Abstract
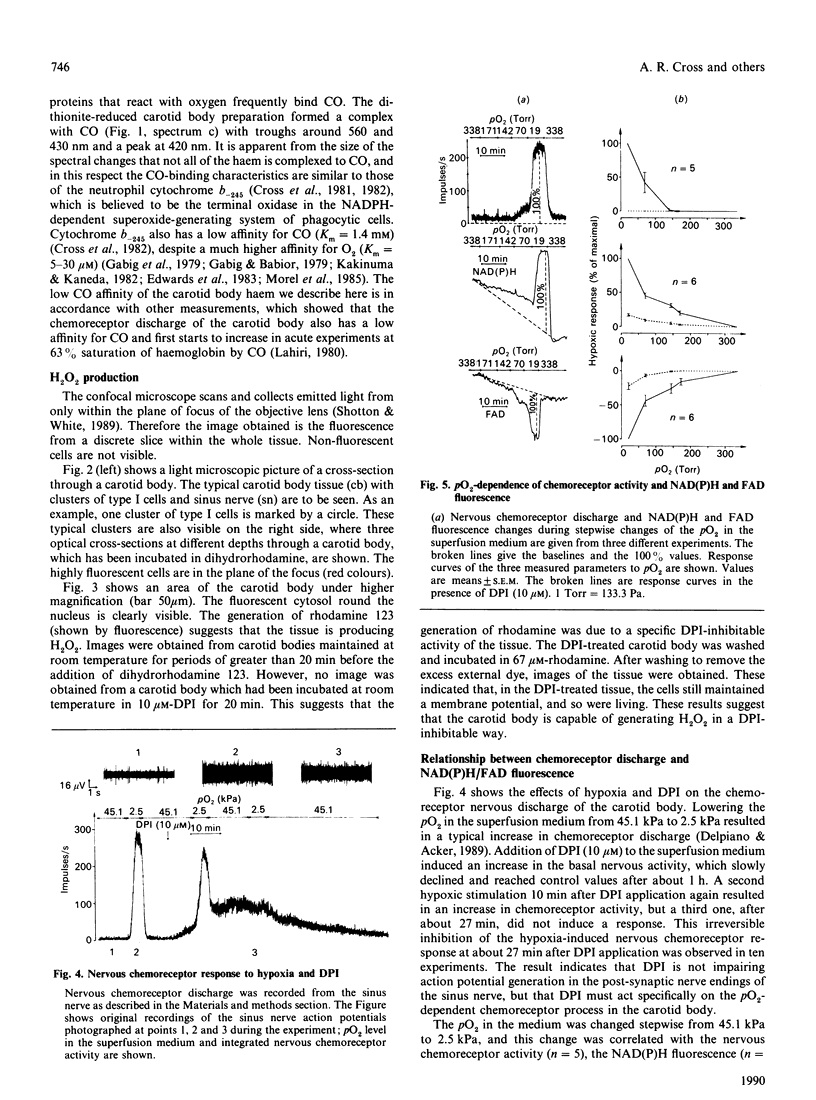
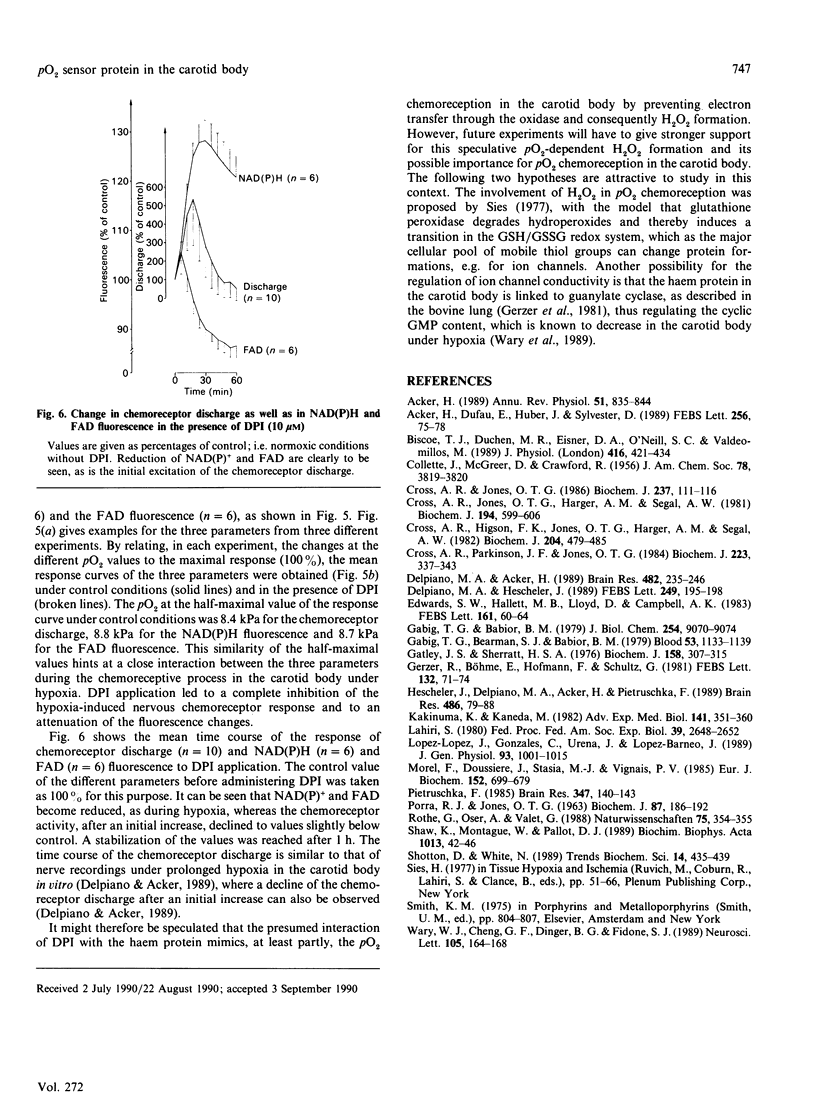
The rat carotid body tissue reveals a photometrically measurable haem signal with absorbance maxima at 560 nm, 518 nm and 425 nm, suggesting the presence of a b-type cytochrome; this was confirmed by pyridine haemochrome and CO spectra. The quantity of cytochrome b was estimated to be 310 pmol.mg of protein-1. This haem is capable of H2O2 formation, which can be inhibited by 10 microM-diphenyliodonium (DPI). The hypoxia-induced increase in nervous chemoreceptor discharge and the reduction of FAD and NAD(P)+ were also inhibited by DPI (10 microM). These results suggest that an oxidase such as the NAD(P)H oxidase of neutrophils may act as a pO2 sensor protein in the rat carotid body, probably inducing the pO2 chemoreceptor process by H2O2 formation.
Full text
PDF
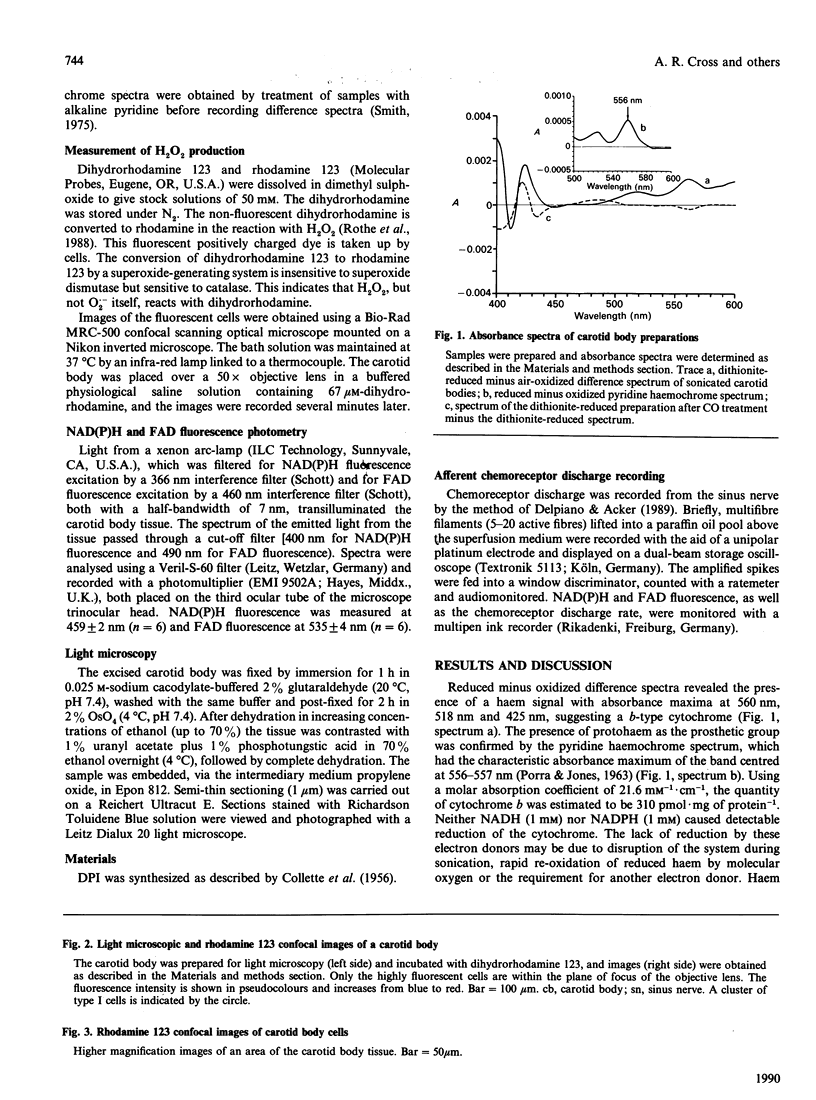
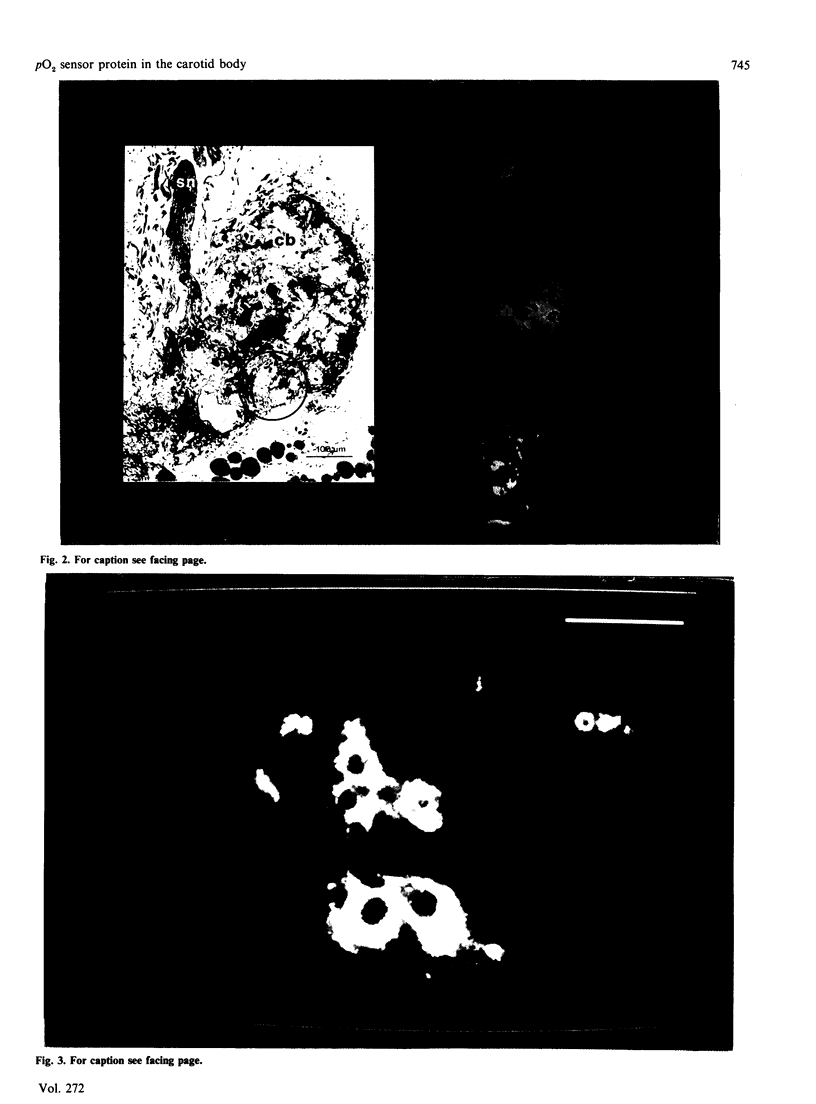
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker H., Dufau E., Huber J., Sylvester D. Indications to an NADPH oxidase as a possible pO2 sensor in the rat carotid body. FEBS Lett. 1989 Oct 9;256(1-2):75–78. doi: 10.1016/0014-5793(89)81721-1. [DOI] [PubMed] [Google Scholar]
- Acker H. PO2 chemoreception in arterial chemoreceptors. Annu Rev Physiol. 1989;51:835–844. doi: 10.1146/annurev.ph.51.030189.004155. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R., Eisner D. A., O'Neill S. C., Valdeolmillos M. Measurements of intracellular Ca2+ in dissociated type I cells of the rabbit carotid body. J Physiol. 1989 Sep;416:421–434. doi: 10.1113/jphysiol.1989.sp017769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Higson F. K., Jones O. T., Harper A. M., Segal A. W. The enzymic reduction and kinetics of oxidation of cytochrome b-245 of neutrophils. Biochem J. 1982 May 15;204(2):479–485. doi: 10.1042/bj2040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T., Harper A. M., Segal A. W. Oxidation-reduction properties of the cytochrome b found in the plasma-membrane fraction of human neutrophils. A possible oxidase in the respiratory burst. Biochem J. 1981 Feb 15;194(2):599–606. doi: 10.1042/bj1940599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J. 1986 Jul 1;237(1):111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Parkinson J. F., Jones O. T. The superoxide-generating oxidase of leucocytes. NADPH-dependent reduction of flavin and cytochrome b in solubilized preparations. Biochem J. 1984 Oct 15;223(2):337–344. doi: 10.1042/bj2230337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpiano M. A., Acker H. Hypoxic and hypercapnic responses of [Ca2+]o and [K+]o in the cat carotid body in vitro. Brain Res. 1989 Mar 20;482(2):235–246. doi: 10.1016/0006-8993(89)91186-4. [DOI] [PubMed] [Google Scholar]
- Delpiano M. A., Hescheler J. Evidence for a PO2-sensitive K+ channel in the type-I cell of the rabbit carotid body. FEBS Lett. 1989 Jun 5;249(2):195–198. doi: 10.1016/0014-5793(89)80623-4. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Hallett M. B., Lloyd D., Campbell A. K. Decrease in apparent Km for oxygen after stimulation of respiration of rat polymorphonuclear leukocytes. FEBS Lett. 1983 Sep 5;161(1):60–64. doi: 10.1016/0014-5793(83)80730-3. [DOI] [PubMed] [Google Scholar]
- Gabig T. G., Babior B. M. The O2(-) -forming oxidase responsible for the respiratory burst in human neutrophils. Properties of the solubilized enzyme. J Biol Chem. 1979 Sep 25;254(18):9070–9074. [PubMed] [Google Scholar]
- Gabig T. G., Bearman S. I., Babior B. M. Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Blood. 1979 Jun;53(6):1133–1139. [PubMed] [Google Scholar]
- Gatley S. J., Sherratt S. A. The effects of diphenyleneiodonium on mitochondrial reactions. Relation of binding of diphenylene[125I]iodonium to mitochondria to the extent of inhibition of oxygen uptake. Biochem J. 1976 Aug 15;158(2):307–315. doi: 10.1042/bj1580307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzer R., Böhme E., Hofmann F., Schultz G. Soluble guanylate cyclase purified from bovine lung contains heme and copper. FEBS Lett. 1981 Sep 14;132(1):71–74. doi: 10.1016/0014-5793(81)80429-2. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Delpiano M. A., Acker H., Pietruschka F. Ionic currents on type-I cells of the rabbit carotid body measured by voltage-clamp experiments and the effect of hypoxia. Brain Res. 1989 May 1;486(1):79–88. doi: 10.1016/0006-8993(89)91280-8. [DOI] [PubMed] [Google Scholar]
- Kakinuma K., Kaneda M. Apparent Km of leukocyte O2 and H2O2 forming enzyme for oxygen. Adv Exp Med Biol. 1982;141:351–360. doi: 10.1007/978-1-4684-8088-7_33. [DOI] [PubMed] [Google Scholar]
- Lahiri S. Role of arterial O2 flow in peripheral chemoreceptor excitation. Fed Proc. 1980 Jul;39(9):2648–2652. [PubMed] [Google Scholar]
- López-López J., González C., Ureña J., López-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel F., Doussiere J., Stasia M. J., Vignais P. V. The respiratory burst of bovine neutrophils. Role of a b type cytochrome and coenzyme specificity. Eur J Biochem. 1985 Nov 4;152(3):669–679. doi: 10.1111/j.1432-1033.1985.tb09247.x. [DOI] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 2. An in vestigation of the role offerrochelatase in the biosynthesis of various haem prosthetic groups. Biochem J. 1963 Apr;87:186–192. doi: 10.1042/bj0870186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietruschka F. Calcium influx in cultured carotid body cells is stimulated by acetylcholine and hypoxia. Brain Res. 1985 Nov 11;347(1):140–143. doi: 10.1016/0006-8993(85)90901-1. [DOI] [PubMed] [Google Scholar]
- Rothe G., Oser A., Valet G. Dihydrorhodamine 123: a new flow cytometric indicator for respiratory burst activity in neutrophil granulocytes. Naturwissenschaften. 1988 Jul;75(7):354–355. doi: 10.1007/BF00368326. [DOI] [PubMed] [Google Scholar]
- Shaw K., Montague W., Pallot D. J. Biochemical studies on the release of catecholamines from the rat carotid body in vitro. Biochim Biophys Acta. 1989 Sep 4;1013(1):42–46. doi: 10.1016/0167-4889(89)90125-0. [DOI] [PubMed] [Google Scholar]
- Shotton D., White N. Confocal scanning microscopy: three-dimensional biological imaging. Trends Biochem Sci. 1989 Nov;14(11):435–439. doi: 10.1016/0968-0004(89)90096-0. [DOI] [PubMed] [Google Scholar]
- Wang W. J., Cheng G. F., Dinger B. G., Fidone S. J. Effects of hypoxia on cyclic nucleotide formation in rabbit carotid body in vitro. Neurosci Lett. 1989 Oct 23;105(1-2):164–168. doi: 10.1016/0304-3940(89)90030-x. [DOI] [PubMed] [Google Scholar]