Abstract
Objective
To evaluate the feasibility of using a multigene signature to tailor individualised adjuvant therapy for patients with operable triple negative breast cancer.
Design
Randomised, multicentre, open label, phase 3 trial.
Setting
7 cancer centres in China between 3 January 2016 and 17 July 2023.
Participants
Female patients aged 18-70 years with early triple negative breast cancer after definitive surgery.
Interventions
After risk stratification using the integrated signature, patients at high risk were randomised (1:1) to receive an intensive adjuvant treatment comprising four cycles of docetaxel, epirubicin, and cyclophosphamide followed by four cycles of gemcitabine and cisplatin (arm A; n=166) or a standard treatment of four cycles of epirubicin and cyclophosphamide followed by four cycles of docetaxel (arm B; n=170). Patients at low risk received the same adjuvant chemotherapy as arm B (arm C; n=168).
Main outcome measures
The primary endpoint was disease-free survival in the intention-to-treat analysis for arm A versus arm B. Secondary endpoints included disease-free survival for arm C versus arm B, recurrence-free survival, overall survival, and safety.
Results
Among the 504 enrolled patients, 498 received study treatment. At a median follow-up of 45.1 months, the three year disease-free survival rate was 90.9% for patients in arm A and 80.6% for patients in arm B (hazard ratio 0.51, 95% confidence interval (CI) 0.28 to 0.95; P=0.03). The three year recurrence-free survival rate was 92.6% in arm A and 83.2% in arm B (hazard ratio 0.50, 95% CI 0.25 to 0.98; P=0.04). The three year overall survival rate was 98.2% in arm A and 91.3% in arm B (hazard ratio 0.58, 95% CI 0.22 to 1.54; P=0.27). The rates of disease-free survival (three year disease-free survival 90.1% v 80.6%; hazard ratio 0.57, 95% CI 0.33 to 0.98; P=0.04), recurrence-free survival (three year recurrence-free survival 94.5% v 83.2%; 0.42, 0.22 to 0.81; P=0.007), and overall survival (three year overall survival 100% v 91.3%; 0.14, 0.03 to 0.61; P=0.002) were significantly higher in patients in arm C than in those in arm B with the same chemotherapy regimen. The incidence of grade 3-4 treatment related adverse events were 64% (105/163), 51% (86/169), and 54% (90/166) for arms A, B, and C, respectively. No treatment related deaths occurred.
Conclusions
The multigene signature showed potential for tailoring adjuvant chemotherapy for patients with operable triple negative breast cancer. Intensive regimens incorporating gemcitabine and cisplatin into anthracycline/taxane based therapy significantly improved disease-free survival with manageable toxicity.
Trial registration
ClinicalTrials.gov NCT02641847.
Introduction
Triple negative breast cancer, characterised by the absence of expression of oestrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2), accounts for 15-20% of all invasive breast cancers and is associated with a high risk of early recurrence and mortality.1 2 Adjuvant anthracycline/taxane based chemotherapy is a standard treatment for early stage triple negative breast cancer, but approximately 20-40% of patients experience disease recurrence.3 4 5 Therefore, an urgent need exists for more effective strategies to optimise adjuvant therapy for triple negative breast cancer. Treatment of triple negative breast cancer has been challenging owing to the molecular heterogeneity of the disease, which leads to inconsistent responses and outcomes following current treatment approaches. Advances in high throughput technologies have led to the development of multigene signatures, including Oncotype DX, Mammaprint, and HER2DX, for predicting prognosis and guiding adjuvant therapy.6 7 8 9 Nevertheless, signatures specific to triple negative breast cancer are scarce and lack validation in prospective clinical trials.10 11 12
In our previous research, we developed an integrated mRNA-lncRNA signature (three mRNAs: FCGR1A, RSAD2, and CHRDL1; two lncRNAs: HIF1A-AS2 and AK124454) that could effectively classify patients with triple negative breast cancer into groups at high risk or low risk for disease recurrence.13 Importantly, our preliminary data suggest that the patients at high risk derived less benefit from taxane based chemotherapy. This finding supports the notion of integrating non-cross resistant platinum containing agents into standard adjuvant chemotherapy for patients at high risk. Given the strong pre-clinical evidence for a synergistic effect of cisplatin with gemcitabine,14 15 and the high objective response rate observed with this doublet as first line treatment in a phase 3 trial of metastatic triple negative breast cancer,16 we hypothesised that patients with high risk, early stage triple negative breast cancer identified using the multigene signature would benefit from the addition of gemcitabine and cisplatin to the standard anthracycline/taxane based regimen in the adjuvant setting.
In this context, we did a phase 3 trial (BCTOP-T-A01) to compare an intensive adjuvant regimen—four cycles of docetaxel, epirubicin, and cyclophosphamide followed by four cycles of gemcitabine and cisplatin—with the anthracycline/taxane containing standard regimen (epirubicin and cyclophosphamide followed by docetaxel), in patients with high risk, early stage triple negative breast cancer identified using the multigene signature. Additionally, the trial aimed to prospectively validate the prognostic value of the signature in patients treated with a uniform adjuvant chemotherapy regimen of epirubicin and cyclophosphamide followed by docetaxel. Here, we present the results of this trial after a median follow-up time of 45.1 months.
Methods
Study design and patients
This prospective, multicentre, open label, phase 3 trial was conducted across seven cancer centres in China (supplementary table A). Eligible participants were women aged 18-70 years with newly diagnosed, operable, unilateral invasive triple negative breast cancer with clear margins after primary surgery and pathologically confirmed regional node positive disease or node negative disease with a primary tumour diameter >10 mm. The status of oestrogen receptor, progesterone receptor, and HER2 was confirmed locally through immunohistochemical analysis, with oestrogen receptor/progesterone receptor negative status defined as <1% nuclear staining and HER2 negative status defined as an immunohistochemical score of 0 or 1 or an immunohistochemical score of 2 without HER2 amplification.17 18 Other inclusion criteria were Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1, normal organ function, and ability to start study treatments within eight weeks after surgery. We excluded patients if they had received preoperative anticancer therapy or had distant metastases. Detailed inclusion and exclusion criteria are provided in the protocol (supplementary material). Written informed consent was obtained from all patients.
Sample preparation
Samples from the resected tumours were obtained from every woman who participated in the trial. The fresh resected tumour samples were preserved in RNAlater (Thermo Fisher Scientific) and shipped on ice to a central laboratory for further analysis. Total RNA was isolated from the samples by using the RNeasy Plus Mini Kit (Qiagen). The purity and quantity of total RNA were estimated by measuring the absorbance at 260 nm (A260) and 280 nm (A280) using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). RNase-free water was used as a blank control. When the A260/A280 ratio was between 1.9 and 2.1, the extracted RNA was determined to be pure and was used in subsequent experiments.
Quantitative real time polymerase chain reaction assay
The expression of mRNAs and lncRNAs constituting the multigene signature (mRNAs: FCGR1A, RSAD2, and CHRDL1; lncRNAs: HIF1A-AS2 and AK124454) was measured using quantitative real time polymerase chain reaction, as previously reported.13 cDNA was synthesised using the PrimeScript RT reagent kit (Takara Bio Inc, Otsu, Japan) and SYBR Premix Ex Taq kit (Takara Bio Inc, Otsu, Japan). The ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA) was used for quantitative real time polymerase chain reaction analysis. All experiments were conducted by following the standard protocol provided by the manufacturer. U6 was used as the reference gene. The expressions of the five RNAs were normalised to U6 expression for the calculation of the risk score for recurrence, as previously described.13
Randomisation and masking
On the basis of the recurrence score, patients at high risk were randomised (1:1) to receive either intensive adjuvant chemotherapy (four cycles of docetaxel, epirubicin, and cyclophosphamide followed by four cycles of gemcitabine and cisplatin; arm A) or standard adjuvant chemotherapy (four cycles of epirubicin and cyclophosphamide followed by docetaxel; arm B). Patients at low risk (arm C) were assigned to receive the same chemotherapy regimen as arm B. Randomisation was generated centrally with a block size of four. This process was carried out with a computer generated random allocation sequence prepared by an independent statistician. The investigators sent the random assignment forms by fax to the Clinical Research Coordination Office at Fudan University Shanghai Cancer Centre (Shanghai, China). After applying the inclusion and exclusion criteria to each patient, the study coordinator sent the details for the allocated treatment group to the investigator by fax. The study was open label, and allocation was unmasked to patients and investigators. However, all outcome assessors (for example, radiologists and laboratory personnel), data collectors, and analysts were blinded to treatment assignments.
Procedures
The patients in arm A received four cycles of epirubicin 75 mg/m2, cyclophosphamide 500 mg/m2, and docetaxel 75 mg/m2 on day 1 every three weeks, followed by four cycles of gemcitabine 1250 mg/m2 on days 1 and 8 and cisplatin 75 mg/m2 on day 1 every three weeks. In both arms B and C, chemotherapy consisted of four cycles of epirubicin 90 mg/m2 and cyclophosphamide 600 mg/m2 on day 1 every three weeks, followed by four cycles of docetaxel 100 mg/m2 on day 1 every three weeks). Treatment was continued until the maximum number of cycles was reached, the disease progressed, the patient withdrew, or unacceptable toxicity occurred or at the discretion of the investigator. Post-chemotherapy radiotherapy was administered according to guidelines from the National Comprehensive Cancer Network, St Gallen International Consensus, and Chinese Breast Cancer Society and implemented according to institutional protocols.19 20
All patients received primary prophylaxis with pegylated recombinant human granulocyte colony stimulating factor. Patients were permitted up to two dose reductions (set at 75% and 50% of the initial dosage, respectively) and a dose delay of up to 14 days for managing adverse events. The dose was reduced in the event of grade 4 neutropenia lasting for three or more days, febrile neutropenia, grade 4 thrombocytopenia or bleeding related to thrombocytopenia, grade 3 anaemia, grade 3/4 non-haematological toxicities, and other toxicities deemed by the investigator to necessitate dose reduction. If the whole blood count was low, day 8 gemcitabine could be given at a reduced dose or postponed for up to seven days to allow recovery; otherwise it was discontinued. Patients could discontinue a single chemotherapy agent in the combination if a severe adverse event was judged to be related to that specific agent (for example, grade ≥2 pneumonitis or haemolytic uraemic syndrome for gemcitabine; creatinine clearance <30 mL/min or grade 3/4 neurotoxicity for cisplatin). Additional details on dose modification, pre-medication, and supportive care are specified in the protocol.
Clinical and laboratory assessments were required before each cycle and within four weeks after completion of chemotherapy. Physical examination, breast ultrasonography, and abdominal ultrasonography were performed every three months during years 1 and 2, every six months during years 3-5, and yearly thereafter. Mammography and computed tomography of the chest were performed yearly.
Outcomes
The primary endpoint was disease-free survival for arm A versus arm B. The secondary endpoints included disease-free survival for arm B versus arm C, recurrence-free survival, overall survival, and safety. Events used for the analysis of the endpoint of disease-free survival included locoregional or distant recurrence, invasive contralateral cancer, second primary malignancy, or death from any cause, whichever occurred first. Events used for the analysis of the endpoint of recurrence-free survival included first instance of invasive breast cancer recurrence or death. Events used for the analysis of the endpoint of overall survival were death from any cause. All events were measured from the date of random assignment.
The study was originally designed with recurrence-free survival for arm A versus arm B as the primary endpoint (version 1.0; 14 May 2015). In August 2023, after completion of patient enrolment but without any comparative analysis, our data monitoring committee recommended that the primary endpoint be changed from recurrence-free survival to disease-free survival (originally a secondary endpoint), owing to a lower than expected event number for recurrence-free survival, making observation of the required number of events within a reasonable timeframe unlikely; in addition, disease-free survival serves as a more widely adopted primary endpoint in most large scale trials of adjuvant breast cancer treatment.21 22
The protocol (version 1.2; 16 August 2023) and statistical analysis plan (version 1.1; 16 August 2023) were amended accordingly. The updated documents, including a protocol amendment list, are provided in the supplementary material. No results from the BCTOP-T-A01 trial were available at the time of this amendment; we did no data analyses until the data cut-off for this report.
Safety assessments included evaluations of the incidence and severity of adverse events as per National Cancer Institute Common Toxicity Criteria version 4.0. These were conducted up to 30 days from the last dose of chemotherapy.
Statistical analysis
We based the sample size calculation for the original primary endpoint of recurrence-free survival on the requirement for sufficient patients at high risk to test the superiority of intensive over standard adjuvant chemotherapy. We needed a total of 106 recurrence-free survival events for patients at high risk to detect an improvement in the three year recurrence-free survival rate of 12%; the detailed sample size calculation is available in the study protocol. For the sample size calculation for disease-free survival, we updated the hypothesis to reflect an 11% difference in the three year disease-free survival rate from 79% to 90% (hazard ratio 0.45) with the intensive regimen versus the standard regimen.23 24 We estimated the period of enrolment and follow-up needed at 36 and 24 months, respectively. On the basis of a 1:1 randomisation ratio and an assumed 9% drop-out rate, 335 patients at high risk with 50 disease-free survival events would provide 80% power at a significance level of 5% for the two sided log-rank test. We also enrolled 168 patients at low risk who received standard treatment at a 1:1 ratio alongside patients at high risk on the same regimen, totalling 503 participants required. We used the intention-to-treat population for efficacy analyses. We assessed safety and toxicity in patients who received at least one dose of study treatment. We estimated survival outcomes by using the Kaplan-Meier method and compared them with log-rank tests. We built Cox models to control for intergroup prognostic variables and estimate hazard ratios and 95% confidence intervals (CIs). We also tested interaction of treatment with clinicopathological factors and analysed subgroups by menopausal status, histological grade, T stage, nodal status, lymphovascular invasion, and Ki-67 index. We plotted time dependent receiver operating characteristic curves and calculated areas under the curve to assess the efficacy of the signature compared with conventional clinicopathological factors in patients receiving standard chemotherapy.
For the primary endpoint, we considered P values to be significant at a two sided significance level of 5%. We present nominal P values for other endpoints without adjustment for multiplicity. We used SPSS version 22.0 for statistical analyses.
Patient and public involvement
Patients were not involved in the design or implementation of the trial as this was not customary in China at the time of study design. In addition, to keep the confidentiality of clinical data, the patients were not involved in data analysis, interpretation, or writing up of the results. Although patients and the public were not directly involved in this trial, mainly owing to training restrictions, we informed patients about the trial and ensured their awareness of its purpose and content during recruitment. We also invited a member of the public to review our manuscript after submission and communicated the results to patients who expressed an interest during clinic visits.
Results
Patients’ characteristics
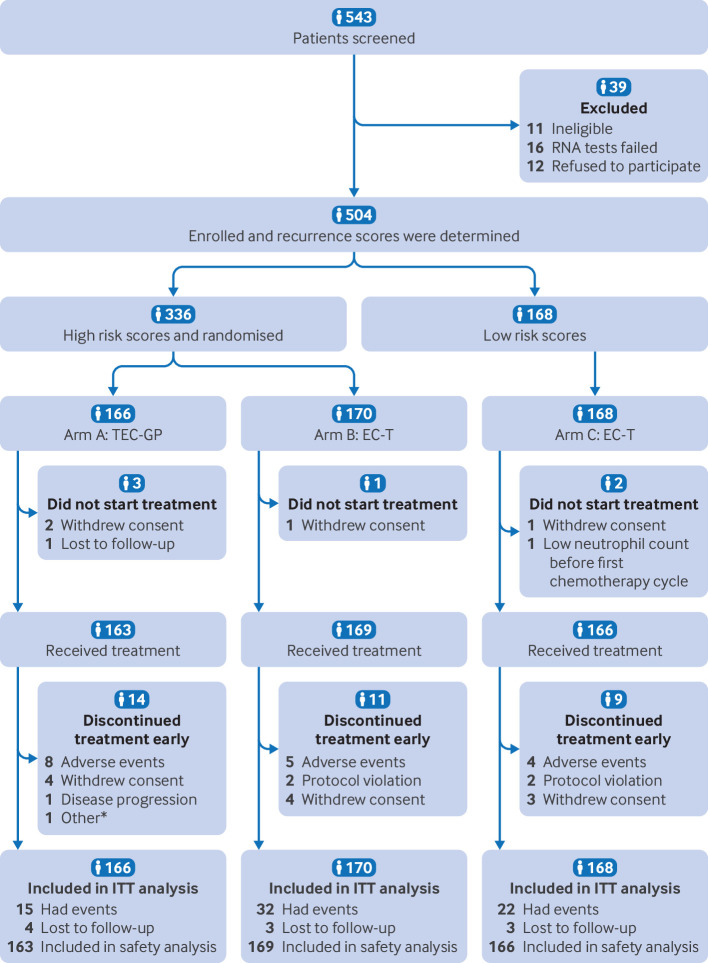
Between 3 January 2016 and 17 July 2023, 504 patients were enrolled in the study, of whom 336 were classified as being at high risk and 168 as at low risk (fig 1). The baseline characteristics of the patients at high risk were well balanced between arms A and B (table 1). In the high risk cohort, the overall median age was 52 (range 27-69) years; 279 (83%) patients had a primary tumour size of greater than 2 cm, and 105 (31%) patients had four or more involved lymph nodes (44 (13%) patients had ≥10 positive nodes). Most patients in both arms had undergone a mastectomy (99%; 333/336) and axillary lymph node dissection (80%; 270/336) and had received radiation therapy (69%; 231/336). Compared with patients at low risk in arm C, a numerically higher proportion of patients at high risk had poor prognostic features (that is, larger tumour sizes and greater axillary node involvement). For example, among women at high risk, 70% (116/166) in arm A and 69% (118/170) in arm B had lymph node involvement compared with 17% (29/168) in arm C.
Fig 1.
Flowchart of study. *Unspecified reasons. EC-T=epirubicin and cyclophosphamide followed by docetaxel; ITT-intention-to-treat; TEC-GP=docetaxel, epirubicin, and cyclophosphamide followed by gemcitabine and cisplatin
Table 1.
Characteristics of patients in intention-to-treat population at baseline. Values are numbers (percentages) unless stated otherwise
| Characteristic | High risk | Low risk | ||
|---|---|---|---|---|
| Arm A: TEC-GP (n=166) | Arm B: EC-T (n=170) | Arm C: EC-T (n=168) | ||
| Median (range) age, years | 51 (27-68) | 54 (27-69) | 53 (22-69) | |
| Menopausal status: | ||||
| Premenopausal | 73 (44) | 72 (42) | 67 (40) | |
| Postmenopausal | 93 (56) | 98 (58) | 101 (60) | |
| Histological grade: | ||||
| I-II | 22 (13) | 22 (13) | 35 (21) | |
| III | 144 (87) | 148 (87) | 133 (79) | |
| Pathologic tumour size: | ||||
| pT1 | 25 (15) | 32 (19) | 62 (37) | |
| pT2 | 132 (80) | 131 (77) | 106 (63) | |
| pT3 | 9 (5) | 7 (4) | 0 (0) | |
| No of positive lymph nodes: | ||||
| 0 | 50 (30) | 52 (31) | 139 (83) | |
| 1-3 | 60 (36) | 69 (41) | 29 (17) | |
| 4-9 | 32 (19) | 29 (17) | 0 (0) | |
| ≥10 | 24 (14) | 20 (12) | 0 (0) | |
| Lymphovascular invasion: | ||||
| Negative | 55 (33) | 61 (36) | 127 (76) | |
| Positive | 111 (67) | 109 (64) | 41 (24) | |
| Ki-67 index: | ||||
| <30% | 20 (12) | 18 (11) | 28 (17) | |
| ≥30% | 146 (88) | 152 (89) | 140 (83) | |
| Primary surgery: | ||||
| Mastectomy | 164 (99) | 169 (99) | 165 (98) | |
| Breast conservation | 2 (1) | 1 (<1) | 3 (2) | |
| Axillary surgery: | ||||
| SLNB | 33 (20) | 33 (19) | 95 (57) | |
| ALND | 133 (80) | 137 (81) | 73 (43) | |
| Adjuvant radiation: | ||||
| Yes | 116 (70) | 115 (68) | 27 (16) | |
| No | 50 (30) | 55 (32) | 141 (84) | |
| BRCA1/2 genes: | ||||
| Deleterious variant | 9 (5) | 7 (4) | 7 (4) | |
| No deleterious variant | 64 (39) | 69 (41) | 45 (27) | |
| Unknown | 93 (56) | 94 (55) | 116 (69) | |
| HRR related genes: | ||||
| Deleterious variant | 11 (7) | 13 (8) | 8 (5) | |
| No deleterious variant | 62 (37) | 63 (37) | 44 (26) | |
| Unknown | 93 (56) | 94 (55) | 116 (69) | |
| HER2 status: | ||||
| 0 | 60 (36) | 67 (39) | 79 (47) | |
| 1+~2+ | 106 (64) | 103 (61) | 89 (53) | |
ALND=axillary lymph node dissection; EC-T=epirubicin and cyclophosphamide followed by docetaxel; HER2=human epidermal growth factor receptor 2; HRR=homologous recombination repair; SLNB=sentinel lymph node biopsy; TEC-GP=docetaxel, epirubicin, and cyclophosphamide followed by gemcitabine and cisplatin.
Approximately 99% (498/504) of patients received at least one cycle of assigned chemotherapy (fig 1), with most completing chemotherapy treatment as specified in the protocol (91% (149/163) of patients in arm A, 93% (158/169) in arm B, and 95% (157/166) in arm C). The proportion of patients needing dose reductions was 31% (51/163), 15% (25/169), and 14% (23/166) respectively. Cycle delays occurred in 29% (47/163), 20% (33/169), and 22% (37/166), respectively. Haematological toxicity was the most frequent reason for cycle delay.
Efficacy outcomes in patients at high risk
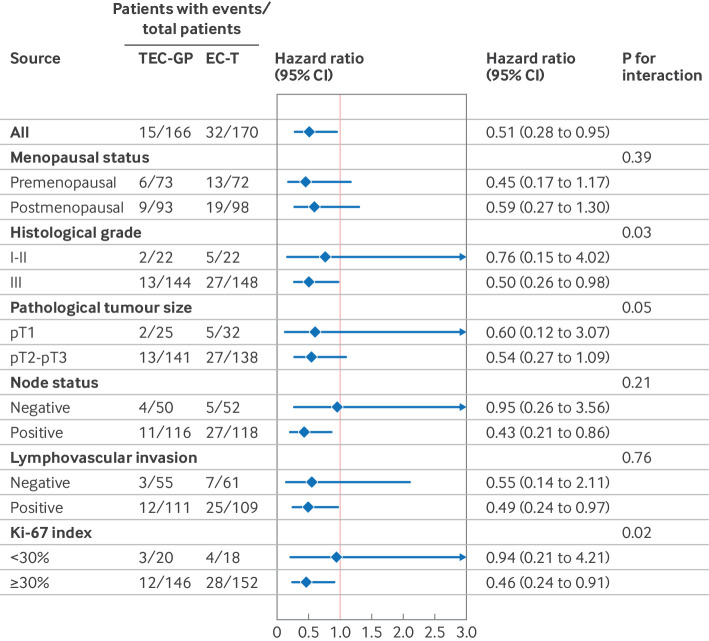
As of the data cut-off date of 25 December 2023, the median follow-up time was 45.1 months. A total of 47 (14%) disease-free survival events occurred among 336 patients at high risk, including 36 locoregional or distant relapses, eight second primary malignancies, one contralateral breast cancer, and two deaths. Table 2 shows the distribution of the first disease-free survival events by treatment arm. The primary endpoint of three year disease-free survival rate (originally a secondary endpoint) was 90.9% for arm A and 80.6% for arm B (a 10.3 percentage point difference; fig 2, top). Disease-free survival was significantly higher among patients receiving intensive chemotherapy than in those receiving standard therapy (hazard ratio 0.51, 95% CI 0.28 to 0.95; P=0.03). The three year recurrence-free survival rate (the original primary endpoint) was higher in arm A than in arm B (92.6% v 83.2%; hazard ratio 0.50, 95% CI 0.25 to 0.98; P=0.04; fig 2, middle). As of data cut-off, overall survival events were recorded in a total of 18 (5%) patients in arm A and B. Preliminary data at this early time point indicated a trend favouring arm A over arm B in overall survival (three year overall survival rate 98.2% v 91.3%; hazard ratio 0.58, 95% CI 0.22 to 1.54; P=0.27; fig 2, bottom); adequate assessment of the efficacy of intensive chemotherapy in terms of overall survival will require long term follow-up and more events. Exploratory forest plot analyses for disease-free survival in the intention-to-treat population showed hazard ratios that consistently favoured the intensive chemotherapy regimen (fig 3).
Table 2.
First disease-free survival event by treatment
| Event | High risk | Low risk | ||
|---|---|---|---|---|
| Arm A: TEC-GP (n=166) | Arm B: EC-T (n=170) | Arm C: EC-T (n=168) | ||
| Any event | 15 | 32 | 22 | |
| Local and regional recurrence | 1 | 5 | 5 | |
| Distant metastasis: | 10 | 20 | 7 | |
| Lung | 6 | 7 | 4 | |
| Liver | 1 | 7 | 1 | |
| Bone | 5 | 8 | 2 | |
| Brain | 2 | 3 | 1 | |
| Other | 2 | 1 | 2 | |
| Second primary malignancy | 3 | 5 | 7 | |
| Contralateral breast cancer | 0 | 1 | 2 | |
| Death | 1 | 1 | 1 | |
EC-T=epirubicin and cyclophosphamide followed by docetaxel; TEC-GP=docetaxel, epirubicin, and cyclophosphamide followed by gemcitabine and cisplatin.
Fig 2.
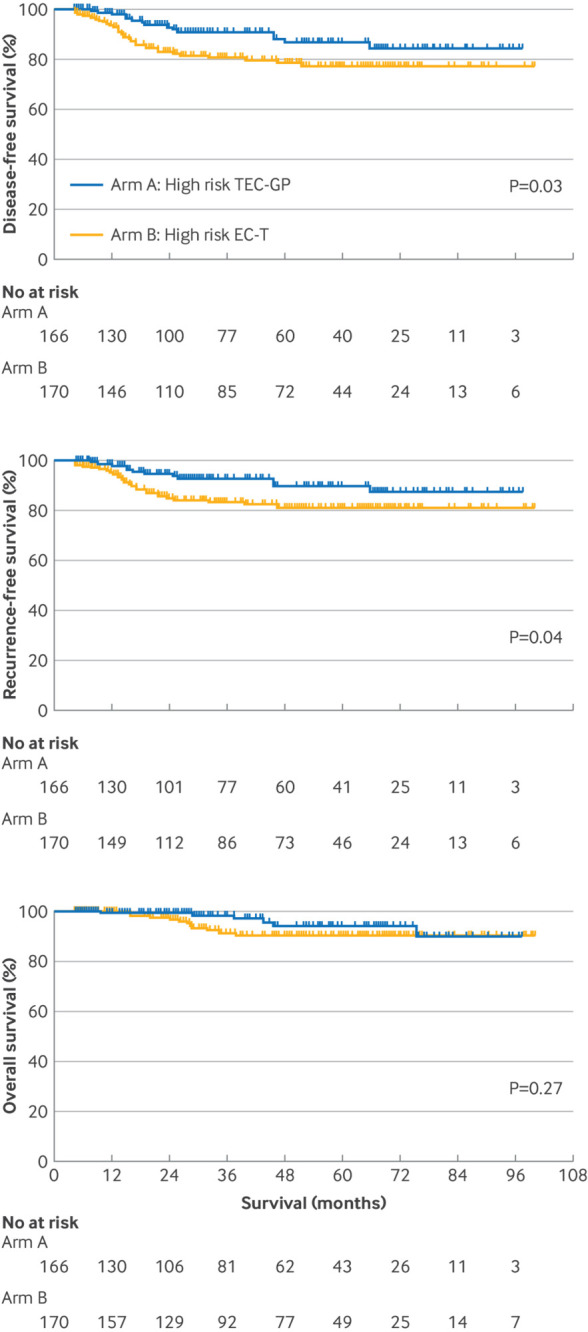
Clinical outcomes among patients with high risk score. Kaplan-Meier plots show disease-free survival (top), recurrence-free survival (middle), and overall survival (bottom) for patients with high risk score. EC-T=epirubicin and cyclophosphamide followed by docetaxel; TEC-GP=docetaxel, epirubicin, and cyclophosphamide followed by gemcitabine and cisplatin
Fig 3.
Exploratory subgroup analyses for disease-free survival among patients with high risk score. CI=confidence interval; EC-T=epirubicin and cyclophosphamide followed by docetaxel; TEC-GP=docetaxel, epirubicin, and cyclophosphamide followed by gemcitabine and cisplatin
Validation of signature
Among the 168 patients classified as having low risk triple negative breast cancer, 22 (13%) disease-free survival events occurred (table 2). The three year disease-free survival, recurrence-free survival, and overall survival rates for patients in arm C were 90.1%, 94.5%, and 100%, respectively. Patients classified as being at low risk had significantly higher rates of disease-free survival (hazard ratio 0.57, 95% CI 0.33 to 0.98; P=0.04), recurrence-free survival (0.42, 0.22 to 0.81; P=0.007), and overall survival (0.14, 0.03 to 0.61; P=0.002) than did patients at high risk receiving the same standard chemotherapy (supplementary figure A). Analysis of areas under the receiver operating characteristic curves indicated that the integrated signature was more effective than the traditional clinicopathological factors, including TNM stage, tumour grade, Ki-67, and age in predicting disease-free survival, recurrence-free survival, and overall survival at three years (supplementary figure B).
Safety
All 498 patients in the safety population had at least one treatment related adverse event (table 3). The overall incidence of grade 3 or 4 treatment related adverse events was 105 (64%) of 163 patients in the arm A, 86 (51%) of 169 patients in arm B, and 90 (54%) of 166 patients in arm C. The most common treatment related adverse events of grade 3 or 4 were haematological toxicities in all arms; grade 3 or 4 events occurring more frequently in arm A (percentage point difference ≥2% versus either arm B or arm C) included neutropenia, thrombocytopenia, febrile neutropenia, anaemia, nausea, and vomiting. Overall, 22 treatment related serious adverse events occurred: nine (6%) in arm A (febrile neutropenia and infection (n=3 each); thrombosis, thrombocytopenia, and diarrhoea (n=1 each)), seven (4%) in arm B (febrile neutropenia (n=2); liver dysfunction, vomiting, rash, infection, and severe neutropenia n=1 each)), and six (4%) in arm C (febrile neutropenia (n=2); allergic reaction, thrombocytopenia, liver dysfunction, and vomiting (n=1 each)). Discontinuation of study treatment due to toxicity was recorded in eight (5%), five (3%), and four (2%) patients, respectively, in arms A, B, and C. In randomised patients who received study treatment, incidence of grade ≥3 treatment related adverse events was significantly higher in arm A than in arm B; however, we found no significant difference in the incidence of treatment related serious adverse events or treatment related adverse events leading to dose discontinuation between arms A and B (supplementary table B). No treatment related deaths occurred in the study.
Table 3.
Most common treatment related adverse events according to treatment arms. Values are numbers (percentages)
| Adverse events* | High risk | Low risk | ||||||
|---|---|---|---|---|---|---|---|---|
| Arm A: TEC-GP (n=163) | Arm B: EC-T (n=169) | Arm C: EC-T (n=166) | ||||||
| All grades | Grade 3-4 | All grades | Grade 3-4 | All grades | Grade 3-4 | |||
| Any | 163 (100) | 105 (64) | 169 (100) | 86 (51) | 166 (100) | 90 (54) | ||
| Haematological | ||||||||
| Neutropenia | 154 (94) | 96 (59) | 149 (88) | 81 (48) | 159 (96) | 77 (46) | ||
| Anaemia | 137 (84) | 9 (6) | 137 (81) | 3 (2) | 146 (88) | 3 (2) | ||
| Thrombocytopenia | 103 (63) | 23 (14) | 91 (54) | 4 (2) | 85 (51) | 3 (2) | ||
| Febrile neutropenia | 16 (10) | 16 (10) | 7 (4) | 7 (4) | 8 (5) | 8 (5) | ||
| Non-haematological | ||||||||
| Fatigue | 138 (85) | 15 (9) | 131 (78) | 11 (7) | 126 (76) | 14 (8) | ||
| Nausea | 135 (83) | 11 (7) | 136 (80) | 6 (4) | 141 (85) | 8 (5) | ||
| Myalgia | 109 (67) | 4 (2) | 127 (75) | 9 (5) | 127 (77) | 9 (5) | ||
| Insomnia | 91 (56) | 3 (2) | 47 (28) | 2 (1) | 44 (27) | 1 (<1) | ||
| Vomiting | 67 (41) | 14 (9) | 57 (34) | 7 (4) | 67 (40) | 8 (5) | ||
| Diarrhoea | 61 (37) | 4 (2) | 59 (35) | 6 (4) | 63 (38) | 7 (4) | ||
| Peripheral sensory neuropathy | 58 (36) | 2 (1) | 65 (38) | 4 (2) | 71 (43) | 5 (3) | ||
| SGPT increased | 49 (30) | 4 (2) | 72 (43) | 3 (2) | 76 (46) | 4 (2) | ||
| Stomatitis | 42 (26) | 3 (2) | 39 (23) | 4 (2) | 40 (24) | 4 (2) | ||
| Constipation | 42 (26) | 0 (0) | 34 (20) | 1 (<1) | 24 (14) | 0 (0) | ||
| Hand-foot skin reaction | 42 (26) | 0 (0) | 39 (23) | 4 (2) | 40 (24) | 4 (2) | ||
| SGOT increased | 36 (22) | 3 (2) | 47 (28) | 2 (1) | 52 (31) | 2 (1) | ||
| Rash | 31 (19) | 1 (<1) | 44 (26) | 6 (4) | 49 (30) | 8 (5) | ||
| Oedema limbs | 23 (14) | 0 (0) | 31 (18) | 0 (0) | 33 (20) | 0 (0) | ||
| Infection: | ||||||||
| Infection without neutropenia | 17 (10) | 5 (3) | 25 (15) | 6 (4) | 20 (12) | 5 (3) | ||
| Infection with neutropenia | 7 (4) | 7 (4) | 4 (2) | 4 (2) | 5 (3) | 5 (3) | ||
| Pain (other than musculoskeletal) | 16 (10) | 2 (1) | 22 (13) | 8 (5) | 23 (14) | 6 (4) | ||
| Epistaxis | 12 (7) | 0 (0) | 15 (9) | 1 (<1) | 17 (10) | 0 (0) | ||
| Creatinine increased | 12 (7) | 0 (0) | 8 (5) | 0 (0) | 10 (6) | 0 (0) | ||
| Hearing impaired | 11 (7) | 0 (0) | 10 (6) | 0 (0) | 6 (4) | 0 (0) | ||
| Allergic reaction | 10 (6) | 1 (<1) | 9 (5) | 2 (1) | 11 (7) | 1 (<1) | ||
| Hyperglycaemia | 8 (5) | 0 (0) | 13 (8) | 1 (<1) | 10 (6) | 0 (0) | ||
| Cardiac failure | 7 (4) | 0 (0) | 5 (3) | 1 (<1) | 7 (4) | 1 (<1) | ||
| Syncope | 3 (2) | 3 (2) | 3 (2) | 3 (2) | 2 (1) | 2 (1) | ||
| Thrombosis | 1 (<1) | 1 (<1) | 4 (2) | 2 (1) | 2 (1) | 1 (<1) | ||
EC-T=epirubicin and cyclophosphamide followed by docetaxel; SGOT=serum glutamic oxaloacetic transaminase; SGPT=serum glutamic pyruvic transaminase; TEC-GP=docetaxel, epirubicin, and cyclophosphamide followed by gemcitabine and cisplatin.
Treatment related adverse events of grade 1-2 occurring in ≥5% of patients and all events of grade ≥3 occurring in treatment arms are listed. No grade 5 treatment related adverse events occurred.
Discussion
The assessment of the risk of recurrence of breast cancer after therapy of curative intent has traditionally relied on clinical and histological evaluations. However, given the disease heterogeneity and the absence of well defined molecular targets, optimised adjuvant strategies are needed. This phase 3 trial marks a pivotal advance, showing for the first time the feasibility of using multigene signatures to tailor individualised adjuvant therapy for patients with operable triple negative breast cancer. The results showed that the addition of gemcitabine and cisplatin to anthracycline/taxane based adjuvant chemotherapy led to significantly improved disease-free survival (hazard ratio 0.51) compared with standard anthracycline/taxane based chemotherapy in patients with high risk triple negative breast cancer identified using the integrated mRNA-lncRNA signature. The benefits for disease-free survival with the intensive chemotherapy were consistent across all patient subgroups. In addition, this study provides independent external validation of the prognostic value of the integrated signature in a uniformly treated population.
Comparison with other studies
After we had designed and initiated our trial, the KEYNOTE-522 study showed that adding pembrolizumab to neoadjuvant chemotherapy, followed by adjuvant pembrolizumab, significantly improved pathological complete response rates and event-free survival for high risk, early stage triple negative breast cancer.25 Nevertheless, controversies remain regarding the optimal chemotherapy partners and treatment duration with immunotherapy. In addition, the US Food and Drug Administration approved olaparib as adjuvant treatment for patients with high risk BRCA1/2 mutated triple negative breast cancer, on the basis of the findings of the OlympiA study. However, the reported prevalence of BRCA1/2 variants in unselected patients with triple negative breast cancer is low at 11.2%.26 These data highlight the urgent need for more effective treatment strategies to optimise adjuvant therapy for the broad population of patients with triple negative breast cancer. Our multigene signature might serve as a prognostic tool to guide clinical decisions. For example, patients at low risk may benefit from de-escalated chemotherapy (such as a platinum-free regimen) in combination with immunotherapy during the neoadjuvant phase. Moreover, those with low risk disease who have achieved a pathological complete response following neoadjuvant chemoimmunotherapy might be exempted from adjuvant immunotherapy. Further research is needed to explore the applicability of this multigene signature across various clinical settings.
Identifying molecular characteristics specific to triple negative breast cancer subtypes to distinguish patients with different prognoses is crucial for tailoring treatment. Various multigene signatures have been proposed for triple negative breast cancer, such as the 44 gene DNA damage immune response signature, exploiting an RNA based signature to differentiate patients with different prognoses.12 Although these models have enhanced our understanding of the heterogeneity of triple negative breast cancer and facilitated clinical research, prospective evidence on their performance is scarce. This study applied the integrated mRNA-lncRNA signature to patients prospectively and tailored adjuvant treatment strategies on the basis of the risk classification indicated by the signature. The results independently validated the prognostic value of the signature, confirming its clinical feasibility in guiding precision treatment. Importantly, the stratification schema based on the multigene signature could be iteratively updated with advances in drug development, highlighting its potential for widespread application.
A major focus of this trial was to explore the efficacy of intensive chemotherapy in patients with high risk triple negative breast cancer. We hypothesised that augmenting standard anthracycline/taxane based therapy with non-cross resistant agents would further improve patients’ outcomes. In this trial, intensive chemotherapy significantly improved disease-free survival compared with standard chemotherapy (90.9% v 80.6%; P=0.03), particularly reducing the risk of distant metastases (10 v 20 events). These results are remarkable, considering that the control arm received the standard eight cycles of anthracycline/taxane based chemotherapy. Previous attempts to improve outcomes by adding additional chemotherapeutic agents to anthracyclines, taxanes, and cyclophosphamide in unselected patients have not been successful.27 28 29 Distinct from previous intensive treatment strategies mentioned above, the intensive chemotherapy regimen used in our trial was specifically tailored to patients at high risk identified using our integrated signature, in contrast to the inclusion of “all-comers” in other trials. This study provides evidence that the multigene signature could be used to effectively identify patients for intensive treatment.
This BCTOP-T-A01 trial tested the hypothesis that the gemcitabine and cisplatin doublet regimen would improve the prognosis of patients with high risk triple negative breast cancer. Our results indicated a 10.3% absolute improvement in disease-free survival compared with standard chemotherapy. Although platinum containing regimens have shown clinical benefits in both metastatic and preoperative settings,16 24 25 26 their value as adjuvant treatment remains debatable. A recent meta-analysis showed that platinum based chemotherapy using carboplatin in the adjuvant or neoadjuvant setting improved disease-free survival and overall survival in patients with triple negative breast cancer.30 Nevertheless, none of the studies included in the meta-analysis assessed the benefits of incorporating platinum in a standard anthracycline containing regimen in the adjuvant setting, which could potentially optimise outcomes for patients at high risk. Two ongoing large scale randomised trials, NRG BR-003 (ClinicalTrials.gov: NCT02488967) and CITRINE (Carboplatin Intensified Chemotherapy for TRIple NEgative Breast Cancer; NCT04296175), will shed further light regarding the benefits of platinum in the adjuvant setting for patients with triple negative breast cancer.
In addition to defining a subgroup of patients with a high risk of recurrence who will benefit from intensive adjuvant therapy, defining a subgroup in which treatment can be safely de-escalated with a minimal risk of recurrence is equally crucial. To this end, our results indicated an overall promising prognosis in patients who were classified as being at low risk, with survival rates (three year disease-free survival 90.1%; three year overall survival 100%) substantially higher than those previously reported in the ECOG 1199 trial for a general triple negative breast cancer population (three year disease-free survival of 73% and three year overall survival of 82%) treated with the same schedule and dosage of epirubicin and cyclophosphamide followed by docetaxel.31 Notably, 15% of patients in arm C were lymph node positive and 65% had T2-3 disease. Our findings might underscore the need for a cautious approach to de-escalating adjuvant therapy for patients in the low risk category, even if they have high risk clinicopathological factors. This observation is of clinical importance because adjuvant chemotherapy is uniformly administered in routine clinical practice despite the existence of distinct biological subgroups. Identifying patients destined for favourable outcomes opens the door to the exploration of de-escalation treatment strategies, such as shorter chemotherapy regimens or non-anthracycline based approaches.
The spectrum of adverse events associated with the intensive chemotherapy regimen were consistent with those reported for gemcitabine plus cisplatin and anthracycline/taxane respectively, with no new safety concerns identified.16 32 As expected, an increased incidence of grade 3 or 4 treatment related adverse events was seen among patients in the intensive chemotherapy group compared with patients in the standard chemotherapy group (64% v 51%; P=0.01), primarily driven by haematological toxicities including neutropenia, febrile neutropenia, and thrombocytopenia. Neutropenia and febrile neutropenia were effectively managed with standard supportive measures. Although the incidence of febrile neutropenia was higher among patients treated with intensive chemotherapy (despite the administration of primary prophylaxis with granulocyte colony stimulating factor) than among those treated with standard chemotherapy (10% v 4%; P=0.04), grade 3 or 4 infection with neutropenia was comparable in the two arms (4% v 2%). In addition, thrombocytopenia was effectively managed through dose modifications or the use of thrombopoietin or interleukin 11. No patient in our study needed transfusion. Importantly, the addition of gemcitabine plus cisplatin did not increase the incidence of serious adverse events or compromise the patients’ ability to receive chemotherapy, with a comparable proportion of patients in the two arms completing the full number of cycles per protocol (91% v 94%; P=0.47). The generally manageable safety profile with intensive treatment facilitated the maximisation of exposure to treatment to achieve favourable long term cancer related outcomes in a potentially curable disease setting,33 34 and it supported a clinically significant improvement in disease-free survival in patients at high risk, compared with standard treatment.
Limitations of study
This study has several limitations. Firstly, the primary endpoint was amended after completion of enrolment. This amendment aimed to expedite the trial’s primary completion within a reasonable and relevant timeframe, following the data monitoring committee’s recommendation under blinding. Importantly, the required event number for a fully powered analysis of disease-free survival was achievable without affecting the sample size, on the basis of assumptions of similar between group differences (arm A versus arm B) in rates of three year recurrence-free survival and disease-free survival. The results showed significant differences in both disease-free survival and recurrence-free survival, with the degree of absolute and relative benefit being highly consistent. Secondly, despite the finding that dose dense chemotherapy can reduce both recurrence of and mortality from breast cancer,35 it is not widely used, and a treatment schedule of once every three weeks was considered standard when the trial was first designed in 2015. Thirdly, updated data from the ECOG 1199 trial suggest that epirubicin and cyclophosphamide, followed by weekly paclitaxel, may be the optimal regimen for treating triple negative breast cancer.31 However, evidence directly comparing epirubicin and cyclophosphamide followed by docetaxel against epirubicin and cyclophosphamide followed by weekly paclitaxel is lacking, and the former regimen remains the recommended choice for triple negative breast cancer. Fourthly, the study was confined to Chinese patients, and validation trials for extrapolation to other ethnic groups are warranted. Finally, we used an open label design in our study owing to the nature of the interventions. However, all outcome assessors as well as data collectors and analysts were masked to treatment assignment to reduce the potential for open label bias.
Conclusions
The results of our study indicate that the integrated mRNA-lncRNA signature had potential to tailor adjuvant chemotherapy for patients with operable triple negative breast cancer. Intensive regimens incorporating gemcitabine and cisplatin led to significantly improved disease-free survival compared with standard anthracycline/taxane based therapy in a well defined subgroup of patients with operable triple negative breast cancer—namely, those classified as being at high risk on the basis of the signature. Despite a higher incidence of adverse events, primarily haematological, the safety profile of intensive chemotherapy was manageable. This study is not the end of our research, and the tailored treatment regimen based on the integrated signature will be iteratively updated with advances in drug development. The feasibility of this stratification method makes it highly promising for guiding precision treatment for patients with early triple negative breast cancer.
What is already known on this topic
The prognosis of early stage triple negative breast cancer is unsatisfactory, and a need for optimisation of adjuvant therapy remains
A prospectively validated signature for triple negative breast cancer that can predict prognosis and guide adjuvant treatments is lacking
An integrated mRNA-lncRNA signature has been previously developed to provide prognostic information in triple negative breast cancer
What this study adds
This study is the first to use a multigene signature to tailor individualised adjuvant therapy for patients with operable triple negative breast cancer
The intensive chemotherapy regimen significantly improved disease-free survival compared with standard chemotherapy in patients identified as being at high risk
The prognostic value of the multigene signature was prospectively validated, with better survival outcomes in patients at low risk than in those at high risk receiving the same standard therapy
Acknowledgments
We are grateful to the patients and their families who contributed to this study. We also thank Yi-Rong Liu for his assistance in designing the trial and Xiu-Zhi Wu and Yu Shen for their assistance in editing the manuscript.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary figures and tables
Web appendix: Study protocol
Web appendix: Statistical analysis plan
Contributors: MH, Y-ZJ, and YG contributed equally (joint first authors). Z-HW and Z-MS contributed equally (joint last authors). MH, Y-ZJ, YG, Z-HW, and Z-MS contributed to the study design. MH, Y-ZJ, YG, LF, X-YL, YL, L-CT, XY, Y-FH, G-HD, G-YL, K-DY, JW, X-HZ, D-YF, C-GS, Z-GZ, K-JW, Z-HW, and Z-MS contributed to the collection and assembly of data. MH, Y-ZJ, YG, LF, X-YL, YL, L-CT, MM, Z-HW, and Z-MS contributed to the analysis and interpretation of data. MM did the statistical analysis. MH, Y-ZJ, YG, Z-HW, and Z-MS drafted the manuscript. All authors contributed to revision of the manuscript and approved the final draft. Z-HW and Z-MS are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was supported by grants from the National Key Research and Development Project of China (2020YFA0112304), the National Natural Science Foundation of China (91959207, 92159301, 82103369), the Science and Technology Commission of Shanghai Municipality (22Y11912800), the Shanghai Key Laboratory of Breast Cancer (12DZ2260100), and the SHDC Municipal Project for Developing Emerging and Frontier Technology in Shanghai Hospitals (SHDC12021103). This study is supported by CSPC Pharmaceutical Co Ltd, Shijiazhuang, China. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from the National Key Research and Development Project of China, National Natural Science Foundation of China, Science and Technology Commission of Shanghai Municipality, Shanghai Key Laboratory of Breast Cancer, SHDC Municipal Project for Developing Emerging and Frontier Technology in Shanghai Hospitals, and CSPC Pharmaceutical Co Ltd, Shijiazhuang, China, for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Transparency: The corresponding author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Participating sites were informed of the results. The results will be communicated to study participants who express an interest during clinic visits. Dissemination to the public will be achieved through media outreach.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This study was approved by the independent ethics committee of all participating institutions and was conducted in adherence with Good Clinical Practice and the Declaration of Helsinki.
Data availability statement
Requests for individual de-identified participant data that underlie the results reported in this article will be considered. Qualified researchers should submit a proposal to the corresponding author outlining the reasons for requesting the data. The leading clinical site will check whether the request is subject to any intellectual property obligations. No custom code was used for data analysis in this study.
References
- 1. Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 2. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010;363:1938-48. 10.1056/NEJMra1001389 [DOI] [PubMed] [Google Scholar]
- 3. Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol 2006;24:5652-7. 10.1200/JCO.2006.06.5664 [DOI] [PubMed] [Google Scholar]
- 4. Tan DS, Marchió C, Jones RL, et al. Triple negative breast cancer: molecular profiling and prognostic impact in adjuvant anthracycline-treated patients. Breast Cancer Res Treat 2008;111:27-44. 10.1007/s10549-007-9756-8 [DOI] [PubMed] [Google Scholar]
- 5. De Laurentiis M, Cancello G, D’Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol 2008;26:44-53. 10.1200/JCO.2007.11.3787 [DOI] [PubMed] [Google Scholar]
- 6. Sparano JA, Gray RJ, Makower DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med 2018;379:111-21. 10.1056/NEJMoa1804710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cardoso F, van’t Veer LJ, Bogaerts J, et al. MINDACT Investigators . 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med 2016;375:717-29. 10.1056/NEJMoa1602253 [DOI] [PubMed] [Google Scholar]
- 8. Prat A, Guarneri V, Pascual T, et al. Development and validation of the new HER2DX assay for predicting pathological response and survival outcome in early-stage HER2-positive breast cancer. EBioMedicine 2022;75:103801. 10.1016/j.ebiom.2021.103801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalinsky K, Barlow WE, Gralow JR, et al. 21-Gene Assay to Inform Chemotherapy Benefit in Node-Positive Breast Cancer. N Engl J Med 2021;385:2336-47. 10.1056/NEJMoa2108873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Criscitiello C, Bayar MA, Curigliano G, et al. A gene signature to predict high tumor-infiltrating lymphocytes after neoadjuvant chemotherapy and outcome in patients with triple-negative breast cancer. Ann Oncol 2018;29:162-9. 10.1093/annonc/mdx691 [DOI] [PubMed] [Google Scholar]
- 11. Kleivi Sahlberg K, Bottai G, Naume B, et al. A serum microRNA signature predicts tumor relapse and survival in triple-negative breast cancer patients. Clin Cancer Res 2015;21:1207-14. 10.1158/1078-0432.CCR-14-2011 [DOI] [PubMed] [Google Scholar]
- 12. Sharma P, Barlow WE, Godwin AK, et al. Validation of the DNA Damage Immune Response Signature in Patients With Triple-Negative Breast Cancer From the SWOG 9313c Trial. J Clin Oncol 2019;37:3484-92. 10.1200/JCO.19.00693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang YZ, Liu YR, Xu XE, et al. Transcriptome Analysis of Triple-Negative Breast Cancer Reveals an Integrated mRNA-lncRNA Signature with Predictive and Prognostic Value. Cancer Res 2016;76:2105-14. 10.1158/0008-5472.CAN-15-3284 [DOI] [PubMed] [Google Scholar]
- 14. Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Peters GJ. Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res 1996;2:521-30. [PubMed] [Google Scholar]
- 15. van Moorsel CJ, Kroep JR, Pinedo HM, et al. Pharmacokinetic schedule finding study of the combination of gemcitabine and cisplatin in patients with solid tumors. Ann Oncol 1999;10:441-8. 10.1023/A:1008301522349 [DOI] [PubMed] [Google Scholar]
- 16. Hu XC, Zhang J, Xu BH, et al. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 2015;16:436-46. 10.1016/S1470-2045(15)70064-1 [DOI] [PubMed] [Google Scholar]
- 17. Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784-95. 10.1200/JCO.2009.25.6529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolff AC, Hammond MEH, Hicks DG, et al. American Society of Clinical Oncology. College of American Pathologists . Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997-4013. 10.1200/JCO.2013.50.9984 [DOI] [PubMed] [Google Scholar]
- 19.Network NCC. Breast Cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 20. The Society of Breast Cancer China Anti-Cancer Association. Breast Oncology Group of the Oncology Branch of the Chinese Medical Association . Guidelines for breast cancer diagnosis and treatment by China Anti-cancer Association (2024 edition). China Oncology 2023;33:1092-187. [Google Scholar]
- 21. Tolaney SM, Garrett-Mayer E, White J, et al. Updated Standardized Definitions for Efficacy End Points (STEEP) in Adjuvant Breast Cancer Clinical Trials: STEEP Version 2.0. J Clin Oncol 2021;39:2720-31. 10.1200/JCO.20.03613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 2007;25:2127-32. 10.1200/JCO.2006.10.3523 [DOI] [PubMed] [Google Scholar]
- 23. Wang X, Wang SS, Huang H, et al. South China Breast Cancer Group (SCBCG) . Effect of Capecitabine Maintenance Therapy Using Lower Dosage and Higher Frequency vs Observation on Disease-Free Survival Among Patients With Early-Stage Triple-Negative Breast Cancer Who Had Received Standard Treatment: The SYSUCC-001 Randomized Clinical Trial. JAMA 2021;325:50-8. 10.1001/jama.2020.23370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Varshavsky-Yanovsky AN, Goldstein LJ. Role of Capecitabine in Early Breast Cancer. J Clin Oncol 2020;38:179-82. 10.1200/JCO.19.02946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmid P, Cortes J, Pusztai L, et al. KEYNOTE-522 Investigators . Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020;382:810-21. 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 26. Couch FJ, Hart SN, Sharma P, et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol 2015;33:304-11. 10.1200/JCO.2014.57.1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lluch A, Barrios CH, Torrecillas L, et al. GEICAM Spanish Breast Cancer Group. CIBOMA (Iberoamerican Coalition for Research in Breast Oncology) LACOG (Latin American Cooperative Oncology Group) . Phase III Trial of Adjuvant Capecitabine After Standard Neo-/Adjuvant Chemotherapy in Patients With Early Triple-Negative Breast Cancer (GEICAM/2003-11_CIBOMA/2004-01). J Clin Oncol 2020;38:203-13. 10.1200/JCO.19.00904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Swain SM, Tang G, Geyer CE, Jr, et al. Definitive results of a phase III adjuvant trial comparing three chemotherapy regimens in women with operable, node-positive breast cancer: the NSABP B-38 trial. J Clin Oncol 2013;31:3197-204. 10.1200/JCO.2012.48.1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wardley AM, Hiller L, Howard HC, et al. tAnGo Trial Collaborators . tAnGo: a randomised phase III trial of gemcitabine in paclitaxel-containing, epirubicin/cyclophosphamide-based, adjuvant chemotherapy for early breast cancer: a prospective pulmonary, cardiac and hepatic function evaluation. Br J Cancer 2008;99:597-603. 10.1038/sj.bjc.6604538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mason SR, Willson ML, Egger SJ, Beith J, Dear RF, Goodwin A. Platinum chemotherapy for early triple-negative breast cancer. Breast 2024;75:103712. 10.1016/j.breast.2024.103712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 2008;358:1663-71. 10.1056/NEJMoa0707056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang B, Sun T, Zhao Y, et al. A randomized phase 3 trial of Gemcitabine or Nab-paclitaxel combined with cisPlatin as first-line treatment in patients with metastatic triple-negative breast cancer. Nat Commun 2022;13:4025. 10.1038/s41467-022-31704-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Swain SM, Jeong JH, Geyer CE, Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med 2010;362:2053-65. 10.1056/NEJMoa0909638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chirivella I, Bermejo B, Insa A, et al. Optimal delivery of anthracycline-based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res Treat 2009;114:479-84. 10.1007/s10549-008-0018-1 [DOI] [PubMed] [Google Scholar]
- 35. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet 2019;393:1440-52. 10.1016/S0140-6736(18)33137-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary figures and tables
Web appendix: Study protocol
Web appendix: Statistical analysis plan
Data Availability Statement
Requests for individual de-identified participant data that underlie the results reported in this article will be considered. Qualified researchers should submit a proposal to the corresponding author outlining the reasons for requesting the data. The leading clinical site will check whether the request is subject to any intellectual property obligations. No custom code was used for data analysis in this study.