Abstract
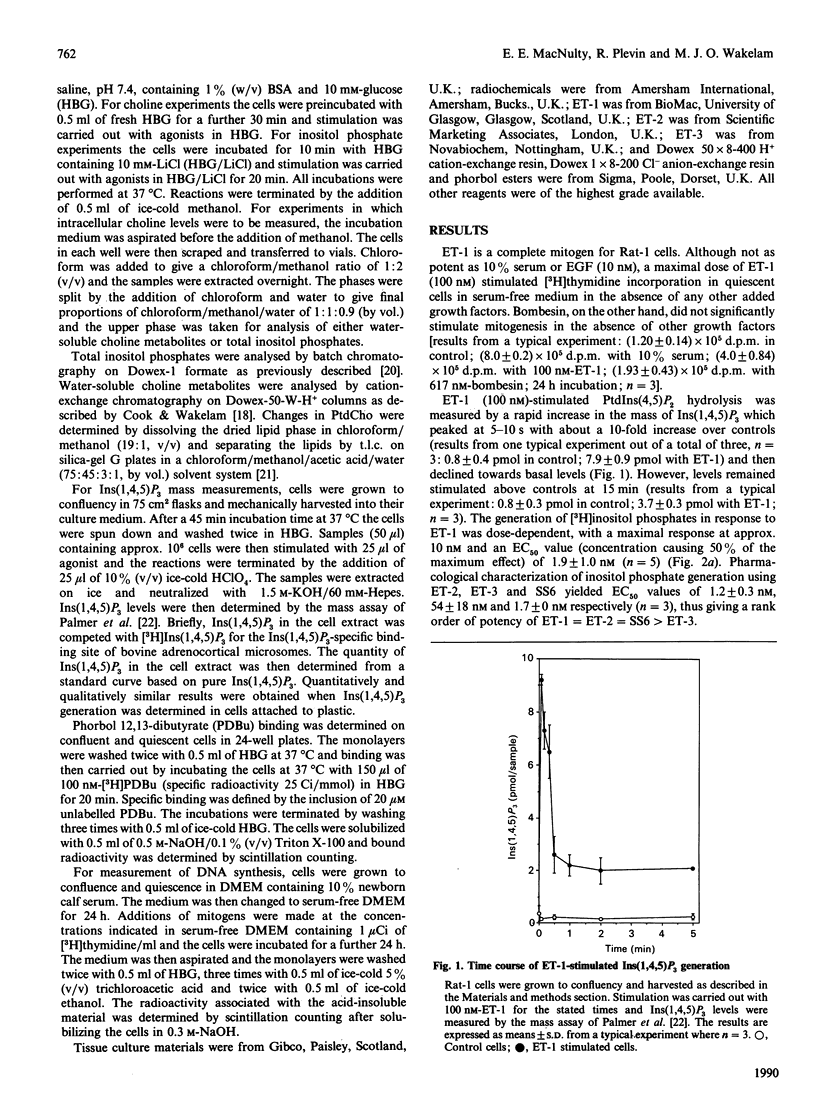
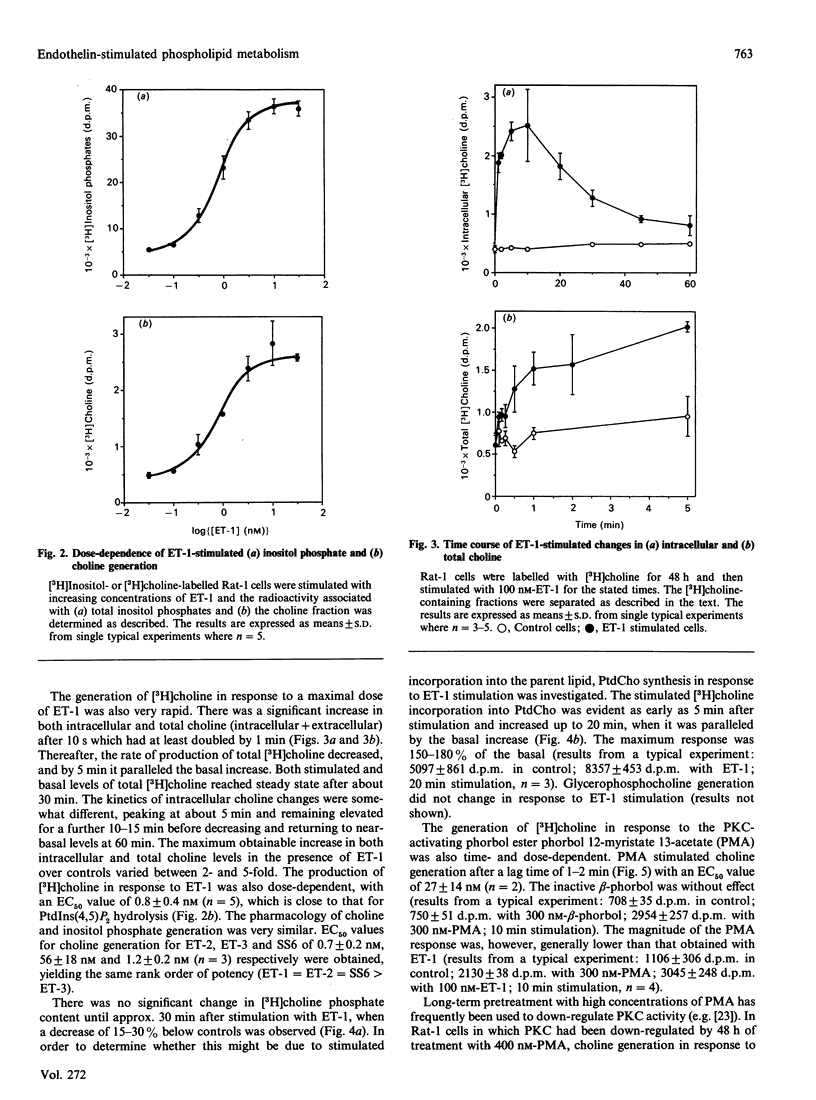
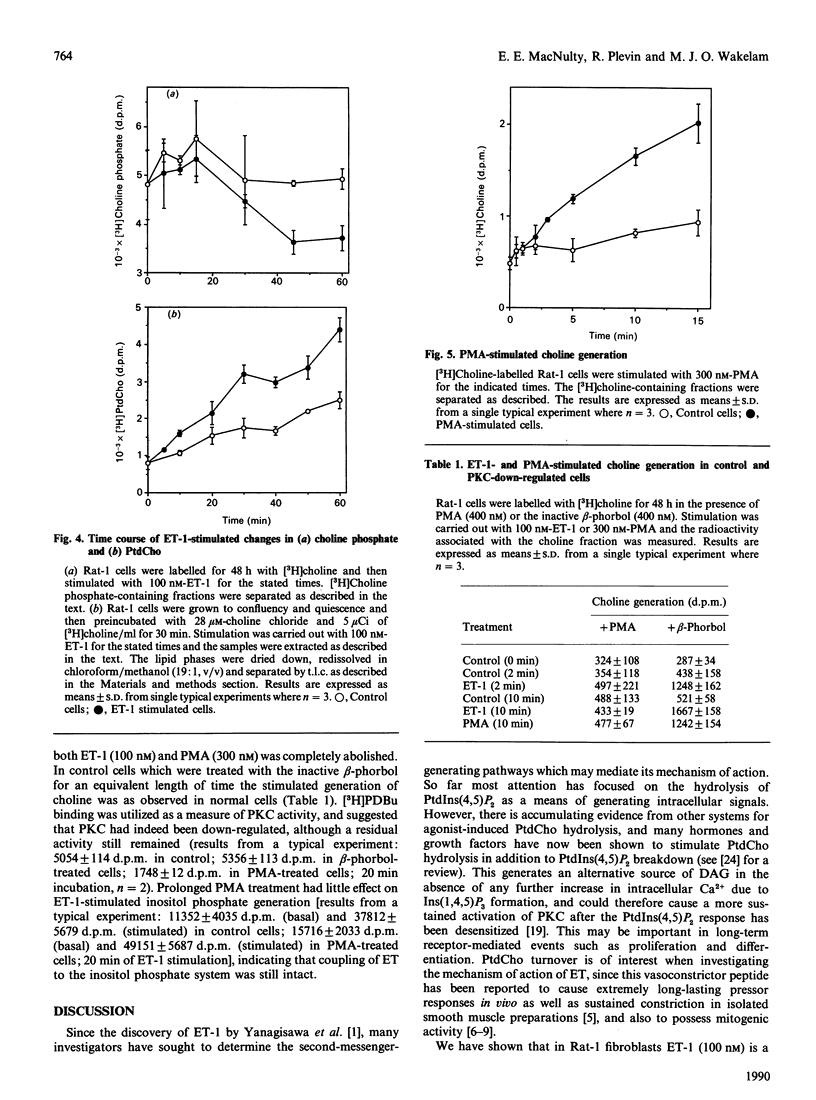
The mitogenic activity of endothelin and its ability to stimulate PtdIns(4,5)P2 and phosphatidylcholine turnover in Rat-1 fibroblasts was studied. Stimulated incorporation of [3H]thymidine occurred in the absence of any other added growth factors. The endothelins stimulated rapid generation of both Ins(1,4,5)P3 and choline. Endothelin-1 and endothelin-2 were equipotent in stimulating both responses, but endothelin-3 was less potent. Endothelin-1-stimulated Ins(1,4,5)P3 generation reached a maximum at 5 s and then declined; however, the response was long-lived, with a 4.5-fold elevation over basal still observed after 15 min. Endothelin-stimulated choline generation was observed with no increase in choline phosphate; indeed, the apparent level of this metabolite fell after 30 min of stimulation, presumably due to the observed stimulation of phosphatidylcholine synthesis. The endothelin-stimulated increase in choline generation was abolished in cells where protein kinase C was down-regulated. However, endothelin-stimulated choline generation was greater than that observed in response to a protein kinase C-activating phorbol ester, raising the possibility that the peptide activates phospholipase D by both protein kinase C-dependent and -independent mechanisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bocckino S. B., Blackmore P. F., Wilson P. B., Exton J. H. Phosphatidate accumulation in hormone-treated hepatocytes via a phospholipase D mechanism. J Biol Chem. 1987 Nov 5;262(31):15309–15315. [PubMed] [Google Scholar]
- Brown K. D., Littlewood C. J. Endothelin stimulates DNA synthesis in Swiss 3T3 cells. Synergy with polypeptide growth factors. Biochem J. 1989 Nov 1;263(3):977–980. doi: 10.1042/bj2630977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabot M. C., Welsh C. J., Cao H. T., Chabbott H. The phosphatidylcholine pathway of diacylglycerol formation stimulated by phorbol diesters occurs via phospholipase D activation. FEBS Lett. 1988 Jun 6;233(1):153–157. doi: 10.1016/0014-5793(88)81374-7. [DOI] [PubMed] [Google Scholar]
- Cabot M. C., Welsh C. J., Zhang Z. C., Cao H. T. Evidence for a protein kinase C-directed mechanism in the phorbol diester-induced phospholipase D pathway of diacylglycerol generation from phosphatidylcholine. FEBS Lett. 1989 Mar 13;245(1-2):85–90. doi: 10.1016/0014-5793(89)80197-8. [DOI] [PubMed] [Google Scholar]
- Cook S. J., Palmer S., Plevin R., Wakelam M. J. Mass measurement of inositol 1,4,5-trisphosphate and sn-1,2-diacylglycerol in bombesin-stimulated Swiss 3T3 mouse fibroblasts. Biochem J. 1990 Jan 15;265(2):617–620. doi: 10.1042/bj2650617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Analysis of the water-soluble products of phosphatidylcholine breakdown by ion-exchange chromatography. Bombesin and TPA (12-O-tetradecanoylphorbol 13-acetate) stimulate choline generation in Swiss 3T3 cells by a common mechanism. Biochem J. 1989 Oct 15;263(2):581–587. doi: 10.1042/bj2630581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K., Kasuya Y., Matsuki N., Takuwa Y., Kurihara H., Ishikawa T., Kimura S., Yanagisawa M., Masaki T. Endothelin activates the dihydropyridine-sensitive, voltage-dependent Ca2+ channel in vascular smooth muscle. Proc Natl Acad Sci U S A. 1989 May;86(10):3915–3918. doi: 10.1073/pnas.86.10.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling K. K., Tsuda T., Alexander R. W. Endothelin stimulates diacylglycerol accumulation and activates protein kinase C in cultured vascular smooth muscle cells. J Biol Chem. 1989 May 15;264(14):8237–8240. [PubMed] [Google Scholar]
- Hirata Y., Yoshimi H., Takata S., Watanabe T. X., Kumagai S., Nakajima K., Sakakibara S. Cellular mechanism of action by a novel vasoconstrictor endothelin in cultured rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1988 Aug 15;154(3):868–875. doi: 10.1016/0006-291x(88)90220-3. [DOI] [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Kimura S., Kasuya Y., Miyauchi T., Goto K., Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog Y., Ambar I., Sokolovsky M., Kochva E., Wollberg Z., Bdolah A. Sarafotoxin, a novel vasoconstrictor peptide: phosphoinositide hydrolysis in rat heart and brain. Science. 1988 Oct 14;242(4876):268–270. doi: 10.1126/science.2845579. [DOI] [PubMed] [Google Scholar]
- Kloog Y., Bousso-Mittler D., Bdolah A., Sokolovsky M. Three apparent receptor subtypes for the endothelin/sarafotoxin family. FEBS Lett. 1989 Aug 14;253(1-2):199–202. doi: 10.1016/0014-5793(89)80958-5. [DOI] [PubMed] [Google Scholar]
- Kolesnick R. N., Paley A. E. 1,2-Diacylglycerols and phorbol esters stimulate phosphatidylcholine metabolism in GH3 pituitary cells. Evidence for separate mechanisms of action. J Biol Chem. 1987 Jul 5;262(19):9204–9210. [PubMed] [Google Scholar]
- Koseki C., Imai M., Hirata Y., Yanagisawa M., Masaki T. Autoradiographic distribution in rat tissues of binding sites for endothelin: a neuropeptide? Am J Physiol. 1989 Apr;256(4 Pt 2):R858–R866. doi: 10.1152/ajpregu.1989.256.4.R858. [DOI] [PubMed] [Google Scholar]
- Lee T. S., Chao T., Hu K. Q., King G. L. Endothelin stimulates a sustained 1,2-diacylglycerol increase and protein kinase C activation in bovine aortic smooth muscle cells. Biochem Biophys Res Commun. 1989 Jul 14;162(1):381–386. doi: 10.1016/0006-291x(89)92008-1. [DOI] [PubMed] [Google Scholar]
- Marsden P. A., Danthuluri N. R., Brenner B. M., Ballermann B. J., Brock T. A. Endothelin action on vascular smooth muscle involves inositol trisphosphate and calcium mobilization. Biochem Biophys Res Commun. 1989 Jan 16;158(1):86–93. doi: 10.1016/s0006-291x(89)80180-9. [DOI] [PubMed] [Google Scholar]
- Martin T. W., Michaelis K. P2-purinergic agonists stimulate phosphodiesteratic cleavage of phosphatidylcholine in endothelial cells. Evidence for activation of phospholipase D. J Biol Chem. 1989 May 25;264(15):8847–8856. [PubMed] [Google Scholar]
- Milligan G., Wakelam M. J. G-proteins and second messengers in mitogenesis. Prog Growth Factor Res. 1989;1(3):161–177. doi: 10.1016/0955-2235(89)90009-4. [DOI] [PubMed] [Google Scholar]
- Muldoon L. L., Rodland K. D., Forsythe M. L., Magun B. E. Stimulation of phosphatidylinositol hydrolysis, diacylglycerol release, and gene expression in response to endothelin, a potent new agonist for fibroblasts and smooth muscle cells. J Biol Chem. 1989 May 25;264(15):8529–8536. [PubMed] [Google Scholar]
- Nakaki T., Nakayama M., Yamamoto S., Kato R. Endothelin-mediated stimulation of DNA synthesis in vascular smooth muscle cells. Biochem Biophys Res Commun. 1989 Feb 15;158(3):880–883. doi: 10.1016/0006-291x(89)92804-0. [DOI] [PubMed] [Google Scholar]
- Pai J. K., Siegel M. I., Egan R. W., Billah M. M. Activation of phospholipase D by chemotactic peptide in HL-60 granulocytes. Biochem Biophys Res Commun. 1988 Jan 15;150(1):355–364. doi: 10.1016/0006-291x(88)90528-1. [DOI] [PubMed] [Google Scholar]
- Palmer S., Hughes K. T., Lee D. Y., Wakelam M. J. Development of a novel, Ins(1,4,5)P3-specific binding assay. Its use to determine the intracellular concentration of Ins(1,4,5)P3 in unstimulated and vasopressin-stimulated rat hepatocytes. Cell Signal. 1989;1(2):147–156. doi: 10.1016/0898-6568(89)90004-1. [DOI] [PubMed] [Google Scholar]
- Price B. D., Morris J. D., Hall A. Stimulation of phosphatidylcholine breakdown and diacylglycerol production by growth factors in Swiss-3T3 cells. Biochem J. 1989 Dec 1;264(2):509–515. doi: 10.1042/bj2640509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resink T. J., Scott-Burden T., Bühler F. R. Activation of phospholipase A2 by endothelin in cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):279–286. doi: 10.1016/s0006-291x(89)80209-8. [DOI] [PubMed] [Google Scholar]
- Resink T. J., Scott-Burden T., Weber E., Bühler F. R. Phorbol ester promotes a sustained down-regulation of endothelin receptors and cellular responses to endothelin in human vascular smooth muscle cells. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1213–1219. doi: 10.1016/0006-291x(90)90995-y. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Saida K., Mitsui Y., Ishida N. A novel peptide, vasoactive intestinal contractor, of a new (endothelin) peptide family. Molecular cloning, expression, and biological activity. J Biol Chem. 1989 Sep 5;264(25):14613–14616. [PubMed] [Google Scholar]
- Simonson M. S., Wann S., Mené P., Dubyak G. R., Kester M., Nakazato Y., Sedor J. R., Dunn M. J. Endothelin stimulates phospholipase C, Na+/H+ exchange, c-fos expression, and mitogenesis in rat mesangial cells. J Clin Invest. 1989 Feb;83(2):708–712. doi: 10.1172/JCI113935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M., Inagami T., Hare G. M., Johns J. A. Endothelin action: Inhibition by a protein kinase C inhibitor and involvement of phosphoinositols. Biochem Biophys Res Commun. 1989 Jan 16;158(1):170–176. doi: 10.1016/s0006-291x(89)80193-7. [DOI] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Yanagisawa M., Yamashita K., Masaki T. A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem. 1989 May 15;264(14):7856–7861. [PubMed] [Google Scholar]
- Van Renterghem C., Vigne P., Barhanin J., Schmid-Alliana A., Frelin C., Lazdunski M. Molecular mechanism of action of the vasoconstrictor peptide endothelin. Biochem Biophys Res Commun. 1988 Dec 30;157(3):977–985. doi: 10.1016/s0006-291x(88)80970-7. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Miyazaki H., Kondoh M., Masuda Y., Kimura S., Yanagisawa M., Masaki T., Murakami K. Two distinct types of endothelin receptors are present on chick cardiac membranes. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1252–1259. doi: 10.1016/0006-291x(89)91377-6. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Masaki T. Endothelin, a novel endothelium-derived peptide. Pharmacological activities, regulation and possible roles in cardiovascular control. Biochem Pharmacol. 1989 Jun 15;38(12):1877–1883. doi: 10.1016/0006-2952(89)90484-x. [DOI] [PubMed] [Google Scholar]


