Abstract
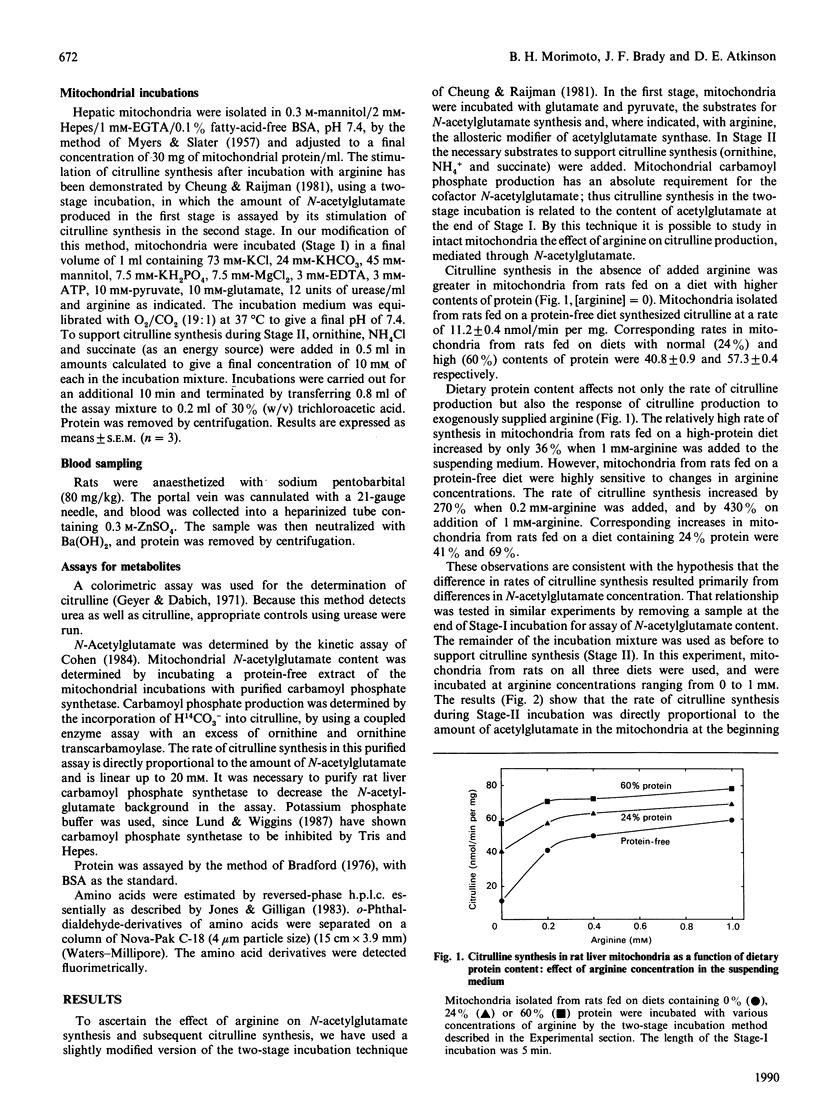
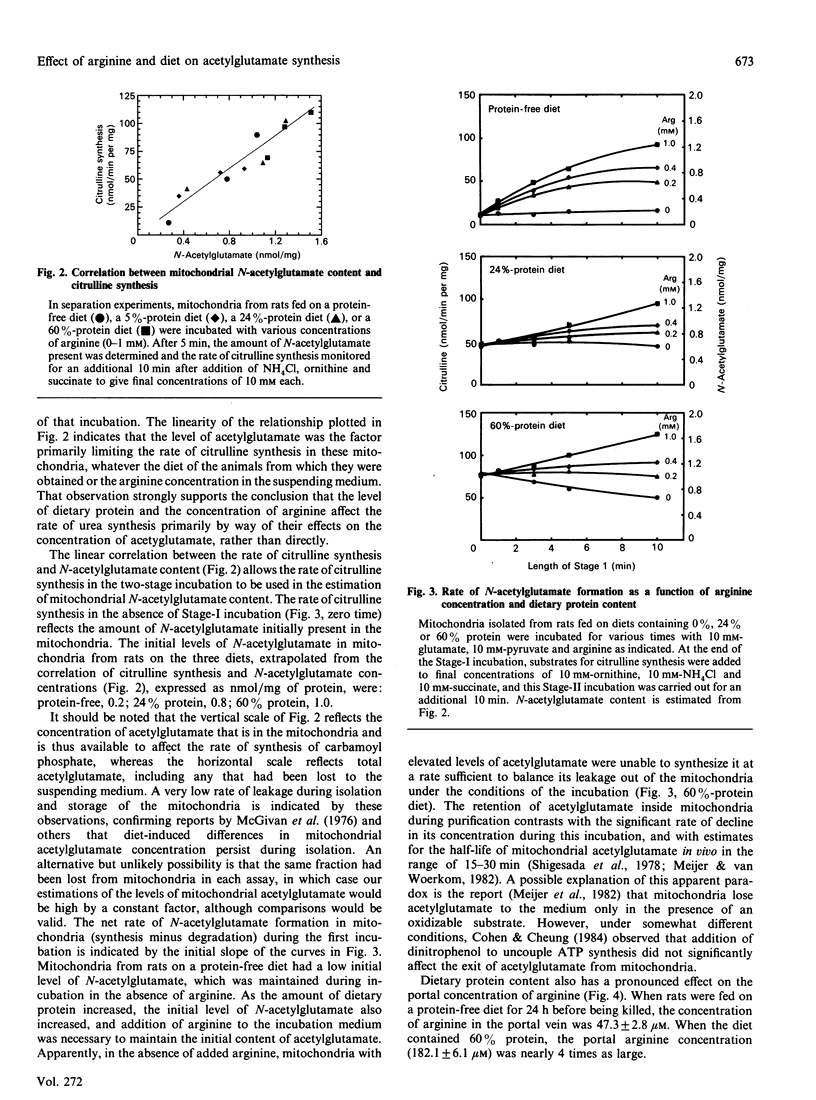
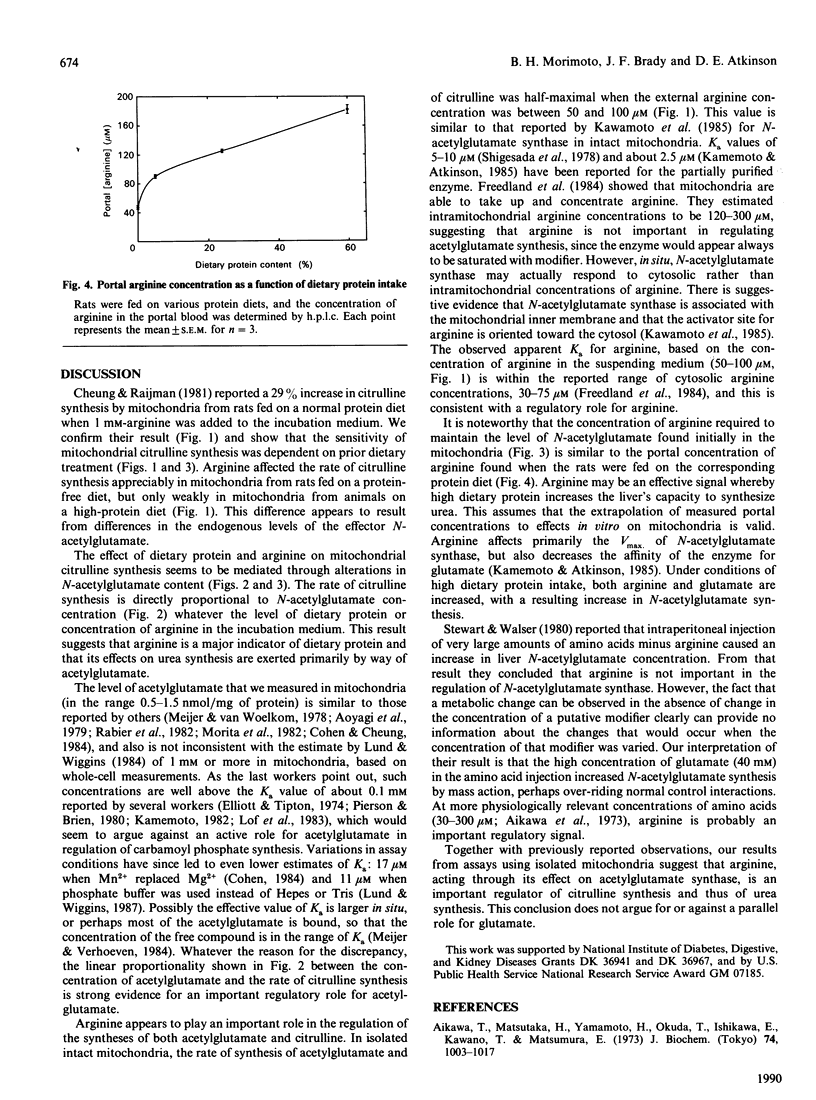
Increases in dietary protein have been reported to increase the rate of citrulline synthesis and the level of N-acetylglutamate in liver. We have confirmed this effect of diet on citrulline synthesis in rat liver mitochondria and show parallel increases in N-acetylglutamate concentration. The magnitude of the effect of arginine in the suspending medium on citrulline synthesis was also dependent on dietary protein content. Mitochondria from rats fed on a protein-free diet initially contained low levels of N-acetylglutamate, and addition of arginine increased the rate of its synthesis. Citrulline synthesis and acetylglutamate content in these mitochondria increased more than 5-fold when 1 mM-arginine was added. A diet high in protein results in mitochondria with increased N-acetylglutamate and a high rate of citrulline synthesis; 1 mM-arginine increased citrulline synthesis in such mitochondria by only 36%. The concentration of arginine in portal blood was 47 microM in rats fed on a diet lacking protein, and 182 microM in rats fed on a diet containing 60% protein, suggesting that arginine may be a regulatory signal to the liver concerning the dietary protein intake. The rates of citrulline synthesis were proportional to the mitochondrial content of acetylglutamate in mitochondria obtained from rats fed on diets containing 0, 24, or 60% protein, whether incubated in the absence or presence of arginine. Although the effector concentrations are higher than the Ka for the enzymes, these results support the view that concentrations of both arginine and acetylglutamate are important in the regulation of synthesis of citrulline and urea. Additionally, the effects of dietary protein level (and of arginine) are exerted in large part by way of modulation of the concentration of acetylglutamate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa T., Matsutaka H., Yamamoto H., Okuda T., Ishikawa E. Gluconeogenesis and amino acid metabolism. II. Inter-organal relations and roles of glutamine and alanine in the amino acid metabolism of fasted rats. J Biochem. 1973 Nov;74(5):1003–1017. [PubMed] [Google Scholar]
- Aoyagi K., Mori M., Tatibana M. Inhibition of urea synthesis by pent-4-enoate associated with decrease in N-acetyl-L-glutamate concentration in isolated rat hepatocytes. Biochim Biophys Acta. 1979 Nov 1;587(4):515–521. doi: 10.1016/0304-4165(79)90005-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheung C. W., Raijman L. Arginine, mitochondrial arginase, and the control of carbamyl phosphate synthesis. Arch Biochem Biophys. 1981 Jul;209(2):643–649. doi: 10.1016/0003-9861(81)90324-6. [DOI] [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W. Differential effects of N-acetylglutamate on citrulline synthesis by coupled and uncoupled mitochondria. Arch Biochem Biophys. 1984 Oct;234(1):31–44. doi: 10.1016/0003-9861(84)90321-7. [DOI] [PubMed] [Google Scholar]
- Cohen N. S. N-acetylglutamate-independent activity of carbamyl phosphate synthetase (ammonia): implications for the kinetic assay of acetylglutamate. Arch Biochem Biophys. 1984 Jul;232(1):38–46. doi: 10.1016/0003-9861(84)90519-8. [DOI] [PubMed] [Google Scholar]
- Elliott K. R., Tipton K. F. Kinetic studies of bovine liver carbamoyl phosphate synthetase. Biochem J. 1974 Sep;141(3):807–816. doi: 10.1042/bj1410807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland R. A., Crozier G. L., Hicks B. L., Meijer A. J. Arginine uptake by isolated rat liver mitochondria. Biochim Biophys Acta. 1984 Dec 20;802(3):407–412. doi: 10.1016/0304-4165(84)90357-x. [DOI] [PubMed] [Google Scholar]
- Geyer J. W., Dabich D. Rapid method for determination of arginase activity in tissue homogenates. Anal Biochem. 1971 Feb;39(2):412–417. doi: 10.1016/0003-2697(71)90431-3. [DOI] [PubMed] [Google Scholar]
- Jones B. N., Gilligan J. P. o-Phthaldialdehyde precolumn derivatization and reversed-phase high-performance liquid chromatography of polypeptide hydrolysates and physiological fluids. J Chromatogr. 1983 Aug 26;266:471–482. doi: 10.1016/s0021-9673(01)90918-5. [DOI] [PubMed] [Google Scholar]
- Kamemoto E. S., Atkinson D. E. Modulation of the activity of rat liver acetylglutamate synthase by pH and arginine concentration. Arch Biochem Biophys. 1985 Nov 15;243(1):100–107. doi: 10.1016/0003-9861(85)90777-5. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Sonoda T., Ohtake A., Tatibana M. Stimulatory effect of arginine on acetylglutamate synthesis in isolated mitochondria of mouse and rat liver. Biochem J. 1985 Dec 1;232(2):329–334. doi: 10.1042/bj2320329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lof C., Cohen M., Vermeulen L. P., van Roermund C. W., Wanders R. J., Meijer A. J. Properties of carbamoyl-phosphate synthetase (ammonia) in rat-liver mitochondria made permeable with toluene. Eur J Biochem. 1983 Sep 15;135(2):251–258. doi: 10.1111/j.1432-1033.1983.tb07645.x. [DOI] [PubMed] [Google Scholar]
- Lund P., Wiggins D. Inhibition of carbamoyl-phosphate synthase (ammonia) by Tris and Hepes. Effect on Ka for N-acetylglutamate. Biochem J. 1987 Apr 1;243(1):273–276. doi: 10.1042/bj2430273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund P., Wiggins D. Is N-acetylglutamate a short-term regulator of urea synthesis? Biochem J. 1984 Mar 15;218(3):991–994. doi: 10.1042/bj2180991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS D. K., SLATER E. C. The enzymic hydrolysis of adenosine triphosphate by liver mitochondria. I. Activities at different pH values. Biochem J. 1957 Dec;67(4):558–572. doi: 10.1042/bj0670558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivan J. D., Bradford N. M., Mendes-Mourão J. The regulation of carbamoyl phosphate synthase activity in rat liver mitochondria. Biochem J. 1976 Feb 15;154(2):415–421. doi: 10.1042/bj1540415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer A. J., Van Woerkom G. M. Turnover of N-acetylglutamate in isolated rat hepatocytes. Biochim Biophys Acta. 1982 Nov 17;721(3):240–246. doi: 10.1016/0167-4889(82)90075-1. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., Van Woerkom G. M., Wanders R. J., Lof C. Transport of N-acetylglutamate in rat-liver mitochondria. Eur J Biochem. 1982 May 17;124(2):325–330. doi: 10.1111/j.1432-1033.1982.tb06595.x. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., Verhoeven A. J. N-acetylglutamate and urea synthesis. Biochem J. 1984 Oct 15;223(2):559–560. doi: 10.1042/bj2230559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer A. J., van Woerkom G. M. Control of the rate of citrulline synthesis by short-term changes in N-acetylglutamate levels in isolated rat-liver mitochondria. FEBS Lett. 1978 Feb 1;86(1):117–121. doi: 10.1016/0014-5793(78)80111-2. [DOI] [PubMed] [Google Scholar]
- Morita T., Mori M., Tatibana M. Regulation of N-acetyl-L-glutamate degradation in mammalian liver. J Biochem. 1982 Feb;91(2):563–569. doi: 10.1093/oxfordjournals.jbchem.a133728. [DOI] [PubMed] [Google Scholar]
- Pierson D. L., Brien J. M. Human carbamylphosphate synthetase I. Stabilization, purification, and partial characterization of the enzyme from human liver. J Biol Chem. 1980 Aug 25;255(16):7891–7895. [PubMed] [Google Scholar]
- Powers S. G. Regulation of rat liver carbamyl phosphate synthetase I. Inhibition by metal ions and activation by amino acids and other chelating agents. J Biol Chem. 1981 Nov 10;256(21):11160–11165. [PubMed] [Google Scholar]
- Rabier D., Briand P., Petit F., Parvy P., Kamoun P., Cathelineau L. Acute effects of glucagon on citrulline biosynthesis. Biochem J. 1982 Sep 15;206(3):627–631. doi: 10.1042/bj2060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki T., Katsunuma T., Sase M. Regulation of urea synthesis in rat liver. Changes of ornithine and acetylglutamate concentrations in the livers of rats subjected to dietary transitions. J Biochem. 1977 Aug;82(2):551–558. [PubMed] [Google Scholar]
- Shigesada K., Aoyagi K., Tatibana M. Role of acetylglutamate in ureotelism. Variations in acetylglutamate level and its possible significance in control of urea synthesis in mammalian liver. Eur J Biochem. 1978 Apr 17;85(2):385–391. doi: 10.1111/j.1432-1033.1978.tb12250.x. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Chock P. B. Interconvertible enzyme cascades in metabolic regulation. Curr Top Cell Regul. 1978;13:53–95. doi: 10.1016/b978-0-12-152813-3.50007-0. [DOI] [PubMed] [Google Scholar]
- Stewart P. M., Walser M. Short term regulation of ureagenesis. J Biol Chem. 1980 Jun 10;255(11):5270–5280. [PubMed] [Google Scholar]


