Abstract
BACKGROUND
Subjective cognitive decline (SCD) has been recognized as a potential risk stage for progression to Alzheimer's disease (AD), while glymphatic dysfunction is considered an important characteristic of AD. We hypothesize that glymphatic dysfunction occurs during the SCD stage, aiming to discover potential biomarkers for SCD.
METHODS
Participants from two independent studies, Sino Longitudinal Study on Cognitive Decline (SILCODE, n = 654) and the Alzheimer's Disease Neuroimaging Initiative (ADNI, n = 650), representing different ethnicities and disease stages, were included to assess glymphatic function using diffusion tensor image analysis along the perivascular space (DTI‐ALPS).
RESULTS
Abnormal glymphatic function occurs during the SCD stage, with the ALPS index demonstrating excellent classification performance for SCD and normal controls (area under the receiver operating characteristic curve [AUC]SILCODE = 0.816, AUCADNI = 0.797). Lower ALPS index indicates higher risk of cognitive progression, which is negatively correlated with Subjective Cognitive Decline Questionnaire 9 scores and amyloid positron emission tomography burden.
DISSCUSION
Our study suggests the ALPS index has the potential to serve as a biomarker for SCD.
Highlights
Glymphatic function characterized by the analysis along the perivascular space (ALPS) index becomes abnormal in subjective cognitive decline (SCD), the earliest symptomatic manifestation and preclinical stage of Alzheimer's disease (AD).
The ALPS index demonstrates excellent classification performance for SCD and normal controls in the East Asian and Western cohorts.
Participants with a lower ALPS index show a higher risk of clinical progression.
The ALPS index is closely associated with serval cognitive scales and amyloid beta burden.
Keywords: Alzheimer's disease, biomarker, diffusion tensor image analysis along the perivascular space, glymphatic, subjective cognitive decline
1. INTRODUCTION
Alzheimer's disease (AD) is a progressive neurodegenerative disease that is irreversible. 1 Many studies have shown that drug clinical trials fail because treatment is initiated too late. Early diagnosis and early intervention are critical for controlling the progression of the disease. Subjective cognitive decline (SCD) is the earliest symptomatic manifestation and preclinical stage of AD. 2 Research based on biomarkers has found that SCD has characteristic pathological changes similar to AD, further suggesting that SCD is a high risk for AD. 3 The risk ratio of SCD developing into dementia in the future is approximately 1.63 to 3.01. 4 , 5 Therefore, SCD is an important gateway for AD prevention and treatment. 3
AD is characterized by progressive cognitive decline, reflecting potential amyloid beta (Aβ) depositions, tau protein depositions, synaptic loss, and neurodegeneration. 6 , 7 , 8 The glymphatic system is a novel mechanism of waste removal in the brain. It refers to the flow of cerebrospinal fluid (CSF) into the cerebral parenchyma along the periarterial space, and then the interstitial fluid transports the metabolic waste to the meningeal lymphatic vessels and deep cervical lymph nodes. 9 Recent research has shown that the cerebral glymphatic system is involved in the clearance of ≈ 55% to 65% of Aβ protein in the brain of mice. 10 Successive further studies have indicated that the glymphatic dysfunction is considered a common pathway for AD. 11 , 12 , 13
Currently, neurologists have applied neuroimaging techniques to quantify glymphatic dysfunction. Diffusion tensor image (DTI) analysis along the perivascular space (ALPS) index has been used to evaluate the function of the glymphatic system, and is considered a biomarker for disease progression or treatment assessment in patients with AD. 13 , 14 Previous studies have found a close correlation between the ALPS index and several AD biomarkers, including CSF Aβ42, as well as Aβ deposition, tau entanglement, and glucose metabolism on positron emission tomography (PET). 15 , 16 It has also been observed that the decreased ALPS index shows significant consistency with various AD‐related multiple cognitive functioning performances. 15 , 17 However, whether the ALPS index can be used as a biomarker in the SCD stage is still unknown. It is important to explore the glymphatic activity alterations in SCD individuals and the relationship to cognitive decline.
Therefore, the aim of this study is to investigate whether the ALPS index, which characterizes glymphatic function, has the potential to serve as an imaging biomarker for the SCD stage. We hypothesize that glymphatic dysfunction occurs during the SCD stage, potentially contributing to cognitive progression.
2. MATERIALS AND METHODS
2.1. Participants
We included 1304 participants from two independent studies, the Sino Longitudinal Study on Cognitive Decline (SILCODE, ClinicalTrials.gov identifier: NCT03370744) from China and the Alzheimer's Disease Neuroimaging Initiative (ADNI, http://adni.loni.usc.edu/). We investigated the potential of the ALPS index as a biomarker for SCD and its relationship to clinical and pathological features of AD for each cohort.
SILCODE is a project aimed at the diagnosis of SCD and mild cognitive impairment (MCI) in the early stage of AD using multimodal data, including basic clinical information, neuropsychological assessment, biological markers, and neuroimaging. 18 The SILCODE study was approved by the local ethics committee of Xuanwu Hospital of Capital Medical University (2017[046]). Informed consent was signed by all participants. ADNI, established by Dr. Michael W. Weiner, is a comprehensive, multicenter research project backed by diverse organizations and funds, including the National Institute on Aging (NIA) and the Food and Drug Administration (FDA). Its objective is to develop and investigate a range of biomarkers—clinical, imaging, and biochemical—to aid in the early detection and monitoring of AD, with open access for researchers.
The current analyses included data from 654 SILCODE participants (215 normal controls [NC], 194 SCD, 153 MCI, and 92 AD) and 650 ADNI participants (197 NC, 191 SCD, 158 MCI, and 104 AD) after quality control. Both studies were approved by the responsible ethics committees and radiation protection authorities. All participants provided written informed consent.
2.2. Inclusion criterion
SILCODE: NC participants were volunteers without any concerns about cognitive decline and whose neuropsychologic test scores were in the normal range. The entry criterion of SCD referred to the conceptual framework proposed by Jessen et al. in 2014 19 and our previous references, 18 , 20 including (1) self‐reported experience of persistent decline in memory compared to a previous state (within the last 5 years), (2) persistent concerns about memory changes, and (3) performance within the normal range on all clinical scales (adjusted for age, sex, and education; versions suitable for the Chinese). The diagnosis of MCI was based on a neuropsychological method. The diagnostic criteria for MCI meet the Jak/Bondi diagnostic criteria for MCI: (1) at least two scores on the same cognitive domain (memory, language, or executive function) on the neuropsychological test are both impaired to a degree ≥ 1.0 standard deviation (SD); (2) one score on each cognitive domain (memory, language, executive function) of the neuropsychological test was impaired to a degree ≥ 1.0 SD; (3) Functional Activity Questionnaire (FAQ) score ≥ 9, indicating impairment in the ability to perform independently at least three or more activities of daily living. MCI can be diagnosed when one of the above conditions is met. 21 AD refers to the guidelines from NIA–Alzheimer's Association workgroups. 22 , 23 Participants were excluded if they had a history of stroke, brain damage, severe anemia, syphilis infection, or other conditions. 22 The diagnoses were checked by experienced neurologists.
ADNI: Participants are classified according to the pre‐established criteria by ADNI, as outlined below: NC participants are defined as having no subjective cognitive complaints and normal performance on cognitive scales; MCI and AD must have subjective cognitive complaints: MCI participants have Mini‐Mental State Examination (MMSE) scores ranging from 24 to 30, while AD scores range from 20 to 26; Clinical Dementia Rating (CDR) score are 0.5, and for AD they are ≥ 0.5; Additionally, ADNI recruited a group of elderly individuals with SCD, defined as subjectively reflecting cognitive issues, with self‐complaints exceeding the predefined cut‐off on memory ratings assessed by the Cognitive Change Index. To obtain more detailed diagnostic criteria, please refer to the procedure manual of ADNI (http://www.adni‐info.org).
Participants included in the study must undergo DTI sequence scanning, along with providing basic demographic information (i.e., age, sex, education). Due to the sensitivity of the ALPS index to the anterior commissure–posterior commissure (AC‐PC) line, images of subjects with AC‐PC line exceeding the horizon by more than 20° were excluded. 15 , 24 A detailed ADNI and SILCODE participants screening process is provided in Figures S1 and S2 in supporting information.
RESEARCH IN CONTEXT
Systematic review: Glymphatic dysfunction characterized by analysis along the perivascular space (ALPS) index has been validated in the Alzheimer's disease (AD) continuum. According to our literature review on PubMed, few studies have simultaneously been conducted in Asian and Western cohorts, and no prior research has been found to explore the performance of glymphatic function in subjective cognitive decline (SCD).
Interpretation: We demonstrate that glymphatic system dysfunction occurs earliest in SCD and is most severe in AD, a finding validated across both cohorts (East Asian and Western). For the first time, we suggest the potential of the ALPS index as a biomarker for SCD, indicating that a lower ALPS index is associated with a higher risk of cognitive progression, closely linked to cognitive performance and amyloid beta burden.
Future directions: Future research to assess the glymphatic system more accurately and non‐invasively is imperative. This holds significance for effective diagnosis and prognosis of SCD, whether in Western or Eastern populations.
2.3. Image acquisition
Two scan parameters were used for the data in SILCODE. Three hundred five images were acquired on an integrated simultaneous 3.0 T MR system (Signa, GE Healthcare) at Xuanwu Hospital of Capital Medical University. DTI scans were collected axially with a single‐shot spin‐echo diffusion‐weighted echo planar imaging sequence. The parameters were as follows: 30 gradient directions and 5 b0 images (b = 1000 s/mm2), flip angle = 90°, field of view (FOV) = 256 × 256 × 256, matrix = 112 × 112, repetition time = 16,500 ms, echo time = 95.6 ms, slice number = 70, slice thickness = 2 mm. Three hundred forty‐nine images were acquired using a 3.0 T Trio Siemens scanner at Xuanwu Hospital, Capital Medical University. Using an echo planar imaging sequence in 32 independent, non‐collinear directions of a b‐value = 1000 s/mm2, and one additional image with no diffusion weighting (b = 0), repetition time = 11,000 ms, echo time = 98 ms, flip angle = 90°, FOV = 256 mm × 256 mm, matrix = 128 × 128, slice number = 60, and slice thickness = 2 mm.
Participants in ADNI come from different centers; all of the diffusion images were acquired at 3.0 T MR system, primarily conducted on GE, SIMENS, and Philips machines. A total of 56 sites were included in this study based on the information provided by ADNI. The five major imaging acquisition parameters used are listed in Supplementary Material 1 in supporting information. For all detailed information on the imaging acquisition of ADNI, please refer to the description in the imaging protocol column of the ADNI manual (http://adni.loni.usc.edu/methods/mri‐tool/mri‐analysis/).
2.4. MRI imaging and assessment of ALPS index
Diffusion image pre‐processing was based on FMRIB Software Library (FSL) version 5.0 (Oxford Centre for Functional MRI of the Brain; https://fsl.fmrib.ox.ac.uk/fsl/), including eddy currents and motion correction, skull stripping, tensor fitting, and normalization to Montreal Neurological Institute (MNI) space. Using the Johns Hopkins University International Consortium of Brain Mapping DTI‐81 White Matter atlas (JHU ICBM‐DTI‐81 Atlas), we extracted fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) for each participant as reference DTI metrics.
Based on the JHU ICBM‐DTI‐81 Atlas overlayed on the FA map, we located the associated fiber of the lateral ventricle body (superior longitudinal fasciculus [SLF]), and the projection fiber (superior corona radiata [SCR]). 25 , 26 The SLF and SCR white matter fiber tracts are oriented orthogonally to the medullary vessels (X direction), which serve as the principal pathways for waste transport in the body of the lateral ventricles. Therefore, selecting these two fiber tracts helps to mitigate the influence of larger white matter fiber bundles on the diffusion of water molecules in the X direction. 25 This approach allows us to independently investigate the diffusivity within the medullary vessels and along the perivascular spaces, thereby assessing the activity of the glymphatic system. The region of interest (ROI) was defined as a spherical region with a radius of 5 mm placed at the corresponding locations of SCR and SLF in the left and right brains. The location and validation of ROI were performed by two experienced clinicians. These ROIs were then applied to all participants and used to extract diffusivity values of the Dxx, Dyy, and Dzz to calculate the ALPS index (Figure 1). The calculation formula of ALPS index is shown in the Figure 1. The MD in the ROI of the projection area and the associated area in the X direction (Dxxproj, Dxxassoc) is used as the numerator, and the MD in the ROI of the projection area in the Y direction (Dyyproj) and the associated area in the Z direction (Dzzassoc) is used as the denominator. 25 Then, we calculated the ALPS index of the left ROI (ALPS‐index‐L), and right ROI (ALPS‐index‐R) and averaged them (ALPS‐index‐Bi).
FIGURE 1.
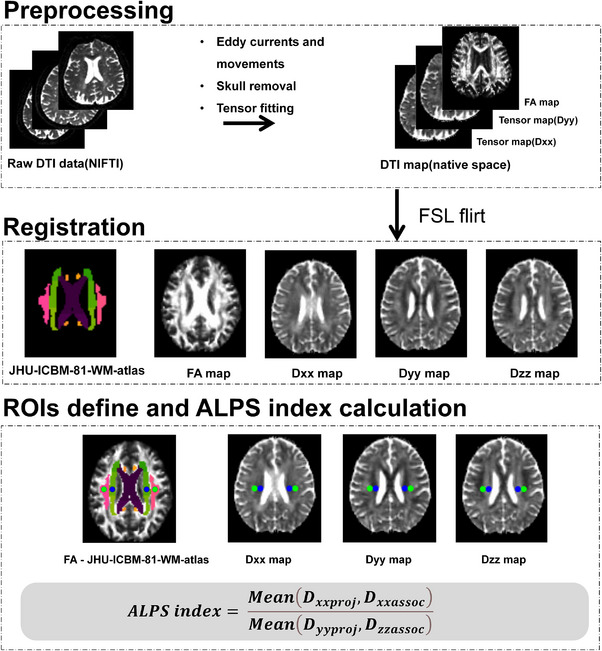
Image pre‐processing flow and ALPS index calculation. ALPS, analysis along the perivascular space; DTI, diffusion tensor imaging; FA, fractional anisotropy; JHU ICBM DTI‐81 WM atlas, Johns Hopkins University International Consortium of Brain Mapping DTI‐81 White Matter atlas
Because the SILCODE cohort used two different imaging acquisition parameters and the ADNI cohort included participants from multiple sites, we performed batch effect correction on both cohorts separately before conducting any statistical analyses to mitigate the impact of multi‐site variations on the ALPS index. We used the ComBat algorithm, a widely used method for removing unwanted effects introduced by multi‐site. 27 This algorithm has been demonstrated to effectively reduce multi‐site effects in DTI images. 28
2.5. PET imaging
A subset of participants from the NC and SCD groups in the ADNI cohort underwent florbetapir (AV45) PET scans, and the provided PET images were included in this study. The preprocessing of AV45 PET images was conducted using SPM12 (https://www.fil.ion.ucl.auk/spm/software/spm12/), a software package commonly used for analyzing and interpreting functional neuroimaging data. The processing steps primarily involved registering the PET images to T1‐weighted images, followed by normalization to the MNI space. Subsequently, an 8‐mm full width half‐maximum Gaussian kernel was applied. The amyloid deposition was quantified using standardized uptake value ratio (SUVR), which was determined by the region tracer uptake normalized to the whole cerebellum based on the Anatomical Automatic Labeling (AAL) template (area: 91‐116). 29
2.6. Statistical analysis
In this study, statistical analysis was conducted using IBM SPSS statistics v26.0 (IBM Corp) and GraphPad Prism 9.0.0 for Windows (GraphPad Software, www.graphpad.com). All analyses were conducted separately for the SILCODE and ADNI cohorts. A P < 0.05 was considered significant unless otherwise stated.
2.6.1. Sample characteristics
The chi‐square test was used for group comparisons of the categorical variable sex. Continuous variables including demographic information and neuropsychological tests were compared between groups with the use of two‐sample t tests. All between‐group tests were two‐sample t tests between SCD groups and other groups (NC, MCI, AD).
2.6.2. Receiver operating characteristic analysis
We used the receiver operating characteristic curve (ROC) to evaluate the ability of the ALPS index as an early biomarker of SCD. Classification experiments were based on the generalized linear mixed model, with age, sex, and education level included as covariates. Multi‐site effects are incorporated as random effects in model construction. The area under the ROC curve (AUC) values were calculated based on the predicted results from the model and were determined by the true positive rate and false positive rate. First, we investigated the classification ability of the ALPS index for distinguishing SCD from NC in both the ADNI and SILCODE cohorts separately. Second, considering the influence of Aβ, we further subdivided SCD participants into Aβ negative (Aβ–) and Aβ positive (Aβ+). The Aβ status of each participant was determined based on established cut‐off points (global SUVR of AV45 > 1.11, normalized to the whole cerebellum). 30 Similarly, we used the ALPS index to classify the Aβ status in SCD. Results were compared to traditional DTI metrics (FA, AD, RD, MD).
2.6.3. Group comparisons along the AD continuum
We explored the trend and difference of the ALPS index among the NC group, SCD Aβ– group, and SCD Aβ+ group. In addition, we examined changes of the ALPS index along the AD continuum in two cohorts. Analysis of variance (ANOVA) was used to analyze differences between groups. The Gaussian distribution of the data was assessed using the Shapiro–Wilk test and by examining quantile‐quantile plots. When the data followed a Gaussian distribution, we used standard ANOVA to test for differences between groups. If the data did not follow a Gaussian distribution, we used the Kruskal–Wallis test to assess group differences. The post hoc test of multiple comparisons used the Benjamini–Hochberg false discovery rate (FDR) test.
2.6.4. Survival analysis
A subset of participants from the SCD and NC groups in the ADNI cohort provided follow‐up data, which aided us in evaluating the ALPS index for detecting cognitive decline and prognosis. Survival analysis was performed using the Kaplan–Meier method, and differences in survival were evaluated through a log‐rank test. We constructed two models, each aimed at exploring the predictive ability of the ALPS index for cognitive progression in NC and SCD groups. In model construction, the ALPS index was defined as a continuous variable, and high‐ and low‐risk categories were delineated based on within‐group baseline medians (NC: 1.20; SCD: 1.11). Multivariable analysis was used to predict risk factors for cognitive progression. Age, education, sex, and apolipoprotein E (APOE) ε4 were included as covariates to adjust for their potential confounding effects. Taking the site as the random intercept to account for clustering effect, a Cox frailty model was constructed to obtain the hazard ratio of the ALPS index. Note that due to the collinearity issues when including ALPS‐index‐L/R/Bi simultaneously in the regression model, we opted to use ALPS‐index‐Bi as the primary indicator in this analysis.
2.6.5. Correlation analysis
The correlation between the ALPS index and neuropsychological scales and Aβ deposition was calculated by partial correlation analysis. Age, sex, and education were regressed as covariates. The P values were corrected by FDR tests.
We also investigated the association between the ALPS index and amyloid deposition at the voxel level. This voxel‐wise analysis was implemented based on multiple regression in SPM12. Age, sex, education, and APOE ε4 status were included as covariates in this model. Clusters with voxel number > 200 and P < 0.001 were considered significant.
2.6.6. Mediation analysis
Mediation analysis was used to examine whether Aβ burden mediated the pathway from glymphatic function to cognitive decline. The mediation model used is a three‐variable regression model, with the ALPS index as the independent variable, cognitive performance as the dependent variable, and Aβ deposition as the mediator. Age, sex, education level, and APOE status are included as covariates. The significance of the mediation effect was tested using bootstrap with 5000 replications and 95% confidence interval (CI). A CI obtained from the bootstrapped distribution that does not contain 0 was considered significant. The percentage of mediation effect (PM) was used to measure the weight of the ALPS index in the total effect and was calculated by dividing the indirect effect by the total effect. This analysis was based on the PROCESS macro.
3. RESULTS
3.1. Demographic data
The demographic information data of all participants are summarized in Table 1. The two cohorts differed in age and education level, but within each cohort there were no significant differences in age, sex, or education between the SCD and NC groups. In the ADNI cohort, there were differences in age and education level between the AD and SCD groups. In SILCODE, the 9‐item version of the subjective cognitive decline questionnaire (SCD‐9) scores of the SCD group were significantly different from those of the NC group (P < 0.001). However, there was no difference in Montreal Cognitive Assessment (MoCA) Basic (MoCA‐B) and MMSE scores between SCD and NC group. In ADNI, Aβ SUVRs were significantly different between the SCD and NC groups (P < 0.001), but no difference was present in MoCA and MMSE scores.
TABLE 1.
Demographic data of participants.
| SILCODE | ADNI | |||||||
|---|---|---|---|---|---|---|---|---|
| NC | SCD | MCI | AD | NC | SCD | MCI | AD | |
| N | 215 | 194 | 153 | 92 | 197 | 191 | 158 | 104 |
| Age | 67.03 ± 6.18 | 66.84 ± 5.97 | 67.80 ± 9.86 | 68.87 ± 9.67 | 72.20 ± 8.86 | 72.36 ± 6.80 | 73.23 ± 7.39 | 74.86 ± 8.11 c ,** |
| Sex (M/F) | 85/130 | 74/120 | 79/74 | 39/53 | 78/119 | 84/107 | 91/67 b ,*** | 68/36 c ,*** |
| Education | 11.60 ± 3.81 | 11.69 ± 2.94 | 10.93 ± 4.12 | 10.59 ± 4.67 | 16.74 ± 2.32 | 16.85 ± 2.25 | 16.25 ± 2.42 | 15.38 ± 2.18 c ,** |
| MoCA‐B | 25.82 ± 2.86 | 26.01 ± 2.34 | 19.82 ± 3.9 b ,*** | 12.00 ± 5.17 c ,*** | / | / | / | / |
| MoCA | / | / | / | / | 26.19 ± 2.61 | 25.89 ± 2.49 | 22.35 ± 3.48 b ,*** | 17.06 ± 4.30 c ,*** |
| SCD‐9 | 3.59 ± 2.23 a ,*** | 5.57 ± 1.50 | 5.24 ± 1.92 b ,*** | 6.13 ± 2.05 c ,*** | / | / | / | / |
| MMSE | 28.57 ± 1.70 | 28.34 ± 1.65 | 24.42 ± 3.75 b ,*** | 17.83 ± 5.58 c ,*** | 28.98 ± 1.25 | 29.12 ± 1.12 | 27.33 ± 2.10 b ,*** | 22.90 ± 2.68 c ,*** |
| SUVR of AV45 | / | / | / | / | 1.104 ± 0.16 a ,* | 1.145 ± 0.192 | / | / |
| Follow‐up data | / | / | / | / | N = 79 | N = 66 | / | / |
| Visits | / | / | / | / | 3 (3–9) | 3 (3–9) | / | / |
| Duration (years) | / | / | / | / | 4.9 (0.5–10.3) | 4.2 (1.0–9.1) | / | / |
Note: Data are presented as mean ± SD.
Abbreviations: AD, Alzheimer's disease; ADNI, Alzheimer's Disease Neuroimaging Initiative; ALPS, analysis along the perivascular space; AV45, florbetapir; MCI, mild cognitive impairment; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; MoCA‐B, Montreal Cognitive Assessment Basic; NC, normal control; SCD, subjective cognitive decline; SCD‐9, 9‐item subjective cognitive decline questionnaire; SD, standard deviation; SILCODE, Sino Longitudinal Study on Cognitive Decline; SUVR, standardized uptake value ratio.
NC versus SCD group.
SCD versus MCI group.
SCD versus AD group.
* P < 0.05, ** P < 0.01, *** P < 0.001.
3.2. Tensor map of ALPS index
We selected diffusion images of four subjects from each cohort for example presentation, representing different disease stages (NC‐SCD‐MCI‐AD, Figure 2). In SILCODE, the diffusivity of projection and associated fiber in the X direction (Dxx map) diffusivity in the NC case is higher than that in SCD, MCI, and AD cases (brighter). The diffusivity of projection fibers in Y direction (Dyy map) and associated fibers in the Z direction (Dzz map) in the NC case were slightly lower than those in MCI and AD cases (darker). Similar results can be seen in the cases of the ADNI cohort. These abnormal diffusivity alterations may be caused by the damage of the glymphatic system, which could be reflected by decreased ALPS index. As shown in the case of NC‐SCD‐MCI‐AD, the cognitive function (MoCA scores) decreased sequentially, and this trend of cognitive dysfunction was reflected by the ALPS index. The age and education of the cases remained consistent across the respective cohorts.
FIGURE 2.
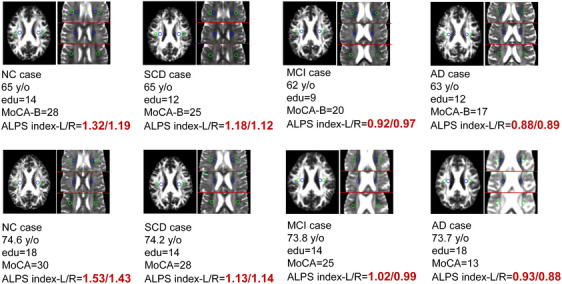
Diffusion images and ROI locations of typical cases from two cohorts. AD, Alzheimer's disease; ADNI, Alzheimer's Disease Neuroimaging Initiative; ALPS, analysis along the perivascular space; edu, education; FA, fractional anisotropy; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; MoCA‐B, Montreal Cognitive Assessment Basic; NC, normal control; SCD, subjective cognitive decline; SILCODE, Sino Longitudinal Study on Cognitive Decline; y/o, years old
3.3. ALPS index distinguishes between SCD and NC
Evaluating the performance of DTI metrics in distinguishing between NC and SCD subjects (Figure 3A, B), we found excellent diagnostic performances of the ALPS index. In both cohorts, the ALPS index consistently achieved the highest AUC (AUC = 0.793/0.768/0.816 in SILCODE, AUC = 0.797/0.770/0.794 in ADNI), whereas conventional DTI metrics showed lower discriminatory ability (AUC < 0.745).
FIGURE 3.
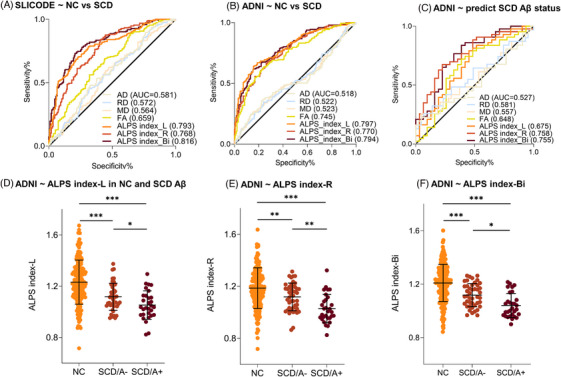
Performance of ALPS index for distinguishing NC from SCD and predicting the Aβ status of SCD. ROC results of ALPS index and conventional DTI metrics distinguish NC and SCD in SILCODE (A) and ADNI (B). C, ALPS index and conventional DTI metrics predict the Aβ status of SCD in ADNI. Trends of ALPS index among NC, SCD A−, and SCD A+ groups in the left (D), right (E), and bilateral (F). “−” indicates negative; “+” indicates positive. * P < 0.05, ** P < 0.01, *** P < 0.001. A, amyloid; Aβ, amyloid beta; AD, axial diffusivity; ADNI, Alzheimer's Disease Neuroimaging Initiative; ALPS, analysis along the perivascular space; AUC, area under the curve; DTI, diffusion tensor imaging; FA, fractional anisotropy; MD, mean diffusivity; NC, normal control; RD, radial diffusivity; ROC, receiver operating characteristic curve; SCD, subjective cognitive decline; SILCODE, Sino Longitudinal Study on Cognitive Decline
We next examined whether the ALPS index could predict Aβ status in the SCD group (Figure 3C). Results also showed the highest classification performance of the ALPS index to distinguish Aβ+ from Aβ– participants in the SCD group (AUC = 0.758). Compared to Aβ–‐ participants, the Aβ+ participants consistently showed decreased glymphatic function as reflected by lower ALPS index, regardless of ALPS‐index‐L, R, and Bi (Figure 3D–F).
3.4. Alterations of the ALPS index along the AD continuum
Next, we explored disease‐related changes of the ALPS index along the AD continuum. The results showed that there was a downward trend in the ALPS index along the disease continuum in both cohorts, and the ALPS index reached the lowest level in the AD group. In SILCODE, the differences between SCD and MCI (ALPS‐index‐L/Bi: P = 0.001/0.004) as well as AD (ALPS‐index‐L/R/Bi: P = 0.0003/0.040/0.0008) reached significance levels, while the differences between MCI and AD did not (Figure 4A–C). In ADNI, we also observed significant differences between the SCD and AD groups. Notably, unlike SILCODE, in ADNI only ALPS‐index‐R can show significant differences between SCD and MCI (P = 0.0251), while differences between SCD and AD can only be revealed by ALPS‐index‐R/Bi (Figure 4D‐F).
FIGURE 4.
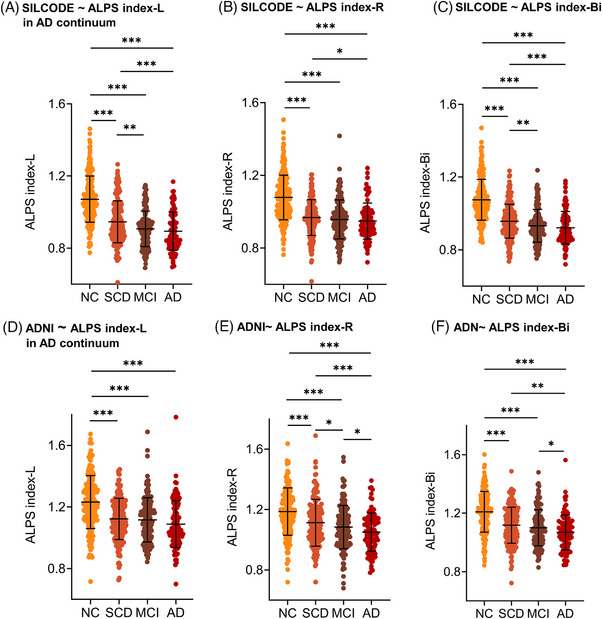
Trend of ALPS index along the AD continuum. A–C, Results in SILCODE. D–F, Results in ADNI. * P < 0.05, ** P < 0.01, *** P < 0.001. AD, Alzheimer's disease; ADNI, Alzheimer's Disease Neuroimaging Initiative; ALPS, analysis along the perivascular space; MCI, mild cognitive impairment; NC, normal control; SCD, subjective cognitive decline; SILCODE, Sino Longitudinal Study on Cognitive Decline
3.5. The predictive effect of the ALPS index on the cognitive impairment
In the NC and SCD groups of the ADNI cohort, a total of 145 participants had follow‐up data. Among those without cognitive complaints (n = 79), there were a total of 11 (13.9%) participants who experienced cognitive decline, including 9 participants progressing to MCI and 2 participants progressing to AD. In the SCD group (n = 66), a total of 13 (19.7%) participants experienced clinical progression during follow‐up, including 12 participants progressing to MCI and 1 participant progressing to AD. Table S1 in supporting information provides detailed information for the participants with follow‐up.
Based on the Cox regression model, in the SCD group, the results indicate that a lower ALPS index is associated with an increased risk of cognitive progression, with age, education, and sex as covariates (Figure 5A, P = 0.002). Furthermore, the result of Kaplan–Meier curve showed that ALPS index had excellent performance in predicting cognitive decline (hazard ratio [HR; 95% CI]: 0.240 [0.0804–0.7283], P = 0.0193, in the low‐rank test, Figure 5B). Notably, these analyses were then repeated in baseline NC participants, yielding similar results (Figure 5C, P < 0.001; Figure 5D, HR [95% CI]: 0.1019 [0.03125–0.3323], P = 0.0073).
FIGURE 5.
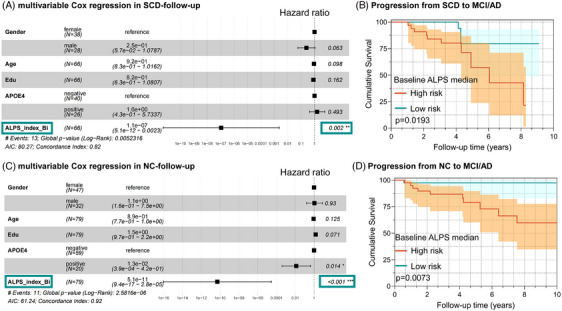
Cox model with hazard ratio and Kaplan–Meier survival curves. Cox model predicting the cognitive decline risk of ALPS index after adjustment for age, years of education, sex in SCD and NC participants (A), (C). Associations of ALPS index with risk of clinical progression, the Kaplan–Meier curves of clinical progression to MCI/AD dementia in SCD and NC participants (B), (D). AD, Alzheimer's disease; AIC, Akaike information criterion; ALPS, analysis along the perivascular space; APOE, apolipoprotein E; edu, education; MCI, mild cognitive impairment; NC, normal control; SCD, subjective cognitive decline.
3.6. Correlation between ALPS index and clinical scales
In SILCODE, the results of partial correlation analysis showed that there was a significant negative correlation between SCD‐9 and ALPS‐index‐L/Bi (r = −0.199, P = 0.002; r = −0.183 P = 0.004, FDR corrected; Figure 6A). We found that ALPS‐index‐L/Bi had a greater correlation with MoCA‐B (r = 0.213, P = 0.001; r = 0.179, P = 0.005; Figure 6B). However, the correlations were not found between ALPS‐index‐R and SCD‐9, MoCA‐B. Similar results were found in ADNI: there were significant correlations between the ALPS index and MoCA score in the NC and SCD group (r = 0.122, P = 0.017; r = 0.150, P = 0.003; r = 0.145, P = 0.004; Figure 6C).
FIGURE 6.
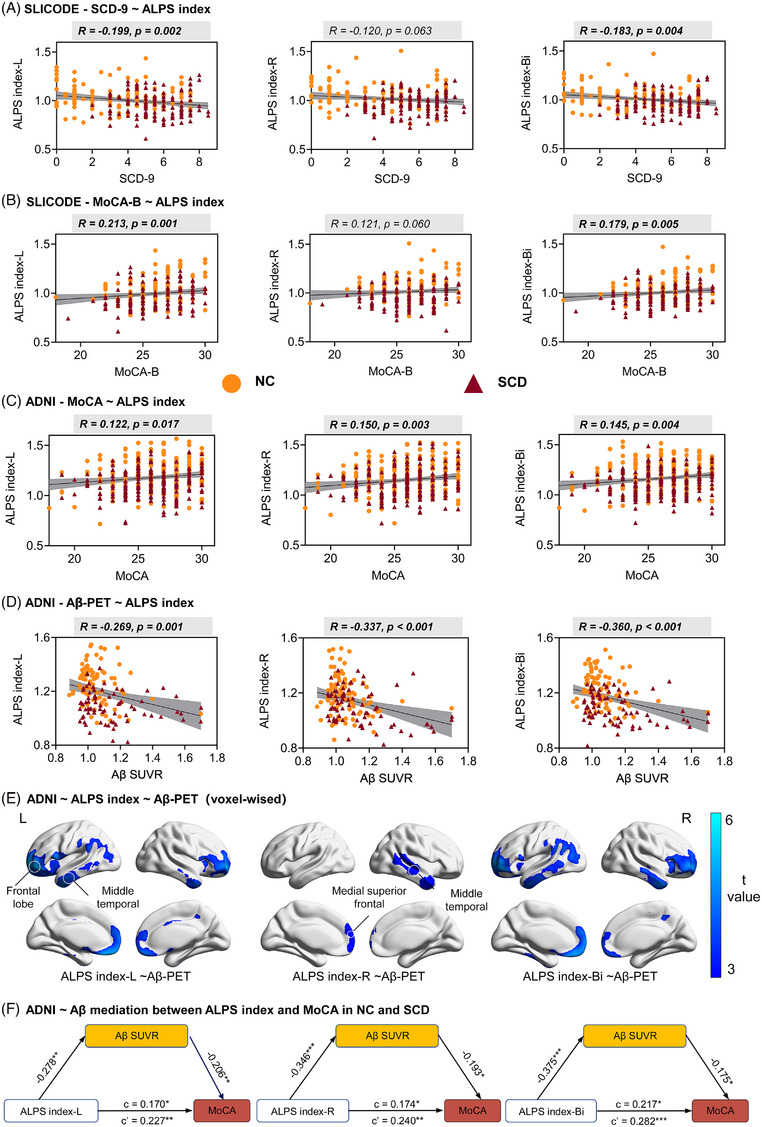
Correlation between ALPS index and neuropsychological tests and Aβ deposition: (A) correlation between ALPS index and SCD‐9 in SILCODE; (B) correlation between ALPS index and MoCA‐B in SILCODE; (C) correlation between ALPS index and MoCA in ADNI; (D) correlation between ALPS index and SUVRs of AV45 in ADNI; (E) voxel‐wise analysis of the association between ALPS index and Aβ PET; (F) mediation effect of Aβ deposition between ALPS index and MoCA scores in ADNI. * P < 0.05, ** P < 0.01. Aβ, amyloid beta; AD, Alzheimer's disease; ADNI, Alzheimer's Disease Neuroimaging Initiative; ALPS, analysis along the perivascular space; AV45: florbetapir; c, direct effect; c', indirect effect; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; MoCA‐B, Montreal Cognitive Assessment Basic; NC, normal control; PET, positron emission tomography; SCD, subjective cognitive decline; SCD‐9, 9‐item subjective cognitive decline questionnaire; SILCODE, Sino Longitudinal Study on Cognitive Decline; SUVR, standardized uptake value ratio
We next measured the relationship between glymphatic and Aβ deposition. Including data from the NC and SCD participants from the ADNI cohort, we found a significant negative correlation between ALPS index and SUVR of AV45, especially in ALPS‐index‐Bi (r = −0.360, P < 0.001; Figure 6D). Results of voxel‐wise analysis showed significant negative correlation between ALPS index and SUVR of AV45. Significant voxels are primarily distributed in the frontal lobe (e.g., middle frontal gyrus, superior frontal gyrus). Similarly, there is a significant negative correlation between Aβ burden and ALPS‐index‐R, with significant voxels distributed in regions such as the left medial superior frontal and right middle temporal gyrus (Figure 6E).
3.7. The ALPS index causes cognitive decline through Aβ burden
We further explored whether SUVR of AV45 mediates the cognitive performance decline caused by glymphatic dysfunction by conducting a mediation model. The results showed that the SUVR of AV45 showed significant mediation effects between ALPS index and MoCA scores after correcting for multiple covariates (i.e., age, sex, education). The mediation effect of SUVR of AV45 on the cognitive decline caused by ALPS‐index‐R is the most significant (Figure 6F, PM = 27.9%; total effect c' = 0.240, P = 0.003; direct effect c = 0.171, P = 0.039; indirect effect β = 0.067; [95% CI] = 0.0030–0.1375).
4. DISCUSSION
In this study, we explored whether the ALPS index could be used as a biomarker for SCD. The results of ROC, Cox regression, and survival analysis demonstrate that the ALPS index serves as a risk factor for cognitive decline in SCD; patients with a lower ALPS index have a higher risk of cognitive decline. There is a significant association between lower ALPS index and Aβ burden in SCD, further mediation models indicate that the Aβ burden served as a mediator in the relationships between the ALPS index and cognitive performance. Notably, the results of this study are cross‐validated in two independent cohorts from Chinese and American populations.
Previous studies have shown a significantly decreased ALPS index in AD and MCI patients, which is consistent with our findings. 13 , 14 We further confirmed that the ALPS index had begun to decline at the SCD stage. In addition, compared to other DTI metrics, the ALPS index showed better classification performance in distinguishing SCD from NC (AUCSILCODE = 0.816, AUCADNI = 0.797). Although FA and MD diffusion measure maps have often been used to perform AD classification, research about SCD populations have poor classification effects. 31 This may be due to the absence of widespread disorder and significantly abnormal changes in the white matter structure of subjects in the ultra‐early phase of AD. 32 However, the ALPS index indirectly indicates the state of glymphatic function, which may be impaired before white matter structural integrity. It is known that the glymphatic system contributes to the clearance of waste products in the brain. Previous studies reported higher perivascular space volume fraction and fractional volume of free water in white matter in patients with AD. 17 , 33 This impairment in the perivascular space may result from blockage of brain drainage pathways due to the accumulation of Aβ. 34 The accumulation of Aβ may be the cause of perivascular clearance impairment that could be reflected by the ALPS index.
Our study revealed the impairment of the ALPS index in bilateral ROIs. Different from our results, previous studies only showed a decreased ALPS index in the left hemisphere. 25 , 33 Such a discrepancy may be attributed to their small sample size relative to our study. The relatively consistent result is that we found patients with lower ALPS index of the left hemisphere had a higher risk of cognitive decline. These results were consistent across the two cohorts. More differences and a more stable outcome in our study may be due to the ROIs we used determined on a standard template. This method has demonstrated higher inter‐ and intra‐rater correlation coefficients, compared to the manual method.
Furthermore, we observed significant correlations between the ALPS index and multiple cognitive functioning scales, especially in the SCD‐9. Kamagata et al. found that in AD and MCI patients, a lower ALPS index is associated with lower CSF Aβ42, fluorodeoxyglucose PET uptake, and poorer deficits in multiple cognitive domains. 17 This is consistent with our study, but the relationship between the ALPS index and cognitive impairment in SCD has not been reported previously. AD is a continuous spectrum of disease, with SCD as its initial stage. A decreased ALPS index in SCD is further evidence that subtle pathological changes associated with AD have occurred at the SCD stage. 35 Hong et al. observed significant associations between the ALPS index and cognitive domains in AD, including memory, executive function, visual‐spatial, and language. 15 In our study, we found that there was a significant negative correlation between SCD‐9 and ALPS‐index‐L/Bi (r = −0.199, P = 0.002; r = −0.183 P = 0.004, FDR corrected; Figure 6A). This study confirms that more severe cognitive impairment was associated with higher SCD‐9 scores and lower ALPS index.
Previous studies detected correlations between ALPS index and Aβ deposition in the temporal, parietal, and cingulate cortex. 36 These brain areas contribute to AD‐related cognitive dysfunction. We also found a significant association exists between lower ALPS index and Aβ burden. However, further voxel analysis indicates that the ALPS index is mainly associated with Aβ burden in the frontal and temporal lobes. One possible explanation for the slightly different results is that our analysis is based on SCD, which as an earlier clinical stage preceding AD, whereas Ota et al.’s 36 study only included the NC and AD participants. During the prodromal phase of AD, Aβ deposition is not widespread throughout the brain but is present in certain early Aβ‐sensitive regions, such as the frontal lobe and cingulate gyrus. 19 It is noteworthy that recent studies may support our claim. In a recent study that included a larger number of participants in the preclinical stages of AD, both cross‐sectional and longitudinal investigations found a close association between the ALPS index and brain regions considered particularly sensitive and early sites of possible Aβ deposition (i.e., superior frontal, rostral middle frontal, and posterior cingulate gyrus). 16 This suggests that the ALPS index may function as a window to evaluate the abnormal subtle changes of the whole brain at the SCD stage, even though it is an index of local water dynamics calculated from local brain data. Apart from the information on amyloid deposition, an axis of evaluation could be added for glymphatic system abnormalities that are either a cause or a consequence of the deposition. 36
The ALPS index demonstrates the ability to predict the clinical progression of the AD spectrum. Huang et al. successfully validated the effectiveness of the ALPS index in predicting the conversion of cognitively normal participants to MCI/AD, 16 which is consistent with our findings. Our innovation lies in demonstrating that the ALPS index is sensitive not only to cognitive progression in cognitively normal participants but also equally sensitive to cognitive progression in SCD participants.
More interestingly, based on a hypothetical cascade model, Huang et al. suggested that glymphatic dysfunction indicated by the ALPS index might precede significant Aβ deposition and brain atrophy. Our findings support this, as we found in SCD that the ALPS index may influence cognitive decline through Aβ deposition. And, research with AD mouse models has shown that reduced glymphatic transport occurs before extensive Aβ deposition, 37 and other studies indicated that disrupting key glymphatic pathways promotes Aβ accumulation. 9 , 38 Considering these findings, we believe that compared to other biomarkers that are sensitive to AD but may reflect later‐occurring pathologies, the ALPS index, which represents glymphatic function, may play a more significant role in the early detection of AD.
There are several limitations to this study. First, the ALPS index provides an overview of the whole brain glymphatic function. Given the potential regional heterogeneity of AD neuropathology (such as Aβ, tau, and neurodegeneration), future studies should focus on AD‐specific regions and include more AD‐sensitive biomarkers to explore regional glymphatic function. Second, the longitudinal analysis in this study included a small subset of participants from the ADNI cohort, necessitating caution in interpreting these results. Future research should focus on larger, more diverse populations to explore the longitudinal changes in ALPS index and the interactions with cognitive progression and neuropathology. Third, glymphatic function might be affected by circadian rhythm. 12 The effect of different scanning times on the ALPS index may provide additional information of SCD diagnosis.
5. CONCLUSION
Altogether, we demonstrated common changes of the ALPS index in both Asian and Western populations. Our findings suggest the association of glymphatic system impairment with cognitive dysfunctions in the SCD population. Furthermore, we also showed the potential use of the ALPS index as a disease progression biomarker for SCD.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All procedures performed in studies involving human participants were under the ethical standards of the Institutional and National Research Committee and with the 1964 Declaration of Helsinki and its later amendments. Participants from SILCODE all signed informed consent forms. Approval for the study was granted by the Medical Ethics Committee of Xuanwu Hospital, Capital Medical University (ClinicalTrials.gov identifier: NCT03370744). Authorization for participants from ADNI can be accessed at http://www.adni‐info.org for further details.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We extend our sincere gratitude to the researchers within SILCODE and ADNI for their efforts in collecting, organizing, and sharing the data. A complete list of ADNI researchers can be found at https://adni.loni.usc.edu/wp‐content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf. This article was supported by STI2030‐Major Projects (2022ZD021600, 2022ZD0211600 and 2022ZD0211800), National Natural Science Foundation of China (62206165, 62376150, 82020108013, 82272039, 82021002, 81971641, 82001773 and 82327809), Shanghai Industrial Collaborative Innovation Project (No. XTCX‐KJ‐2023‐37), Shanghai Science and Technology Development Funds (Sailing Program, 22YF1413900), Medical Science Research Project of Hebei Province (20221842), Construction Project of Academician Cooperation Key Unit of Hebei Province, Research project of Shanghai Health Commission (2020YJZX0111), Clinical Research Plan of SHDC (SHDC2020CR1038B), National Key R&D Program of China (2022YFC2009902 and 2022YFC2009900), Sino‐German Cooperation Grant (M‐0759), and Shenzhen Bay Scholars Program and Tianchi Scholars Program.
Li Y, Wang L, Zhong J, et al.; Alzheimer's Disease Neuroimaging Initiative . Impaired glymphatic function as a biomarker for subjective cognitive decline: An exploratory dual cohort study. Alzheimer's Dement. 2024;20:6542–6555. 10.1002/alz.14149
Yuxia Li, Luyao Wang, and Jiayi Zhong contributed equally to this study.
Contributor Information
Chuantao Zuo, Email: zuochuantao@fudan.edu.cn.
Jiehui Jiang, Email: jiangjiehui@shu.edu.cn.
REFERENCES
- 1. Dementia: World Health Organization (WHO) . World Health Organization. 2021. Accessed January 15, 2023. https://www.who.int/news‐room/fact‐sheets/detail/dementi
- 2. Rabin LA, Smart CM, Amariglio RE. Subjective cognitive decline in preclinical Alzheimer's disease. Annu Rev Clin Psychol. 2017;13:369‐396. 10.1146/annurev-clinpsy-032816-045136 [DOI] [PubMed] [Google Scholar]
- 3. Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271‐278. 10.1016/S1474-4422(19)30368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peters R, Beckett N, Antikainen R, Rockwood K, Bulpitt CJ, Anstey KJ. Subjective memory complaints and incident dementia in a high risk older adult hypertensive population. Age Ageing. 2019;48(2):253‐259. 10.1093/ageing/afy193 [DOI] [PubMed] [Google Scholar]
- 5. van Wanrooij LL, Richard E, Jongstra S, Moll van Charante EP, van Gool WA. Associations of subjective memory complaints and simple memory task scores with future dementia in the primary care setting. Ann Fam Med. 2019;17(5):412‐418. 10.1370/afm.2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer's disease. Lancet. 2021;397(10284):1577‐1590. 10.1016/S0140-6736(20)32205-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tian M, Zuo C, Civelek AC, et al. International nuclear medicine consensus on the clinical use of amyloid positron emission tomography in Alzheimer's disease. Phenomics. 2022;3(4):375‐389. 10.1007/s43657-022-00068-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahaman YAR, Embaye KS, Huang F, et al. Biomarkers used in Alzheimer's disease diagnosis, treatment, and prevention. Ageing Res Rev. 2022;74:101544. 10.1016/j.arr.2021.101544 [DOI] [PubMed] [Google Scholar]
- 9. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mestre H, Mori Y, Nedergaard M. The brain's glymphatic system: current controversies. Trends Neurosci. 2020;43(7):458‐466. 10.1016/j.tins.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harrison IF, Ismail O, Machhada A, et al. Impaired glymphatic function and clearance of tau in an Alzheimer's disease model. Brain. 2020;143(8):2576‐2593. 10.1093/brain/awaa179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370(6512):50‐56. 10.1126/science.abb8739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsu JL, Wei YC, Toh CH, et al. Magnetic resonance images implicate that glymphatic alterations mediate cognitive dysfunction in Alzheimer disease. Ann Neurol. 2023;93(1):164‐174. 10.1002/ana.26516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhong J, Zhang X, Xu H, et al. Unlocking the enigma: unraveling multiple cognitive dysfunction linked to glymphatic impairment in early Alzheimer's disease. Front Neurosci. 2023;17:1222857. 10.3389/fnins.2023.1222857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hong H, Hong L, Luo X, et al. The relationship between amyloid pathology, cerebral small vessel disease, glymphatic dysfunction, and cognition: a study based on Alzheimer's disease continuum participants. Alzheimers Res Ther. 2024;16(1):43. 10.1186/s13195-024-01407-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang SY, Zhang YR, Guo Y, et al. Glymphatic system dysfunction predicts amyloid deposition, neurodegeneration, and clinical progression in Alzheimer's disease. Alzheimers Dement. 2024;20(5):3251‐3269. 10.1002/alz.13789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamagata K, Andica C, Takabayashi K, et al. Association of MRI indices of glymphatic system with amyloid deposition and cognition in mild cognitive impairment and Alzheimer disease. Neurology. 2022;99(24):e2648‐e2660. 10.1212/WNL.0000000000201300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li X, Wang X, Su L, Hu X, Han Y. Sino Longitudinal Study on Cognitive Decline (SILCODE): protocol for a Chinese longitudinal observational study to develop risk prediction models of conversion to mild cognitive impairment in individuals with subjective cognitive decline. BMJ Open. 2019;9(7):e028188. 10.1136/bmjopen-2018-028188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(6):844‐852. 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen G, Yang K, Du W, Hu X, Han Y. Clinical characteristics in subjective cognitive decline with and without worry: baseline investigation of the SILCODE Study. J Alzheimers Dis. 2019;72(2):443‐454. 10.3233/JAD-190501 [DOI] [PubMed] [Google Scholar]
- 21. Bondi MW, Edmonds EC, Jak AJ, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis. 2014;42(1):275‐289. 10.3233/JAD-140276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263‐269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taoka T, Ito R, Nakamichi R, et al. Reproducibility of diffusion tensor image analysis along the perivascular space (DTI‐ALPS) for evaluating interstitial fluid diffusivity and glymphatic function: CHanges in Alps index on Multiple conditiON acquIsition eXperiment (CHAMONIX) study. Jpn J Radiol. 2022;40(2):147‐158. 10.1007/s11604-021-01187-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI‐ALPS) in Alzheimer's disease cases. Jpn J Radiol. 2017;35(4):172‐178. 10.1007/s11604-017-0617-z [DOI] [PubMed] [Google Scholar]
- 26. Liu X, Barisano G, Shao X, et al. Cross‐vendor test‐retest validation of diffusion tensor image analysis along the perivascular space (DTI‐ALPS) for evaluating glymphatic system function. Aging Dis. 2024;15(4):1885‐1898. doi: 10.14336/AD.2023.0321-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118‐127. 10.1093/biostatistics/kxj037 [DOI] [PubMed] [Google Scholar]
- 28. Fortin JP, Parker D, Tunç B, et al. Harmonization of multi‐site diffusion tensor imaging data. Neuroimage. 2017;161:149‐170. 10.1016/j.neuroimage.2017.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357‐367. 10.1016/S1474-4422(13)70044-9 [DOI] [PubMed] [Google Scholar]
- 30. Joshi AD, Pontecorvo MJ, Clark CM, et al. Performance characteristics of amyloid PET with florbetapir F 18 in patients with Alzheimer's disease and cognitively normal subjects. J Nucl Med. 2012;53(3):378‐384. 10.2967/jnumed.111.090340 [DOI] [PubMed] [Google Scholar]
- 31. Teipel SJ, Kuper‐Smith JO, Bartels C, et al. Multicenter tract‐based analysis of microstructural lesions within the Alzheimer's disease spectrum: association with amyloid pathology and diagnostic usefulness. J Alzheimers Dis. 2019;72(2):455‐465. 10.3233/JAD-190446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Y, Wang Y, Song Z, Fan Y, Gao T, Tang X. Abnormal white matter changes in Alzheimer's disease based on diffusion tensor imaging: a systematic review. Ageing Res Rev. 2023;87:101911. 10.1016/j.arr.2023.101911 [DOI] [PubMed] [Google Scholar]
- 33. Zhang X, Wang Y, Jiao B, et al. Glymphatic system impairment in Alzheimer's disease: associations with perivascular space volume and cognitive function. Eur Radiol. 2024;34(2):1314‐1323. 10.1007/s00330-023-10122-3 [DOI] [PubMed] [Google Scholar]
- 34. Keable A, Fenna K, Yuen HM, et al. Deposition of amyloid β in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy. Biochim Biophys Acta. 2016;1862(5):1037‐1046. 10.1016/j.bbadis.2015.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee YC, Kang JM, Lee H, et al. Subjective cognitive decline and subsequent dementia: a nationwide cohort study of 579,710 people aged 66 years in South Korea. Alzheimers Res Ther. 2020;12(1):52. 10.1186/s13195-020-00618-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ota M, Sato N, Nakaya M, et al. Relationships between the deposition of amyloid‐β and Tau protein and glymphatic system activity in Alzheimer's disease: diffusion tensor image study. J Alzheimers Dis. 2022;90(1):295‐303. 10.3233/JAD-220534 [DOI] [PubMed] [Google Scholar]
- 37. Peng W, Achariyar TM, Li B, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer's disease. Neurobiol Dis. 2016;93:215‐225. 10.1016/j.nbd.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simon M, Wang MX, Ismail O, et al. Loss of perivascular aquaporin‐4 localization impairs glymphatic exchange and promotes amyloid β plaque formation in mice. Alzheimers Res Ther. 2022;14(1):59. 10.1186/s13195-022-00999-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


