Abstract
INTRODUCTION
In this study, we investigated biomarkers in a midlife, racially diverse, at‐risk cohort to facilitate early identification and intervention. We examined neuroimaging measures, including resting state functional magnetic resonance imaging (fMRI), white matter hyperintensity vo (WMH), and hippocampal volumes, alongside cerebrospinal fluid (CSF) markers.
METHODS
Our data set included 76 cognitively unimpaired, middle‐aged, Black Americans (N = 29, F/M = 17/12) and Non‐Hispanic White (N = 47, F/M = 27/20) individuals. We compared cerebrospinal fluid phosphorylated tau141 and amyloid beta (Aβ)42 to fMRI default mode network (DMN) subnetwork connectivity, WMH volumes, and hippocampal volumes.
RESULTS
Results revealed a significant race × Aβ42 interaction in Black Americans: lower Aβ42 was associated with reduced DMN connectivity and increased WMH volumes regions but not in non‐Hispanic White individuals.
DISCUSSION
Our findings suggest that precuneus DMN connectivity and temporal WMHs may be linked to Alzheimer's disease risk pathology during middle age, particularly in Black Americans.
Highlights
Cerebrospinal fluid (CSF) amyloid beta (Aβ)42 relates to precuneus functional connectivity in Black, but not White, Americans.
Higher white matter hyperintensity volume relates to lower CSF Aβ42 in Black Americans.
Precuneus may be a hub for early Alzheimer's disease pathology changes detected by functional connectivity.
Keywords: Alzheimer's disease, cerebrospinal fluid, cerebrovascular disease, functional connectivity, neuroimaging
1. BACKGROUND
Black Americans are 64% more likely to develop Alzheimer's disease (AD) compared to non‐Hispanic White (NHW) Americans. 1 , 2 It is well established that race is a social construct and there is a need to understand how sociocultural factors congregate within these groups to generate biological differences in health outcomes. 3 Defining AD biomarker cutoffs and trajectories across ethno‐racial groups is an imperative step toward eliminating health disparities in diagnostics, disease progression, clinical trial enrollment, and prescription practices. AD biomarkers from both functional 4 and structural 5 magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) studies 6 , 7 show that Black American individuals may exhibit a unique AD biomarker profile.
The default mode network (DMN) is the most well‐characterized functional imaging network in AD research, 8 , 9 and individuals with AD exhibit a more rapid decline in DMN connectivity than those with normal aging. 10 , 11 DMN functional connectivity abnormalities have been reported in the presence of amyloid beta (Aβ)42 pathology in preclinical and dementia cohorts as measured by CSF 12 , 13 and Pittsburgh compound B (PiB) positron emission tomography (PET) imaging. 14 , 15 , 16 Because functional network changes typically precede symptoms in AD, some have proposed the use of longitudinal functional MRI (fMRI) as a biomarker of AD. 17 , 18 However, despite the large number of studies examining the DMN, most samples are from primarily NHW populations. 19 Models of functional connectivity may not be generalizable across ethno‐racial groups. We previously identified that among older Black Americans with and without AD, greater connectivity between the precuneus and regions of the dorsomedial subsystem of the DMN was related to increased CSF total tau, lower CSF Aβ42, and worse cognitive performance 20 (Black American = [43 F/29 M], NHW = [35 F/37 M], MAge = 70.05, standard deviation [SD]Age = 7.67, Atlanta, GA). If we are to use DMN connectivity as a biomarker for AD, we must ensure that it generalizes across ethno‐racial groups, particularly those most affected by AD.
In addition to fMRI, structural MRI identifies regions of atrophy related to AD. 21 , 22 White matter hyperintensity (WMH) volume is typically considered a marker of small vessel cerebrovascular disease 23 and is associated with the risk and progression of dementia. 24 , 25 , 26 Black Americans have higher rates of systemic vascular conditions including hypercholesterolemia, 27 hypertension, 28 and type 2 diabetes, 29 all of which are associated with white matter abnormalities. 30 , 31 , 32 , 33 Studies show that Black Americans have larger WMH volumes than their NHW counterparts. 34 , 35 Howell et al. found that in older individuals on the AD spectrum, increases in WMH volume were associated with poorer cognitive performance in Black Americans than in NHW participants. 6 Studies that investigated lobe‐specific or regional WMH volumes found that parietal lobe volumes are most consistently associated with dementia, 36 and that parietal WMHs were more strongly associated with cortical thinning in areas that overlap with typical patterns on AD neurodegeneration. 37 Few studies have investigated regional WMH volumes with an eye toward potential ethno‐racial disparities.
Hippocampal atrophy is perhaps the most common MRI finding in individuals with AD. 38 Hippocampal volume relates to both CSF Aβ42 39 and CSF total and hyperphosphorylated tau (p‐tau). 40 Factors associated with lower hippocampal volume, aside from AD pathology, include racial discrimination, 41 stress, 42 and vascular conditions 43 (e.g., hypertension 44 and type 2 diabetes 45 ), all of which are reported to be more prevalent in Black Americans. 46 , 47 , 48 , 49 , 50 , 51 While there is little evidence that hippocampal atrophy occurs as early as midlife in the presence of AD pathology, 52 , 53 the reported ethnocultural disparities and the relatively few studies comparing hippocampal volume and CSF AD biomarkers in midlife warrant further analysis in diverse cohorts.
Recently, the field has embraced the need to analyze AD biomarkers in diverse cohorts to reduce health disparities. The “Black American” ethno‐racial identity is a useful, yet simplistic, indicator of a typical conglomeration of factors including cardiovascular conditions, lower educational quality, apolipoprotein E (APOE) ε4 status, and lower socio‐economic status, that place Black Americans at a greater risk for AD. In this study, we compare DMN connectivity, regional WMH volumes, and hippocampal volumes to CSF markers of AD in a midlife cohort of racially diverse individuals with a family history of AD.
2. METHODS
2.1. Participants
Participants included cognitively unimpaired, middle‐aged, self‐identified Non‐Hispanic Black and NHW Americans with a parental history of AD 54 enrolled in the Association Between Cardiovascular Risk and Preclinical Alzheimer's Disease Pathology (ASCEND) study (Black Americans = 29, NHW = 47, PI Wharton). Full cohort demographics are displayed in Table 1. None of the individuals we enrolled were bilingual, and none identified as Hispanic. As previously described, we enrolled 82 middle‐aged to older adult children of persons with AD (median age for Black Americans = 58, NHW = 60). AD diagnosis of family member was either autopsy confirmed or probable AD as defined by National Institute of Neurological and Communicative Disorders and Stroke‐Alzheimer's Disease and Related Disorders Association criteria and verified using the validated Dementia Questionnaire (DQ) and medical records when available. Participants were recruited from the Emory Alzheimer's Disease Research Center (ADRC) clinical cohort, physician referral, and through community events and received $100 compensation.
TABLE 1.
Demographic data and biomarker levels.
| Black American (n = 29) | Non‐Hispanic White (n = 47) | |
|---|---|---|
| Age | 60.1 ± 7.8 | 58.5 ± 6.1 |
| Sex | ||
| %Male/%Female | 39.62/60.38 | 44.07/55.93 |
| APOE ε4 (%) | 38.5% | 36.8% |
| CSF Aβ42 | 712 ± 162 | 700 ± 197 |
| CSF t‐tau | 219 ± 96.4 | 333 ± 176** |
| CSF p‐tau | 39.1 ± 14.12 | 52.73 ± 21.98** |
| Hippocampal volume, cm3 | 7.36 ± 0.62 | 7.68 ± 0.85 |
| History of diabetes (%) | 7.4%* | 0% |
| History of hypertension (%) | 57.1%* | 34.00% |
| History of hyperlipidemia (%) | 45% | 45.9% |
| Total cardiovascular risk score (%) | 0 = 25%/1 = 35%/2 = 35%/3 = 5% | 0 = 37.8%/1 = 37.8%/2 = 24.4%/3 = 0% |
| WMH volume | 3.10 ± 2.80 | 1.89 ± 0.78 |
| National Area Deprivation Index | 57 ± 24.17** | 33.1 ± 22.05 |
| Highest level of completed education (%) | 15/41/44 | 18/38/44 |
| High school or GED/college/postgraduate |
Abbreviations: Aβ, amyloid beta; APOE, apolipoprotein E: CSF, cerebrospinal fluid; GED, General Educational Development; p‐tau, phosphorylated tau; SD, standard deviation; t‐tau, total tau; WMH, white matter hyperintensity.
= significantly different between groups at p < 0.05, ** p < 0.001.
Baseline results and methods have been described previously, 54 but briefly, participants underwent a detailed interview for demographic information, medical and medication history including vascular risk factors (coronary artery disease, congestive heart failure, atrial fibrillation, hypertension, hyperlipidemia, diabetes, suspected transient ischemic attack) and other medical comorbidities (e.g., cancer). Participants had a biological parent with AD and were 45 to 65 years old at baseline. Exclusion criteria included significant neurologic disease, history of significant head trauma, major untreated depression, history of alcohol or substance abuse, and current diagnosis of AD or mild cognitive impairment. Twenty Black participants underwent lumbar puncture (LP), and of these participants, 16 of them also underwent an MRI. Reasons for fluctuating participant numbers are that individuals who have a contraindication for MRI may still be eligible for an LP, and LPs are occasionally unsuccessful. In Table S1 in supporting information, we have included the demographic makeup of individuals with CSF, LP, and MRI data.
RESEARCH IN CONTEXT
Systematic review: We reviewed literature using traditional (e.g., PubMed) sources. We know that Black Americans are at an increased risk of developing Alzheimer's disease (AD), but if racial differences related to imaging modalities exist is unclear. We investigated whether there is consistency in the reported relationships among cerebrospinal fluid (CSF) markers of AD and imaging.
Interpretation: Evidence suggested that there would be differences in relationships between CSF and neuroimaging modalities between races, but studies investigating ethno‐racial differences and neuroimaging are limited in preclinical and at‐risk populations.
Future directions: The article proposes a framework for the generation of new hypotheses and the conduct of additional studies. Examples include further understanding: (a) the relationships between amyloid beta 42 and functional connectivity even in middle age; (b) the role of race in the modification of CSF to neuroimaging biomarker relationships; and (c) if white matter hyperintensities and functional neuroimaging measures are independently related CSF markers of AD.
2.2. CSF collection
CSF was collected after an 8‐hour overnight fast and according with the International Society for Biological and Environmental Repositories Best Practices guidelines. 55 Approximately 22 mL of CSF was collected using sterile polypropylene collection tubes. Participants underwent LP to collect CSF for Aβ and tau, and markers of vascular dysfunction and inflammatory cytokines and chemokines, as previously described. 54 Participants also underwent blood draw for analysis of (1) RAS function, including angiotensin‐converting enzyme activity, (2) plasma inflammatory markers, and (3) APOE genotyping. CSF Aβ40, Aβ42, and p‐tau concentrations were measured by Lumipulse technology (Fujirebio). 56 , 57 Samples were assayed in two batches by experienced and board‐certified laboratory technicians. Intra‐assay coefficients of variation were < 10% for all three analytes.
2.3. Cardiovascular risk score
Using an approach described in previous analyses, we created a cardiovascular risk score to understand the relationship between cardiovascular risk factors and our variables of interest. 6 , 20 We created this score by coding presence of three common cardiovascular risk factors (hypertension, type 2 diabetes, hyperlipidemia) with either a “0” for not present, or “1” for present. We then summed the scores for a total cardiovascular risk score (minimum 0, maximum 3). Table S2 in supporting information includes the number of individuals with all combinations of cardiovascular conditions.
2.4. Area Deprivation Index
We generated national Area Deprivation Index (ADI) scores as a proxy measure of socio‐economic status using the Neighborhood Atlas. 58 Scores were generated from participant residential zip codes.
2.5. Neuroimaging data
All participants were scanned on a MAGNETOM PrismaFit 3T MRI machine at the Emory Center for Systems Imaging. Scanning protocol included a T1‐weighted 3D magnetization‐prepared rapid acquisition gradient echo sequence (repetition time [TR]/inversion time [TI]/echo time [TE] = 2300/800/2.89msec, flip angle = 8°, matrix = 256 × 256 × 176, and voxel size = 1 × 1 × 1mm3), a 4.25‐minute eyes‐open resting state fMRI scan (TR/TE = 3000 ms/32 ms, flip angle = 90°, field of view [FOV] = 200 × 200 mm2, acquisition matrix = 220 × 220 × 144, voxel size = 2 × 2 × 2 mm3, slice = 48, time point = 170), and a T2‐weighted fluid‐attenuated inversion recovery (FLAIR) sequence (TR/TE = 2480 ms/364 ms, flip angle = 90°, FOV = 250 × 250 mm2, acquisition matrix = 250 × 250 × 160, voxel size = 1 × 1 × 1 mm3, slice = 160).
2.6. fMRI data preprocessing
We used a standard preprocessing pipeline using the DPARSFA toolbox. 59 The first 10 time points were removed, scans were slice time corrected, manually reoriented where necessary, realigned for head motion, normalized to Montreal Neurological Institute space, and smoothed using the DARTEL algorithm. 60 , 61 We then performed nuisance covariate regression (Friston's 24 parameter head motion regressors, CSF, white matter). We further removed motion confounds using ICA‐AROMA 62 and applied a high‐pass filter. We performed a seed‐based analysis using the regions described by Andrews‐Hanna et al. 63 in the three DMN subnetworks, namely, (1) the medial frontal and temporal lobe subsystem (temporal pole [TP], temporal parietal junction [TPJ], lateral temporal cortex [LTC], dorsomedial prefrontal cortex [dmPFC], and anterior medial prefrontal cortex [amPFC]), (2) the medial temporal lobe (MTL) subsystem (hippocampal formation, parahippocampal cortex, retrosplenial cortex, posterior inferior parietal lobule, ventromedial prefrontal cortex), and (3) the midline core (posterior cingulate and precuneus). As we were interested in precuneus to dorsomedial subsystem connectivity, we used five measures of pairwise connectivity between the precuneus and the following regions: amPFC, dmPFC, TPJ, LTC, and temporal pole, depicted in Figure 1.
FIGURE 1.
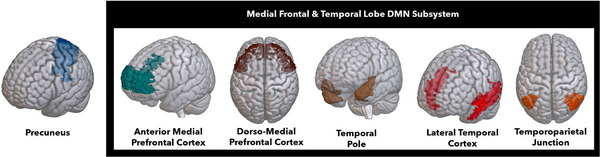
Regions of interest for the functional connectivity analysis. DMN, default mode network.
2.7. Regional WMH analysis
Regional WMH volumes were derived from T2‐weighted FLAIR images. 64 Briefly, each participant's FLAIR image was brain extracted and intensity normalized. Images were then processed through a high‐pass filter at the mode of the distribution of the image voxel intensity values. A half Gaussian mixture model was fit to the log‐transformed histogram of the intensity values of each image. The Gaussian distribution that encapsulated the highest intensity values defined the hyperintense voxels and was labeled. Any cluster of labeled voxels that comprised fewer than five voxels was removed from the mask. The labeled images were visually inspected and false positives removed. The number of labeled voxels was summed and multiplied by voxel dimensions to yield a total volume in cm3.
2.8. Hippocampal volumes
T1‐weighted images were processed with FreeSurfer (version 6). 65 We obtained hippocampal and total intracranial volume and calculated hippocampal volume as a proportion of total intracranial volume.
2.9. Statistical analyses
We constructed three separate multiple multivariate regression models, one for each modality: functional connectivity, hippocampal volumes, and WMH volumes. Outcome variables for the functional connectivity models were precuneus to dmPFC, TPJ, LTC, TP, and amPFC. The independent variables were race, CSF Aβ42, and CSF p‐tau, and covariates were sex, age, and mean framewise displacement (functional connectivity models only). We included race × CSF Aβ42, and race × p‐tau interaction terms. We corrected for multiple comparisons using a Bonferroni correction in the “p.adjust” package in R and report the corrected p‐values in the results table. 66 Our regression results depict only individuals who had complete data for our variables of interest; thus, sample size may vary marginally for each regression analysis.
Because we did not have statistical power to include all our variables of interest in one model, we conducted follow‐up analyses to determine whether any risk factor variables associated with race that could potentially explain any racial differences that we identified. In these models, we added ADI, APOE ε4 status, and cardiovascular risk score.
3. RESULTS
3.1. Functional connectivity
Summary results of the functional connectivity analyses are displayed in Table 2 and Figure 2.
TABLE 2.
Functional connectivity race × Aβ42 summary table.
| Precuneus to lateral temporal cortex | |||
|---|---|---|---|
| Estimate | CI | p | |
| Race | 0.75 | −0.30 to 0.85 | 0.15 |
| Aβ42 | 0.00001 | −0.20 to 0.32 | 0.31 |
| Race × Aβ42 | −0.001 | −0.04 to 0.05 | 0.26 |
| Black | 0.001 | −2.45 | 0.88 |
| White | −0.0003 | −0.001 to 0.0001 | 0.18 |
| Precuneus to dmPFC | |||
|---|---|---|---|
| Estimate | CI | p | |
| Race | 1.02 | 0.20 to 1.92 | 0.04 |
| Aβ42 | 0.001 | 0.0002 to 0.002 | 0.06 |
| Race × Aβ42 | −0.001 | −0.003 to 0.001 | 0.06 |
| Black | −0.0003 | −0.003 to 0.002 | 0.8 |
| White | −0.0001 | −0.001 to 0.001 | 0.82 |
| Precuneus to temporal parietal junction | |||
|---|---|---|---|
| Estimate | CI | p | |
| Race | 1.05 | 0.30 to 1.80 | 0.01 |
| Aβ42 | 0.01 | 0.00 to 0.01 | 0.02 |
| Race × Aβ42 | −0.001 | −0.002 to −0.0001 | 0.03 |
| Black | 0.001 | −0.001 to 0.003 | 0.31 |
| White | 0.19 | −0.15 to 0.53 | 0.27 |
| Precuneus to temporal pole | |||
|---|---|---|---|
| Estimate | CI | p | |
| Race | 0.39 | 0.03 to 0.75 | 0.04 |
| Aβ42 | 0 | 0.00 to 0.00 | 0.04 |
| Race × Aβ42 | 0 | −0.00 to −0.00 | 0.03 |
| Black | 0.001 | 0.0001 to 0.002 | 0.05 |
| White | −0.0003 | −0.001 to 0.0001 | 0.18 |
| Precuneus to amPFC connectivity | |||
|---|---|---|---|
| Estimate | CI | p | |
| Race | 1.06 | 0.20 to 1.92 | 0.02 |
| Aβ42 | 0.001 | 0.0002 to 0.002 | 0.02 |
| Race × Aβ42 | −0.001 | −0.003 to −0.0002 | 0.03 |
| Black | 0.001 | 0.0001 to 0.002 | 0.04 |
| White | −0.0001 | −0.0014 | 0.62 |
Note: Bold text = significant at P < 0.05.
Abbreviations: Aβ, amyloid beta; aMPFC, anterior medial prefrontal cortex; CI, confidence interval, dmPFC, dorsomedial prefrontal cortex.
FIGURE 2.
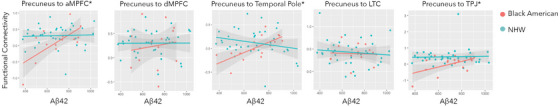
Functional connectivity to Aβ42. Red = Black American participants; Green = non‐Hispanic White participants. Aβ, amyloid beta; amPFC, anterior medial prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; LTC, lateral temporal cortex; NHW, non‐Hispanic White; PCC, posterior cingulate cortex; TPJ, temporal parietal junction.
3.2. Precuneus to amPFC connectivity
There was a significant race x Aβ42 interaction (B = −0.001, t[0.29, 45] = −2.29, P = 0.04). Lower Aβ42 levels were associated with lower connectivity, but only for Black American participants (Black Americans: B = 0.001, t[4,11] = 2.39, P = 0.04, NHW: B = −0.0001, t[4,47] = 0.64, P = 0.62). There was not a significant main effect or interaction for p‐tau. Full model results are included in Table S3 in supporting information.
3.3. Precuneus to TP connectivity
There was a significant race × Aβ42 interaction (B = −0.001, t[6, 45] = −2.29, P = 0.03). Lower Aβ42 levels were associated with lower connectivity, but only for Black American participants (Black Americans: B = 0.001, t[4,11] = 1.98, p = 0.04, NHW: B = −0.0001, t[4,47] = −1.37, P = 0.18). There was not a significant main effect or interaction for p‐tau. Full model results are included in Table S4 in supporting information.
3.4. Precuneus to TPJ connectivity
Lower Aβ42 levels were associated with lower connectivity (main effect of Aβ42, B = 0.002, t[0.29, 45] = 2.57, P = 0.01). There was a significant race × Aβ42 interaction (B = −0.001, t[0.29, 45] = −2.22, P = 0.03) such that within Black participants, lower Aβ42 was related to lower connectivity (Black Americans: B = 0.001, t[4,11] = 1.06, P = 0.31, NHW: B = 0.001, t[4,47] = 0.39, P = 0.70), but this relationship within group was not statistically significant. There was not a significant main effect or interaction for p‐tau. Full model results are included in Table S5 in supporting information.
3.5. Precuneus to dmPFC
Lower Aβ42 levels were associated with lower connectivity (main effect of Aβ42, B = 0.002, t[0.29, 45] = 2.57, P = 0.01). There was a significant race × Aβ42 interaction (B = −0.001, t[0.29, 45] = −2.22, P = 0.03) such that within Black participants, lower Aβ42 was related to lower connectivity (Black Americans: B = 0.001, t[4,11] = 1.06, P = 0.31, NHW: B = 0.001, t[4,47] = −1.64, P = 0.11), but this relationship within group was not statistically significant. There was not a significant main effect or interaction for p‐tau. Full model results are included in Table S6 in supporting information.
3.6. Precuneus to LTC connectivity
There was not a significant main effect or interaction of race (B = 0.75, 1, t[0.29, 45) = 1.36, P = 0.15), Aβ42(B = 0.00001, 1, t[0.29, 45] = 0.11, P = 0.31), or p‐tau. Full model results are included in Table S7 in supporting information.
3.7. White matter hyperintensities and AD CSF biomarkers
Summary results of the WMH analyses are displayed in Table 3 and Figure 3.
TABLE 3.
White matter hyperintensity race × Aβ42 summary table.
| Frontal lobe WMH volumes | |||
|---|---|---|---|
| Estimates | CI | p | |
| Race | 0.17 | −0.98 to 1.33 | 0.76 |
| Aβ42 | −0.001 | −0.003 to 0.001 | 0.33 |
| Race × Aβ42 | 0.001 | −0.001 to 0.003 | 0.40 |
| Black | −0.03 | −0.11 to 0.04 | 0.25 |
| White | <0.001 | −0.0005 to 0.001 | |
| Temporal lobe WMH volumes | |||
|---|---|---|---|
| Estimates | CI | p | |
| Race | −1.20 | −1.89 to −0.52 | 0.001 |
| Aβ42 | − 0.00 | −0.00 to −0.00 | 0.01 |
| Race × Aβ42 | 0.00 | 0.00 to 0.00 | 0.01 |
| Black | −0.18 | − 0.01 to 0.005 | 0.050 |
| White | 0.0003 | −0.0001 to 0.0001 | 0.52 |
| Parietal lobe WMH volumes | |||
|---|---|---|---|
| Estimates | CI | p | |
| Race | 0.14 | −0.24 to 0.53 | 0.46 |
| Aβ42 | −0.002 | −0.003 to −0.002 | <0.001 |
| Race × Aβ42 | 0.002 | 0.002 to 0.003 | <0.001 |
| Black | −0.17 | −0.01 to −0.0055 | 0.05 |
| White | 0.00003 | −0.0001 to 0.0001 | 0.47 |
| Occipital lobe WMH volumes | |||
|---|---|---|---|
| Estimates | CI | p | |
| Race | −0.56 | −0.96 to −0.16 | 0.007 |
| Aβ42 | −0.0002 | −0.00003 to 0.00004 | 0.18 |
| Race × Aβ42 | 0.001 | 0.0002 to 0.01 | 0.031 |
| Black | −0.05 | −0.01 to −0.0055 | 0.05 |
| White | 0.00003 | −0.0001 to 0.0001 | 0.47 |
Note: bold values indicate p < 0.05.
Abbreviations: Aβ, amyloid beta; CI, confidence interval; WMH, white matter hyperintensity.
FIGURE 3.
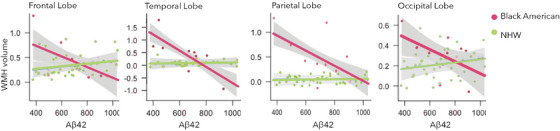
White matter hyperintensity volumes to Aβ42. Red = Black American participants; Green = non‐Hispanic White participants. Aβ, amyloid beta; WMH, white matter hyperintensity.
For parietal lobe WMH volumes, there was a race × Aβ42 interaction (B = 0.002, t[5,38] = 2.55, P = 0.05) such that within Black participants, lower levels of CSF Aβ42 were related to higher parietal lobe WMH volumes (Black Americans: B = −0.17, t[2,14] = −1.35, P = 0.05, NHW: B = 0.00003, t[3,31] = 1.72, P = 0.47). Additionally, we observed a significant race × Aβ42 occipital lobe WMH volumes (B = 0.001, t[4,45] = 2.24, P = 0.05, Black Americans: B = −0.05, t[2,14] = −1.25, P = 0.05, NHW: B = 0.00003, t[3,31] = 1.72, P = 0.47), and temporal lobe WMH volumes (Black Americans: B = −0.18, t[2,14] = −1.89, P = 0.01, NHW: B = 0.0003, t[3,31] = 0.89, P = 0.52) interaction. Condensed model results are displayed in Table 3, and full model results in Tables S8‐11 in supporting information.
3.8. Hippocampal volume and AD CSF biomarkers
There was not a statistically significant relationship between hippocampal volume and CSF Aβ42 and p‐tau.
3.9. analyses with ethno‐racial comorbidities
In our follow‐up analyses, cardiovascular risk score, ADI, and APOE ε4 status were not significantly related to our outcome variables of interest. Results are included in supplemental tables.
4. DISCUSSION
The purpose of this study was to identify the relationships between AD CSF biomarkers and AD neuroimaging biomarkers in a middle‐aged, cognitively normal cohort of individuals with a parental history of AD. We identified an interaction between AD CSF biomarker levels, precuneus functional connectivity, and WMHs such that CSF Aβ42 was more strongly correlated with lower connectivity and increased WMH volumes in Black participants than in NHW participants.
Functional neuroimaging measures were only related to AD CSF biomarkers in Black participants. More specifically, lower CSF Aβ42 in Black participants was related to lower connectivity between the posterior cingulate cortex (PCC)/precuneus and regions of the medial frontal DMN subsystem. By contrast, no significant relationship between AD CSF biomarkers and DMN connectivity was observed in NHW participants. Previous research using this subsystem approach identified connectivity alterations between the PCC and the frontal and temporal lobe subsystems in NHW individuals with AD. 20 , 67 , 68 Precuneus connectivity is typically lower in individuals with AD, and lower precuneus connectivity correlates with increased amyloid deposition. 69 , 70 , 71 The precuneus also seems to be an initial site of amyloid accumulation in healthy aging. 72 , 73 Future research should investigate whether the CSF concentrations of Aβ42 are related to increased Aβ42 deposition in the precuneus and if there are ethno‐racial–specific relationships between connectivity and regional amyloid deposition.
In an independent cohort of older Black Americans and NHW participants with and without AD, we previously identified race‐related differences similar to those reported in the current work, but in the opposite direction: 20 greater DMN connectivity was related to greater AD biomarker burden between the precuneus and regions of the MTL/frontal subsystem among Black Americans. Comparisons across the two studies are difficult to make, as age and presence of AD change connectivity; however, the fact that we identified a race‐related difference in connectivity to biomarker relationships in both cohorts does suggest that racial differences in brain biomarker relationships may be generalizable to other cohorts. A possible explanation for the fact that we observed the opposite relationship between two cohorts could be that the AD cohort included older adults, many of whom were on the dementia spectrum. Connectivity profiles can change throughout the lifetime, with some studies identifying both increased 74 and decreased 10 , 11 connectivity between default network nodes over the course of aging and AD. It could be that there are other regions more affected by amyloid in the later stages of the disease that then cause a compensatory mechanism within the DMN. 75 , 76
Potential explanations for race‐related differences may include differences in the prevalence of cardiovascular and sociocultural factors including stress and access to resources including health care, and, in our case, uneven distribution of sex across racial groups. Here we controlled for cardiovascular risk factors, APOE ε4 status, and socio‐economic status, and when we performed the same analyses within only women, we found that the relationships were still consistent. Potential explanations for these differences that we did not analyze include educational quality, diet, and exercise levels, which have been reported to affect connectivity and may show racial disparities, potentially due to access to resources and higher stress. Our sample is also unique in that participants were highly educated. Typically, only ≈ 26% of Black Americans across the United States have a bachelor's degree, compared to 41% in our sample. It is possible that we would see even more pronounced differences in brain biomarker relationships in a sample with a lower percentage of people with higher education. Black Americans, particularly females, typically experience a greater number of adverse childhood experiences. We did not account for this in our analysis, which could explain some of the racial differences we identified.
4.1. White matter hyperintensity volumes
This study is one of a few to include both WMHs and AD CSF biomarkers, particularly with attention to regional specificity, in a racially diverse, middle‐aged cohort at high risk for AD by virtue of parental history. We identified a relationship between CSF Aβ levels and WMHs such that within cognitively healthy Black Americans, lower CSF Aβ42 was associated with increased WMH volumes in the parietal, temporal, and occipital lobes. We did not identify these same relationships in NHW participants. There is growing evidence that amyloid itself can cause WMHs, and lower CSF Aβ42 is often found to be related to increased WMH burden. 77 , 78
Amyloid deposition in preclinical and healthy aging populations is not well characterized. CSF Aβ42 is typically lower in AD, but it does not always correlate with AD symptoms. Current models suggest that amyloid accumulation begins as early as 10 to 20 years before symptom onset, and that as Aβ reaches pathological loads, it plateaus and tau serves as a more accurate marker of disease trajectory. Although this is a small sample, the difference in our results for Black and NHW participants suggests that other health factors may underlie the disparate disease trajectories for these two groups.
Vascular health may play a role in the association between increased WMHs and AD CSF biomarkers observed in Black participants in this study. Black Americans demonstrate greater incidence and prevalence of cardiovascular diseases, including diabetes, hypertension, hypercholesterolemia, and obesity compared to NHW individuals. 51 , 79 Black Americans also have a greater WMH burden compared NHW individuals, a neuroimaging finding often considered to be an indicator of cerebrovascular burden and a finding we replicated in this cohort. 80 , 81 Although we did not observe a significant difference in amyloid deposition between groups in this study, higher vascular burden may result in a stronger relationship between amyloid deposition and WMH volumes for Black Americans. 82 Research has shown that cerebral amyloid angiopathy, which is the deposition of amyloid in cerebral blood vessels, may partially explain the relationship between amyloid and WMH volumes. 77 , 83 However, some study samples have neglected to show significant differences in cerebral amyloid angiopathy in Black Americans, underscoring the potential role of other determinants of health in dementia pathology for these groups. 84 While not measured in this study, it is possible that Black participants had increased vascular, compared to parenchymal, amyloid burden.
While we did not identify a significant relationship between cardiovascular risk score and our functional connectivity measures, it is possible that unique combinations of the vascular risk scores could explain some of the relationships that we identified. Hypertension, hyperlipidemia, and type 2 diabetes mellitus all predict worse AD brain biomarker outcomes; the combination of these factors and MRI biomarkers has not been thoroughly investigated. There is not a consensus about the effect of the combination of cardiovascular conditions on AD risk. Ruthirakuhan et al. found that hypertension + type 2 diabetes does not significantly increase AD risk more than hypertension alone. 85 Nakamura et al. reported that hypertension + hyperlipidemia did not increase dementia risk in a cohort of older Japanese adults. 86 Dyslipidemia and type 2 diabetes mellitus likely synergize to increase risk of AD, but we are unaware of an analysis of the effect of the combination of these two factors on brain biomarkers. 87 Research suggests that APOE ε4 status may modify the effects of these risk factors on AD risk, and should be considered in analysis of these factors. 88 , 89 , 90 Future analyses will investigate the effect of individual risk factors and all combinations of these risk factors on MRI and CSF biomarkers.
Disparities in central and peripheral vascular health are, in part, likely the result of lifetime exposure to systemic disadvantages in wealth, housing, education, neighborhood characteristics, access to health care, and racial discrimination. 91 , 92 These stressors appear to activate a physiological response in combination with epigenetic changes that “weather” the body and accelerate premature aging. For instance, chronic stress leads to hyperactivation of the hypothalamic–pituitary adrenal axis through the overproduction of glucocorticoids including cortisol which, over time, triggers neuroinflammation and hyperglycemia that collectively induce oxidative stress and ultimately cause neuronal dysfunction. 93 High glucocorticoid concentrations are associated with increased Aβ deposition, which correlates with lower CSF Aβ42, and worse cognitive outcomes across measures of learning and memory. 94 , 95 Taken together, weathering may partially explain our finding of an association between elevated WMH volumes and low CSF Aβ42 in otherwise cognitively healthy Black participants but not NHW participants.
4.2. Hippocampal volumes
The lack of relationships between hippocampal volumes and CSF Aβ42 and tau is not surprising. Atrophy in the presence of amyloid and tau typically does not occur until well after symptoms of AD have begun. 96 , 97 , 98 In AD, hyperphosphorylated tau—historically seen as the cause of MTL atrophy—typically emerges in the temporal lobe and then spreads from these MTL structures to the rest of the cortex. 99 , 100 Although the cognitively normal individuals in this study are at risk for AD, their pathology levels may not reach clinically significant thresholds required to cause atrophy. Our results support previous work that Black Americans do not exhibit higher rates of hippocampal atrophy, as we did not identify any racial differences in hippocampal volumes, and that these volumes do not typically exhibit vulnerability to AD pathology until late life. 36
4.3. Limitations
We were not able to analyze the interaction of race and sex on our variables of interest because of the uneven distribution of men across the two ethno‐racial groups and individual cardiovascular conditions. Even though we still identified a significant interaction between races, the lower number of Black men limited our ability to analyze sex at a more granular level. We know that sex interacts with inflammatory pathways that can contribute to the development of AD, 101 , 102 and can affect the relationship between AD and WMH. As data collection and recruitment in this group are ongoing, we hope to explore the intersection of female sex and Black American race on the trajectory of the neuroimaging and CSF AD biomarkers.
Our sample size limited the number of explanatory variables that we could include in our analyses such as sex, adverse childhood experiences, and individual vascular conditions. Only individuals with complete datasets for each regression analysis were included in each model, further decreasing our sample size and limiting our statistical power. While our community‐based recruitment methods help ensure that our samples are not collected out of convenience, we did not collect data from every stratum of society including individuals from rural areas, a high percentage of individuals with no higher education, bilingual individuals, and so on. Future work will analyze adverse childhood experiences, stress, and perceived discrimination and their relationship to the brain biomarker disparities we identified. Furthermore, multicohort meta‐analyses and comparisons will allow us to investigate the generalizability of our findings.
Analysis of racial differences by category grossly generalizes across the diversity of the human condition. Race is likely a proxy measure for a variety of comorbid factors and experiences that converge to create an increased risk for dementia. However, because we can observe differences between these ethno‐racial groups, further studies can investigate the nature of these disparities in hopes of then developing interventions to address unique needs of those most at risk for AD.
5. CONCLUSION
CSF Aβ42 correlated with both precuneus functional connectivity and WMH volume in a cohort of Black Americans at risk for AD. This finding, consistent with relationships identified in individuals with AD, was not explained by potentially confounding variables such as ADI and cardiovascular risk factors alone. This work supports the notion that racial disparities in brain to biomarker relationships cannot be solely explained by disparities in vascular health, and that the degree to which someone experiences a combination of stressors more prevalent in their racial group may influence the relationship between amyloid and neurological function. Future research will investigate longitudinal changes in these biomarkers and their relationships to identify mechanisms linking amyloid to both neurological activity and WMH.
AUTHOR CONTRIBUTIONS
M. Misiura is the primary author of the manuscript responsible for much of the content, analytical design, neuroimaging analyses, and communication between authors. C. Munkombwe performed the WMH and CSF analyses and assisted with table consolidation. K. Igwe performed the WMH quantification under the supervision of A. M. Brickman. D. D. Verble assisted with data entry, participation recruitment, data collection, and data transfer. K. D. S. Likos assisted with data entry, participation recruitment, data collection, and data transfer. L. Minto authored portions of the manuscript. A. Bartlett authored portions of the manuscript. H. Zetterberg performed CSF analyses. J. Turner oversaw initial neuroimaging analyses. V. Dotson authored portions of this manuscript. A. M. Brickman oversaw the WMH quantification and provided feedback on manuscript drafts. W. T. Hu is a Wharton lab collaborator who assisted with data transfer and initial liasing between M. Misiura and W. Wharton. W. Wharton was the principal investigator of the ASCEND cohort study, provided feedback on manuscript and analyses, and authored portions of the manuscript.
CONFLICT OF INTEREST STATEMENT
HZ has served on scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). AMB holds an inventor a patent for white matter hyperintensity quantification (US patent# 9867566). Author disclosures are available in the supporting information. MM, CM, KI, DL, LM, AB, JAT, VMD, WTH, WW have nothing to disclose. Author disclosures are available in the supporting information.
CONSENT STATEMENT
This study was approved by the Emory IRB and participants consented to share their data.
CONSENT FOR PUBLICATION
All participants gave their consent for their de‐identified data to be used for the analyses for this publication.
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
This project was supported by the National Institute on Aging and R01AG066203.
Misiura M, Munkombwe C, Igwe K, et al. Neuroimaging correlates of Alzheimer's disease biomarker concentrations in a racially diverse high‐risk cohort of middle‐aged adults. Alzheimer's Dement. 2024;20:5961–5972. 10.1002/alz.14051
DATA AVAILABILITY STATEMENT
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Steenland K, Tan Y, Wingo T, Shi L, Xiao S, Wharton W. The effect of race and co‐morbidities on Alzheimer's disease based on Medicare data. Alzheimers Dement J Alzheimers Assoc. 2023;19:1858‐1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lines LM, Wiener JM, Racial and Ethnic Disparities in Alzeimer's Disease: A Literature Review . (2014).
- 3. Gleason CE, Zuelsdorff M, Gooding DC, et al. Alzheimer's disease biomarkers in Black and non‐Hispanic White cohorts: a contextualized review of the evidence. Alzheimers Dement. 2021;18(8):1545‐1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carmichael O, Newton R. Brain MRI findings related to Alzheimer's disease in older African American adults. Prog Mol Biol Transl Sci. 2019;165:3‐23. [DOI] [PubMed] [Google Scholar]
- 5. Zahodne L, Manly J, Narkhede A, et al. Structural MRI predictors of late‐life cognition differ across African Americans, Hispanics, and Whites. Curr Alzheimer Res. 2015;12:632‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howell JC, Watts KD, Parker MW, et al. Race modifies the relationship between cognition and Alzheimer's disease cerebrospinal fluid biomarkers. Alzheimers Res Ther. 2017;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garrett SL, Mcdaniel D, Obideen M, et al. Racial disparity in cerebrospinal fluid amyloid and tau biomarkers and associated cutoffs for mild cognitive impairment. JAMA Netw Open. 2019;2:e1917363‐e1917363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koch W, Teipel S, Mueller S, et al. Diagnostic power of default mode network resting state fMRI in the detection of Alzheimer's disease. Neurobiol Aging. 2012;33:466‐478. [DOI] [PubMed] [Google Scholar]
- 9. Hafkemeijer A, van der Grond J, Rombouts SARB. Imaging the default mode network in aging and dementia. Imaging Brain Aging Neurodegener Dis. 2012;1822:431‐441. [DOI] [PubMed] [Google Scholar]
- 10. Jones DT, Machulda MM, Vemuri P, et al. Age‐related changes in the default mode network are more advanced in Alzheimer disease. Neurology. 2011;77:1524‐1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greicius MD, Srivastava G, Reiss AL, Menon V. Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637‐4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Millar PR, Ances BM, Gordon BA, et al. Evaluating resting‐state BOLD variability in relation to biomarkers of preclinical Alzheimer's disease. Neurobiol Aging. 2020;96:233‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ingala S, Tomassen J, Collij LE, et al. Amyloid‐driven disruption of default mode network connectivity in cognitively healthy individuals. Brain Commun. 2021;3:fcab201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hasani SA, Mayeli M, Salehi MA, Barzegar Parizi R. A systematic review of the association between amyloid‐β and τ pathology with functional connectivity alterations in the Alzheimer dementia spectrum utilizing PET scan and rsfMRI. Dement Geriatr Cogn Disord Extra. 2021;11:78‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Myers N, Pasquini L, Göttler J, et al. Within‐patient correspondence of amyloid‐β and intrinsic network connectivity in Alzheimer's disease. Brain J Neurol. 2014;137:2052‐2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koch K, Myers NE, Göttler J, et al. Disrupted intrinsic networks link amyloid‐β pathology and impaired cognition in prodromal Alzheimer's disease. Cereb Cortex N Y N 1991. 2015;25:4678‐4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Damoiseaux JS. Resting‐state fMRI as a biomarker for Alzheimer's disease? Alzheimers Res Ther. 2012;4:8‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teipel SJ. DTI and resting state fMRI as biomarker of Alzheimer's disease: present state and perspectives. Alzheimers Dement J Alzheimers Assoc. 2010;6:S169. [Google Scholar]
- 19. Ballard EL, Gwyther LP, Edmonds HL. Challenges and opportunities: recruitment and retention of African Americans for Alzheimer disease research: lessons learned. Alzheimer Dis Assoc Disord. 2010;24:S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Misiura MB, Howell JC, Wu J, et al. Race modifies default mode connectivity in Alzheimer's disease. Transl Neurodegener. 2020;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brickman AM, Muraskin J, Zimmerman ME. Structural neuroimaging in Alzheimer's disease: do white matter hyperintensities matter? Dialogues Clin Neurosci. 2009;11:181‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lombardi G, Crescioli G, Cavedo E, et al. Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer's disease in people with mild cognitive impairment. Cochrane Database Syst Rev. 2020;3(3):CD009628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Leeuw F‐E. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abraham HMA, Wolfson L, Moscufo N, Guttmann CRG, Kaplan RF, White WB. Cardiovascular risk factors and small vessel disease of the brain: blood pressure, white matter lesions, and functional decline in older persons. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2016;36:132‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gaubert M, Lange C, Garnier‐Crussard A, et al. Topographic patterns of white matter hyperintensities are associated with multimodal neuroimaging biomarkers of Alzheimer's disease. Alzheimers Res Ther. 2021;13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Y‐Li, Chen W, Cai W‐J, et al. Associations of white matter hyperintensities with cognitive decline: a longitudinal study. J Alzheimers Dis JAD. 2020;73:759‐768. [DOI] [PubMed] [Google Scholar]
- 27. Toft‐Nielsen F, Emanuelsson F, Benn M. Familial hypercholesterolemia prevalence among ethnicities‐systematic review and meta‐analysis. Front Genet. 2022;13:840797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferdinand KC, Yadav K, Nasser SA, et al. Disparities in hypertension and cardiovascular disease in blacks: the critical role of medication adherence. J Clin Hypertens Greenwich Conn. 2017;19:1015‐1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brancati FL, Kao WHL, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in african american and white adults: the atherosclerosis risk in communities study. JAMA. 2000;283:2253‐2259. [DOI] [PubMed] [Google Scholar]
- 30. Cohen JI, Cazettes F, Convit A. Abnormal cholesterol is associated with prefrontal white matter abnormalities among obese adults, a diffusion tensor imaging study. Neuroradiol J. 2011;1:989‐997. [PMC free article] [PubMed] [Google Scholar]
- 31. Tong X‐K, Trigiani LJ, Hamel E. High cholesterol triggers white matter alterations and cognitive deficits in a mouse model of cerebrovascular disease: benefits of simvastatin. Cell Death Dis. 2019;10:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Divers J, Hugenschmidt C, Sink KM, et al. Cerebral white matter hyperintensity in African Americans and European Americans with type 2 diabetes. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2013;22:e46‐e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van Dijk EJ, Breteler MMB, Schmidt R, et al. The association between blood pressure, hypertension, and cerebral white matter lesions. Hypertension. 2004;44:625‐630. [DOI] [PubMed] [Google Scholar]
- 34. Morrison C, Dadar M, Manera AL, Collins DL. Racial differences in white matter hyperintensity burden in older adults. Neurobiol Aging. 2023;122:112‐119. [DOI] [PubMed] [Google Scholar]
- 35. Nyquist PA, Bilgel MS, Gottesman R, et al. Extreme deep white matter hyperintensity volumes are associated with African American race. Cerebrovasc Dis Basel Switz. 2014;37:244‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brickman AM, Provenzano FA, Muraskin J, et al. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Arch Neurol. 2012;69:1621‐1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rizvi B, Lao PJ, Chesebro AG, et al. Association of regional white matter hyperintensities with longitudinal Alzheimer‐like pattern of neurodegeneration in older adults. JAMA Netw Open. 2021;4:e2125166‐e2125166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Henneman WJP, Sluimer JD, Barnes J, et al. Hippocampal atrophy rates in Alzheimer disease. Neurology. 2009;72:999‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stricker NH, Dodge HH, Dowling NM, Han SD, Erosheva EA, Jagust WJ. CSF biomarker associations with change in hippocampal volume and precuneus thickness: implications for the Alzheimer's pathological cascade. Brain Imaging Behav. 2012;6:599‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Souza LC, Chupin M, Lamari F, et al. CSF tau markers are correlated with hippocampal volume in Alzheimer's disease. Neurobiol Aging. 2012;33:1253‐1257. [DOI] [PubMed] [Google Scholar]
- 41. Zahodne LB, Sharifian N, Kraal AZ, et al. Longitudinal associations between racial discrimination and hippocampal and white matter hyperintensity volumes among older Black adults. Soc Sci Med. 2023;316:114789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rahman MM, Callaghan CK, Kerskens CM, Chattarji S, O'mara SM. Early hippocampal volume loss as a marker of eventual memory deficits caused by repeated stress. Sci Rep. 2016;6:29127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Elcombe EL, Lagopoulos J, Duffy SL, et al. Hippocampal volume in older adults at risk of cognitive decline: the role of sleep, vascular risk, and depression. J Alzheimers Dis. 2015;44:1279‐1290. [DOI] [PubMed] [Google Scholar]
- 44. Fiford CM, Nicholas JM, Biessels GJ, Lane CA, Cardoso MJ, Barnes J. High blood pressure predicts hippocampal atrophy rate in cognitively impaired elders. Alzheimers Dement Diagn Assess Dis Monit. 2020;12:e12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hirabayashi N, Hata J, Ohara T, et al. Association between diabetes and hippocampal atrophy in elderly Japanese: the Hisayama study. Diabetes Care. 2016;39:1543‐1549. [DOI] [PubMed] [Google Scholar]
- 46. Lincoln KD, Nguyen AW. Biopsychosocial risk profiles among African American and non‐Hispanic white adults: findings from the health and retirement study. J Gerontol A Biol Sci Med Sci. 2022;77:e82‐e88. [DOI] [PubMed] [Google Scholar]
- 47. Klonoff EA, Landrine H, Ullman JB. Racial discrimination and psychiatric symptoms among Blacks. Cultur Divers Ethnic Minor Psychol. 1999;5:329‐339. [Google Scholar]
- 48. Carden KD, Mcduffie DL, Murry K, Bui C, Allen RS. Minority stress process among older Black Americans: the role of age, perceived discrimination, and anxiety. Aging Ment Health. 2021;26(4):1‐9. doi: 10.1080/13607863.2021.1904380 [DOI] [PubMed] [Google Scholar]
- 49. Robins JL, Kliewer W. Stress and coping profiles and cardiometabolic risk in low‐income African American women. J Womens Health 2002. 2019;28:636‐645. [DOI] [PubMed] [Google Scholar]
- 50. Bancks MP, Kershaw K, Carson AP, Gordon‐Larsen P, Schreiner PJ, Carnethon MR. Association of modifiable risk factors in young adulthood with racial disparity in incident type 2 diabetes during middle adulthood. JAMA. 2017;318:2457‐2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carnethon MR, Pu J, Howard G, et al. Cardiovascular health in African Americans: a scientific statement from the American heart association. Circulation. 2017;136:e393‐e423. [DOI] [PubMed] [Google Scholar]
- 52. Van De Pol LA, Hensel A, Barkhof F, Gertz HJ, Scheltens P, Van Der Flier WM. Hippocampal atrophy in Alzheimer disease: age matters. Neurology. 2006;66:236‐238. [DOI] [PubMed] [Google Scholar]
- 53. Okonkwo OC, Xu G, Dowling NM, et al. Family history of Alzheimer disease predicts hippocampal atrophy in healthy middle‐aged adults. Neurology. 2012;78:1769‐1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kumar VV, Huang H, Zhao L, et al. Baseline results: the association between cardiovascular risk and preclinical Alzheimer's disease pathology (ASCEND) study. J Alzheimers Dis. 2020;75:109‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paul S. ISBER best practices recommendations for biobanks and biorepositories. Biobankingcom. 2020. Accessed May 23, 2023. https://www.biobanking.com/isber‐best‐practices‐recommendations‐for‐biobanks‐and‐biorepositories/ [Google Scholar]
- 56. Gobom J, Parnetti L, Rosa‐Neto P, et al. Validation of the LUMIPULSE automated immunoassay for the measurement of core AD biomarkers in cerebrospinal fluid. Clin Chem Lab Med. 2022;60:207‐219. [DOI] [PubMed] [Google Scholar]
- 57. Leitão MJ, Silva‐Spínola A, Santana I, et al. Clinical validation of the Lumipulse G cerebrospinal fluid assays for routine diagnosis of Alzheimer's disease. Alzheimers Res Ther. 2019;11:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kind AJH, Buckingham WR. Making neighborhood‐disadvantage metrics accessible — the neighborhood atlas. N Engl J Med. 2018;378:2456‐2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yan C, Zang Y. DPARSF: a MATLAB toolbox for ‘pipeline’ data analysis of resting‐state fMRI. Front Syst Neurosci. 2010;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Komatsu J, Matsunari I, Samuraki M, et al. Optimization of DARTEL settings for the detection of Alzheimer disease. Am J Neuroradiol. 2018;39:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95‐113. [DOI] [PubMed] [Google Scholar]
- 62. Pruim RHR, Mennes M, Van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA‐AROMA: a robust ICA‐based strategy for removing motion artifacts from fMRI data. NeuroImage. 2015;112:267‐277. [DOI] [PubMed] [Google Scholar]
- 63. Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional‐anatomic fractionation of the brain's default network. Neuron. 2010;65:550‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brown PJ, Roose SP, O'boyle KR, et al. Frailty and its correlates in adults with late life depression. Am J Geriatr Psychiatry. 2020;28:145‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fischl B. FreeSurfer. 20 YEARS FMRI. 2012;62:774‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. R: Adjust P‐values for Multiple Comparisons. Accessed February 15, 2023. https://search.r‐project.org/CRAN/refmans/POSTm/html/p.adjust.html
- 67. Qi H, Liu H, Hu H, He H, Zhao X. Primary disruption of the memory‐related subsystems of the default mode network in Alzheimer's disease: resting‐state functional connectivity MRI study. Front Aging Neurosci. 2018;10:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Celone KA, Calhoun VD, Dickerson BC, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222‐10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Teipel SJ, Wohlert A, Metzger C, et al. Multicenter stability of resting state fMRI in the detection of Alzheimer's disease and amnestic MCI. NeuroImage Clin. 2017;14:183‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yokoi T, Watanabe H, Yamaguchi H, et al. Involvement of the precuneus/posterior cingulate cortex is significant for the development of Alzheimer's disease: a PET (THK5351, PiB) and Resting fMRI Study. Front Aging Neurosci. 2018;10:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer's disease. NeuroImage. 2018;169:302‐311. [DOI] [PubMed] [Google Scholar]
- 72. Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446‐452. [DOI] [PubMed] [Google Scholar]
- 73. Rodrigue KM, Kennedy KM, Devous MD, et al. β‐Amyloid burden in healthy aging. Neurology. 2012;78:387‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Penalba‐Sánchez L, Oliveira‐Silva P, Sumich AL, Cifre I. Increased functional connectivity patterns in mild Alzheimer's disease: a rsfMRI study. Front Aging Neurosci. 2023;14:1037347. doi: 10.3389/fnagi.2022.1037347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hafkemeijer A, Altmann‐Schneider I, Oleksik AM, et al. Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connect. 2013;3:353‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen H, Sheng X, Luo C, et al. The compensatory phenomenon of the functional connectome related to pathological biomarkers in individuals with subjective cognitive decline. Transl Neurodegener. 2020;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Walsh P, Sudre CH, Fiford CM, et al. CSF amyloid is a consistent predictor of white matter hyperintensities across the disease course from aging to Alzheimer's disease. Neurobiol Aging. 2020;91:5‐14. [DOI] [PubMed] [Google Scholar]
- 78. Graff‐Radford J, Arenaza‐Urquijo EM, Knopman DS, et al. White matter hyperintensities: relationship to amyloid and tau burden. Brain. 2019;142:2483‐2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics‐2023 update: a report from the American heart association. Circulation. 2023;147:e93‐e621. [DOI] [PubMed] [Google Scholar]
- 80. Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65:1053‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Butts B, Huang H, Hu WT, et al. sPDGFRβ and neuroinflammation are associated with AD biomarkers and differ by race: the ASCEND Study. Alzheimers Dement. 2024;20(2):1175‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Roseborough A, Ramirez J, Black SE, Edwards JD. Associations between amyloid β and white matter hyperintensities: a systematic review. Alzheimers Dement. 2017;13:1154‐1167. [DOI] [PubMed] [Google Scholar]
- 83. Cerebral amyloid angiopathy burden associated with leukoaraiosis: A positron emission tomography/magnetic resonance imaging study—Gurol ‐ 2013 ‐ Annals of Neurology—Wiley Online Library. Accessed October 10, 2023. https://onlinelibrary.wiley.com/doi/abs/10.1002/ana.23830 [DOI] [PMC free article] [PubMed]
- 84. Kamara DM, Gangishetti U, Gearing M, et al. Cerebral amyloid angiopathy: similarity in African‐Americans and Caucasians with Alzheimer's disease. J Alzheimers Dis. 2018;62:1815‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ruthirakuhan M, Swardfager W, Xiong L, et al. Investigating the impact of hypertension with and without diabetes on Alzheimer's disease risk: a clinico‐pathological study. Alzheimers Dement. 2024;20(4):2766‐2778. doi: 10.1002/alz.13717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nakamura Y, :Kabayama M, Godai K, et al. Longitudinal association of hypertension and dyslipidemia with cognitive function in community‐dwelling older adults: the SONIC study. Hypertens Res. 2023;46:1829‐1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Carlsson CM. Type 2 diabetes mellitus, dyslipidemia, and Alzheimer's disease. J Alzheimers Dis JAD. 2010;20:711‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Evans RM, Emsley CL, Gao S, et al. Serum cholesterol, APOE genotype, and the risk of Alzheimer's disease: a population‐based study of African Americans. Neurology. 2000;54:240. [DOI] [PubMed] [Google Scholar]
- 89. Hendrie HC, Murrell J, Baiyewu O, et al. APOE ε4 and the risk for Alzheimer disease and cognitive decline in African Americans and Yoruba. Int Psychogeriatr IPA. 2014;26:977‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Husain MA, Laurent B, Plourde M. APOE and Alzheimer's disease: from lipid transport to physiopathology and therapeutics. Front Neurosci. 2021;15:630502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. TO DENIGRATE, IGNORE, OR DISRUPT: Racial Inequality in Health and the Impact of a Policy‐induced Breakdown of African American Communities | Du Bois Review: Social Science Research on Race | Cambridge Core. https://www.cambridge.org/core/journals/du‐bois‐review‐social‐science‐research‐on‐race/article/to‐denigrate‐ignore‐or‐disrupt‐racial‐inequality‐in‐health‐and‐the‐impact‐of‐a‐policyinduced‐breakdown‐of‐african‐american‐communities/6E8565ECC036B7F8456AE0E23261AE9C
- 92. Geronimus AT, Hicken M, Keene D, Bound J. ‘Weathering’ and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96:826‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Matos TM, Souza‐Talarico JND. How stress mediators can cumulatively contribute to Alzheimer's disease an allostatic load approach. Dement Neuropsychol. 2019;13:11‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tatomir A, Micu C, Crivii C. The impact of stress and glucocorticoids on memory. Clujul Med. 2014;87:3‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Green KN, Billings LM, Roozendaal B, Mcgaugh JL, Laferla FM. Glucocorticoids increase amyloid‐β and tau pathology in a mouse model of Alzheimer's disease. J Neurosci. 2006;26:9047‐9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Insel PS, Mormino EC, Aisen PS, Thompson WK, Donohue MC. Neuroanatomical spread of amyloid β and tau in Alzheimer's disease: implications for primary prevention. Brain Commun. 2020;2:fcaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Adi Kurnia Susanto T, Pua E, Zhou J, Cognition, Brain Atrophy, and Cerebrospinal Fluid Biomarkers Changes from Preclinical to Dementia Stage of Alzheimer's Disease and the Influence of Apolipoprotein E . 2014;vol. 45. [DOI] [PubMed] [Google Scholar]
- 98. Apostolova LG, :Hwang KS, Andrawis JP, et al. 3D PIB and CSF biomarker associations with hippocampal atrophy in ADNI subjects. Neurobiol Aging. 2010;31:1284‐1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sabuncu MR. The dynamics of cortical and hippocampal atrophy in Alzheimer disease. Arch Neurol. 2011;68:1040‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Braak H, Braak E. Frequency of stages of Alzheimer‐related lesions in different age categories. Neurobiol Aging. 1997;18:351‐357. [DOI] [PubMed] [Google Scholar]
- 101. Scheyer O, Rahman A, Hristov H, et al. Female sex and Alzheimer's risk: the menopause connection. J Prev Alzheimers Dis. 2018;5:225‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ocañas SR, Pham KD, Cox JEJ, et al. Microglial senescence contributes to female‐biased neuroinflammation in the aging mouse hippocampus: implications for Alzheimer's disease. Neurology. 2023;54(1):240‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.


