Abstract
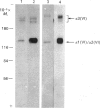
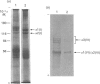
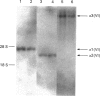
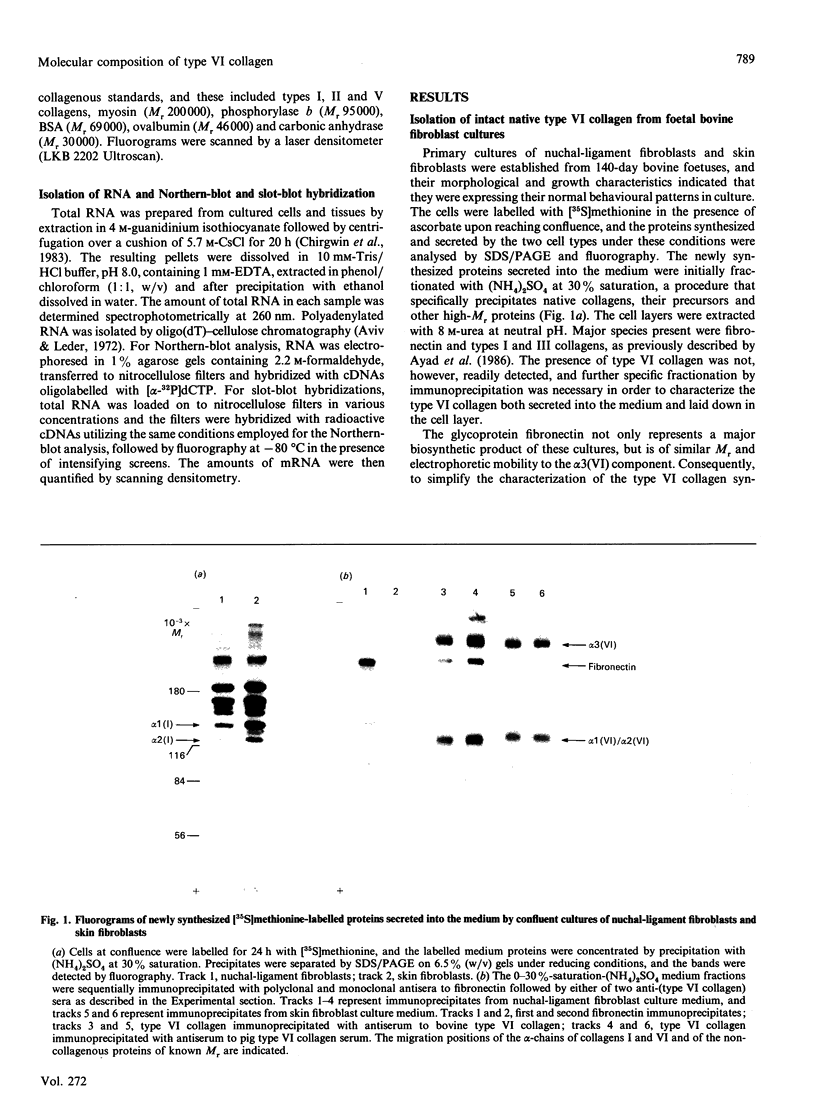
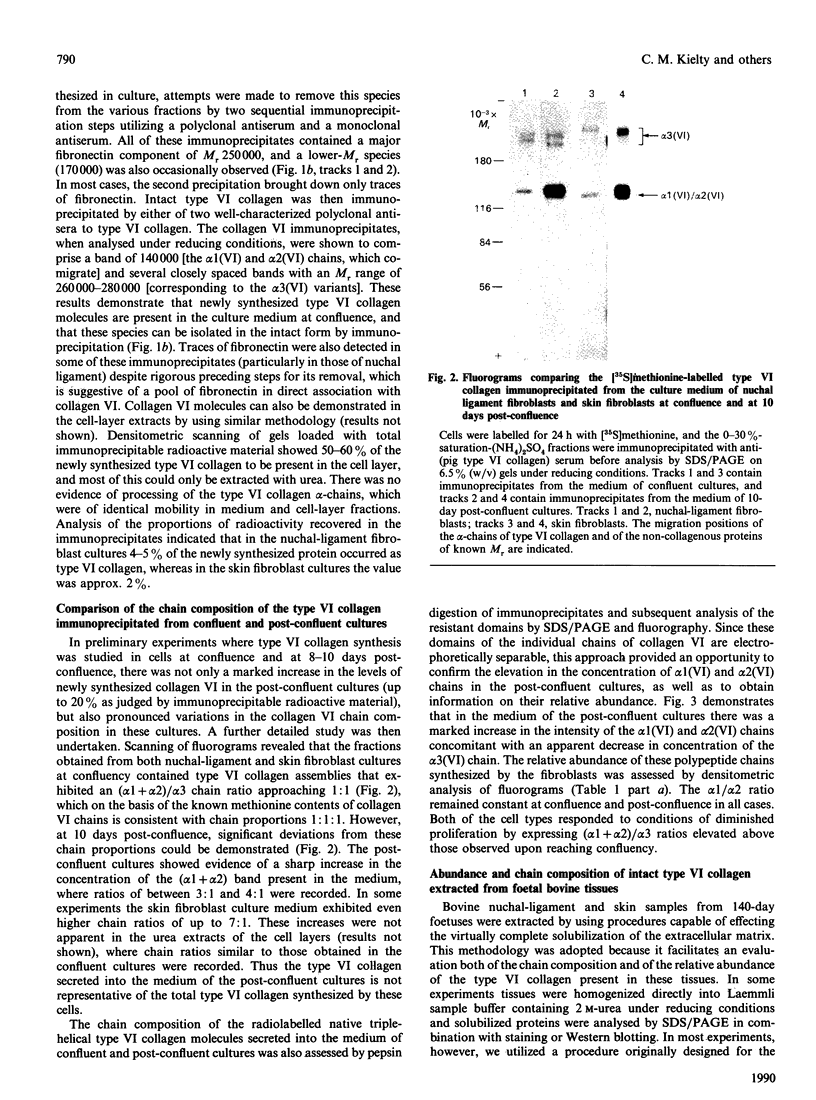
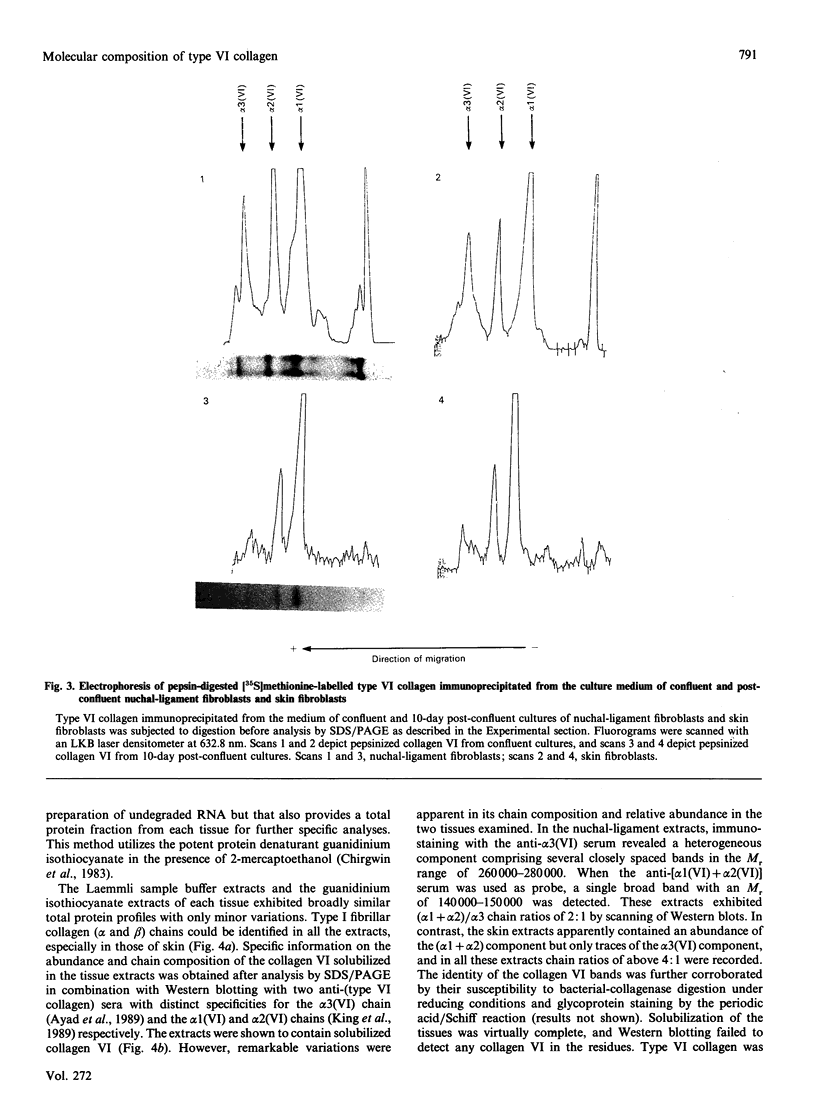
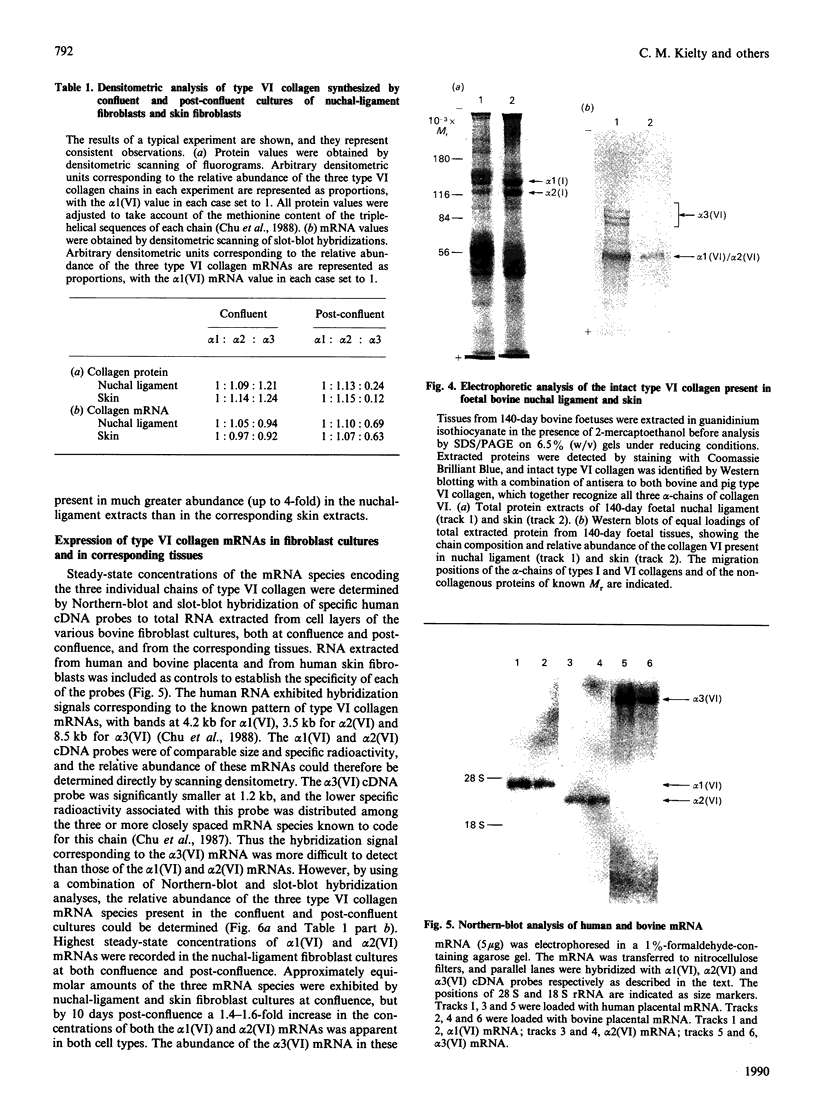
The chain composition and relative abundance of type VI collagen synthesized by cells cultured from foetal bovine nuchal ligament and skin were compared with those of the type VI collagen present in these foetal tissues. Immunoprecipitation of intact collagen VI from medium and cell layers of nuchal ligament fibroblasts and skin fibroblasts at confluence revealed collagen type VI molecules with a chain composition consistent with an [alpha 1(VI)alpha 2(VI)alpha 3(VI)] monomeric assembly. Maintenance of cells in a post-confluent quiescent state promoted a marked phenotypic change in these ratios, with increased concentrations of assemblies composed of equimolar ratios of alpha 1(VI) and alpha 2(VI) chains detected in the medium of these cultures. Analysis of steady-state concentrations of mRNA for alpha 1(VI) and alpha 2(VI) chains revealed these species to be present in increased abundance at post-confluence in all the cultures, but no corresponding increase was observed in the alpha 3(VI) mRNA. In order to assess the physiological significance of these observations, the chain composition of the collagen VI content of the corresponding foetal tissues was assessed by Western blotting after extraction in guanidinium isothiocyanate under reducing conditions. Extracts of nuchal ligament revealed a collagen VI chain composition consistent with a heterotrimeric chain assembly. In contrast, the skin extracts revealed an abundance of alpha 1(VI) and alpha 2(VI) chains with only traces of the alpha 3(VI) chain detected. Increased equimolar concentrations of the alpha 1(VI)-chain and alpha 2(VI)-chain mRNAs in skin again reflected the increased concentrations of these polypeptide chains. Type VI collagen was present in greater abundance both in the nuchal ligament and in the corresponding nuchal-ligament fibroblast cultures. The results indicate that the chain composition of type VI collagen is subject to modulation at the level of transcription as a result of variations in the proliferative state of the cells, and demonstrate that different isoforms of collagen VI occur in foetal development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aumailley M., Mann K., von der Mark H., Timpl R. Cell attachment properties of collagen type VI and Arg-Gly-Asp dependent binding to its alpha 2(VI) and alpha 3(VI) chains. Exp Cell Res. 1989 Apr;181(2):463–474. doi: 10.1016/0014-4827(89)90103-1. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad S., Chambers C. A., Berry L., Shuttleworth C. A., Grant M. E. Type VI collagen and glycoprotein MFPI are distinct components of the extracellular matrix. Biochem J. 1986 May 15;236(1):299–302. doi: 10.1042/bj2360299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad S., Evans H., Weiss J. B., Holt L. Type VI collagen but not type V collagen is present in cartilage. Coll Relat Res. 1984 Mar;4(2):165–168. doi: 10.1016/s0174-173x(84)80023-0. [DOI] [PubMed] [Google Scholar]
- Ayad S., Marriott A., Morgan K., Grant M. E. Bovine cartilage types VI and IX collagens. Characterization of their forms in vivo. Biochem J. 1989 Sep 15;262(3):753–761. doi: 10.1042/bj2620753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaldo P., Colombatti A. The carboxyl terminus of the chicken alpha 3 chain of collagen VI is a unique mosaic structure with glycoprotein Ib-like, fibronectin type III, and Kunitz modules. J Biol Chem. 1989 Dec 5;264(34):20235–20239. [PubMed] [Google Scholar]
- Bonaldo P., Russo V., Bucciotti F., Doliana R., Colombatti A. Structural and functional features of the alpha 3 chain indicate a bridging role for chicken collagen VI in connective tissues. Biochemistry. 1990 Feb 6;29(5):1245–1254. doi: 10.1021/bi00457a021. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bruns R. R. Beaded filaments and long-spacing fibrils: relation to type VI collagen. J Ultrastruct Res. 1984 Nov;89(2):136–145. doi: 10.1016/s0022-5320(84)80010-6. [DOI] [PubMed] [Google Scholar]
- Bruns R. R., Press W., Engvall E., Timpl R., Gross J. Type VI collagen in extracellular, 100-nm periodic filaments and fibrils: identification by immunoelectron microscopy. J Cell Biol. 1986 Aug;103(2):393–404. doi: 10.1083/jcb.103.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chu M. L., Conway D., Pan T. C., Baldwin C., Mann K., Deutzmann R., Timpl R. Amino acid sequence of the triple-helical domain of human collagen type VI. J Biol Chem. 1988 Dec 15;263(35):18601–18606. [PubMed] [Google Scholar]
- Chu M. L., Mann K., Deutzmann R., Pribula-Conway D., Hsu-Chen C. C., Bernard M. P., Timpl R. Characterization of three constituent chains of collagen type VI by peptide sequences and cDNA clones. Eur J Biochem. 1987 Oct 15;168(2):309–317. doi: 10.1111/j.1432-1033.1987.tb13422.x. [DOI] [PubMed] [Google Scholar]
- Chu M. L., Pan T. C., Conway D., Kuo H. J., Glanville R. W., Timpl R., Mann K., Deutzmann R. Sequence analysis of alpha 1(VI) and alpha 2(VI) chains of human type VI collagen reveals internal triplication of globular domains similar to the A domains of von Willebrand factor and two alpha 2(VI) chain variants that differ in the carboxy terminus. EMBO J. 1989 Jul;8(7):1939–1946. doi: 10.1002/j.1460-2075.1989.tb03598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., Zhang R. Z., Pan T. C., Stokes D., Conway D., Kuo H. J., Glanville R., Mayer U., Mann K., Deutzmann R. Mosaic structure of globular domains in the human type VI collagen alpha 3 chain: similarity to von Willebrand factor, fibronectin, actin, salivary proteins and aprotinin type protease inhibitors. EMBO J. 1990 Feb;9(2):385–393. doi: 10.1002/j.1460-2075.1990.tb08122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombatti A., Ainger K., Colizzi F. Type VI collagen: high yields of a molecule with multiple forms of alpha 3 chain from avian and human tissues. Matrix. 1989 Jun;9(3):177–185. doi: 10.1016/s0934-8832(89)80048-4. [DOI] [PubMed] [Google Scholar]
- Cooper A. R., Kurkinen M., Taylor A., Hogan B. L. Studies on the biosynthesis of laminin by murine parietal endoderm cells. Eur J Biochem. 1981 Sep;119(1):189–197. doi: 10.1111/j.1432-1033.1981.tb05593.x. [DOI] [PubMed] [Google Scholar]
- Cornah M. S., Meachim G., Parry E. W. Banded structures in the matrix of human and rabbit nucleus pulposus. J Anat. 1970 Sep;107(Pt 2):351–362. [PMC free article] [PubMed] [Google Scholar]
- Crawford S. W., Featherstone J. A., Holbrook K., Yong S. L., Bornstein P., Sage H. Characterization of a type VI collagen-related Mr-140 000 protein from cutis-laxa fibroblasts in culture. Biochem J. 1985 Apr 15;227(2):491–502. doi: 10.1042/bj2270491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Hessle H., Klier G. Molecular assembly, secretion, and matrix deposition of type VI collagen. J Cell Biol. 1986 Mar;102(3):703–710. doi: 10.1083/jcb.102.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatamochi A., Aumailley M., Mauch C., Chu M. L., Timpl R., Krieg T. Regulation of collagen VI expression in fibroblasts. Effects of cell density, cell-matrix interactions, and chemical transformation. J Biol Chem. 1989 Feb 25;264(6):3494–3499. [PubMed] [Google Scholar]
- Heckmann M., Aumailley M., Hatamochi A., Chu M. L., Timpl R., Krieg T. Down-regulation of alpha 3(VI) chain expression by gamma-interferon decreases synthesis and deposition of collagen type VI. Eur J Biochem. 1989 Jul 1;182(3):719–726. doi: 10.1111/j.1432-1033.1989.tb14884.x. [DOI] [PubMed] [Google Scholar]
- Jander R., Rauterberg J., Glanville R. W. Further characterization of the three polypeptide chains of bovine and human short-chain collagen (intima collagen). Eur J Biochem. 1983 Jun 1;133(1):39–46. doi: 10.1111/j.1432-1033.1983.tb07427.x. [DOI] [PubMed] [Google Scholar]
- Keene D. R., Engvall E., Glanville R. W. Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J Cell Biol. 1988 Nov;107(5):1995–2006. doi: 10.1083/jcb.107.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King I. A., Tabiowo A., Fryer P. R., Pope F. M. A type VI collagen-related glycopolypeptide is the major concanavalin A-binding component in pig skin. Biochem J. 1989 Jan 1;257(1):79–86. doi: 10.1042/bj2570079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller E., Winterhalter K. H., Trueb B. The globular domains of type VI collagen are related to the collagen-binding domains of cartilage matrix protein and von Willebrand factor. EMBO J. 1989 Apr;8(4):1073–1077. doi: 10.1002/j.1460-2075.1989.tb03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Levick J. R., McDonald J. N. Microfibrillar meshwork of the synovial lining and associated broad banded collagen: a clue to identity. Ann Rheum Dis. 1990 Jan;49(1):31–36. doi: 10.1136/ard.49.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittig M., Lütjen-Drecoll E., Rauterberg J., Jander R., Mollenhauer J. Type-VI collagen in the human iris and ciliary body. Cell Tissue Res. 1990 Feb;259(2):305–312. doi: 10.1007/BF00318453. [DOI] [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986 Dec;103(6 Pt 1):2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trüeb B., Schaeren-Wiemers N., Schreier T., Winterhalter K. H. Molecular cloning of chicken type VI collagen. Primary structure of the subunit alpha 2(VI)-pepsin. J Biol Chem. 1989 Jan 5;264(1):136–140. [PubMed] [Google Scholar]
- Trüeb B., Schreier T., Bruckner P., Winterhalter K. H. Type VI collagen represents a major fraction of connective tissue collagens. Eur J Biochem. 1987 Aug 3;166(3):699–703. doi: 10.1111/j.1432-1033.1987.tb13568.x. [DOI] [PubMed] [Google Scholar]
- Trüeb B., Winterhalter K. H. Type VI collagen is composed of a 200 kd subunit and two 140 kd subunits. EMBO J. 1986 Nov;5(11):2815–2819. doi: 10.1002/j.1460-2075.1986.tb04573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D., Bernard M., Gargano S., Ramirez F. The pro alpha 2(V) collagen gene is evolutionarily related to the major fibrillar-forming collagens. Nucleic Acids Res. 1987 Jan 12;15(1):181–198. doi: 10.1093/nar/15.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. J., Eyre D. R., Slayter H. S. Type VI collagen of the intervertebral disc. Biochemical and electron-microscopic characterization of the native protein. Biochem J. 1987 Dec 1;248(2):373–381. doi: 10.1042/bj2480373. [DOI] [PMC free article] [PubMed] [Google Scholar]