Abstract
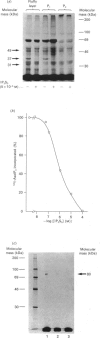
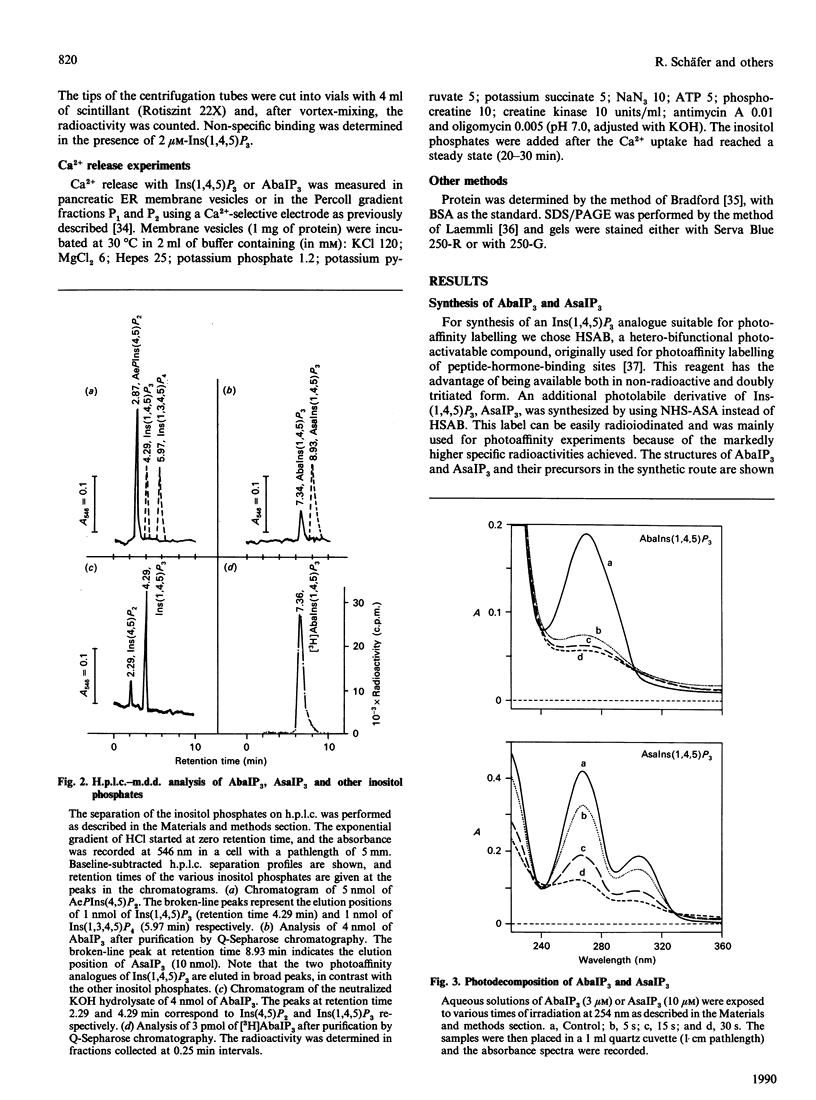
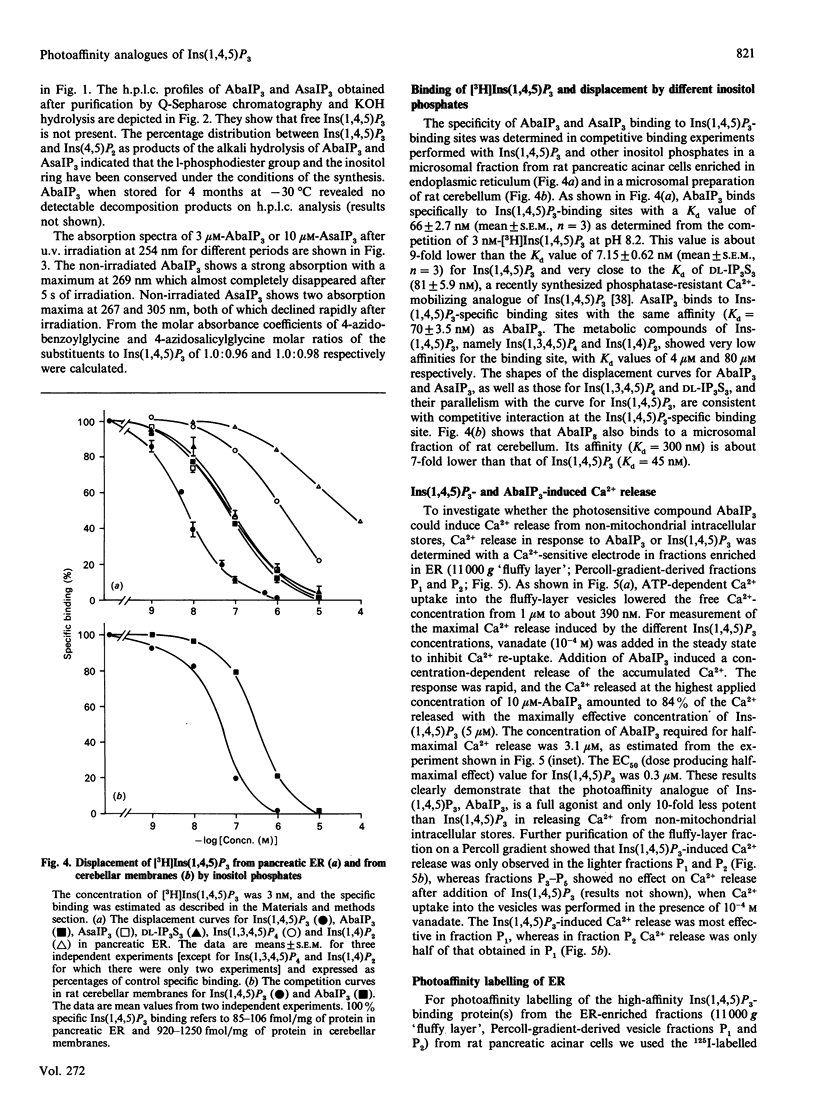
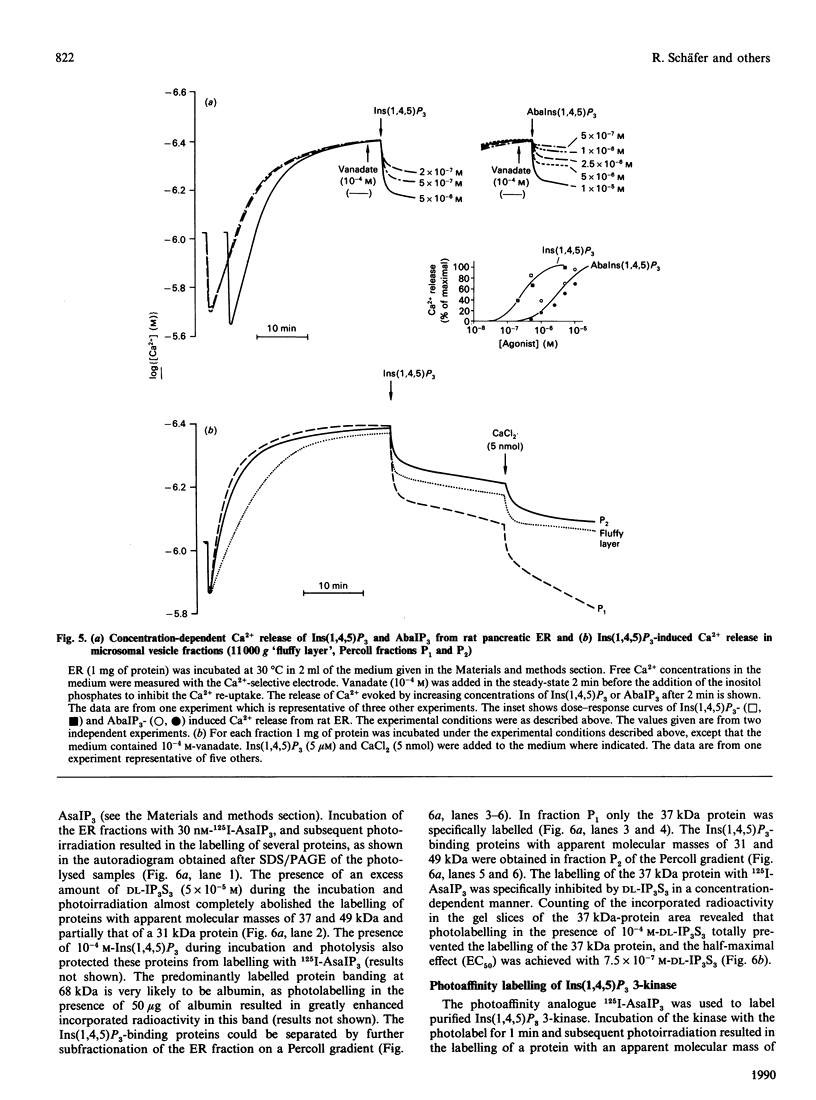
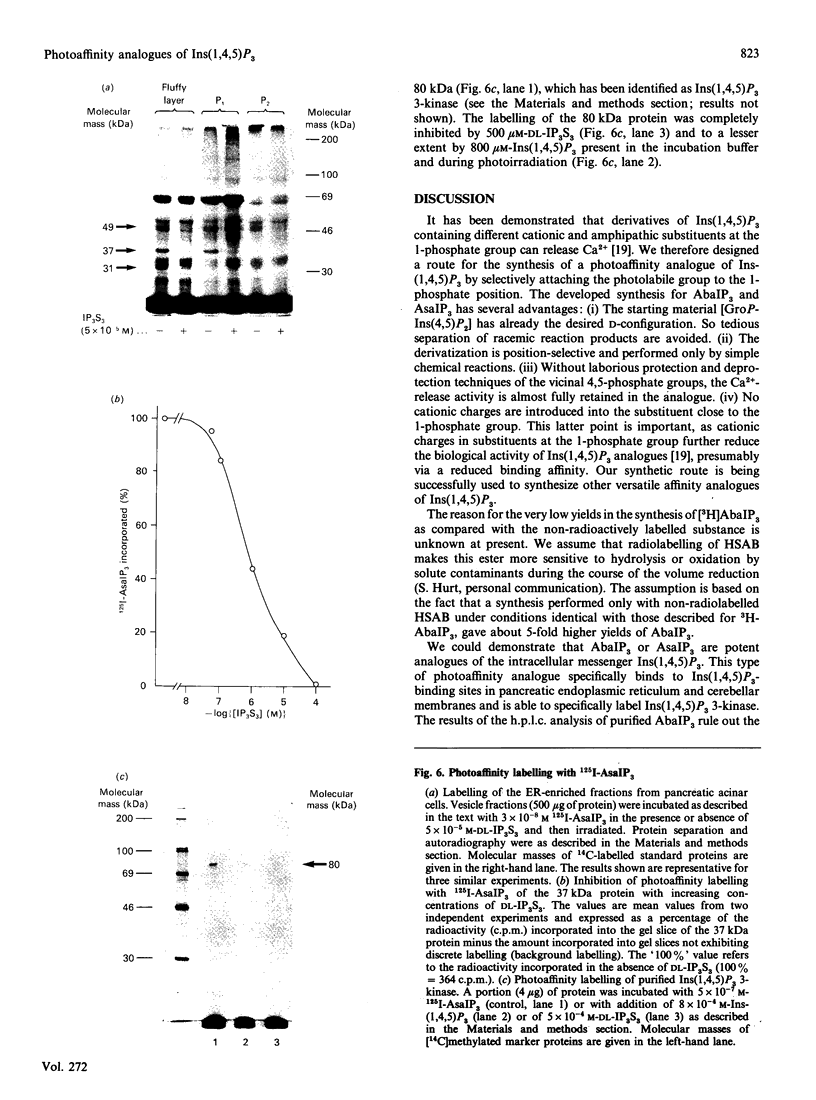
We have synthesized two photolabile arylazido-analogues of Ins(1,4,5)P3 selectively substituted at the 1-phosphate group for determination of Ins(1,4,5)P3-binding proteins. These two photoaffinity derivatives, namely N-(4-azidobenzoyl)aminoethanol-1-phospho-D-myo-inositol 4,5-bisphosphate (AbaIP3) and N-(4-azidosalicyl)aminoethanol-1-phospho-D-myo-inositol 4,5-bisphosphate (AsaIP3), bind to high affinity Ins(1,4,5)P3-specific binding sites at a 9-fold lower affinity (Kd = 66 and 70 nM) than Ins(1,4,5)P3 (Kd = 7.15 nM) in a fraction from rat pancreatic acinar cells enriched in endoplasmic reticulum (ER). Other inositol phosphates tested showed comparable (DL-myo-inositol 1,4,5-trisphosphothioate, Kd = 81 nM) or much lower affinities for the binding sites [Ins(1,3,4,5)P4, Kd = 4 microM; Ins(1,4)P2, Kd = 80 microM]. Binding of AbaIP3 was also tested on a microsomal preparation of rat cerebellum [Kd = 300 nM as compared with Ins(1,4,5)P3, Kd = 45 nM]. Ca2+ release activity of the inositol derivatives was tested with AbaIP3. It induced a rapid and concentration-dependent Ca2+ release from the ER fraction [EC50 (dose producing half-maximal effect) = 3.1 microM] being only 10-fold less potent than Ins(1,4,5)P3 (EC50 = 0.3 microM). From the two radioactive labelled analogues ([3H]AbaIP3 and 125I-AsIP3) synthesized, the radioiodinated derivative was used for photoaffinity labelling. It specifically labelled three proteins with apparent molecular masses of 49, 37 and 31 kDa in the ER-enriched fraction. By subfractionation of this ER-enriched fraction on a Percoll gradient the 37 kDa Ins(1,4,5)P3 binding protein was obtained in a membrane fraction which showed the highest effect in Ins(1,4,5)P3-inducible Ca2+ release (fraction P1). The other two Ins(1,4,5)P3-binding proteins, of 49 and 31 kDa, were obtained in fraction P2, in which Ins(1,4,5)P3-induced Ca2+ release was half of that obtained in fraction P1. We conclude from these data that the 37 kDa and/or the 49 and 31 kDa proteins are involved in Ins(1,4,5)P3-induced Ca2+ release from the ER of rat pancreatic acinar cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baukal A. J., Guillemette G., Rubin R., Spät A., Catt K. J. Binding sites for inositol trisphosphate in the bovine adrenal cortex. Biochem Biophys Res Commun. 1985 Dec 17;133(2):532–538. doi: 10.1016/0006-291x(85)90939-8. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol trisphosphate formation and calcium mobilization in Swiss 3T3 cells in response to platelet-derived growth factor. Biochem J. 1984 Aug 15;222(1):195–201. doi: 10.1042/bj2220195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Ehrlich B. E., Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988 Dec 8;336(6199):583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Supattapone S., Snyder S. H. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989 Nov 2;342(6245):87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- Galardy R. E., Craig L. C., Jamieson J. D., Printz M. P. Photoaffinity labeling of peptide hormone binding sites. J Biol Chem. 1974 Jun 10;249(11):3510–3518. [PubMed] [Google Scholar]
- Guillemette G., Balla T., Baukal A. J., Spät A., Catt K. J. Intracellular receptors for inositol 1,4,5-trisphosphate in angiotensin II target tissues. J Biol Chem. 1987 Jan 25;262(3):1010–1015. [PubMed] [Google Scholar]
- Henne V., Mayr G. W., Grabowski B., Koppitz B., Söling H. D. Semisynthetic derivatives of inositol 1,4,5-trisphosphate substituted at the 1-phosphate group. Effects on calcium release from permeabilized guinea-pig parotid acinar cells and comparison with binding to aldolase A. Eur J Biochem. 1988 May 16;174(1):95–101. doi: 10.1111/j.1432-1033.1988.tb14067.x. [DOI] [PubMed] [Google Scholar]
- Imamura K., Schulz I. Phosphorylated intermediate of (Ca2+ + K+)-stimulated Mg2+-dependent transport ATPase in endoplasmic reticulum from rat pancreatic acinar cells. J Biol Chem. 1985 Sep 15;260(20):11339–11347. [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Ishimatsu T., Kimura Y., Ikebe T., Yamaguchi K., Koga T., Hirata M. Possible binding sites for inositol 1,4,5-trisphosphate in macrophages. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1173–1180. doi: 10.1016/s0006-291x(88)81263-4. [DOI] [PubMed] [Google Scholar]
- Koppitz B., Vogel F., Mayr G. W. Mammalian aldolases are isomer-selective high-affinity inositol polyphosphate binders. Eur J Biochem. 1986 Dec 1;161(2):421–433. doi: 10.1111/j.1432-1033.1986.tb10462.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Maeda N., Niinobe M., Mikoshiba K. A cerebellar Purkinje cell marker P400 protein is an inositol 1,4,5-trisphosphate (InsP3) receptor protein. Purification and characterization of InsP3 receptor complex. EMBO J. 1990 Jan;9(1):61–67. doi: 10.1002/j.1460-2075.1990.tb08080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Deckmyn H., Ross T. S., Bross T. E., Ishii H., Bansal V. S., Wilson D. B. The metabolism of phosphoinositide-derived messenger molecules. Science. 1986 Dec 19;234(4783):1519–1526. doi: 10.1126/science.3024320. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Mayr G. W. A novel metal-dye detection system permits picomolar-range h.p.l.c. analysis of inositol polyphosphates from non-radioactively labelled cell or tissue specimens. Biochem J. 1988 Sep 1;254(2):585–591. doi: 10.1042/bj2540585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery G. A., Südhof T. C., Takei K., De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989 Nov 9;342(6246):192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- Nahorski S. R., Potter B. V. Molecular recognition of inositol polyphosphates by intracellular receptors and metabolic enzymes. Trends Pharmacol Sci. 1989 Apr;10(4):139–144. doi: 10.1016/0165-6147(89)90165-x. [DOI] [PubMed] [Google Scholar]
- Nunn D. L., Potter B. V., Taylor C. W. Molecular target sizes of inositol 1,4,5-trisphosphate receptors in liver and cerebellum. Biochem J. 1990 Jan 15;265(2):393–398. doi: 10.1042/bj2650393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polokoff M. A., Bencen G. H., Vacca J. P., deSolms S. J., Young S. D., Huff J. R. Metabolism of synthetic inositol trisphosphate analogs. J Biol Chem. 1988 Aug 25;263(24):11922–11927. [PubMed] [Google Scholar]
- Putney J. W., Jr Formation and actions of calcium-mobilizing messenger, inositol 1,4,5-trisphosphate. Am J Physiol. 1987 Feb;252(2 Pt 1):G149–G157. doi: 10.1152/ajpgi.1987.252.2.G149. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Meldolesi J., Milner T. A., Satoh T., Supattapone S., Snyder S. H. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature. 1989 Jun 8;339(6224):468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- Shanahan M. F., Wadzinski B. E., Lowndes J. M., Ruoho A. E. Photoaffinity labeling of the human erythrocyte monosaccharide transporter with an aryl azide derivative of D-glucose. J Biol Chem. 1985 Sep 15;260(20):10897–10900. [PubMed] [Google Scholar]
- Spät A., Fabiato A., Rubin R. P. Binding of inositol trisphosphate by a liver microsomal fraction. Biochem J. 1986 Feb 1;233(3):929–932. doi: 10.1042/bj2330929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Streb H., Bayerdörffer E., Haase W., Irvine R. F., Schulz I. Effect of inositol-1,4,5-trisphosphate on isolated subcellular fractions of rat pancreas. J Membr Biol. 1984;81(3):241–253. doi: 10.1007/BF01868717. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Streb H., Schulz I. Regulation of cytosolic free Ca2+ concentration in acinar cells of rat pancreas. Am J Physiol. 1983 Sep;245(3):G347–G357. doi: 10.1152/ajpgi.1983.245.3.G347. [DOI] [PubMed] [Google Scholar]
- Strupish J., Cooke A. M., Potter B. V., Gigg R., Nahorski S. R. Stereospecific mobilization of intracellular Ca2+ by inositol 1,4,5-triphosphate. Comparison with inositol 1,4,5-trisphosphorothioate and inositol 1,3,4-trisphosphate. Biochem J. 1988 Aug 1;253(3):901–905. doi: 10.1042/bj2530901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Takazawa K., Passareiro H., Dumont J. E., Erneux C. Purification of bovine brain inositol 1,4,5-trisphosphate 3-kinase. Identification of the enzyme by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. Biochem J. 1989 Jul 15;261(2):483–488. doi: 10.1042/bj2610483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W., Berridge M. J., Cooke A. M., Potter B. V. Inositol 1,4,5-trisphosphorothioate, a stable analogue of inositol trisphosphate which mobilizes intracellular calcium. Biochem J. 1989 May 1;259(3):645–650. doi: 10.1042/bj2590645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. W., Putney J. W., Jr Size of the inositol 1,4,5-trisphosphate-sensitive calcium pool in guinea-pig hepatocytes. Biochem J. 1985 Dec 1;232(2):435–438. doi: 10.1042/bj2320435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenod F., Kemmer T. P., Christian A. L., Schulz I. Characterization of MgATP-driven H+ uptake into a microsomal vesicle fraction from rat pancreatic acinar cells. J Membr Biol. 1989 Mar;107(3):263–275. doi: 10.1007/BF01871941. [DOI] [PubMed] [Google Scholar]
- Walker J. W., Somlyo A. V., Goldman Y. E., Somlyo A. P., Trentham D. R. Kinetics of smooth and skeletal muscle activation by laser pulse photolysis of caged inositol 1,4,5-trisphosphate. Nature. 1987 May 21;327(6119):249–252. doi: 10.1038/327249a0. [DOI] [PubMed] [Google Scholar]
- Willcocks A. L., Potter B. V., Cooke A. M., Nahorski S. R. Myo-inositol(1,4,5)trisphosphorothioate binds to specific [3H]inositol(1,4,5)trisphosphate sites in rat cerebellum and is resistant to 5-phosphatase. Eur J Pharmacol. 1988 Oct 11;155(1-2):181–183. doi: 10.1016/0014-2999(88)90420-7. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Supattapone S., Wilson V. S., Snyder S. H. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem. 1987 Sep 5;262(25):12132–12136. [PubMed] [Google Scholar]