Abstract
Background
With the increasing resistance to antimicrobial agents, susceptibility‐guided tailored therapy has been emerging as an ideal strategy for Helicobacter pylori treatment. However, susceptibility‐guided tailored therapy requires additional cost, time consumption, and invasive procedure (endoscopy) and its superiority over empirical quadruple therapy as the first‐line H. pylori treatment remains unclear.
Aims
To compare the efficacy of culture‐based susceptibility‐guided tailored versus empirical concomitant therapy as the first‐line Helicobacter pylori treatment.
Methods
This open‐label, randomized trial was performed in four Korean institutions. A total of 312 Patients with H. pylori‐positive culture test and naïve to treatment were randomly assigned in a 3:1 ratio to either culture‐based susceptibility‐guided tailored therapy (clarithromycin‐based or metronidazole‐based triple therapy for susceptible strains or bismuth quadruple therapy for dual‐resistant strains, n = 234) or empirical concomitant therapy (n = 78) for 10 days. Eradication success was evaluated by 13C‐urea breath test at least 4 weeks after treatment.
Results
Prevalence of dual resistance to both clarithromycin and metronidazole was 8%. H. pylori eradication rates for tailored and concomitant groups were 84.2% and 83.3% by intention‐to‐treat analysis (p = 0.859), respectively, and 92.9% and 91.5% by per‐protocol analysis, respectively (p = 0.702), which were comparable between the two groups. However, eradication rates for dual‐resistant strains were significantly higher in the tailored group than in the concomitant group. All adverse events were grade 1 or 2 based on the Common Terminology Criteria for Adverse Events and the incidence was significantly lower in the tailored group. The proportion of patients discontinuing treatment for adverse events was comparable between the two groups (2.1% vs. 2.6%).
Conclusions
The culture‐based susceptibility‐guided tailored therapy failed to show superiority over the empirical concomitant therapy in terms of eradication rate. Based on these findings, the treatment choice in clinical practice would depend on the background rate of antimicrobial resistance, availability of resources and costs associated with culture and susceptibility testing.
Keywords: antibiotic resistance, biopsy, cost, EGDS, endoscopy, esophagogastroduodenoscopy, H. pylori, histology, microbiology, quadruple therapy
Key Summary.
Summarize the established knowledge on this subject
Concomitant therapy achieved eradication success of over 90% in Korea and all the regions of Europe.
With the increasing resistance to antimicrobial agents, susceptibility‐guided tailored therapy has been emerging as an ideal strategy for Helicobacter pylori treatment.
Susceptibility‐guided tailored therapy requires additional cost and time consumption and its superiority over empirical quadruple therapy as the first‐line H. pylori treatment remains unclear.
What are the significant and/or new findings of this study?
The culture‐based susceptibility‐guided tailored therapy was not superior to the locally effective empirical concomitant therapy in terms of eradication rate as the first‐line treatment for H. pylori (92.9% and 91.5% by per‐protocol analysis, respectively).
All adverse events were grade 1 or 2 based on Common Terminology Criteria for Adverse Events (CTCAE) and the incidence was significantly lower in the tailored group than in the concomitant group. However, the proportion of patients discontinuing treatment for adverse events was comparable between the two groups (2.6% for the concomitant group and 2.1% for the tailored group).
INTRODUCTION
The eradication effect of Helicobacter pylori treatment has been steadily decreasing worldwide, mainly due to increasing resistance to antimicrobial agents. 1 , 2 , 3 , 4 Eradication rates with clarithromycin‐based triple therapy have dropped below 80% in North America, Europe and Asia. Therefore, there is a pressing need to optimize management strategy. 5 , 6 , 7 , 8 , 9 Recent European, US, and Korean guidelines recommended the empirical bismuth quadruple therapy or the concomitant quadruple therapy for 10–14 days as the first‐line H. pylori treatment in areas with high (>15%) clarithromycin resistance to overcome antimicrobial resistance. 10 , 11 , 12 According to European registry (Hp‐EuReg), concomitant therapy is the second most commonly used regimen in Europe; in contrast, bismuth quadruple therapy is used in less than 1% of registered cases. 7 Concomitant therapy is the only therapy other than bismuth quadruple therapy that consistently achieved eradication success over 90% in all the regions of Europe. 7 In a recent nationwide study in Korea, empirical 10‐day concomitant therapy also showed acceptable per‐protocol (PP) eradication rate of over 90%. 6 The main drawback of concomitant therapy using three antibiotics is the possibility of antibiotic overuse.
Theoretically, susceptibility‐guided tailored therapy can prevent resistance‐associated treatment failure and limit the emergence of antibiotic resistance by avoiding the use of unnecessary antibiotics. 10 , 11 Therefore, susceptibility‐guided tailored therapy would be an ideal strategy for H. pylori eradication, especially in East Asia where the prevalence of antimicrobial resistance and gastric cancer is high, and a scope‐and‐treat strategy (upper gastrointestinal endoscopy followed by treatment) has been widely accepted. 12 , 13 , 14 , 15 In recent Korean studies, PP eradication rates of culture‐based susceptibility‐guided therapy were over 95% despite high antimicrobial resistance. 16 , 17 However, standard methods of susceptibility testing require additional cost, time consumption, and invasive procedure (endoscopy), which hampers its generalizability. Noninvasive susceptibility testing of stools by polymerase chain reaction (PCR) is currently limited to clarithromycin. In addition, recent meta‐analyses reported that susceptibility‐guided tailored therapy was not better than empirical quadruple therapy with and without bismuth either in first‐line or in rescue treatment of H. pylori infection. 18 , 19 To date, few randomized studies have compared the culture‐based susceptibility‐guided tailored therapy and the empirical concomitant therapy as the first‐line treatment for H. pylori.
In the present study, we aimed to compare the efficacy and tolerability of culture‐based susceptibility‐guided tailored therapy versus empirical concomitant therapy as the first‐line treatment for H. pylori in a region with high antimicrobial resistance.
MATERIALS AND METHODS
Trial conduct
The present study was designed as a multicenter, open‐label, randomized trial comparing the efficacy of culture‐based susceptibility‐guided tailored therapy versus empirical concomitant therapy. This study was conducted between April 2020 and January 2023 at four institutions (Asan Medical Center, Samsung Medical Center, Gangneung Asan Hospital, and Samsung Changwon Hospital) across three geographic areas of Korea (Seoul, Gangwon, and Kyungsang). Informed consent was obtained from all subjects. The study protocol was approved by the institutional review board of each participating hospital. This trial was performed in accordance with the ethical principles of the Declaration of Helsinki and registered with CRIS (KCT0004877).
Patients
H. pylori treatment‐naïve patients were assessed for eligibility when upper endoscopy was required to evaluate the cause of any abdominal symptom or for screening or workup of upper gastrointestinal neoplasm and indications for the H. pylori eradication according to Korean guidelines were met. 12 During upper endoscopy, at least two gastric biopsies were taken from the gastric antrum and body, respectively, for rapid urease test and H. pylori culture. H. pylori culture was performed when rapid urease test showed positive results for H. pylori infection. Exclusion criteria included negative H. pylori culture, history of previous H. pylori eradication therapy, age <19 years, previous gastric or esophageal surgery, allergy to any of the study medications, gastrointestinal bleeding, pregnancy or lactation, and severe comorbidity precluding study therapy. Patients were excluded if they took a proton pump inhibitor, potassium competitive acid blocker, or histamine‐2 blocker within 4 weeks before upper endoscopy or if they needed to take these drugs within 4 weeks before randomization or during the study period from the timing of randomization to 13C‐urea breath test. Patients were also excluded if they took antibiotics or bismuth within 4 weeks before upper endoscopy or if they needed to take these drugs after undergoing upper endoscopy. Patients who could not discontinue steroid treatment during the study period were also excluded.
Isolation of H. pylori and antimicrobial susceptibility testing
For H. pylori culture, two biopsy specimens (one from antrum and one from body) were taken from each patient using standard‐sized biopsy forceps during an upper endoscopy. These samples were placed in a sterile Eppendorf tube and stored at −80°C. These frozen samples were delivered to a central laboratory at Asan Medical Center, Seoul, Korea for H. pylori culture. Isolation of H. pylori and antimicrobial susceptibility testing process using the serial two‐fold agar dilution method have been described in detail elsewhere. 2 , 20 The resistance cutoff values for clarithromycin and metronidazole were ≥0.5 mg/L and >8 mg/L, respectively, based on The European Committee on Antimicrobial Susceptibility Testing ver 10.0 (EUCAST). The subject was considered to have a resistant infection when any strain was resistant to antibiotics.
Randomization
Figure 1 shows a flow chart of the present study. Subjects with positive H. pylori cultures were randomized to receive culture‐based susceptibility‐guided tailored therapy or empirical non‐bismuth concomitant quadruple therapy for 10 days in a 3:1 ratio. Randomization was done with computerized random number sequence generated by an independent statistician. All investigators were masked to the randomization sequence and an independent research assistant notified the study staff of the randomization sequence. As this study was open‐labeled, subjects were not blinded. The technicians who performed culture, antimicrobial susceptibility testing, or urea breath testing were blinded to treatment allocation.
FIGURE 1.
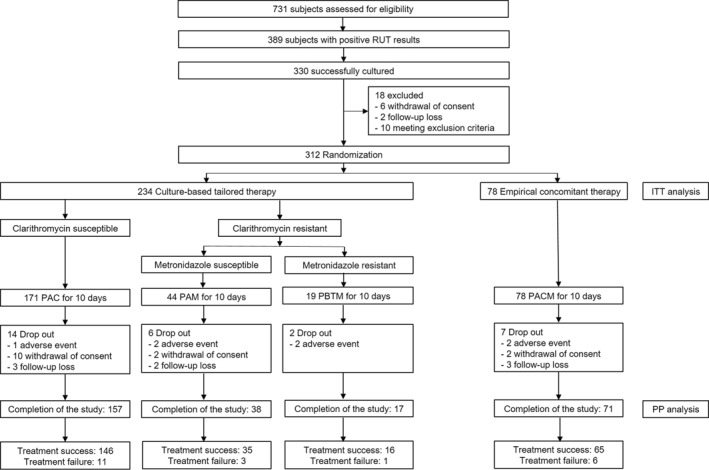
A flow diagram for the trial. RUT, rapid urease test; ITT, intention‐to‐treat; PP, per‐protocol; PAC, lansoprazole, amoxicillin, and clarithromycin; PAM, lansoprazole, amoxicillin, and metronidazole; PBTM, lansoprazole, bismuth potassium citrate, tetracycline and metronidazole; PACM, lansoprazole, amoxicillin, clarithromycin, and metronidazole.
Trial intervention
Patients treated with empirical concomitant therapy received lansoprazole 30 mg twice a day, amoxicillin 1 g twice a day, clarithromycin 500 mg twice a day, and metronidazole 500 mg twice a day for 10 days (PACM). Culture‐based susceptibility‐guided tailored therapy was based on antimicrobial susceptibility to clarithromycin and metronidazole: 1) if the strain was susceptible to both clarithromycin and metronidazole, patients received lansoprazole 30 mg twice a day, amoxicillin 1 g twice a day, and clarithromycin 500 mg twice a day for 10 days (PAC) 2) if the strain was susceptible to clarithromycin, but resistant to metronidazole, patients also received lansoprazole 30 mg twice a day, amoxicillin 1 g twice a day, and clarithromycin 500 mg twice a day for 10 days (PAC) 3) if the strain was susceptible to metronidazole, but resistant to clarithromycin, patients took lansoprazole 30 mg twice a day, amoxicillin 1 g twice a day, and metronidazole 500 mg twice a day for 10 days (PAM) 4) if the strain was resistant to both clarithromycin and metronidazole, patients received lansoprazole 30 mg twice a day, bismuth potassium citrate 300 mg four times a day, tetracycline 500 mg four times a day and metronidazole 500 mg three times a day for 10 days (PBTM). 21
To check compliance with the study protocol and adverse events, telephone monitoring was performed by an independent research assistant 12–15 days after patients started taking study medication. When patients reported any adverse event during telephone monitoring, the investigators inquired them and assessed the severity using the 1 to 4 grading system based on the Common Terminology Criteria for Adverse Events (CTCAE) V.5.0. 22 The patients were also asked to grade the severity of adverse events according to the influence on their daily activities, experienced as “mild” (discomfort with no interruption of their daily activities), “moderate” (discomfort affecting their daily activities) or “severe” (severe interruption of their daily activities). Patients were considered to have demonstrated poor compliance if they took less than 80% of the total medication prescribed.
At least 4 weeks after the completion of therapy, 13C‐urea breath test was performed to evaluate eradication success. H. pylori eradication was defined as a negative urea breath test.
Study outcomes
The primary endpoint of this study was the H. pylori eradication rate in the major treatment groups (culture‐based susceptibility‐guided tailored therapy vs. empirical concomitant therapy). The secondary endpoints were the eradication rates with each of the individual components of the culture‐based tailored therapy and the frequency and severity of adverse events.
Statistical analysis
This study was a superiority‐design trial comparing strategies. The sample size estimation was based on previous trials performed in Korea. In a recent Korean randomized study, the intention to treat (ITT) eradication rate of culture‐based susceptibility‐guided tailored therapy was 94.7% as the first‐line H. pylori treatment. 17 In another Korean nationwide randomized study, the ITT eradication rate of empirical concomitant therapy was 81.2% as the first‐line therapy. 6 We calculated the sample size to detect a difference of 13% in the eradication rate between the culture‐based susceptibility‐guided tailored therapy (assuming 94% eradication rate) and the empirical concomitant therapy (assuming 81% eradication rate) with a power of 90% and a significance level of 0.05 (an alpha of 0.05, two sided) in a ratio of 3:1. With this calculation, at least 210 subjects in culture‐based tailored therapy and 70 subjects in empirical concomitant therapy would be required to show superiority of culture‐based tailored therapy over empirical concomitant therapy. Taking into consideration a dropout rate of 10%, at least 312 subjects (234 for culture‐based tailored therapy and 78 for empirical concomitant therapy) were expected to be recruited for the study.
The H. pylori eradication rate of each group was assessed using ITT and PP analyses. Subjects lost to follow‐up were scored as treatment failures in the ITT analysis. Subjects with poor compliance, withdrawal of consent, or lost follow‐up were excluded from the PP analysis. Comparative superiority of the two groups was assessed through the derivation of a two‐sided 95% confidence interval (CI) of difference based on the H. pylori eradication rate. Between‐group differences were evaluated using Student's t test for continuous variables and Pearson's χ 2 test or Fisher's exact test for categorical variables, as appropriate, and were analyzed with the SPSS software (version 28). All p values were two‐sided, and were considered statistically significant if less than 0.05.
RESULTS
Baseline characteristics
A flow diagram for the trial is provided in Figure 1. A total of 389 subjects with positive rapid urease test results underwent H. pylori culture test. H. pylori culture was successful in 330 subjects. Among them, 312 subjects were finally enrolled and randomly assigned to either culture‐based susceptibility‐guided tailored therapy or empirical concomitant therapy in a ratio of 3:1. The baseline characteristics were well balanced between the two groups, including demographic data, clinical characteristics and prevalence of antimicrobial resistance (Table 1 for ITT analysis; Table S1 for PP analysis). Among the randomized subjects, resistance rates to clarithromycin and metronidazole were 26.9% (84/312) and 23.1% (72/312), respectively. Dual resistance to both clarithromycin and metronidazole was observed in 8.0% (25/312) of the study subjects.
TABLE 1.
Baseline characteristics of intention‐to‐treat analysis population.
| Culture‐based tailored therapy | Empirical concomitant therapy | p value | |
|---|---|---|---|
| (n = 234) | (n = 78) | ||
| Age | 0.739 | ||
| Mean ± SD | 60.1 ± 10.2 | 60.5 ± 10.8 | |
| Range | 28–82 | 31–79 | |
| Gender | 0.225 | ||
| Male (%) | 141 (60.3) | 53 (67.9) | |
| Female (%) | 93 (39.7) | 25 (32.1) | |
| BMI | |||
| Mean ± SD | 24.3 ± 3.1 | 24.2 ± 2.6 | 0.767 |
| Smoking | 0.725 | ||
| Never‐smoker (%) | 149 (63.7) | 46 (59.0) | |
| Ex‐smoker (%) | 56 (23.9) | 22 (28.2) | |
| Current‐smoker (%) | 29 (12.4) | 10 (12.8) | |
| Alcohol drinking | 0.800 | ||
| Never‐drinker (%) | 97 (41.5) | 29 (37.2) | |
| Ex‐drinker (%) | 62 (26.5) | 22 (28.2) | |
| Current‐drinker (%) | 75 (32.1) | 27 (34.6) | |
| Indication for eradication | 0.365 | ||
| Atrophic gastritis (%) | 79 (33.8) | 23 (29.5) | |
| Gastric ulcer (%) | 8 (3.4) | 4 (5.1) | |
| Duodenal ulcer (%) | 8 (3.4) | 6 (7.7) | |
| Gastric neoplasm (%) | 139 (59.4) | 45 (57.7) | |
| Clarithromycin | 0.556 | ||
| Susceptible (%) | 169 (72.2) | 59 (75.6) | |
| Resistant (%) | 65 (27.8) | 19 (24.4) | |
| Metronidazole | 0.352 | ||
| Susceptible (%) | 183 (78.2) | 57 (73.1) | |
| Resistant (%) | 51 (21.8) | 21 (26.9) | |
| Dual‐susceptible (%) | 137 (58.5) | 44 (56.4) | 0.741 |
| Dual‐resistant (%) | 19 (8.1) | 6 (7.7) | 0.904 |
Abbreviation: BMI, body mass index.
H. pylori eradication rates
The H. pylori eradication rates of each therapy group are summarized in Table 2. In the ITT analysis, the H. pylori eradication rate was 84.2% (95% CI 79.1% to 88.4%) for the culture‐based tailored group and 83.3% (95% CI 73.9%–90.3%) for the empirical concomitant group. In the PP analysis, the H. pylori eradication rate was 92.9% (95% CI 88.9%–95.8%) for the culture‐based tailored group and 91.5% (95% CI 83.4%–96.4%) for the empirical concomitant group. Therefore, in Korean regions with high antimicrobial resistance, culture‐based susceptibility‐guided tailored therapy was not superior to locally effective empirical concomitant therapy in terms of eradication rate as the first‐line treatment for H. pylori.
TABLE 2.
H. pylori eradication rates of each therapy group and eradication rates according to each treatment regimen and antimicrobial susceptibility.
| Culture‐based tailored therapy | Empirical concomitant therapy | p value | ||
|---|---|---|---|---|
| Intention‐to‐treat | 84.2% (197/234) | 83.3% (65/78) | 0.859 | |
| 95% CI | 79.1%–88.4% | 73.9%–90.3% | ||
| Per‐protocol | 92.9% (197/212) | 91.5% (65/71) | 0.702 | |
| 95% CI | 88.9%–95.8% | 83.4%–96.4% | ||
| Resistance pattern (Per‐protocol) | ||||
| Clarithromycin | metronidazole | |||
| Susceptible | susceptible | 94.5% (120/127; PAC) | 97.5% (39/40; PACM) | 0.681 |
| Susceptible | resistant | 86.7% (26/30; PAC) | 100% (14/14; PACM) | 0.290 |
| Resistant | susceptible | 92.1% (35/38; PAM) | 90.9% (10/11; PACM) | 1.000 |
| Resistant | resistant | 94.1% (16/17; PBTM) | 33.3% (2/6; PACM) | 0.008 |
Abbreviations: CI, confidence interval; PAC, lansoprazole, amoxicillin, and clarithromycin; PACM, lansoprazole, amoxicillin, clarithromycin, and metronidazole; PAM, lansoprazole, amoxicillin, and metronidazole; PBTM, lansoprazole, bismuth potassium citrate, tetracycline and metronidazole.
H. pylori eradication rates according to antimicrobial susceptibility
We performed subgroup analysis to investigate the influence of antimicrobial resistance on the H. pylori eradication rate in the PP analysis population (Table 2). In the metronidazole‐resistant strain (n = 67), the eradication rate in the culture‐based tailored group was comparable to that in the empirical concomitant group (89.4% vs. 80.0%, p = 0.434). In the clarithromycin‐resistant strain (n = 72), however, the eradication rate in the culture‐based tailored group was significantly higher than that in the empirical concomitant group (92.7% vs. 70.6%, p = 0.029). Clarithromycin‐resistant strain consists of clarithromycin‐resistant/metronidazole‐susceptible H. pylori strain and dual‐resistant H. pylori strain. In the PP analysis population, the eradication rate of the PAM regimen for clarithromycin‐resistant/metronidazole‐susceptible strain in the culture‐based tailored group was comparable to that of the PACM regimen in the empirical concomitant group (92.1% vs. 90.9%). In contrast, the eradication rate of the PBTM regimen for the dual‐resistant strain in the culture‐based tailored group was significantly higher than that of the PACM regimen in the empirical concomitant group (94.1% vs. 33.3%, p = 0.008). The eradication rates of the PAC regimen for the clarithromycin‐susceptible strain in the culture‐based tailored group were comparable to those of the PACM regimen in the empirical concomitant group regardless of metronidazole‐susceptibility (94.5% vs. 97.5% and 86.7% vs. 100%, respectively).
Adverse events
The adverse events in each therapy group are shown in Table 3. The proportion of subjects experiencing moderate grade (discomfort affecting their daily activities) of adverse events was significantly higher in the empirical concomitant group (34.7%) than in the culture‐based tailored group (12.2%). No patient complained of a severe grade of adverse events in either group. Taste alteration, constipation, and dizziness were significantly more frequent in the empirical concomitant group than those in the culture‐based tailored group. All adverse events were grade 1 or 2 based on CTCAE and disappeared after the eradication therapy ceased. Two subjects in the empirical concomitant group (2.6%, 2/78) and five in the culture‐based tailored group (2.1%, 5/234) discontinued the eradication treatment because of adverse events (Figure 1). No subjects were hospitalized because of adverse events.
TABLE 3.
Adverse events in each therapy group.
| Culture‐based tailored therapy | Empirical concomitant therapy | p value | |
|---|---|---|---|
| (n = 229) | (n = 75) | ||
| Influence on daily activity | <0.001 | ||
| None | 124 (54.1) | 21 (28.0) | |
| Mild | 77 (33.6) | 28 (37.3) | |
| Moderate | 28 (12.2) | 26 (34.7) | |
| Taste alteration (%) | 49 (21.4) | 34 (45.3) | <0.001 |
| Grade 2 | 10 | 16 | |
| Diarrhea (%) | 40 (17.5) | 17 (22.7) | 0.317 |
| Grade 2 | 12 | 7 | |
| Constipation (%) | 3 (1.3) | 6 (8.0) | 0.008 |
| Grade 2 | 0 | 3 | |
| Abdominal pain (%) | 11 (4.8) | 8 (10.7) | 0.095 |
| Grade 2 | 3 | 3 | |
| Nausea (%) | 20 (8.7) | 9 (12.0) | 0.403 |
| Grade 2 | 3 | 4 | |
| Vomiting (%) | 4 (1.7) | 2 (2.7) | 0.639 |
| Grade 2 | 1 | 1 | |
| Bloating (%) | 5 (2.2) | 4 (5.3) | 0.232 |
| Grade 2 | 1 | 0 | |
| Belching (%) | 0 (0.0) | 2 (2.7) | 0.060 |
| Grade 2 | 0 | 0 | |
| Acid reflux (%) | 4 (1.7) | 4 (5.3) | 0.106 |
| Grade 2 | 0 | 1 | |
| Chest distress (%) | 3 (1.3) | 3 (4.0) | 0.162 |
| Grade 2 | 2 | 2 | |
| Dizziness (%) | 7 (3.1) | 11 (14.7) | 0.001 |
| Grade 2 | 3 | 6 | |
| Fatigue (%) | 10 (4.4) | 8 (10.7) | 0.086 |
| Grade 2 | 3 | 5 | |
| Skin rash (%) | 5 (2.2) | 0 (0.0) | 0.338 |
| Grade 2 | 0 | 0 |
DISCUSSION
To the best of our knowledge, this is the first randomized study comparing the empirical concomitant therapy and the culture‐based susceptibility‐guided tailored therapy where treatment regimens were optimized according to the presence of resistance to multiple antibiotics. In the present study, the culture‐based susceptibility‐guided tailored therapy was not superior to the locally effective empirical concomitant therapy in terms of eradication rate as the first‐line treatment for H. pylori. The incidence of adverse events was significantly lower in the tailored group than in the concomitant group. However, the proportion of patients discontinuing treatment for adverse events was comparable between the two groups (2.6% for the concomitant group and 2.1% for the tailored group). Furthermore, no patients complained of a severe grade of adverse events in either group and all adverse events disappeared after the eradication therapy ceased.
Two recent Korean randomized studies compared the empirical concomitant therapy and the PCR‐based susceptibility‐guided tailored therapy as the first‐line treatment for H. pylori. 23 , 24 In both studies, treatment regimens in the PCR‐based tailored therapy group were determined only according to the presence of genotypic clarithromycin resistance alone, which implied a limitation in treatment optimization. Both studies showed comparable H. pylori eradication rates between the concomitant group and the tailored group, which was consistent with the present study. In contrast to Korean studies using the PCR method, a Chinese randomized study by Zhou et al. 25 compared the culture‐based susceptibility‐guided tailored therapy with the empirical concomitant therapy as the first‐line treatment for H. pylori. Although they used the culture‐based E‐test, treatment regimens in the tailored therapy group were determined only according to the presence of phenotypic clarithromycin resistance. In this study, the PP eradication rate of the concomitant group (87.4%) was significantly lower than that of the tailored group (93.3%). This discrepancy between Korean and Chinese studies might be mainly due to the difference in treatment regimens and the prevalence of dual resistance to both clarithromycin and metronidazole. It is well known that concomitant therapy is ineffective against dual resistant strains and recent guidelines recommended against concomitant therapy if the prevalence of dual resistance is over 15%. 11 In the study by Zhou et al., 25 the dual resistance rate was up to 35.3% and H. pylori eradication rate against dual resistant strains was 75.9% for the concomitant group in the PP analysis, which was significantly lower than 90.4% in the tailored group. Dual resistance rates in other countries were far lower than those in China. The prevalence of dual resistance in Korea, the US, and Europe was reported to be 7.1%, 10.5% and 13.4%, respectively. 2 , 4 , 7 In the present study, the dual resistance rate was 8.0%.
Given the need for additional resources and costs and the comparable eradication rates reported in the present and previous studies, 23 , 24 evidence might be currently insufficient to support the superiority of susceptibility‐guided tailored therapy over empirical concomitant therapy in clinical practice. Although concomitant therapy carries the risk of antibiotic overuse, recent large studies showed that the prevalence of antibiotic resistance of E. coli and K. pneumoniae transiently increased at week 2 but returned to the basal state at week 8 and 1 year after completion of concomitant therapy and clarithromycin‐based triple therapy as the first‐line treatment. The authors of these studies concluded that the short‐term increase in antibiotic resistance after eradication therapy was reversible. 26 , 27 Practically speaking, empirical concomitant therapy can be preferred in clinical practice for its relative simplicity and comparable eradication rate, especially in areas where the prevalence of dual resistance to clarithromycin and metronidazole is less than 15%, such as Korea, US, and Europe. 2 , 4 , 7 , 23 , 24 A large Italian study reported that empirical sequential therapy achieved an eradication rate of 83.1% against dual resistant strain. 28 Given these data, empirical sequential therapy as well as susceptibility‐guided tailored therapy might be considered as a treatment option in areas where the prevalence of dual resistance is high.
This study design has several advantages. First, the study was a large randomized controlled trial conducted in multiple institutions. Second, the antibacterial susceptibility of H. pylori was confirmed in all enrolled patients, and treatments were performed accordingly. However, owing to the open‐label nature of the study design, the lack of blinding might have influenced the outcomes such as adverse events. To minimize the bias, the technicians who performed culture, antimicrobial susceptibility testing, or urea breath testing were blinded to treatment allocation. Based on previous trials performed in Korea, 6 , 17 the study was designed and powered as a superiority trial. In contrast to our initial assumption, however, we found that the culture‐based susceptibility‐guided tailored therapy was not better than the empirical concomitant therapy. Therefore, non‐inferiority designed trials are required to confirm this result. Performing telephone monitoring after treatment completion and not using a symptom diary during the study period might have affected the accuracy in reporting compliance or adverse events. Finally, we only randomized patients undergoing endoscopy with a successful culture of H. pylori. Therefore, ITT analysis did not include the number of patients with culture failure or who did not give consent to an invasive endoscopy, which might lead to an overestimation of H. pylori eradication rate in the culture‐based tailored group. Even in the optimal conditions, culture sensitivity is usually below 90%. 18 , 19
In conclusion, both culture‐based susceptibility‐guided tailored therapy and empirical concomitant therapy provided acceptable H. pylori eradication rates and safety profiles as the first‐line treatment for H. pylori despite high rates of antimicrobial resistance. As evidence might be currently insufficient to support the superiority of culture‐based susceptibility‐guided tailored therapy over empirical concomitant therapy, the treatment choice in clinical practice would depend on the background rate of antimicrobial resistance, availability of resources and costs associated with culture and susceptibility testing.
AUTHOR CONTRIBUTIONS
Jeong Hoon Lee and Byung‐Hoon Min: Conception and design, analysis and interpretation of data, and drafting the manuscript. Eun Jeong Gong, Jun Young Kim, Hee Kyong Na., Ji Yong Ahn., Do Hoon Kim, Kee Don Choi, Yang Won Min, Hyuk Lee, and Jun Haeng Lee: Acquisition of data and critical revision of the manuscript for important intellectual content. Hwoon‐Yong Jung. and Jae J. Kim: Conception and design, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content. All authors had access to the study data and reviewed and approved the final version of the article, including the authorship list.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
CLINICAL TRIAL REGISTRATION
CRIS (KCT0004877), https://cris.nih.go.kr/.
Supporting information
Table S1
ACKNOWLEDGMENTS
This work was supported by the Korean College of Helicobacter and Upper Gastrointestinal Research Foundation Grant (KCHUGR‐201902505). The funder had no role in the design and conduct of the study: collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Lee JH, Min B‐H, Gong EJ, Kim JY, Na HK, Ahn JY, et al. Culture‐based susceptibility‐guided tailored versus empirical concomitant therapy as first‐line Helicobacter pylori treatment: a randomized clinical trial. United European Gastroenterol J. 2024;12(7):941–50. 10.1002/ueg2.12609
[Correction added on 3 July 2024, after first online publication: Eun Jeong Gong’s affiliation has been updated.]
Jeong Hoon Lee and Byung‐Hoon Min These two authors contributed equally as first authors.
Hwoon‐Yong Jung Guarantor of the article.
Contributor Information
Hwoon‐Yong Jung, Email: hyjung@amc.seoul.kr.
Jae J. Kim, Email: jjkim@skku.edu.
DATA AVAILABILITY STATEMENT
Anonymized data will be made available upon reasonable request to the chief investigator. Proposals will be reviewed and approved by the chief investigator and collaborators based on scientific merit. After approval of a proposal or request, data can be shared through a secure online platform after signing a data access agreement.
REFERENCES
- 1. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59(8):1143–1153. 10.1136/gut.2009.192757 [DOI] [PubMed] [Google Scholar]
- 2. Lee JH, Ahn JY, Choi KD, Jung H, Kim JM, Baik GH, et al. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: a prospective multicenter study. Helicobacter. 2019;24(4):e12592. 10.1111/hel.12592 [DOI] [PubMed] [Google Scholar]
- 3. Graham DY. Implications of the paradigm shift in management of Helicobacter pylori infections. Therap Adv Gastroenterol. 2023;16:17562848231160858. 10.1177/17562848231160858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hulten KG, Lamberth LB, Kalfus IN, Graham DY. National and regional US antibiotic resistance to Helicobacter pylori: lessons from a clinical trial. Gastroenterology. 2021;161(1):342–344e341. 10.1053/j.gastro.2021.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Argueta EA, Alsamman MA, Moss SF, D'Agata EMC. Impact of antimicrobial resistance rates on eradication of Helicobacter pylori in a US population. Gastroenterology. 2021;160(6):2181–2183e2181. 10.1053/j.gastro.2021.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim BJ, Lee H, Lee YC, Jeon SW, Kim GH, Kim HS, et al. Ten‐day concomitant, 10‐day sequential, and 7‐day triple therapy as first‐line treatment for Helicobacter pylori infection: a nationwide randomized trial in Korea. Gut Liver. 2019;13(5):531–540. 10.5009/gnl19136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nyssen OP, Bordin D, Tepes B, Pérez‐Aisa Á, Vaira D, Caldas M, et al. European Registry on Helicobacter pylori management (Hp‐EuReg): patterns and trends in first‐line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut. 2021;70(1):40–54. 10.1136/gutjnl-2020-321372 [DOI] [PubMed] [Google Scholar]
- 8. Suzuki S, Gotoda T, Kusano C, Ikehara H, Ichijima R, Ohyauchi M, et al. Seven‐day vonoprazan and low‐dose amoxicillin dual therapy as first‐line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut. 2020;69(6):1019–1026. 10.1136/gutjnl-2019-319954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishibashi F, Suzuki S, Nagai M, Mochida K, Morishita T. Optimizing Helicobacter pylori treatment: an updated review of empirical and susceptibility test‐based treatments. Gut Liver. 2023;17(5):684–697. 10.5009/gnl220429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212–239. 10.1038/ajg.2016.563 [DOI] [PubMed] [Google Scholar]
- 11. Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;71(9):1724–1762. 10.1136/gutjnl-2022-327745 [DOI] [Google Scholar]
- 12. Jung HK, Kang SJ, Lee YC, Yang HJ, Park SY, Shin CM, et al. Evidence‐based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Gut Liver. 2021;15(2):168–195. 10.5009/gnl20288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, et al. Fifth Chinese national consensus report on the management of Helicobacter pylori infection. Helicobacter. 2018;23(2):e12475. 10.1111/hel.12475 [DOI] [PubMed] [Google Scholar]
- 14. Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta‐analysis in world health organization regions. Gastroenterology. 2018;155(5):1372–1382e1317. 10.1053/j.gastro.2018.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jun JK, Choi KS, Lee HY, Suh M, Park B, Song SH, et al. Effectiveness of the Korean national cancer screening program in reducing gastric cancer mortality. Gastroenterology. 2017;152(6):1319–1328e1317. 10.1053/j.gastro.2017.01.029 [DOI] [PubMed] [Google Scholar]
- 16. Lee JW, Kim N, Nam RH, Lee SM, Kwon YH, Sohn SD, et al. Favorable outcomes of culture‐based Helicobacter pylori eradication therapy in a region with high antimicrobial resistance. Helicobacter. 2019;24(2):e12561. 10.1111/hel.12561 [DOI] [PubMed] [Google Scholar]
- 17. Park CS, Lee SM, Park CH, Koh HR, Jun CH, et al. Pretreatment antimicrobial susceptibility‐guided vs. clarithromycin‐based triple therapy for Helicobacter pylori eradication in a region with high rates of multiple drug resistance. Am J Gastroenterol. 2014;109(10):1595–1602. 10.1038/ajg.2014.222 [DOI] [PubMed] [Google Scholar]
- 18. Nyssen OP, Espada M, Gisbert JP. Empirical vs. Susceptibility‐guided treatment of Helicobacter pylori infection: a systematic review and meta‐analysis. Front Microbiol. 2022;13:913436. 10.3389/fmicb.2022.913436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gingold‐Belfer R, Niv Y, Schmilovitz‐Weiss H, Levi Z, Boltin D. Susceptibility‐guided versus empirical treatment for Helicobacter pylori infection: a systematic review and meta‐analysis. J Gastroenterol Hepatol. 2021;36(10):2649–2658. 10.1111/jgh.15575 [DOI] [PubMed] [Google Scholar]
- 20. Gong EJ, Ahn JY, Kim JM, Lee SM, Na HK, Lee JH, et al. Genotypic and phenotypic resistance to clarithromycin in Helicobacter pylori strains. J Clin Med. 2020;9(6):1930. 10.3390/jcm9061930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Graham DY, Moss SF. Antimicrobial susceptibility testing for Helicobacter pylori is now widely available: when, how, why. Am J Gastroenterol. 2022;117(4):524–528. 10.14309/ajg.0000000000001659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Rogak LJ, et al. Validity and reliability of the US national cancer institute's patient‐reported outcomes version of the Common Terminology criteria for adverse events (PRO‐CTCAE). JAMA Oncol. 2015;1(8):1051–1059. 10.1001/jamaoncol.2015.2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ong S, Kim SE, Kim JH, Yi NH, Kim TY, Jung K, et al. Helicobacter pylori eradication rates with concomitant and tailored therapy based on 23S rRNA point mutation: a multicenter randomized controlled trial. Helicobacter. 2019;24(5):e12654. 10.1111/hel.12654 [DOI] [PubMed] [Google Scholar]
- 24. Choi YI, Chung JW, Kim KO, Kwon KA, Kim YJ, et al. Tailored eradication strategy vs concomitant therapy for Helicobacter pylori eradication treatment in Korean patients. World J Gastroenterol. 2021;27(31):5247–5258. 10.3748/wjg.v27.i31.5247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou L, Zhang J, Song Z, Li Y, Qian J, et al. Tailored versus triple plus bismuth or concomitant therapy as initial Helicobacter pylori treatment: a randomized trial. Helicobacter. 2016;21(2):91–99. 10.1111/hel.12242 [DOI] [PubMed] [Google Scholar]
- 26. Liou JM, Chen CC, Chang CM, Fang YJ, Bair MJ, Chen PY, et al. Long‐term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open‐label, randomised trial. Lancet Infect Dis. 2019;19(10):1109–1120. 10.1016/S1473-3099(19)30272-5 [DOI] [PubMed] [Google Scholar]
- 27. Liou JM, Jiang XT, Chen CC, Luo JC, Bair MJ, Chen PY, et al. Second‐line levofloxacin‐based quadruple therapy versus bismuth‐based quadruple therapy for Helicobacter pylori eradication and long‐term changes to the gut microbiota and antibiotic resistome: a multicentre, open‐label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2023;8(3):228–241. 10.1016/S2468-1253(22)00384-3 [DOI] [PubMed] [Google Scholar]
- 28. Gatta L, Scarpignato C, Fiorini G, Belsey J, Saracino IM, Ricci C, et al. Impact of primary antibiotic resistance on the effectiveness of sequential therapy for Helicobacter pylori infection: lessons from a 5‐year study on a large number of strains. Aliment Pharmacol Ther. 2018;47(9):1261–1269. 10.1111/apt.14597 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Anonymized data will be made available upon reasonable request to the chief investigator. Proposals will be reviewed and approved by the chief investigator and collaborators based on scientific merit. After approval of a proposal or request, data can be shared through a secure online platform after signing a data access agreement.


