validating that XFZYT can improve the erectile function of DIED rats by affecting the level of ET-1 and then improving the erectile function.
In recent years, the over-activation of the PKC pathway in a DM hyperglycemic environment has been a research hotspot, as it induces vascular endothelial dysfunction and causes the formation of vascular complications (Klymenko et al., 2014). Studies have shown that the extracellular hyperglycemic environment can activate the PKC pathway from multiple pathways, among which the classical pathway, calcium-sensitive receptor (CaSR), is affected by the hyperglycemic environment by coupling to phospholipase C (PLC) via the Gq protein, which further catabolizes phosphatidylinositol-4,5-bisphosphate (PIP2) to produce diethylacylglycerol (DAG) and inositol triphosphate (IP3). IP3 binds with a high-affinity receptor on the cell nucleus and opens calcium channels to release nuclear calcium into the cytoplasm, which together with DAG activates PKC on the inner surface of the plasma membrane (Das Evcimen & King, 2007; Williams & Nadler, 2007). In addition, PKC activation increases reduced coenzyme oxidase (NOX)-mediated oxygen radical production, decreases NO bioavailability, and increases the release of ET-1, thereby affecting vascular endothelial function and endothelium-dependent vasodilatory dysfunction (Kizub et al., 2010). This investigation indicated that the CaSR, PLCβ1 and PKCβ protein and mRNA levels in the penile tissues of DIED model rats were increased than those in the blank control group, and after XFZYT treatment, the CaSR, PLCβ1 and PKCβ protein and mRNA levels were significantly decreased, which proved that XFZYT could improve vascular endothelium function by regulating the relevant molecules of the CaSR/PLC/PKC pathway and then improve the frequency of erections in DIED rats.
The Raf–MEK–ERK is a representative MAPK (mitogen-activated protein kinase) signaling pathway that induces cell growth and differentiation. Recently, it was discovered that the MEK/ERK pathway is closely associated with diabetic complications, such as diabetic cataracts (Chen et al., 2014; Ullah et al., 2022). The MEK/ERK signaling pathway has been associated with the contractile function of vascular smooth muscle and causes VSMC dysfunction, vasoconstriction, and vascular remodeling (Feng et al., 2016). The RSK is a serine/threonine family of proteins, which are important effector molecules downstream of the Raf–MEK–ERK cascade signaling pathway. RSK activation by growth factors, neurotransmitters, and other stimulatory factors initiates multiple consecutive phosphorylation regulatory sites in the RSKs via ERK1/2, which modulate substrate protein phosphorylation to exert biologically active roles and play important roles in the regulation of cell growth and proliferation, cell cycle and protein expression (Anjum et al., 2005; Nam et al., 2008; Smith et al., 2005). This analysis found that the protein and mRNA levels of MEK1, ERK1, and RSK1 were significantly elevated in the DIED model group than in the blank control group, and were significantly decreased after XFZYT treatment, confirming that XFZYT can diastole the blood vessels by inhibiting the MEK/ERK/RSK pathway, which in turn improves the erectile function in DIED rats. CaSR/PLC/PKC and MEK/ERK/RSK pathways affect cell proliferation (Han et al., 2023; P. Wang et al., 2009), and the proliferation of vascular endothelial cells is closely related to DIED, this study did not detect the related indexes, which is a shortcoming. In the future, further in-depth research will be conducted to provide a certain reference basis for the clinical treatment of diabetic ED.
Conclusion
In summary, this study indicated that XFZYT could promote vascular endothelial diastole by regulating the relevant molecules of CaSR/PLC/PKC and MEK/ERK/RSK pathways, which could be a mechanism that improves erectile function in DIED rats. It is worth noting that there is little literature exploring DIED from CaSR/PLC/PKC with MEK/ERK/RSK pathway, which is our innovation.
Acknowledgments
All the authors of the manuscript are immensely grateful to the foundations for their valuable support.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Henan Province Chinese Medicine Discipline Leading Talent Training Project, Henan Province Chinese Medicine Scientific Research Special Project (2019JDZX2028, 2023ZY3004), China Association of Chinese Medicine Young Talent Support Project (CACM-2022-QNRC2-B13), Henan Province Key Research and Development and Promotional Special Project (Scientific and Technological Tackling; 212102311127), and Henan Science and Technology Research and Development Programme Joint Fund (Advantageous Discipline Cultivation Category) Project (232301420075), and 2024 Henan Province Postdoctoral Research Project Funding(HN2024086).
ORCID iDs: Mingzhao Zhang  https://orcid.org/0009-0007-5294-9735
https://orcid.org/0009-0007-5294-9735
Jisheng Wang  https://orcid.org/0000-0002-3332-076X
https://orcid.org/0000-0002-3332-076X
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Ali H., Rustam R., Aprilia D., Arizal C., Gusadri I. B., Utami P. R. (2019). Upregulation of SCUBE2 expression in dyslipidemic type 2 diabetes mellitus is associated with endothelin-1. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 13(5), 2869–2872. 10.1016/j.dsx.2019.07.058 [DOI] [PubMed] [Google Scholar]
- Anita L., Yin G. N., Hong S. S., Kang J. H., Gho Y. S., Suh J. K., Ryu J. K. (2022). Pericyte-derived extracellular vesicle-mimetic nanovesicles ameliorate erectile dysfunction via lipocalin 2 in diabetic mice. International Journal of Biological Sciences, 18(9), 3653–3667. 10.7150/ijbs.72243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum R., Roux P. P., Ballif B. A., Gygi S. P., Blenis J. (2005). The tumor suppressor DAP kinase is a target of RSK-mediated survival signaling. Current Biology, 15(19), 1762–1767. 10.1016/j.cub.2005.08.050 [DOI] [PubMed] [Google Scholar]
- Bergman R. N., Stefanovski D., Kim S. P. (2014). Systems analysis and the prediction and prevention of Type 2 diabetes mellitus. Current Opinion in Biotechnology, 28, 165–170. 10.1016/j.copbio.2014.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivalacqua T. J., Champion H. C., Usta M. F., Cellek S., Chitaley K., Webb R. C., Lewis R. L., Mills T. M., Hellstrom W. J., Kadowitz P. J. (2004). RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: A mechanism for diabetes-associated erectile dysfunction. Proceedings of the National Academy of Sciences of the USA, 101(24), 9121–9126. 10.1073/pnas.0400520101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser G. E. (2008). Extracellular calcium as an integrator of tissue function. The International Journal of Biochemistry & Cell Biology, 40(8), 1467–1480. 10.1016/j.biocel.2008.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayetano-Alcaraz A. A., Tharakan T., Chen R., Sofikitis N., Minhas S. (2023). The management of erectile dysfunction in men with diabetes mellitus unresponsive to phosphodiesterase type 5 inhibitors. Andrology, 11(2), 257–269. 10.1111/andr.13257 [DOI] [PubMed] [Google Scholar]
- Chang W., Lajko M., Fawzi A. A. (2018). Endothelin-1 is associated with fibrosis in proliferative diabetic retinopathy membranes. PLOS ONE, 13(1), e0191285. 10.1371/journal.pone.0191285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Ye S., Xiao W., Wang W., Luo L., Liu Y. (2014). ERK1/2 pathway mediates epithelial-mesenchymal transition by cross-interacting with TGFβ/Smad and Jagged/Notch signaling pathways in lens epithelial cells. International Journal of Molecular Medicine, 33(6), 1664–1670. 10.3892/ijmm.2014.1723 [DOI] [PubMed] [Google Scholar]
- Corder R., Douthwaite J. A., Lees D. M., Khan N. Q., Viseu Dos Santos A. C., Wood E. G., Carrier M. J. (2001). Endothelin-1 synthesis reduced by red wine. Nature, 414(6866), 863–864. 10.1038/414863a [DOI] [PubMed] [Google Scholar]
- Cui Y., Lu P., Song G., Liu Q., Zhu D., Liu X. (2016). Involvement of PI3K/Akt, ERK and p38 signaling pathways in emodin-mediated extrinsic and intrinsic human hepatoblastoma cell apoptosis. Food and Chemical Toxicology, 92, 26–37. 10.1016/j.fct.2016.03.013 [DOI] [PubMed] [Google Scholar]
- Das Evcimen N., King G. L. (2007). The role of protein kinase C activation and the vascular complications of diabetes. Pharmacological Research, 55(6), 498–510. 10.1016/j.phrs.2007.04.016 [DOI] [PubMed] [Google Scholar]
- Feng J., Ge S., Zhang L., Che H., Liang C. (2016). Aortic dissection is associated with reduced polycystin-1 expression, an abnormality that leads to increased ERK phosphorylation in vascular smooth muscle cells. European Journal of Histochemistry, 60(4), 2711. 10.4081/ejh.2016.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Li P., Fu X., Huang Z., Yin W. (2023). Aesculetin inhibits proliferation and induces mitochondrial apoptosis in bladder cancer cells by suppressing the MEK/ERK signaling pathway. Anti-Cancer Agents in Medicinal Chemistry, 23(4), 478–487. 10.2174/1871520622666220615142636 [DOI] [PubMed] [Google Scholar]
- Heaton J. P., Varrin S. J., Morales A. (1991). The characterization of a bio-assay of erectile function in a rat model. The Journal of Urology, 145(5), 1099–1102. 10.1016/s0022-5347(17)38543-9 [DOI] [PubMed] [Google Scholar]
- Jain A., Coffey C., Mehrotra V., Flammer J. (2019). Endothelin-1 traps as a potential therapeutic tool: From diabetes to beyond? Drug Discovery Today, 24(9), 1937–1942. 10.1016/j.drudis.2019.07.008 [DOI] [PubMed] [Google Scholar]
- Kizub I. V., Pavlova O. O., Ivanova I. V., Soloviev A. I. (2010). Protein kinase C-dependent inhibition of BK(Ca) current in rat aorta smooth muscle cells following gamma-irradiation. International Journal of Radiation Biology, 86(4), 291–299. 10.3109/09553000903564042 [DOI] [PubMed] [Google Scholar]
- Klymenko K., Novokhatska T., Kizub I., Parshikov A., Dosenko V., Soloviev A. (2014). PKC-δ isozyme gene silencing restores vascular function in diabetic rat. Journal of Basic and Clinical Physiology and Pharmacology, 25(4), 341–349. 10.1515/jbcpp-2013-0147 [DOI] [PubMed] [Google Scholar]
- Li X., Feng J. L., Chen Z. L., Bao B. H., Dai H. H., Meng F. C., Deng S., Wang B., Li H. S., Wang J. S. (2022). Mechanism by which Huoxue Tongluo Qiwei Decoction improves the erectile function of rats with diabetic erectile dysfunction. Journal of Ethnopharmacology, 283, 114674. 10.1016/j.jep.2021.114674 [DOI] [PubMed] [Google Scholar]
- Liu J., Hu D. J., Yan H., Liu J., Ai X., Ren Z., Zeng H., He H., Yang Z. (2018). Attenuated endothelial function is associated with decreased endothelial progenitor cells and nitric oxide in premenopausal diabetic women. Molecular Medicine Reports, 18(5), 4666–4674. 10.3892/mmr.2018.945 [DOI] [PubMed] [Google Scholar]
- Nam H. J., Kim S., Lee M. W., Lee B. S., Hara T., Saya H., Cho H., Lee J. H. (2008). The ERK-RSK1 activation by growth factors at G2 phase delays cell cycle progression and reduces mitotic aberrations. Cellular Signalling, 20(7), 1349–1358. 10.1016/j.cellsig.2008.03.008 [DOI] [PubMed] [Google Scholar]
- Ogurtsova K., da Rocha Fernandes J. D., Huang Y., Linnenkamp U., Guariguata L., Cho N. H., Cavan D., Shaw J. E., Makaroff L. E. (2017). IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Research and Clinical Practice, 128, 40–50. 10.1016/j.diabres.2017.03.024 [DOI] [PubMed] [Google Scholar]
- Sánchez A., Martínez P., Muñoz M., Benedito S., García-Sacristán A., Hernández M., Prieto D. (2014). Endothelin-1 contributes to endothelial dysfunction and enhanced vasoconstriction through augmented superoxide production in penile arteries from insulin-resistant obese rats: Role of ET(A) and ET(B) receptors. British Journal of Pharmacology, 171(24), 5682–5695. 10.1111/bph.12870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Li J., Chen H., Hu Y., Tang L., Wang Y., Zang X., Ma X., Huang G., Zhou X., Tao M., Lv Z., Chen S., Qiu A., Zhuang S., Liu N. (2022). Inhibition of EZH2 suppresses peritoneal angiogenesis by targeting a VEGFR2/ERK1/2/HIF-1α-dependent signaling pathway. The Journal of Pathology, 258(2), 164–178. 10.1002/path.5987 [DOI] [PubMed] [Google Scholar]
- Smith J. A., Poteet-Smith C. E., Xu Y., Errington T. M., Hecht S. M., Lannigan D. A. (2005). Identification of the first specific inhibitor of p90 ribosomal S6 kinase (RSK) reveals an unexpected role for RSK in cancer cell proliferation. Cancer Research, 65(3), 1027–1034. 10.1158/0008-5472.1027.65.3 [DOI] [PubMed] [Google Scholar]
- Tu C., Lu H., Zhou T., Zhang W., Deng L., Cao W., Yang Z., Wang Z., Wu X., Ding J., Xu F., Gao C. (2022). Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials, 286, 121597. 10.1016/j.biomaterials.2022.121597 [DOI] [PubMed] [Google Scholar]
- Ullah R., Yin Q., Snell A. H., Wan L. (2022). RAF-MEK-ERK pathway in cancer evolution and treatment. Seminars in Cancer Biology, 85, 123–154. 10.1016/j.semcancer.2021.05.010 [DOI] [PubMed] [Google Scholar]
- Wang J. S., Li X., Chen Z. L., Feng J. L., Bao B. H., Deng S., Dai H. H., Meng F. C., Wang B., Li H. S. (2021). Effect of leech-centipede medicine on improving erectile function in DIED rats via PKC signalling pathway-related molecules. Journal of Ethnopharmacology, 267, 113463. 10.1016/j.jep.2020.113463 [DOI] [PubMed] [Google Scholar]
- Wang L., Gao P., Zhang M., Huang Z., Zhang D., Deng Q., Li Y., Zhao Z., Qin X., Jin D., Zhou M., Tang X., Hu Y., Wang L. (2017). Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA, 317(24), 2515–2523. 10.1001/jama.2017.7596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Chen L. L., Yan H., Li J. C. (2009). Trichosanthin suppresses HeLa cell proliferation through inhibition of the PKC/MAPK signaling pathway. Cell Biology and Toxicology, 25(5), 479–488. 10.1007/s10565-008-9102-x [DOI] [PubMed] [Google Scholar]
- Williams M. D., Nadler J. L. (2007). Inflammatory mechanisms of diabetic complications. Current Diabetes Reports, 7(3), 242–248. 10.1007/s11892-007-0038-y [DOI] [PubMed] [Google Scholar]
- Wu J., Fang S., Lu K. T., Kumar G., Reho J. J., Brozoski D. T., Otanwa A. J., Hu C., Nair A. R., Wackman K. K., Agbor L. N., Grobe J. L., Sigmund C. D. (2022). Endothelial Cullin3 mutation impairs nitric oxide-mediated vasodilation and promotes salt-induced hypertension. Function (Oxford, England), 3(3), zqac017. 10.1093/function/zqac017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q., Wang D., Li D., Huang J., Ma F., Zhang H., Sheng Y., Zhang C., Ha X. (2023). Protein kinase C: A potential therapeutic target for endothelial dysfunction in diabetes. Journal of Diabetes and its Complications, 37(9), 108565. 10.1016/j.jdiacomp.2023.108565 [DOI] [PubMed] [Google Scholar]
- Yadav A., Mishra R. K. (2023). Sub-chronic restraint stress suppresses sexual potency and erection efficiency by targeting the hypothalamic-pituitary-testicular axis and the nitric oxide/cyclic guanosine monophosphate/phosphodiesterase 5α pathway in adult rats. Neuroendocrinology, 113(4), 442–456. 10.1159/000528131 [DOI] [PubMed] [Google Scholar]
- Zachary I., Gliki G. (2001). Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovascular Research, 49(3), 568–581. 10.1016/s0008-6363(00)00268-6 [DOI] [PubMed] [Google Scholar]