Abstract
Introduction
Air pollution is widely acknowledged as a significant factor in respiratory outcomes, including coughing, wheezing, emergency department (ED) visits, and even death. Although several literature reviews have confirmed the association between air pollution and respiratory outcomes, they often did not standardize associations across different studies and overlooked other increasingly impactful pollutants such as trace metals. Recognizing the importance of consistent comparison and emissions of non-exhaust particles from road traffic, this study aims to comprehensively evaluate the standardized effects of various criteria pollutants and trace metals on respiratory health.
Methods
We conducted a comprehensive meta-analysis of peer-reviewed journal articles on air pollution and respiratory outcomes published between 1 January 2000, and 1 June 2024. The study included children (age < 18 years), adults (age ≥ 18 years), and all age groups exposed to criteria pollutants established by the US Environmental Protection Agency National Ambient Air Quality Standards and over 10 trace metals. Using databases, such as PubMed, MEDLINE, Web of Science Core Collection, and Google Scholar, we identified 579 relevant articles. After rigorous screening and quality assessment using the Newcastle-Ottawa Scale, 50 high-quality studies were included. We converted various reported outcomes (e.g., odds ratios, relative risk, and percent increase) to a standardized odds ratio (OR) for comparability and performed meta-analyses using R 4.4.0 and related packages, ensuring the robustness of our findings.
Results
Our meta-analysis indicated significant associations between air pollutants and respiratory outcomes. For particulate matter with diameter ≤ 2.5 μm (PM2.5), the overall ORs for children, adults, and combined age groups were 1.31, 1.10, and 1.26, respectively, indicating a consistent positive association. Similar positive associations were observed for particulate matter with diameter ≤ 10 μm (PM10) and other pollutants, with children showing higher susceptibility than adults. The analysis of trace metals also showed significant associations; however, these findings require cautious interpretation due to the small number of studies.
Conclusion
Our study supports associations between air pollutants, including non-exhaust trace metals, and respiratory outcomes across different age groups. The findings underscore the need for stringent environmental health policies and further research, especially in regions with higher pollution levels. The future studies should consider long-term and short-term exposures separately and include diverse populations to improve the accuracy and generalizability of the results.
Keywords: air pollutants, trace metals, respiratory disease, meta-analysis, public health
1. Introduction
It is well established that air pollution is associated with respiratory disease outcomes, such as coughing (1–3), wheezing (4–7), emergency department (ED) visits (8, 9), and even death (10–14). Some literature reviews (3, 8, 13, 15–17) have been conducted on those associations, and they essentially confirmed the associations of air pollution exposure with respiratory disease outcomes. However, these literature reviews did not assess many pollutants, such as trace metals that can be considered toxic air contaminants, nor did they assess associations found in different studies on the same scale.
Past analyses typically used different interquartile range increments in assessing an association, making the impact size challenging to compare across studies. Some studies used odds ratios (OR) of an outcome occurring (2–7) while others used percent increase (1, 14) or relative risk (RR) in assessing the association of air pollution exposure with respiratory outcomes (8, 15).
It is well recognized that on-road vehicle traffic contributes significantly to air pollution locally and regionally. On-road vehicles contribute to ambient air pollution from engine emissions, automobile brakes, tire wear, and dust from road surfaces (18). Non-exhaust particles are generated from brakes, tires, clutch, and road surface sources. In areas with higher traffic density and more braking frequency, brake wear is a significant particulate matter (PM) contributor among all non-exhaust pollutant sources (19). Another PM source is road surface wear. Furthermore, particles that already exist in the form of debris at the roadside get resuspended due to the turbulence created by on-road vehicular traffic and contribute to ambient PM generation. Previous studies show over half of the mean PM2.5 (PM with diameter < 2.5 μm) is a cumulative result of on-road motor vehicles (42%) and road dust (12%) (20).
Due to the increasing implementation of tailpipe emission regulations, non-exhaust emissions from tire and brake wear have become increasingly important. The fraction of PM2.5 from non-exhaust sources is expected to increase as electric vehicles replace internal combustion vehicles. Studies have shown that even with zero exhaust emissions from on-road vehicles, traffic will continue contributing to PM10 (PM with diameter < 10 μm) and PM2.5 through non-exhaust emissions (19). Some studies have shown that air pollution from tire and brake wear increases asthma morbidity and mortality risks (10, 21).
Air pollution’s significant health impacts align with the sustainable development goals (SDGs). SDG 3 aims to promote the health and well-being of those directly affected by air pollution contributing to respiratory diseases, such as asthma and lung cancer. The World Health Organization (WHO) highlights that air pollution causes millions of premature deaths annually (22). Furthermore, SDG 12 targets reducing emissions of hazardous chemicals, including criteria pollutants such as PM and toxic trace metals such as lead and mercury, which have severe health impacts. Achieving these goals necessitates stronger air quality regulations, improved monitoring, and the adoption of cleaner technologies across industries and transportation sectors (23). By assessing the effects of criteria pollutants and trace metals on respiratory health through standardized meta-analysis, we contribute to broader efforts aimed at mitigating air pollution’s adverse effects and advancing global health and environmental sustainability goals.
Therefore, it is essential to understand the association between criteria pollutants and trace metals and respiratory disease, where such knowledge is currently limited. In this study, we performed a meta-analysis to assess the effect sizes of the associations between exposures to criteria pollutants, including nitrogen dioxide (NO2), PM2.5, PM10, ozone (O3), sulfur dioxide (SO2), and more than 10 trace metals that can be considered toxic air contaminants and respiratory disease outcomes to shed light on comparative risk.
2. Materials and methods
2.1. Selection of articles for review and data extraction
We conducted a systematic literature review using peer-reviewed journal articles, to expand the literature cited in the background section of this study on the impacts of on-road vehicle emissions, including on-road non-exhaust pollutants, on subacute respiratory disease symptoms. The following interconnected steps were used to complete the review:
-
Determine inclusion criteria that will include the following:
Study population of children (age 18 years), adults (age 18 years), and all age groups.
Study intervention for individuals (1) exposed to air pollution, including NO2, PM2.5, PM10, O3, and SO2, and (2) more than 10 trace metals are considered toxic air contaminants (including aluminum, iron, magnesium, sulfur, nickel, vanadium, chromium, arsenic, manganese, barium, copper, antimony, zinc, and lead).
Study outcomes for respiratory disease in asthma and chronic obstructive pulmonary disease (COPD), such as coughing, wheezing, shortness of breath, ED visits, hospitalizations, exacerbations and mortality, respiratory infections, and lung cancer.
-
Identify the publication’s characteristics for studies:
Published in peer-reviewed journals;
Published between 1 January 2000 and 1 June 2024;
Written in English.
-
Select the proper search databases and engines, including
PubMed;
Medline;
Web of Science Core Collection; and
Google Scholar.
Decide the search terms and selection process.
Table 1 summarizes pollutant categories and search criteria considered for this literature review.
Table 1.
Literature review categories and search terms.
| Category | Search terms |
|---|---|
| Air pollutants | PM2.5, PM10, NO2, O3, SO2, and trace metals |
| Disease | Asthma, COPD, and respiratory disease |
| Years of publication | From 1 January 2000 to 1 June 2024 |
| Publication type | Peer-reviewed journals |
| Publication language | English |
COPD, chronic obstructive pulmonary disease; PM, particulate matter.
The following steps were used to select scientific publications for the literature review:
Use one term from each category and combine them together (+) to create integrated search terms using the search databases and engines listed above.
Merge together the selected publications and remove the duplicates.
Obtain abstracts for the remaining publications selected from Step 2, screen and remove the publications that are not related to the research topic.
Obtain full text for the remaining publications selected from Step 3, screen and remove the publications that are not related to the topic.
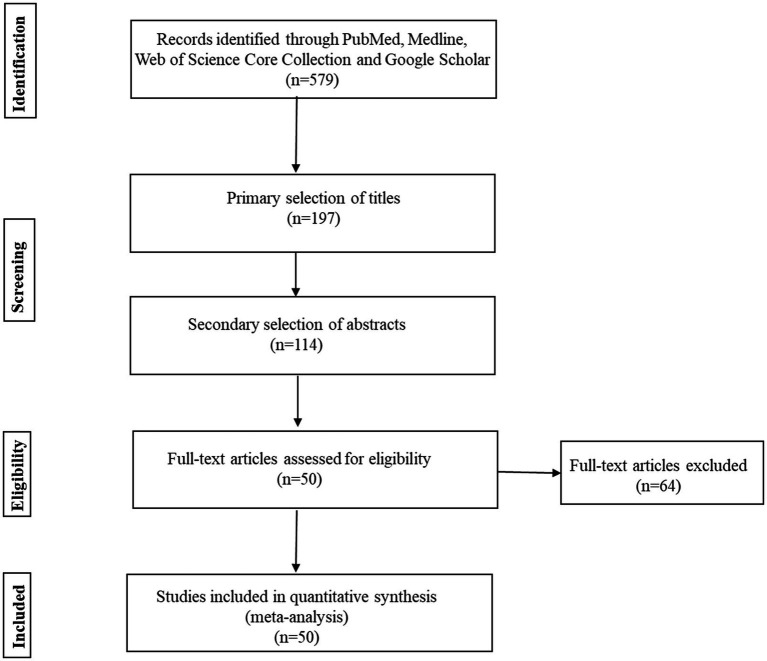
We identified 579 articles considering the search criteria given in Table 1. After screening titles, 197 articles were selected for an abstract search. We evaluated the abstract of each article and selected 114 articles for full-text screening. After checking the risk of bias using the Newcastle–Ottawa scale (NOS) (24), 50 articles were rated as good or high quality (6–9 score; Supplementary Table S37) and included in the final review.
Two independent researchers reviewed the publications and extracted the information. The data of first author, year, location, air pollutant, study design, sample size, study group, and outcome were collected. We investigated the effect of increased exposure to different criteria pollutants, including PM2.5, PM10, NO2, O3, SO2, and trace metals, including aluminum, iron, magnesium, sulfur, nickel, vanadium, chromium, arsenic, manganese, barium, copper, antimony, zinc, and lead. For health outcome measures, we included coughing, wheezing, shortness of breath, ED visits, hospitalizations, exacerbations, and mortality for respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD), respiratory infections, and lung cancer.
2.2. Conversion of outcomes to OR
Not all studies in this systematic review reported the results on the same scale. Estimated outcomes associated with increased exposure to air pollutants are reported in terms of odds ratio (OR), relative risk (RR) or percentage increase (%), and 95% confidence intervals (CI). We standardized all reported results by converting them to odds ratios (ORs) with corresponding confidence intervals (CIs) to facilitate comparison across different studies. By aggregating these standardized effects, we determined the overall impact of each pollutant on respiratory disease outcomes. Since the exposure interval differs for each metal, we did not standardize the reported OR/RR and 95% CI, and they were presented in the same way as reported in the corresponding studies.
To convert the results reported in percentage increase to OR, we exponentiated the reported results and lower/upper CI values from the corresponding studies, as presented in the following set of formulas:
Also, to convert the results reported in RR to OR, the following conversion was used (25):
Where risk0 is the risk of having a positive outcome in the control or unexposed group; similarly, the associated lower and upper C.I.s can be calculated as follows:
2.3. Standardization of exposure increase ranges
Furthermore, not all studies used the same exposure interval to assess the association with respiratory diseases. We standardized all the reported results so that the results were comparable between various studies. To make the results similar between different studies, we standardized the ORs through the following conversions:
where OR(xs) is the standardized OR for pollutant x when its exposure interval is set at xs; xg and OR(xg) represent the standardized exposure interval and associated OR. Based on the potential exposure levels from current studies, we used interquartile ranges of 10 ppb, 10 μg m−3, 10 μg m−3, 10 ppb, and 30 ppb, respectively, for NO2, PM2.5, PM10, SO2, and O3 in standardizing exposure level.
2.4. Statistical analysis
We excluded the studies deemed significant outliers, such as OR greater than 4.0. After standardizing the reported results from studies, effect pooled analysis was performed for each pollutant in three different age categories, including children ( 18 years), adults ( 18 years), and all ages. The R Foundation for Statistical Computing (R 4.4.0) and “outliers,” “metafor” and “meta” packages were used for meta-analysis of the effects, separately, for children, adults, and all ages, for NO2, PM2.5, PM10, SO2, O3, and trace metals. Studies with mixed ages were evaluated separately from those studies focused on just children or adults to avoid the potentially repeated counting of children and adults in the all age category (i.e., we did not pool the effects from children, adults, and all age groups to form a category of all subjects). We conducted a sensitivity analysis to explore the robustness of the meta-analysis results. In this process, we recalculated the overall homogeneity and effect size by systematically removing one study at a time. The heterogeneity was tested using Cochran’s Q test (26, 27) and the I2 value (28, 29). When I2 ≤ 50%, the studies were considered homoscedastic, and the fixed effect model of the meta-analysis was used; when I2 > 50%, the studies were deemed heteroscedastic, and the random effect model of the meta-analysis was used. Publication bias was tested using a regression-based Egger test and funnel plot (30). The significance level was p < 0.05.
3. Results
3.1. Search results and study descriptions
We identified 579 articles considering the search criteria through databases. After screening titles, 197 articles were selected for an abstract search. We screened the abstract of each article and selected 114 articles for full-text assessment. After the full-text assessment, 50 articles were included in the final review and quantitative synthesis (Figure 1).
Figure 1.
Flow diagram summarizing study selection.
The studies were conducted in multiple countries as follows: Australia (1), Belgium (1), Canada (3), China (12), multiple countries in Europe (2), Germany (1), India (1), Iran (2), Italy (4), Israel (1), Japan (3), Mexico (1), The Netherlands (1), Spain (1), South Korea (1), Taiwan (2), Thailand (1), Turkey (1), United Kingdom (1), and United States (12). Among 50 studies,13 studies included children (<18 years), 19 studies included adults (≥18 years), 16 studies included all ages, 2 studies included children (<18 years) and adults (≥18 years), and 1 study included children (<18 years), adults (≥18 years), and all ages (Table 2).
Table 2.
Characteristics of 50 studies included in the meta-analysis.
| First author | Year | Location | Air pollutant | Study design | Sample size | Study group | Outcome |
|---|---|---|---|---|---|---|---|
| De Marco (37) | 2002 | Italy | NO2 | Cross-sectional survey | 18,873 | Adults | Asthma attack, chest tightness, wheezing |
| Sunyer (38) | 2002 | Spain | NO2 | Case-crossover design | 1,078 | All | Mortality due to asthma |
| Gent (39) | 2003 | USA | O3 | Prospective study | 271 | Children | Chest tightness(1 h), Shortness of breath (1 h), Chest tightness(8-h), Shortness of breath (8 h) |
| Mar (2) | 2004 | USA | PM2.5, PM10 | Unknown | 25 | children, adults | Trouble breathing, coughing, sputum production, wheezing |
| Migliaretti (40) | 2004 | Italy | NO2 | Case–control study | 1,060 | Children | Asthma hospitalizations |
| Analitis (41) | 2006 | Europe | PM10 | Unknown | Unknown | All | Respiratory mortality |
| Brauer (5) | 2007 | Netherlands | PM2.5 | PIAMA prospective birth cohort study | ~ 4,000 | Children | Wheezing, doctor-diagnosed asthma, ear/nose/throat infections, flu |
| Smargiassi (42) | 2009 | Canada | SO2 | Time-stratified case-crossover | 3,469 | Children | Asthma emergency department visits, asthma hospitalization |
| Kan (43) | 2010 | Thailand, China | SO2 | Short-term association study | Unknown | Adults | Respiratory mortality |
| Silverman (44) | 2010 | USA | O3 | Unknown | 75,383 | Adults | Asthma HA |
| Weinmayr (3) | 2010 | Germany/Italy | PM10, NO2 | Meta-analysis | 36 | Children | Asthma symptoms, coughing, asthma symptoms |
| Katanoda (45) | 2011 | Japan | PM2.5, NO2, SO2 | Prospective cohort study | 63,520 | All | Mortality due to lung cancer and respiratory diseases |
| Takenoue (6) | 2012 | Japan | NO2 | Meta-analysis | 12 | Children | Asthma development, wheezing |
| Belanger (4) | 2013 | USA | NO2 | Prospective, year-long study | 1,342 | Children | Asthma severity, wheezing, night symptoms due to asthma, medication use due to asthma |
| Kloog (12) | 2013 | USA | PM2.5 | Short-term exposure | 46,8,570 | All | PM-related mortality |
| Raaschou-Nielsen (46) | 2013 | Europe | PM2.5, PM10 | Cohort study | 312,944 | All | Lung cancer, adenocarcinoma |
| Cortez-Lugo (1) | 2015 | Mexico | PM2.5 | Questionnaire | 29 | Adults | COPD cough, COPD phlegm |
| Fan (8) | 2016 | China | PM2.5 | Meta-analysis | 16 | Children, adults, all | Asthma ED |
| Ghozikali (47) | 2016 | Iran | NO2, O3, SO2 | Unknown | Unknown | All | COPD hospitalizations, COPD hospitalization, COPD HA |
| Greenberg (48) | 2016 | Israel | SO2 | Exposure assessment | 137,040 | Children | Asthma severity |
| Hasunuma (49) | 2016 | Japan | NO2 | Nested case–control study | 853 (case),3,409 (control) | Children | Persistence of asthmatic symptoms |
| Mirabelli (50) | 2016 | USA | PM2.5 | Call-back survey | 50,356 | Adults | Any asthma symptoms |
| Pollitt (51) | 2016 | Canada | Aluminum, iron, magnesium, sulfur, nickel, vanadium, Chromium, arsenic, manganese, barium, copper, antimony, zinc | Questionnaire | 217 | Children | Airway inflammation due to asthma |
| Li (15) | 2016a | China | NO2, SO2 | Meta-analysis | 59 | All | COPD exacerbations |
| Li (52) | 2016b | China | SO2 | Unknown | 10,095 | Adults | COPD mortality |
| Day (53) | 2017 | China | O3 | Longitudinal study | 89 | Adults | Pulmonary inflammation |
| Khaniabadi (54) | 2017 | Iran | O3 | Unknown | Unknown | All | Cardiopulmonary mortality, COPD hospitalization |
| Lamichhane (32) | 2018 | India | PM2.5, PM10, NO2 | Unknown | 1,264 | Adults | Reduced lung function due to COPD |
| Magzamen (55) | 2018 | USA | PM10, NO2 | Unknown | 35 | Adults | Inhaler use due to COPD |
| Mao (34) | 2018 | China | Copper, iron | Case–control study | 33 | All | Asthma susceptibility |
| Hansel (56) | 2019 | USA | PM2.5 | Longitudinal study | 484 | Children | Uncontrolled asthma |
| Huang (57) | 2019 | Taiwan | PM2.5 | Exposure assessment | 3,941 | Adults | Susceptibility to COPD |
| Wu (7) | 2019 | USA | Lead | Cross-sectional, population-based study | 5,866 | Children | Risk for active asthma, wheezing |
| Mercan (58) | 2020 | Turkey | SO2 | Unknown | 710 | Adults | Asthma hospitalization, COPD hospitalization |
| Pepper (59) | 2020 | USA | O3 | Randomized controlled trial | 287 | Children, adults | Asthma rescue inhaler use |
| Yu (14) | 2020 | Australia | PM2.5 | Meta-analysis | 242,320 | all | Respiratory mortality |
| Shin (60) | 2021 | Canada | PM2.5, PM10, NO2 | Population-based study | 558,738 | Adults | COPD |
| Yan (61) | 2022 | China | PM2.5 | Retrospective | 39,054 | All | Chronic bronchitis, asthma, COPD, chronic respiratory disease |
| Mebrahtu (62) | 2023 | UK | PM2.5, PM10, NO2 | Retrospective | 124,808 | All | Respiratory illnesses |
| Aron (63) | 2023 | USA | PM2.5 | Time stratified case-crossover | 19,243 | Adults | COPD |
| Kangas (64) | 2023 | Belgium | PM2.5 | Census | 437,340 | Adults | Asthma |
| Marchetti (65) | 2023 | Italy | PM2.5, PM10, NO2 | Multi case–control | 16,173 | Adults | Rhinitis, COPD |
| Wang (66) | 2023 | Taiwan | Arsenic | Cross-sectional | 1,563 | Adults | Lung fibrotic, bronchiectasis |
| Yang (67) | 2024 | China | O3 | Retrospective | 4,574 | all | COPD |
| Stowell (68) | 2024 | USA | O3 | One state case-crossover | 111,71,779 | Children | Allergy, asthma, respiratory disorders, respiratory infections |
| Yu (69) | 2024 | China | Cu, Zn, Se | Survey | 2,807 | Adults | COPD, emphysema, tracheitis |
| Kwon (70) | 2024 | South Korea | NO2 | Survey | 22,387 | Adults | COPD |
| Zhang (71) | 2024 | China | O3 | Prospective cohort | 10,973 | Adults | Asthma |
| Zheng (72) | 2024 | China | PM2.5, PM10 | Multistage probability based | 7,371 | All | Asthma, wheezing, dyspnea |
| Xing (73) | 2024 | China | O3 | Population-based study | 6,537 | all | COPD |
COPD, chronic obstructive pulmonary disease; ED, emergency department; HA, hospital admission; PM, particulate matter; PIAMA, prevention and incidence of asthma and mite allergy.
3.2. Effects of PM2.5 and PM10 exposure increase
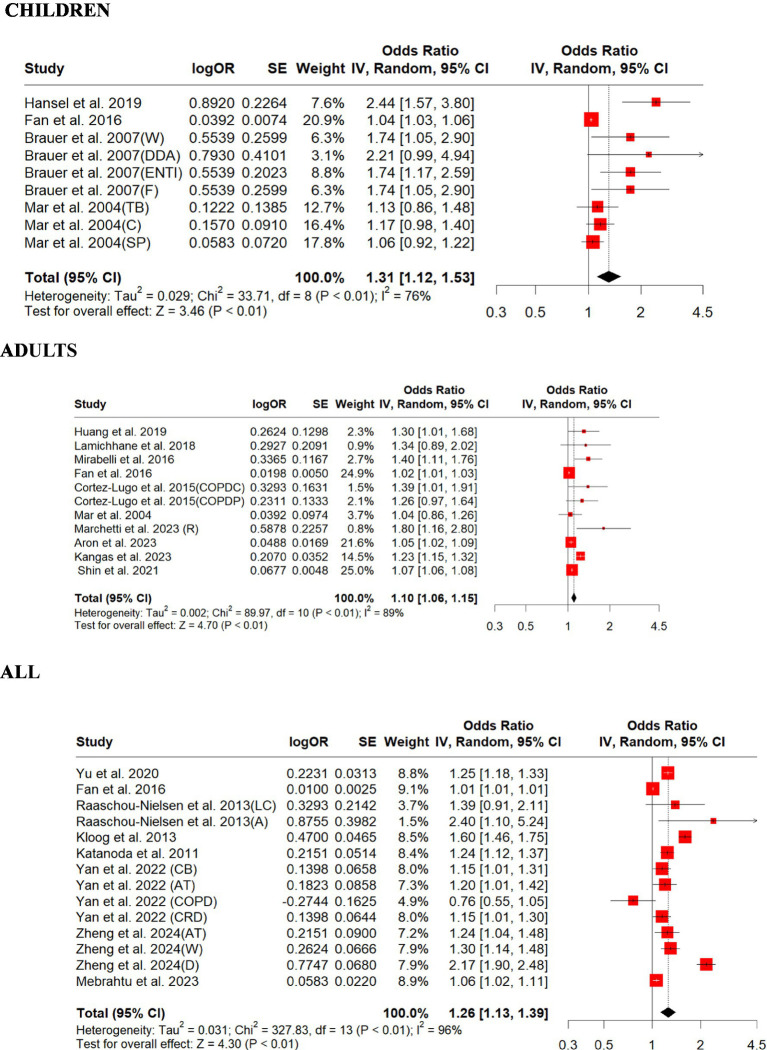
We included 12 PM2.5 and 6 PM10 studies in this review and summarized their findings here. Our standardized effect estimates showed that the associations (ORs) of PM2.5 with respiratory outcomes ranged from 0.76 to 2.44 per 10 μg/m3 increase in exposure (Figure 2). Children had significant overall OR (1.31, 95% CI: 1.12–1.53, p = 0.0005); adults also had significant overall OR (1.10, 95% CI: 1.06–1.15, p = 0.0001; Figure 2). For those studies that did not differentiate children from adults, the overall OR (1.26, 95% CI: 1.13–1.39, p = 0.0001) was significant (Figure 2). There was strong evidence of heterogeneity among these studies (children, Q = heterogeneity χ2 = 33.71, I2 = 76%, p = 0.0001; adults, Q = heterogeneity χ2 = 89.97, I2 = 89%, p = 0.0001; all, Q = heterogeneity χ2 = 327.83, I2 = 96%, p = 0.0001; Figure 2). Egger’s linear regression did not identify publication bias among the studies for adults (Egger’s: p = 0.087), but for children (Egger’s: p = 0.002) and for all (Egger’s: p = 0.003; Supplementary Figure S1). The children’s original model estimate is 0.2714, and the overall sensitivity analysis model estimate is slightly lower at 0.2497 (Supplementary Table S2). Both estimates indicate a positive association of PM2.5 with respiratory outcomes in children. The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain statistically significant regardless of which study is excluded. The original and overall models show consistent results, reinforcing the conclusion that PM2.5 exposure is associated with respiratory disease in children (Supplementary Table S1). The effect size estimate is 0.0960 for both the original and overall models, indicating a consistent positive association of PM2.5 with respiratory outcomes in adults (Supplementary Table S4). The original and overall models show consistent results, reinforcing the conclusion that PM2.5 exposure is associated with respiratory disease in adults (Supplementary Table S3). The effect size estimate is 0.2283 for the original model and slightly lower at 0.2186 for the overall sensitivity analysis model, indicating a consistent positive association of PM2.5 with respiratory disease across all age groups (Supplementary Table S6). The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain statistically significant regardless of which study is excluded. The original and overall models show consistent results, reinforcing the conclusion that PM2.5 exposure is associated with respiratory disease across all age groups (Supplementary Table S5).
Figure 2.
Forest plot of the estimated effects of PM2.5 on respiratory disease. A, adenocarcinoma; AT, asthma; C, coughing; CB, chronic bronchitis; COPDC, COPD cough; COPDP, COPD phlegm; CRD, chronic respiratory disease; D, dyspnea; DDA, doctor-diagnosed asthma; ENTI, ear/nose/throat infections; F, flu; LC, lung cancer; TB, trouble breathing; SP, sputum production; R, rhinitis; W, wheezing.
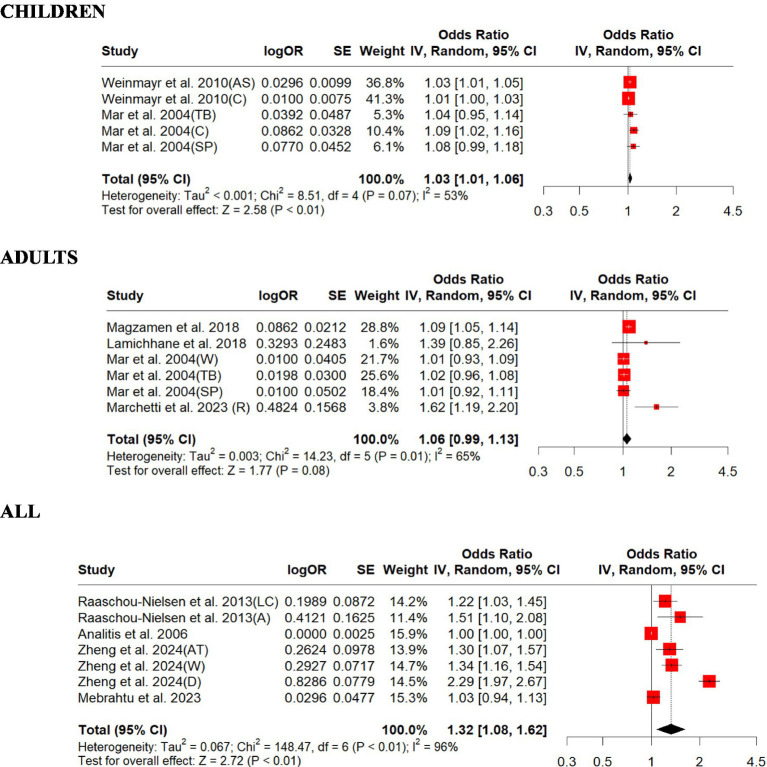
For PM10, the association (ORs) ranged from 1.00 to 2.29 per 10 μg m−3 increase in exposure. Children were found to have a relatively lower overall OR (1.03, 95% CI: 1.01–1.06, p = 0.01) than adults (1.06, 95% CI: 0.99–1.13, p = 0.07; Figure 3). For those studies that did not differentiate children from adults, the overall OR (1.32, 95% CI: 1.08–1.62, p = 0.006) was slightly higher than those for children and adults (Figure 3). There was strong evidence of homogeneity for children (children, Q = heterogeneity χ2 = 8.50, I2 = 53%, p = 0.07), but heterogeneity was found for those studies that did not differentiate all from adults (adults, Q = Heterogeneity χ2 = 14.22, I2 = 65%, p = 0.01; all, Q = heterogeneity χ2 = 148.47, I2 = 96%, p = 0.0001; Figure 3). Egger’s linear regression did identify publication bias among the studies for all (Egger’s: p = 0.04), and children (Egger’s: p = 0.11) but did not identify publication bias for adults (Egger’s: p = 0.46; Supplementary Figure S2). The effect size estimate is 0.0308 for both the original and overall models, indicating a consistent positive effect of PM10 on respiratory outcomes in children (Supplementary Table S8). The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain statistically significant regardless of which study is excluded. The original and overall models show consistent results, reinforcing the conclusion that PM10 exposure is associated with respiratory outcomes in children (Supplementary Table S7). The effect size estimate is 0.0576 for both the original and overall models, indicating a small positive effect of PM10 on respiratory outcomes in adults (Supplementary Table S10). The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain consistent regardless of which study is excluded. However, the original and overall models show that the effect of PM10 on respiratory outcomes in adults is not statistically significant, with confidence intervals that include zero and a p-value slightly above 0.05 (Supplementary Table S9). This suggests that further research might be needed to establish a clearer relationship. The effect size estimate is 0.2798 for both the original and overall models, indicating a moderate positive effect of PM10 on respiratory disease across all age groups (Supplementary Table S12). The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain consistent regardless of which study is excluded. The original and overall models show a statistically significant positive effect of PM10 on respiratory disease across all age groups, with moderate heterogeneity. This suggests a meaningful relationship between PM10 exposure and respiratory disease, warranting further attention and research (Supplementary Table S11).
Figure 3.
Forest plot of the estimated effects of PM10 on respiratory disease. A, adenocarcinoma; AS, asthma symptoms, AT, asthma; C, coughing, D, dyspnea; LC, lung cancer; R, rhinitis; SP, sputum production; TB, trouble breathing; W, wheezing.
3.3. Impacts from nitrogen dioxide exposure increase
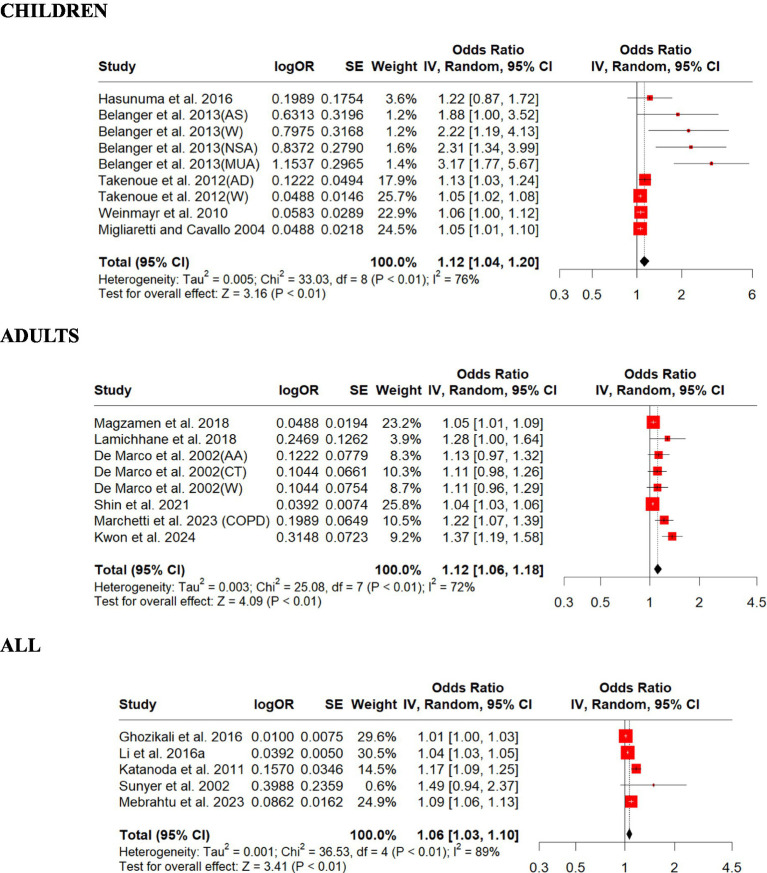
We included 12 studies on NO2 in this review and summarized their findings here. Our standardized estimates showed that NO2 associations (ORs) ranged from 1.01 to 3.17 per 10 ppb increase in exposure. Children were found to have a relatively lower overall OR (1.12, 95% CI: 1.04–1.20, p = 0.001) than adults (1.12, 95% CI: 1.06–1.18, p = 0.0001; Figure 4). The overall all age group OR (1.06, 95% CI: 1.03–1.10, p = 0.0016) was slightly higher than that of children and adults (Figure 4). There was strong evidence of heterogeneity among these studies (children, Q = heterogeneity χ2 = 33.03, I2 = 76%, p = 0.0001; adults, Q = heterogeneity χ2 = 25.08, I2 = 72%, p = 0.0001; all, Q = heterogeneity χ2 = 36.53, I2 = 89%, p = 0.0001; Figure 4). Egger’s linear regression did identify publication bias among the studies for adults (Egger’s: p = 0.008) and children (Egger’s: p = 0.0005) but did not identify publication bias for all (Egger’s: p = 0.24; Supplementary Figure S3). The effect size estimate is 0.1137 for the original model and slightly lower at 0.0911 for the overall sensitivity analysis model, indicating a positive effect of NO2 on respiratory disease in children (Supplementary Table S14). The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain statistically significant regardless of which study is excluded. The original and overall models show consistent results, reinforcing the conclusion that NO2 exposure is associated with respiratory disease in children (Supplementary Table S13). The effect size estimate is 0.1110 for both the original and overall models, indicating a positive effect of NO2 on respiratory disease in adults (Supplementary Table S16). The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain consistent regardless of which study is excluded. The original and overall models show a statistically significant positive effect of NO2 on respiratory disease in adults, with moderate heterogeneity. This suggests a meaningful relationship between NO2 exposure and respiratory disease, warranting further attention and research (Supplementary Table S15). The effect size estimate is 0.0614 for both the original and overall models, indicating a positive effect of NO2 on respiratory disease across all age groups (Supplementary Table S18). The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain consistent regardless of which study is excluded. The original and overall models show a statistically significant positive effect of NO2 on respiratory disease across all age groups, with moderate heterogeneity. This suggests a meaningful relationship between NO2 exposure and respiratory disease, warranting further attention and research (Supplementary Table S17).
Figure 4.
Forest plot of the estimated effects of NO2 on respiratory disease. AA, asthma attack; AD, asthma development; AS, asthma severity; CT, chest tightness; MUA. medication use due to asthma; NSA, night symptoms due to asthma; W, wheezing.
3.4. Impacts of ozone exposure increase
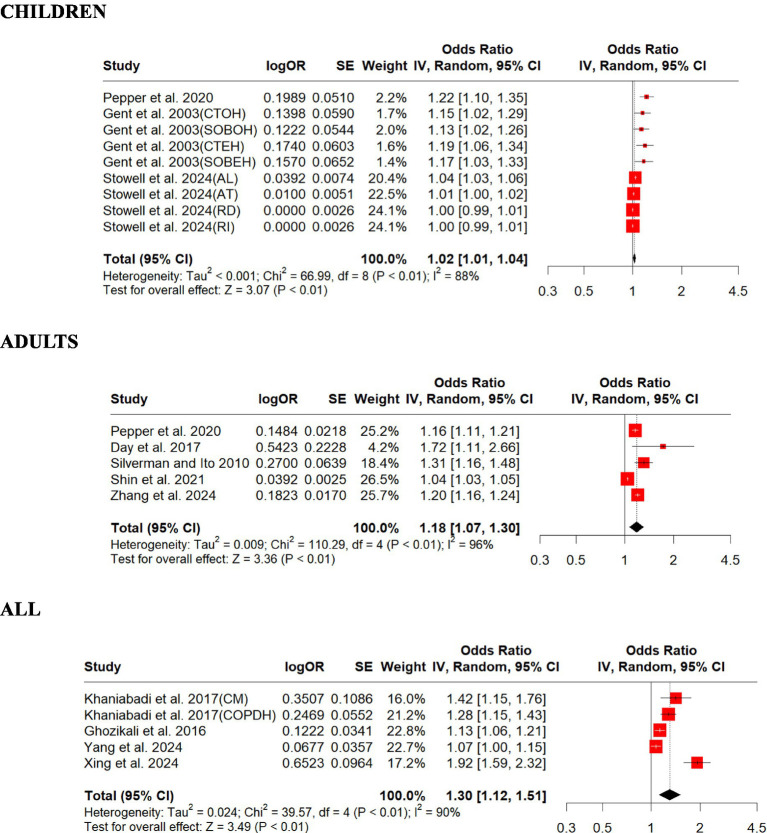
We included 6 studies on O3 in this review and summarized their findings here. Our standardized estimates showed that O3 associations (ORs) ranged from 1.00 to 1.92 per a 30-ppb increase in exposure. Children were found to have lower overall OR (1.02, 95% CI: 1.01–1.04, p = 0.001) compared to adults (1.18, 95% CI: 1.07–1.30, p = 0.001; Figure 5). For those studies that did not differentiate children from adults, all age overall OR (1.30, 95% CI: 1.12–1.51, p = 0.001) was slightly higher than those for children and adults (Figure 5). There was strong evidence of homogeneity among these studies (adults, Q = heterogeneity χ2 = 110.29, I2 = 96%, p = 0.09; all, Q = heterogeneity χ2 = 39.57, I2 = 90%, p = 0.05), but heterogeneity was found for children (children, Q = heterogeneity χ2 = 66.99, I2 = 88%, p = 0.0001; Figure 5). Egger’s linear regression did identify publication bias among the studies for all (Egger’s: p = 0.04) but did not identify publication bias for children (Egger’s: p = 0.5) and adults (Egger’s: p = 0.05; Supplementary Figure S4). The effect size estimate is 0.0244 for both the original and overall models, indicating a small positive effect of O3 on respiratory disease in children (Supplementary Table S20). The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain consistent regardless of which study is excluded. The original and overall models show a statistically significant positive effect of O3 on respiratory disease in children, with moderate heterogeneity. This suggests a meaningful relationship between O3 exposure and respiratory disease, warranting further attention and research (Supplementary Table S19). The effect size estimate is 0.1672 for both the original and overall models, indicating a positive effect of O3 on respiratory disease in adults (Supplementary Table S22). The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain consistent regardless of which study is excluded. The original and overall models show a statistically significant positive effect of O3 on respiratory disease in adults, with high heterogeneity. This suggests a meaningful relationship between O3 exposure and respiratory disease, warranting further attention and research (Supplementary Table S21). The effect size estimate is 0.2643 for both the original and overall models, indicating a positive effect of O3 on respiratory disease across all age groups (Supplementary Table S24). The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain consistent regardless of which study is excluded. The original and overall models show a statistically significant positive effect of O3 on respiratory disease across all age groups, with high heterogeneity. This suggests a meaningful relationship between O3 exposure and respiratory disease, warranting further attention and research (Supplementary Table S23).
Figure 5.
Forest plot of the estimated effects of O3 on respiratory disease. AL, allergy; AT, asthma; CM, cardiopulmonary mortality; COPDH, COPD hospitalization; CTEH, chest tightness (8 h); CTOH, chest tightness (1 h); RD, respiratory disorders; RI, respiratory infections; SOBEH, shortness of breath (8 h); SOBOH, shortness of breath (1 h).
3.5. Impacts from sulfur dioxide exposure increase
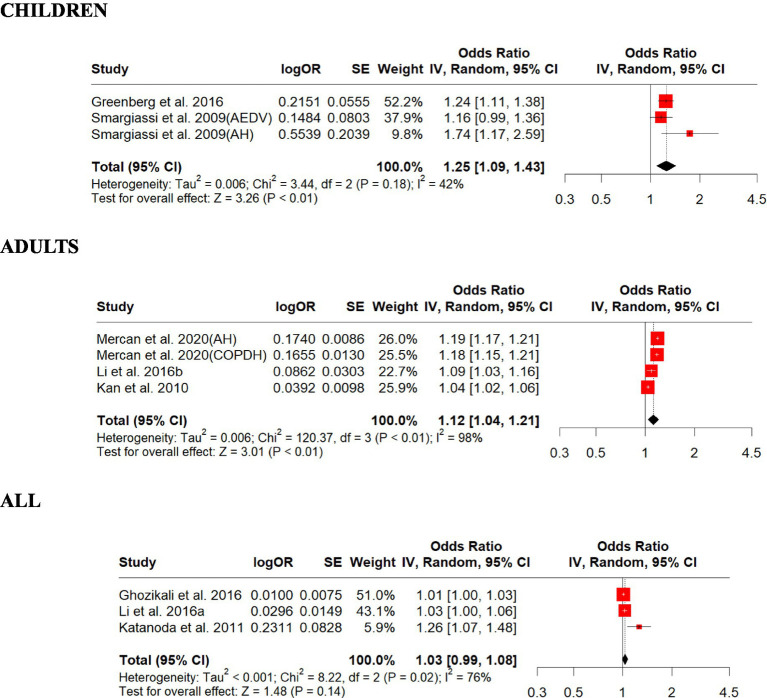
We included eight studies on SO2 in this review and summarized their findings here. Our standardized estimates showed that SO2 associations (ORs) ranged from 1.01 to 1.74 per 10-ppb increase in exposure. Children were found to have the highest overall OR (1.25, 95% CI: 1.09–1.43, p = 0.001) than adults (1.12, 95% CI: 1.04–1.21, p = 0.002; Figure 6). For those studies that did not differentiate children from adults, the overall OR (1.03, 95% CI: 0.99–1.08, p = 0.13) was slightly lower than those for children and adults (Figure 6). There was strong evidence of homogeneity among these studies (children, Q = heterogeneity χ2 = 3.44, I2 = 42%, p = 0.18), but heterogeneity was found for adults and all (adults, Q = Heterogeneity χ2 = 120.37, I2 = 98%, p = 0.0001; all, Q = heterogeneity χ2 = 8.22, I2 = 76%, p = 0 0.01; Figure 6). Egger’s linear regression did identify publication bias among the studies for all (Egger’s: p = 0.023) but did not identify children (Egger’s: p = 0.47) and adults (Egger’s: p = 0.88; Supplementary Figure S5). The effect size estimate is 0.2232 for both the original and overall models, indicating a positive effect of SO2 on respiratory disease in children (Supplementary Table S26). The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain consistent regardless of which study is excluded. The original and overall models show a statistically significant positive effect of SO2 on respiratory disease in children, with moderate heterogeneity. This suggests a meaningful relationship between SO2 exposure and respiratory disease, warranting further attention and research (Supplementary Table S25). The effect size estimate is 0.1171 for both the original and overall models, indicating a positive effect of SO2 on respiratory disease in adults (Supplementary Table S28). The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain consistent regardless of which study is excluded. The original and overall models show a statistically significant positive effect of SO2 on respiratory disease in adults, with high heterogeneity. This suggests a meaningful relationship between SO2 exposure and respiratory disease, warranting further attention and research (Supplementary Table S27). The effect size estimate is 0.0315 for both the original and overall models, indicating a small positive effect of SO2 on respiratory disease across all age groups (Supplementary Table S30). The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain consistent regardless of which study is excluded. However, the original and overall models show that the effect of SO2 on respiratory disease across all age groups is not statistically significant, with confidence intervals that include zero and a p-value above 0.05. This suggests that further research might be needed to establish a more transparent relationship (Supplementary Table S29).
Figure 6.
Forest plot of the estimated effects of SO2 on respiratory disease. AH, asthma hospitalization; AEDV, asthma emergency department visits; COPDH, COPD hospitalization.
3.6. Impacts from trace metals from on-road vehicle emissions
We included three studies on trace metals in this review and summarized their findings here.
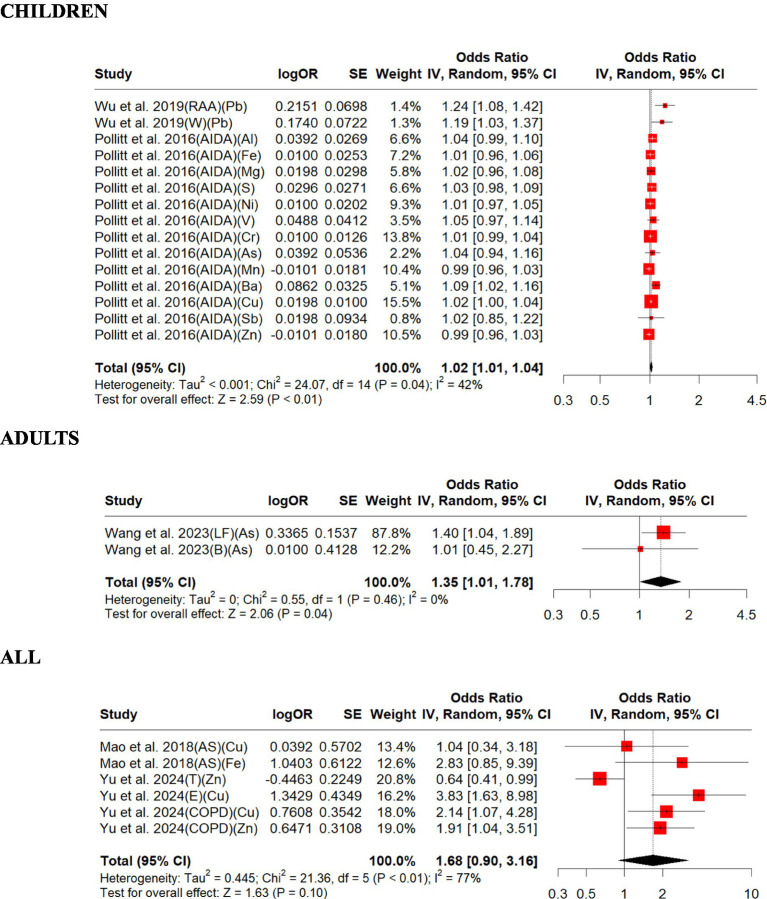
Overall, the studies included in this review support that increased exposures to trace metals are associated with exacerbations in respiratory disease outcomes (ORs from 1.01 to 3.83) except for two cases (ORs = 0.99). The exacerbations in the respiratory disease outcomes in children and all subjects were significantly associated with increased exposure to trace metals. Children were found to have a lower overall OR (1.02, 95% CI: 1.01–1.04, p = 0.009) than all adults (1.35, 95% CI: 1.01–1.78, p = 0.039) and all (1.68, 95% CI: 0.90–3.16, p = 0.104; Figure 7). There was strong evidence of homogeneity for adults (adults, Q = heterogeneity χ2 = 0.55, I2 = 0%, p = 0.45), but heterogeneity found for children and all (children, Q = heterogeneity χ2 = 24.07, I2 = 42%, p = 0.04; all, Q = Heterogeneity χ2 = 21.36, I2 = 77%, p = 0.0007; Figure 7). Egger’s linear regression did not identify publication bias for all (Egger’s: p = 0.15) but for children (Egger’s: p = 0.02; Supplementary Figure S6). The effect size estimate is 0.0220 for both the original and overall models, indicating a small positive effect of trace metals on respiratory disease in children (Supplementary Table S32). The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain consistent regardless of which study is excluded. The original and overall models show a statistically significant positive effect of trace metals on respiratory disease in children, with moderate heterogeneity. This suggests a meaningful relationship between trace metal exposure and respiratory disease, warranting further attention and research (Supplementary Table S31). The effect size estimate is 0.2967 for the original model and slightly higher at 0.3365 for the overall sensitivity analysis model, indicating a positive effect of trace metals on respiratory disease in adults (Supplementary Table S34). The sensitivity analysis shows that the meta-analysis results are robust, as the effect size estimates remain statistically significant regardless of which study is excluded. The original and overall models show a statistically significant positive effect of trace metals on respiratory disease in adults, with no heterogeneity. This suggests a meaningful relationship between trace metal exposure and respiratory disease, warranting further attention and research (Supplementary Table S33). The effect size estimate is 0.5215 for the original model and much lower at 0.0794 for the overall sensitivity analysis model. This indicates that the initial model suggested a moderate positive effect of trace metals on respiratory disease across all age groups, but the sensitivity analysis drastically reduces this effect size (Supplementary Table S36). The sensitivity analysis shows that the meta-analysis results are not robust, as the effect size estimates vary significantly depending on which study is excluded. The original and overall models both indicate that the effect of trace metals on respiratory disease across all age groups is not statistically significant, with confidence intervals that include zero and high heterogeneity. This suggests that there is no clear relationship between trace metal exposure and respiratory disease based on the available data, and further research with more consistent findings is needed (Supplementary Table S35).
Figure 7.
Forest plot of the estimated effects of trace metal on respiratory disease. AIDA, airway inflammation due to asthma; Al, aluminum; As, arsenic; AS, asthma susceptibility; B, bronchiectasis; Ba, barium; Cr, chromium; Cu, copper; E, emphysema; Fe, iron; LF, lung fibrotic; Pb, lead; Mg, magnesium; Mn, manganese; Ni, nickel; RAA, risk for active asthma; S, sulfur; Sb, antimony; T, tracheitis; W, wheezing; Zn, zinc.
4. Discussion
In this meta-analysis, we assessed the impact of exposures to criteria pollutants (NO2, PM2.5, PM10, O3, and SO2) and more than 10 trace metals that can be considered toxic air contaminants on respiratory disease outcomes, including airway inflammation, coughing, wheezing, exacerbations, ED visits, hospitalizations, and mortality. We converted all the study outcomes in RRs and percentage increase to ORs and standardized the impact of exposure increase interval to make the studies comparable. After standardizing the results from different studies, we pooled the effect estimates to report the overall size impact of PM2.5 (Figure 2) and PM10 (Figure 3) on respiratory disease outcomes.
A previous systematic review on the association of major air pollutants with the risk of COPD concludes that even short-term exposures to air pollutants significantly increase the risk of COPD acute exacerbations (15). Furthermore, a recent study found that NO2 or PM2.5 concentration in nine southern Californian communities had been lower than observed in the 1990s and early 2000s; there would have been a corresponding reduction in childhood asthma incidence (31). A meta-analysis study found clear evidence of an association of PM10 exposure with asthma symptoms and, to a lesser extent, with cough and peak expiratory flow (PEF) (3). Another previous study used linear and logistic regression analyses to investigate the associations between chronic exposure to PM10, PM2.5, and NO2 levels and lung function. The study provided evidence that exposure to ambient air pollution adversely affects adult lung function (32). A previous study showed every 5 ppb increase in NO2 exposure above a threshold of 6 ppb was associated with an exposure-dependent rise in the risk of higher asthma severity score, wheezing, night symptoms, and rescue medication use (4). A previous review of the health effects caused by environmental NO2 reported that short-term (24-h) exposure to NO2, even for mean values <50 μg/m3, increased both hospital admission and mortality (13). It also reported that long-term exposure to the NO2 level below the WHO recommended air quality annual mean guideline of 40 μg/m3 was associated with adverse health effects such as respiratory symptoms and diseases, hospitalizations, and mortality (13). A previous study shows that even living in an area with air pollution concentrations below the current US Environmental Protection Agency-National Ambient Air Quality Standard (US EPA-NAAQS), COPD patients suffered an increased risk of exacerbations following short-term exposures to increased concentrations of SO2 (33). Compared with other criteria pollutants, the impact of trace metals on human respiratory health has not been studied much. Based on a previous study, trace elements may be involved in the pathogenesis of asthma (34). Metals in tailpipe and non-tailpipe emissions, such as brake and tire wear, include copper, zinc, antimony, barium, lead, and sulfur (35). A previous study showed that daily exposures to ambient PM2.5 containing trace metals (barium and vanadium) are associated with airway inflammation in asthmatic children. Results from another study suggest that high ambient air PM2.5 containing zinc is associated with increased emergency department visits and hospitalizations for asthma among children living in an urban area (9).
We identified consistently significant associations between all the criteria pollutants and trace metals with a broad range of respiratory disease outcomes. After pooling all the effect estimates, we further identified that the associations were slightly higher for children for exposures to SO2 (1.25 vs. 1.12) and PM2.5 (1.31 vs. 1.10) than for adults but with similar associations for children and adults for exposures to NO2 (1.12 vs. 1.12) while the effects were slightly higher for adults for exposures to PM10 (1.03 vs. 1.06) and O3 (1.02 vs. 1.18) than for children.
Outdoor NO2 concentration is often considered a good marker of traffic-related air pollution (TRAP), and concentrations decline rapidly with increasing distance from highways and major roadways. PM2.5 has smaller spatial gradients than NO2, and regional sources contribute to PM2.5 concentrations. However, traffic remains a major source of PM2.5, especially in high-income countries. SO2 air pollution can be generated from traffic, especially diesel vehicles, and industrial point sources such as coal-fired power plants, rapidly declining with increasing distances from these point sources.
The relatively higher impact of NO2, PM2.5, and SO2 on respiratory disease outcomes for children may be due to both biological and environmental factors. Biologically, children’s lungs are still developing, making them more susceptible to damage from pollutants (36). Environmentally, children are more likely to spend time outdoors and engage in physical activities in areas with high pollutant concentrations, such as near schools and playgrounds in urban settings. This increased exposure during critical growth periods can have both short-term and long-term adverse effects on their respiratory health. Policymakers and stakeholders should adopt strategies to mitigate children’s exposure to air pollution, especially in vulnerable communities. This can include implementing stricter air quality standards around schools and playgrounds, promoting urban planning policies that minimize children’s exposure to high-traffic areas, and encouraging the development of green spaces to act as buffers against pollution. Addressing these factors can help protect children’s health and reduce the burden of respiratory diseases linked to air pollution.
An OR greater than 1.0 indicates an increased risk, while an OR less than 1.0 suggests a protective effect. When ORs are close to 1.0 or when confidence intervals (CIs) include 1.0, it implies a weak or non-significant association. However, even small effect sizes can be meaningful in a public health context due to widespread exposure and the cumulative impact of multiple pollutants. Small increases in risk can lead to significant health impacts across large populations, particularly for vulnerable groups such as children and the older adult. The impact of O3 on respiratory disease outcomes was found to be relatively higher for adults. This may be due to the chemical reactions between O3 and traffic emissions of nitrogen oxides, which lead to higher NO2 concentrations near busy roadways and higher O3 levels farther from traffic. We suspect more adults might have moved to communities with less traffic-related air pollution, thus experiencing greater O3 exposure. Policymakers need to consider these findings to make informed decisions. Non-significant findings should be interpreted cautiously and not used in isolation to justify policy changes. Continuous monitoring and research are essential to understand health impacts better and refine policy interventions. Therefore, the practical implications of our findings are significant for shaping effective public health policies and mitigating respiratory health risks. Therefore, policymakers might consider precautionary measures to limit exposure, especially if the pollutant has other adverse health effects or the population is particularly vulnerable (e.g., children and older adult).
To address the critical need for urban planning and transportation policies that link urban air pollution and respiratory health, as highlighted in this meta-analysis, urban planning must integrate green infrastructure like parks and green roofs, which can absorb pollutants and reduce urban heat island effects. Transportation policies should prioritize the development of public transit options and non-motorized transport to decrease reliance on private vehicles, thereby reducing non-exhaust emissions from road traffic, such as brake and tire wear. Specific policies could include implementing low-emission zones, encouraging the use of electric or hybrid vehicles, and investing in regular road maintenance to reduce road dust resuspension. Regarding climate action, integrated approaches can be designed to reduce emissions from both transportation and industrial sources by promoting renewable energy, electrifying transport systems, and implementing stricter emissions standards. Expanding green urban spaces can serve dual purposes of carbon sequestration and pollution mitigation. This meta-analysis provides critical evidence to inform the development of such integrated policies by quantifying the health impacts of specific pollutants, emphasizing the urgency of reducing emissions to improve public health and climate resilience simultaneously.
4.1. Limitations and the future directions
To maintain an adequate sample size, we combined all studies for each criterion pollutant, regardless of the type of outcome measured, and compared the results across different outcomes. This study assessed the effects of airborne particulate matter (PM2.5 and PM10), NO2, O3, SO2, and trace metals. While this comprehensive approach provides a broad understanding, it introduces considerable heterogeneity, as indicated by the Higgins I2 values. In addition to assessing methodological quality, we evaluated the risk of bias for each study using the Newcastle–Ottawa scale (NOS). The study locations of the 50 included studies were highly heterogeneous, spanning both developed and developing countries across several continents, limiting our findings’ generalizability. It is also worth noting that the impact of trace metals on human respiratory health is understudied, with only five studies included in the meta-analysis. Furthermore, the role of trace metals on adults could barely be assessed due to the limited number of studies focusing on this demographic; most reports concentrated on children or combined age groups.
Although we had five studies in our trace metals analysis, the limited number of studies still requires a cautious interpretation of the results. This small sample size highlights the need for more research to better understand the impact of trace metals on respiratory health. The future studies should focus on expanding the evidence base to ensure more reliable and generalizable findings. More comprehensive research is essential to assess the risks and inform effective public health policies accurately. Another limitation is the lack of information on confounding factors, which could affect the robustness of the results.
The future research should aim to conduct more studies that include specific chemical components of particulate matter, monitor the same individuals over time, and adjust for confounding variables. This approach would enhance our understanding of the relationship between air pollution and respiratory outcomes and provide more accurate data to inform policymakers. Additionally, analyzing long-term and short-term exposures separately could provide more precise risk assessments and clearer insights into specific health outcomes associated with each type of exposure.
5. Conclusion
The findings from this meta-analysis support a positive, robust association between criteria air pollutants, trace metals, and respiratory disease, indicating significant impacts across all age groups, including children and adults. Our study highlights the importance of considering both traditional criteria pollutants and non-exhaust trace metals, which showed significant effects on respiratory health despite being studied in a smaller number of research efforts. This underscores the need to include these often-overlooked pollutants in the future research studies to comprehensively understand the environmental determinants of respiratory disease.
We recommend that the future clinical and epidemiological studies elucidate the potential mechanisms underlying these positive associations. This involves exploring the biological pathways through which air pollutants and trace metals contribute to respiratory conditions. By understanding these mechanisms, more targeted and effective interventions can be developed.
Given our findings, it is crucial to implement rigorous environmental health policies to maintain low levels of air pollution. Such policies are vital to reducing the incidence and severity of respiratory diseases and lowering morbidity and mortality associated with air pollution exposure. Governments should prioritize air quality regulations and ensure strict enforcement to protect public health. A collaborative approach is essential to address the multifaceted nature of air pollution. This involves cooperation between government entities, the automobile industry, the energy sector, and transportation companies. Promoting cleaner fuels, such as natural gas and electricity, can significantly reduce emissions from transportation, one of the significant sources of air pollutants. Additionally, investment in renewable energy sources and sustainable urban infrastructure development are critical components of a comprehensive strategy to combat air pollution.
Further studies are warranted from countries with higher ambient air pollution levels, often facing the most severe health impacts. These studies should provide more comprehensive data on the association between air pollution and respiratory disease, considering different geographic locations’ unique environmental and socioeconomic contexts.
In conclusion, the robust associations identified in this meta-analysis emphasize the urgent need for concerted efforts to mitigate air pollution and its adverse effects on respiratory health. By advancing research, strengthening policies, and fostering cross-sector collaboration, we can achieve substantial progress in reducing air pollution levels and safeguarding public health for the future generations.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the California Air Resources Board (Agreement number 19RD004).
Abbreviations
CI, Confidence interval; COPD, Chronic obstructive pulmonary disease; ED, Emergency department; EPA-NAAQS, Environmental Protection Agency-National Ambient Air Quality Standard; NO2, Nitrogen dioxide; O3, Ozone; OR, Odds ratio; PEF, Peak expiratory flow; PM, Particulate matter; PM10, Particulate matter with a diameter < 10 μm; PM2.5, Particulate matter with a diameter < 2.5 μm; ppb, Part per billion; RR, Relative risk; SO2, Sulfur dioxide; WHO, World Health Organization.
Author contributions
JS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. ES: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. MB: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. JB: Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Conflict of interest
MB is a Propeller Health and ResMed employee and receives salary and stock.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1417450/full#supplementary-material
References
- 1.Cortez-Lugo M, Ramírez-Aguilar M, Pérez-Padilla R, Sansores-Martínez R, Ramírez-Venegas A, Barraza-Villarreal A. Effect of personal exposure to PM2. 5 on respiratory health in a Mexican panel of patients with COPD. Int J Environ Res Public Health. (2015) 12:10635–47. doi: 10.3390/ijerph120910635, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mar TF, Larson TV, Stier RA, Claiborn C, Koenig JQ. An analysis of the association between respiratory symptoms in subjects with asthma and daily air pollution in Spokane, Washington. Inhal Toxicol. (2004) 16:809–15. doi: 10.1080/08958370490506646 [DOI] [PubMed] [Google Scholar]
- 3.Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect. (2010) 118:449–57. doi: 10.1289/ehp.0900844, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belanger K, Holford TR, Gent JF, Hill ME, Kezik JM, Leaderer BP. Household levels of nitrogen dioxide and pediatric asthma severity. Epidemiology. (2013) 24:320. doi: 10.1097/EDE.0b013e318280e2ac, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brauer M, Hoek G, Smit H, De Jongste J, Gerritsen J, Postma DS, et al. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J. (2007) 29:879–88. doi: 10.1183/09031936.00083406, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Takenoue Y, Kaneko T, Miyamae T, Mori M, Yokota S. Influence of outdoor NO2 exposure on asthma in childhood: meta-analysis. Pediatr Int. (2012) 54:762–9. doi: 10.1111/j.1442-200X.2012.03674.x, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Wu K-G, Chang C-Y, Yen C-Y, Lai C-C. Associations between environmental heavy metal exposure and childhood asthma: a population-based study. J Microbiol Immunol Infect. (2019) 52:352–62. doi: 10.1016/j.jmii.2018.08.001, PMID: [DOI] [PubMed] [Google Scholar]
- 8.Fan J, Li S, Fan C, Bai Z, Yang K. The impact of PM2. 5 on asthma emergency department visits: a systematic review and meta-analysis. Environ Sci Pollut Res. (2016) 23:843–50. doi: 10.1007/s11356-015-5321-x, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Hirshon JM, Shardell M, Alles S, Powell JL, Squibb K, Ondov J, et al. Elevated ambient air zinc increases pediatric asthma morbidity. Environ Health Perspect. (2008) 116:826–31. doi: 10.1289/ehp.10759, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorsey TF, Jr, Lafleur AL, Kumata H, Takada H, Herrero-Jimenez P, Thilly WG. Correlations of asthma mortality with traffic-related factors: use of catalytic converters and radial tires. J Occup Environ Med. (2006) 48:1321–7. doi: 10.1097/01.jom.0000236402.08284.15, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Katanoda K, Sobue T, Satoh H, Tajima K, Suzuki T, Nakatsuka H, et al. An association between long-term exposure to ambient air pollution and mortality from lung cancer and respiratory diseases in Japan. J Epidemiol. (2011) 21:1102090211. doi: 10.2188/jea.je20100098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kloog I, Ridgway B, Koutrakis P, Coull BA, Schwartz JD. Long-and short-term exposure to PM2. 5 and mortality: using novel exposure models. Epidemiology. (2013) 24:555. doi: 10.1097/EDE.0b013e318294beaa, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latza U, Gerdes S, Baur X. Effects of nitrogen dioxide on human health: systematic review of experimental and epidemiological studies conducted between 2002 and 2006. Int J Hyg Environ Health. (2009) 212:271–87. doi: 10.1016/j.ijheh.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 14.Yu W, Guo Y, Shi L, Li S. The association between long-term exposure to low-level PM2. 5 and mortality in the state of Queensland, Australia: a modelling study with the difference-in-differences approach. PLoS Med. (2020) 17:e1003141. doi: 10.1371/journal.pmed.1003141, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Sun S, Tang R, Qiu H, Huang Q, Mason TG, et al. Major air pollutants and risk of COPD exacerbations: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. (2016) 11:3079. doi: 10.2147/COPD.S122282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Li G, Tian L, Guo Q, Pan X. Short-term exposure to air pollution and morbidity of COPD and asthma in east Asian area: a systematic review and meta-analysis. Environ Res. (2016) 148:15–23. doi: 10.1016/j.envres.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Wang J, Lu W. Exposure to nitrogen dioxide and chronic obstructive pulmonary disease (COPD) in adults: a systematic review and meta-analysis. Environ Sci Pollut Res. (2018) 25:15133–45. doi: 10.1007/s11356-018-1629-7, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Ren J, Li B, Yu D, Liu J, Ma Z. Approaches to prevent the patients with chronic airway diseases from exacerbation in the haze weather. J Thorac Dis. (2016) 8:E1. doi: 10.3978/j.issn.2072-1439.2015.11.61, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar P, Pirjola L, Ketzel M, Harrison RM. Nanoparticle emissions from 11 non-vehicle exhaust sources–a review. Atmos Environ. (2013) 67:252–77. doi: 10.1016/j.atmosenv.2012.11.011 [DOI] [Google Scholar]
- 20.Grigoratos T, Martini G. Brake wear particle emissions: a review. Environ Sci Pollut Res. (2015) 22:2491–504. doi: 10.1007/s11356-014-3696-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wik A, Dave G. Occurrence and effects of tire wear particles in the environment–a critical review and an initial risk assessment. Environ Pollut. (2009) 157:1–11. doi: 10.1016/j.envpol.2008.09.028, PMID: [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization (WHO) . (2022). Air Quality and Health. Available at: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (Accessed on June 15, 2024).
- 23.United Nations (UN) . (2015). Transforming our world: The 2030 Agenda for Sustainable Development. Available at: https://sdgs.un.org/2030agenda (Accessed on June 15, 2024)
- 24.Wells GA SB, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. (2021).The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://wwwohrica/programs/clinical_epidemiology/oxfordasp (Accessed March 15, 2024).
- 25.Wang Z. Converting odds ratio to relative risk in cohort studies with partial data information. J Stat Softw. (2013) 55:1–11. doi: 10.18637/jss.v055.i05 [DOI] [Google Scholar]
- 26.Greenland S, O'Rourke K. Meta-analysis In: Rothman KJ, editor. Modern epidemiology. 3rd ed. Philadelphia, PA, USA: Lippincott Williams & Wilkins; (2008). 652–82. [Google Scholar]
- 27.Greenland S, Lash TL. Modern epidemiology. Philadelphia, PA, USA: Lippincott Williams & Wilkins; (2008). [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pigott T. Advances in meta-analysis. New York, NY, USA: Springer Science & Business Media; (2012). [Google Scholar]
- 31.Garcia E, Urman R, Berhane K, McConnell R, Gilliland F. Effects of policy-driven hypothetical air pollutant interventions on childhood asthma incidence in southern California. Proc Natl Acad Sci. (2019) 116:15883–8. doi: 10.1073/pnas.1815678116, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamichhane DK, Leem JH, Kim HC. Associations between ambient particulate matter and nitrogen dioxide and chronic obstructive pulmonary diseases in adults and effect modification by demographic and lifestyle factors. Int J Environ Res Public Health. (2018) 15:363. doi: 10.3390/ijerph15020363, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeVries R, Kriebel D, Sama S. Low level air pollution and exacerbation of existing copd: a case crossover analysis. Environ Health. (2016) 15:1–11. doi: 10.1186/s12940-016-0179-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao S, Wu L, Shi W. Association between trace elements levels and asthma susceptibility. Respir Med. (2018) 145:110–9. doi: 10.1016/j.rmed.2018.10.028 [DOI] [PubMed] [Google Scholar]
- 35.Lough GC, Schauer JJ, Park J-S, Shafer MM, DeMinter JT, Weinstein JP. Emissions of metals associated with motor vehicle roadways. Environ Sci Technol. (2005) 39:826–36. doi: 10.1021/es048715f, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Bateson TFSJ, Schwartz J. Children's response to air pollutants. J Toxic Environ Health A. (2008) 71:238–43. doi: 10.1080/15287390701598234, PMID: [DOI] [PubMed] [Google Scholar]
- 37.De Marco R, Poli A, Ferrari M, Accordini S, Giammanco G, Bugiani M, et al. The impact of climate and traffic-related NO2 on the prevalence of asthma and allergic rhinitis in Italy. Clin Exp Allergy. (2002) 32:1405–12. doi: 10.1046/j.1365-2745.2002.01466.x, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Sunyer J, Basagana X, Belmonte J, Anto J. Effect of nitrogen dioxide and ozone on the risk of dying in patients with severe asthma. Thorax. (2002) 57:687–93. doi: 10.1136/thorax.57.8.687, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, et al. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. (2003) 290:1859–67. doi: 10.1001/jama.290.14.1859, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Migliaretti G, Cavallo F. Urban air pollution and asthma in children. Pediatr Pulmonol. (2004) 38:198–203. doi: 10.1002/ppul.20057, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Analitis A, Katsouyanni K, Dimakopoulou K, Samoli E, Nikoloulopoulos AK, Petasakis Y, et al. Short-term effects of ambient particles on cardiovascular and respiratory mortality. Epidemiology. (2006) 17:230–3. doi: 10.1097/01.ede.0000199439.57655.6b, PMID: [DOI] [PubMed] [Google Scholar]
- 42.Smargiassi A, Kosatsky T, Hicks J, Plante C, Armstrong B, Villeneuve PJ, et al. Risk of asthmatic episodes in children exposed to sulfur dioxide stack emissions from a refinery point source in Montreal, Canada. Environ Health Perspect. (2009) 117:653–9. doi: 10.1289/ehp.0800010, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kan H, Wong C-M, Vichit-Vadakan N, Qian Z. Short-term association between sulfur dioxide and daily mortality: the public health and air pollution in Asia (PAPA) study. Environ Res. (2010) 110:258–64. doi: 10.1016/j.envres.2010.01.006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silverman RA, Ito K. Age-related association of fine particles and ozone with severe acute asthma in new York City. J Allergy Clin Immunol. (2010) 125:367–73. doi: 10.1016/j.jaci.2009.10.061, PMID: [DOI] [PubMed] [Google Scholar]
- 45.Katanoda K, Sobue T, Satoh H, Tajima K, Suzuki T, Nakatsuka H, et al. An association between long-term exposure to ambient air pollution and mortality from Lung cancer and respiratory diseases in Japan. J Epidemiol. (2011) 21:132–43. doi: 10.2188/jea.JE20100098, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European study of cohorts for air pollution effects (ESCAPE). Lancet Oncol. (2013) 14:813–22. doi: 10.1016/S1470-2045(13)70279-1, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Ghozikali MG, Heibati B, Naddafi K, Kloog I, Conti GO, Polosa R, et al. Evaluation of chronic obstructive pulmonary disease (COPD) attributed to atmospheric O3, NO2, and SO2 using air Q model (2011–2012 year). Environ Res. (2016) 144:99–105. doi: 10.1016/j.envres.2015.10.030, PMID: [DOI] [PubMed] [Google Scholar]
- 48.Greenberg N, Carel RS, Derazne E, Bibi H, Shpriz M, Tzur D, et al. Different effects of long-term exposures to SO2 and NO2 air pollutants on asthma severity in young adults. J Toxic Environ Health A. (2016) 79:342–51. doi: 10.1080/15287394.2016.1153548, PMID: [DOI] [PubMed] [Google Scholar]
- 49.Hasunuma H, Sato T, Iwata T, Kohno Y, Nitta H, Odajima H, et al. Association between traffic-related air pollution and asthma in preschool children in a national Japanese nested case–control study. BMJ Open. (2016) 6:e010410. doi: 10.1136/bmjopen-2015-010410, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mirabelli MC, Vaidyanathan A, Flanders WD, Qin X, Garbe P. Outdoor PM2. 5, ambient air temperature, and asthma symptoms in the past 14 days among adults with active asthma. Environ Health Perspect. (2016) 124:1882–90. doi: 10.1289/EHP92, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Godri Pollitt KJ, Maikawa CL, Wheeler AJ, Weichenthal S, Dobbin NA, Liu L, et al. Trace metal exposure is associated with increased exhaled nitric oxide in asthmatic children. Environ Health. (2016) 15:1–11. doi: 10.1186/s12940-016-0173-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L, Yang J, Song Y-F, Chen P-Y, Ou C-Q. The burden of COPD mortality due to ambient air pollution in Guangzhou, China. Sci Rep. (2016) 6:25900. doi: 10.1038/srep25900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Day DB, Xiang J, Mo J, Li F, Chung M, Gong J, et al. Association of ozone exposure with cardiorespiratory pathophysiologic mechanisms in healthy adults. JAMA Intern Med. (2017) 177:1344–53. doi: 10.1001/jamainternmed.2017.2842, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khaniabadi YO, Hopke PK, Goudarzi G, Daryanoosh SM, Jourvand M, Basiri H. Cardiopulmonary mortality and COPD attributed to ambient ozone. Environ Res. (2017) 152:336–41. doi: 10.1016/j.envres.2016.10.008, PMID: [DOI] [PubMed] [Google Scholar]
- 55.Magzamen S, Oron AP, Locke ER, Fan VS. Association of ambient pollution with inhaler use among patients with COPD: a panel study. Occup Environ Med. (2018) 75:382–8. doi: 10.1136/oemed-2017-104808, PMID: [DOI] [PubMed] [Google Scholar]
- 56.Hansel NN, Romero KM, Pollard SL, Bose S, Psoter KJ, J Underhill L, et al. Ambient air pollution and variation in multiple domains of asthma morbidity among Peruvian children. Ann Am Thorac Soc. (2019) 16:348–55. doi: 10.1513/AnnalsATS.201807-448OC, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang H-C, Lin FC-F, Wu M-F, Nfor ON, Hsu S-Y, Lung C-C, et al. Association between chronic obstructive pulmonary disease and PM2. 5 in Taiwanese nonsmokers. Int J Hyg Environ Health. (2019) 222:884–8. doi: 10.1016/j.ijheh.2019.03.009, PMID: [DOI] [PubMed] [Google Scholar]
- 58.Mercan Y, Babaoglu UT, Erturk A. Short-term effect of particular matter and sulfur dioxide exposure on asthma and/or chronic obstructive pulmonary disease hospital admissions in Center of Anatolia. Environ Monit Assess. (2020) 192:646. doi: 10.1007/s10661-020-08605-7 [DOI] [PubMed] [Google Scholar]
- 59.Pepper JR, Barrett MA, Su JG, Merchant R, Henderson K, Van Sickle D, et al. Geospatial-temporal analysis of the impact of ozone on asthma rescue inhaler use. Environ Int. (2020) 136:105331. doi: 10.1016/j.envint.2019.105331, PMID: [DOI] [PubMed] [Google Scholar]
- 60.Shin S, Bai L, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, et al. Air pollution as a risk factor for incident chronic obstructive pulmonary disease and asthma. A 15-year population-based cohort study. Am J Respir Crit Care Med. (2021) 203:1138–48. doi: 10.1164/rccm.201909-1744OC, PMID: [DOI] [PubMed] [Google Scholar]
- 61.Yan M, Ge H, Zhang L, Chen X, Yang X, Liu F, et al. Long-term PM2. 5 exposure in association with chronic respiratory diseases morbidity: a cohort study in northern China. Ecotoxicol Environ Saf. (2022) 244:114025. doi: 10.1016/j.ecoenv.2022.114025, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mebrahtu TF, Santorelli G, Yang TC, Wright J, Tate J, McEachan RR. The effects of exposure to NO2, PM2. 5 and PM10 on health service attendances with respiratory illnesses: a time-series analysis. Environ Pollut. (2023) 333:122123. doi: 10.1016/j.envpol.2023.122123, PMID: [DOI] [PubMed] [Google Scholar]
- 63.Aron J, Baldomero AK, Rau A, Fiecas MB, Wendt CH, Berman JD. Individual risk factors of PM2. 5 associated with wintertime mortality in urban patients with COPD. Chest. (2024) 165:825–35. doi: 10.1016/j.chest.2023.10.016, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kangas T, Gadeyne S, Lefebvre W, Vanpoucke C, Rodriguez-Loureiro L. Are air quality perception and PM2. 5 exposure differently associated with cardiovascular and respiratory disease mortality in Brussels? Findings from a census-based study. Environ Res. (2023) 219:115180. doi: 10.1016/j.envres.2022.115180, PMID: [DOI] [PubMed] [Google Scholar]
- 65.Marchetti P, Miotti J, Locatelli F, Antonicelli L, Baldacci S, Battaglia S, et al. Long-term residential exposure to air pollution and risk of chronic respiratory diseases in Italy: the BIGEPI study. Sci Total Environ. (2023) 884:163802. doi: 10.1016/j.scitotenv.2023.163802, PMID: [DOI] [PubMed] [Google Scholar]
- 66.Wang C-W, Chen S-C, Wu D-W, Lin H-H, Chen HC, Hung C-H, et al. Arsenic exposure associated with lung interstitial changes in non-smoking individuals living near a petrochemical complex: a repeated cross-sectional study. Environ Pollut. (2023) 331:121844. doi: 10.1016/j.envpol.2023.121844, PMID: [DOI] [PubMed] [Google Scholar]
- 67.Yang H, Wang Z, Zhou Y, Gao Z, Xu J, Xiao S, et al. Association between long-term ozone exposure and readmission for chronic obstructive pulmonary disease exacerbation. Environ Pollut. (2024) 348:123811. doi: 10.1016/j.envpol.2024.123811, PMID: [DOI] [PubMed] [Google Scholar]
- 68.Stowell JD, Sun Y, Gause EL, Spangler KR, Schwartz J, Bernstein A, et al. Warm season ambient ozone and children’s health in the USA. Int J Epidemiol. (2024) 53:dyae035. doi: 10.1093/ije/dyae035, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu H, Taihong L, Ji Z, Wang S, Liu S, Chen Y, et al. Associations of serum cu, Zn, and se with chronic respiratory diseases in the American adults: Data from NHANES 2013–2016 and a bidirectional Mendelian randomization analysis. Research Square; (2024). [Google Scholar]
- 70.Kwon E, Jin T, You Y-A, Kim B. Joint effect of long-term exposure to ambient air pollution on the prevalence of chronic obstructive pulmonary disease using the Korea National Health and nutrition examination survey 2010–2019. Chemosphere. (2024) 358:142137. doi: 10.1016/j.chemosphere.2024.142137, PMID: [DOI] [PubMed] [Google Scholar]
- 71.Zhang J, Ai B, Guo Y, Chen L, Chen G, Li H, et al. Long-term exposure to ambient ozone and adult-onset asthma: a prospective cohort study. Environ Res. (2024) 252:118962. doi: 10.1016/j.envres.2024.118962, PMID: [DOI] [PubMed] [Google Scholar]
- 72.Zheng XYGS, Hu JX, Meng RL, Xu YJ, Lv YH, Wang Y, et al. Long-term associations of PM1 vs. PM2. 5 and PM10 with asthma and asthma-related respiratory symptoms in middle-aged and elderly population. ERJ Open Res. (2024) 10:1–12. doi: 10.1183/23120541.00972-2023, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xing ZYT, Shi S, Meng X, Chai D, Liu W, Tong Y, et al. Combined effect of ozone and household air pollution on COPD in people aged less than 50 years old. Thorax. (2024) 79:35–42. doi: 10.1136/thorax-2022-219691 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.