Abstract
Objective:
To report our experience for adults undergoing cochlear implantation (CI) for single-sided deafness (SSD).
Methods:
This is a retrospective case series for adults with SSD who underwent CI between January 2013 and May 2021 at our institution. CNC and AzBio speech recognition scores, Tinnitus Handicap Inventory (THI), Speech, Spatial and Qualities of Hearing Scale (SSQ12), datalogging, and the Cochlear Implant Quality of Life (CIQOL)-10 Global measure were utilized.
Results:
Sixty-six adults underwent CI for SSD (median 51.3 years, range 20.0 to 74.3 years), and 57 (86.4%) remained device users at last follow-up. Compared to pre-operative performance, device users demonstrated significant improvement in speech recognition scores and achieved peak performance at six months post-activation for CNC (8.0% increased to 45.6%, p<0.0001) and AzBio in quiet (12.2% increased to 59.5%, p<0.0001). THI was decreased at 6 months post-implantation (58.1 to 14.6, p<0.0001), with 77% of patients reporting improved or resolved tinnitus. Patients demonstrated improved SSQ12 scores as well as the disease-specific CIQOL-10 Global questionnaire. Duration of deafness (DoD) was not associated with significant differences in speech recognition performance. Average daily wear time was positively associated with CNC and AzBio scores as well as post-operative CIQOL-10 scores.
Conclusions:
Herein we present the largest cohort of adult CI recipients with SSD with data on speech recognition scores, tinnitus measures, and SSQ12. Novel insights regarding correlation of datalogging, duration of deafness, and CI-specific quality of life (CIQOL-10) metrics are discussed. Data continue to support CI as an efficacious treatment option for SSD.
Level of Evidence:
IV
Keywords: cochlear implant, single-sided deafness, quality of life, duration of deafness, datalogging, tinnitus
Lay Summary:
Expanding indications for cochlear implants include people with single-sided deafness. Here is our experience with the largest group of such patients to date. There are important improvements in hearing, tinnitus reduction, and quality of life measures!
INTRODUCTION
Single-sided deafness (SSD) is defined as moderate-to-profound sensorineural hearing loss (SNHL) in one ear with normal hearing or mild hearing loss in the contralateral ear. In the United States, the prevalence of acquired SSD is estimated at 0.14%, amounting to nearly 350,000 affected Americans.1 Traditional rehabilitative options for SSD include contralateral routing of signal hearing aids (CROS-HA) and bone conduction hearing devices (BCHD). These options route sound from the poorer-hearing ear to the better-hearing ear via wireless signals and vibratory bone conduction, respectively. Signals from both sides are delivered to the better-hearing ear to overcome the head shadow effect and improve detection of sound arising from the side of the poorer-hearing ear. However, these strategies do not yield improvement in localization, binaural summation, or binaural squelch, which are important for speech understanding in background noise, a common complaint of those with SSD.2 CROS-HA and BCHD devices provide only moderate benefit for speech understanding in spatially-separated speech and noise when the signal is directed at the poorer-hearing ear and/or the noise is directed to the better-hearing ear.3–6
Cochlear implantation (CI) for rehabilitation of SSD offers input to the ear with severe/profound loss and can thus improve spatial hearing, modulate tinnitus, enhance speech recognition in both quiet and noise, and has been shown to improve quality of life (QoL) for these patients.3,7–20 Trials evaluating CI in the setting of adult SSD demonstrate improved sound localization and sentence recognition in noise when the noise was presented from the front or in the better-hearing ear.21 Early studies investigating CI for SSD were focused on effects for incapacitating unilateral tinnitus, with a recent systematic review estimating that 88% of SSD patients experience suppression or improvement of their symptoms after CI.13,22–24 Investigations into subjective quality of hearing performance as measured by the Speech, Spatial and Qualities Questionnaire (SSQ12) demonstrated long-term improvement in speech intelligibility and spatial hearing, and investigations in QoL confirm a significant benefit for patients with SSD.16,18,25–29 This body of research was acknowledged by the United States Food and Drug Administration (FDA) through approval of MED-EL (Innsbruck, Austria) and Cochlear (Sydney, Australia) CI devices for the treatment of SSD and asymmetric hearing loss. These decisions have cemented CI as a mainstream treatment option for SSD.30
Despite a burgeoning knowledge base, many clinical studies regarding CI for SSD are limited by low patient enrollment, differing methodologies for audiometric performance testing, as well as varied assessments for QoL metrics. As a result, information regarding certain aspects of counseling and outcomes for patients with SSD remain scarce or incomplete. Herein, we present speech recognition, tinnitus handicap, and quality of hearing outcomes for the largest single-institution cohort of patients undergoing CI for SSD, to date. In addition, we include our data and observations regarding CI-specific quality of life measures, the role of duration of deafness (DoD), as well as the effect of daily device usage (“datalogging” hours) on performance and QoL. Finally, we aim to better characterize “non-users” identified in our study to inform patient selection and counseling during the hearing rehabilitation process for SSD.
MATERIALS AND METHODS
After approval from our institutional review board (#211355), we performed a retrospective review of patients undergoing CI for SSD between January 2013 and May 2021. Patients implanted prior to FDA approval in July 2019 were considered “off-label”. SSD is defined as a pure-tone average (PTA) of ≥70 dB HL in the poorer ear and PTA ≤30 dB HL in the better ear, with PTA calculated using 500, 1000, 2000, and 4000 Hz thresholds.31 Data including demographics, history, speech recognition assessments (i.e., consonant-nucleus-consonant (CNC) words, AzBio sentences in quiet, and validated patient questionnaires (i.e., SSQ12, THI, CIQOL-10) were collected at the pre-operative, 6-month, 12-month, and most recent encounter. The severity of perceived tinnitus increases with higher THI scores.32 To assess for any change in reported tinnitus handicap, we utilized the recently reported minimal clinical important difference (MCID) for ΔTHI = 7.33 Exclusion criteria included prelingual hearing loss. To characterize the overall cohort undergoing CI for SSD, we included patient demographics from subjects with incomplete records or device non-usage; data regarding outcomes only included CI users with adequate pre- and post-operative data.
Patients underwent audiologic evaluation according to the Minimum Speech Test Battery.34 Speech testing occurred in a sound booth with presentation level of 60 dBA.35,36 Stimuli were presented from a single loudspeaker at 0-degree azimuth (1 m); speech and noise were co-located. In order to isolate the implanted ear in quiet, the opposite ear was appropriately masked for the majority of patients; five Cochlear recipients were tested with a direct connect method and two patients were tested using the plug-and-muff method. Due to the timespan over which data collection occurred (2013 – 2021), our clinic did not yet have a speaker setup to accommodate speech from the front and noise to the better-hearing ear, which we believe to be the more efficacious setup for evaluating these patients. Additionally, speech perception in noise testing requires three channels to appropriately mask the normal hearing ear, which was not available on our audiometers.
Normal distribution was presented with means and standard deviations after Shapiro-Wilk testing, while median values with interquartile range (IQR) were used for non-parametric data. Nominal data were analyzed using the Fisher’s exact test. One-way analysis of variance (ANOVA) and Tukey’s multiple comparisons test were utilized for normally distributed data; adjusted p values were reported with Bonferroni correction. Pearson’s coefficient for normally-distributed sets assessed correlation: ≥0.70 strong, 0.50–0.69 moderate, and <0.50 weak.37 P values <0.05 were statistically significant. Analyses were performed using GraphPad Prism 9.2.0 (GraphPad Software, La Jolla, California).
RESULTS
Demographics and Clinical Characteristics
Sixty-six adult patients with SSD underwent CI (Table 1). Median age at implantation was 51.3 years (range 20.0 to 74.3 years). Thirty-one (47%) were women, and thirty-five (53%) were left ears. Median duration of deafness (DoD) was 1.75 years (range 0.2–32, IQR 0.5–4.3 years). Nine patients (8 of which are current CI users) had a DoD greater than 10 years. User selection included 9 devices (14%) from Advanced Bionics (Valencia, CA), 26 (39%) from Cochlear, and 31 (47%) from MED-EL. Importantly, a non-trivial group of 9 patients (14%) were non-users/explanted at last contact and were not included in analysis; reasons for non-use included magnet retention issues (n=2), lack of perceived tinnitus reduction (n=2), lack of perceived benefit in hearing (n=2), infection/dehiscence (n=2), and magnet pain during ongoing MRI scans (n=1). Incomplete follow-up data resulted from missed appointments as well as current CI users that were implanted/activated at our institution but followed-up elsewhere. Variability in follow-up was potentially due to patient factors, audiologist preference, and the Covid-19 pandemic. Most recent follow-up was at a median of 13.5 months (IQR 9–29.75 months, total range 1–100 months). Thirty-four patients had follow-up beyond the 12-month appointment.
Table 1. Patient Characteristics and Demographics.
| Characteristic | n = 66 |
|---|---|
| Median age (SD) | 51.3 (SD = 13.1) years |
| Gender | |
| Female | 31 (47%) |
| Male | 35 (53%) |
| Laterality | |
| Left | 35 (53%) |
| Right | 31 (47%) |
| Device Status | |
| Users | 57 (86%) |
| Non-Users | 9 (14%) |
| Manufacturer | |
| Advanced Bionics | 9 (14%) |
| Cochlear | 26 (39%) |
| MED-EL | 31 (47%) |
| Etiology | |
| Chronic ear disease | 3 (4.5%) |
| Iatrogenic | 6 (9.1%) |
| Labyrinthitis | 10 (15.2%) |
| Meniere’s | 10 (15.2%) |
| Noise Exposure | 1 (1.5%) |
| Schwannoma (IAC/CPA Vestibular) | 4 (6.1%) |
| Schwannoma (Intralabyrinthine) | 2 (3.0%) |
| Sudden SNHL | 25 (37.9%) |
| Temporal Bone Fracture | 3 (4.5%) |
| Unknown | 2 (3.0%) |
IAC/CPA indicates internal auditory canal/cerebellopontine angle, SD indicates standard deviation.
Speech Recognition Outcomes
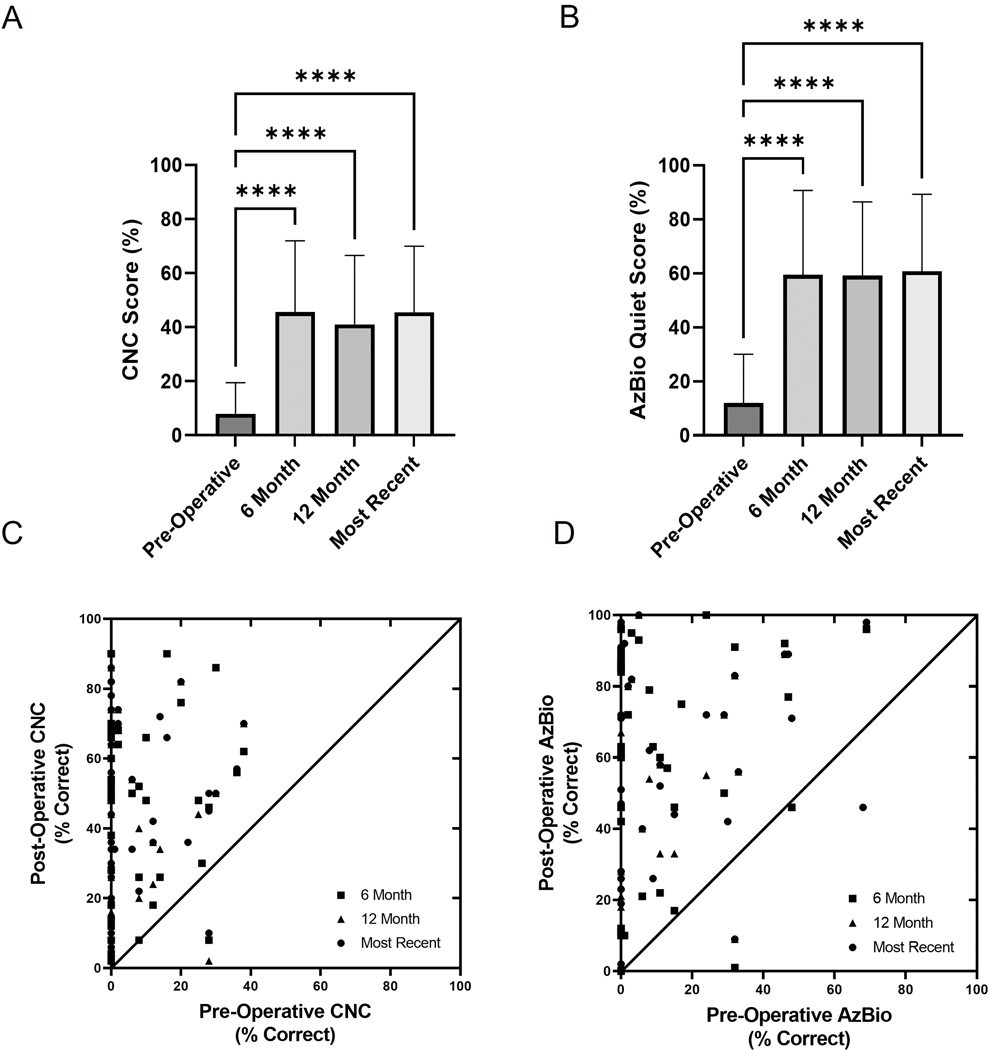
Comparison of CI-only mean speech recognition scores (%) at pre-operative (n=57), 6-month (n=39), 12-month (n=56), and most recent (n=51) audiologic follow-up was performed for CNC and AzBio in quiet (Figure 1A,B). The mean scores were significantly higher than pre-operative scores for CI-only speech recognition measures at the 6-month, 12-month, and most recent time points. CNC improved from mean 8.0% (SD 12) to 45.6% (SD 26.4) at 6-months and 41.1% (SD 25.5) at 12-months (F(3, 170) = 35.75, Bonferroni α = 0.0083, p<0.0001). AzBio in quiet improved from 12.0% (SD 18.1) to 59.4% (SD 31.3 at 6-months and 59.3% (SD 27.2) at 12-months (F(3, 169) = 42.7, Bonferroni α = 0.0083, p<0.0001). Individual pre-operative CNC and AzBio in quiet speech recognition scores were compared to the post-operative time points for CI-only condition in Figure 1C,D. The dashed lines signify the 95% confidence intervals for test-retest reliability on each measure.35,38 There was also no difference for speech recognition scores based on etiology for the SSD on last follow-up (F(5, 45) = 1.231, Bonferroni α = 0.0033, p=0.3104).
FIG. 1.
Multiple comparisons of CI-only mean speech recognition scores (%) at pre-operative, 6-month post-operative, 12-month post-operative, and most recent follow-up time points for A) CNC words and B) AzBio sentences in quiet. Individual pre-operative vs post-operative CNC words and AzBio sentences in quiet are presented in C) and D), respectively. The diagonal line represents equivalence between the two testing intervals. Dashed lines denote the 95% confidence intervals for test-retest reliability on each measure. Asterisks note adjusted significant differences: ****p<0.0001, ***p<0.001, and **p<0.01. Error bars represent one standard deviation. AzBio indicates Arizona Biomedical; CI, cochlear implant; CNC, Consonant-Nucleus-Consonant.
Tinnitus
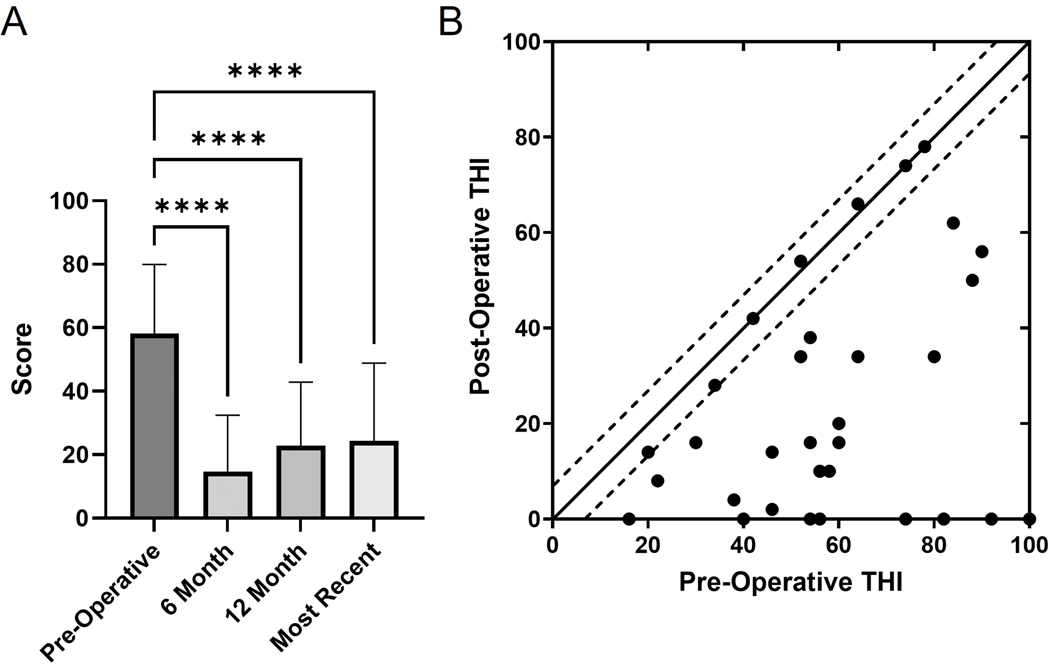
A total of 32 patients completed both pre- and post-implantation THI questionnaires, with each dot representing the pre- and post-implantation scores for an individual patient (Figure 2). Improvement in mean pre-implantation THI score (58.1, SD 21.9) was demonstrated as soon as 6-months postoperatively (14.6, SD 17.8, Bonferroni α = 0.0083, p<0.0001) and was sustained at the 12-month and most recent time points (F(3, 93) = 21.69, Bonferroni α = 0.0083, p<0.0001). An additional 12 patients had subjective tinnitus symptomatology documented and were added to the cohort with THI information to assess overall rate of symptom resolution, improvement, and persistence (Table 2). Complete resolution of tinnitus was classified as a THI score of 0 on the post-operative questionnaire or subjective report of resolution. Clinical improvement in tinnitus was defined as exceeding the MCID of ΔTHI = 7.33 While 18% of patients reported resolution of their tinnitus, an additional 59% reported improvement without resolution of tinnitus, and 23% reported no change in symptoms. One patient had no perceived tinnitus before or after CI.
FIG. 2.
A) Mean Tinnitus Handicap Inventory (THI) scores for the pre-operative, 6-month post-operative, 12-month post-operative, and most recent follow-up time points. Error bars represent one standard deviation. Asterisks note adjusted significant differences: ****p<0.0001. B) Scatter plot of pre-operative THI vs post-operative THI for each user that completed these questionnaires. Each dot represents the pre- and post-implantation scores for an individual patient. The minimal clinically important difference of ΔTHI = 7 is demarcated by dashed lines. Points below the lines demonstrate improvement in THI, while points within the dashed lines signify no MCID in THI.
TABLE 2. Changes in Tinnitus.
| Tinnitus | THI Scores (n=32) | Patient-Reported (n=12) | Overall Change (n=44) |
|---|---|---|---|
| Resolution | 7 (22%) | 1 (8%) | 8 (18%) |
| Improvement | 19 (59%) | 7 (58%) | 26 (59%) |
| No Change | 6 (19%) | 4 (33%) | 10 (23%) |
Changes in tinnitus were either calculated from pre-operative and post-operative THI scores or subjectively through patient-reported symptoms if the full set of THI scores were not available in the medical record. Complete resolution was classified as a THI score of 0 on the post-operative questionnaire. Improvement was defined as an decrease ≥7 THI 33. THI: Tinnitus Handicap Inventory.
Speech, Spatial, and Qualities of Hearing Scale
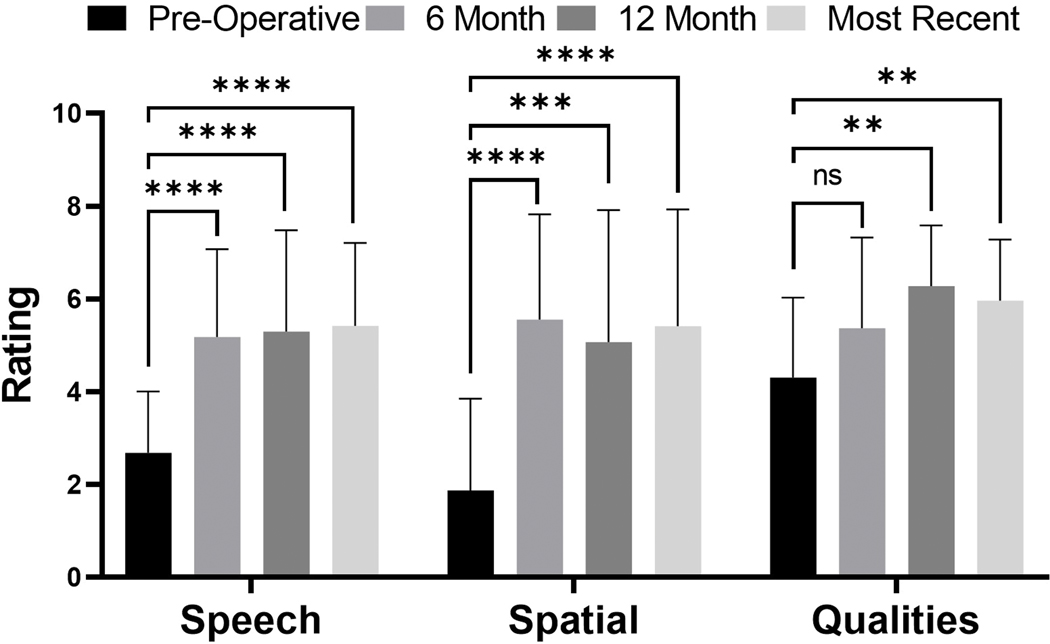
A total of 29 patients completed both pre- and post-implantation SSQ12 questionnaires. Figure 3 demonstrates patient scores for the speech, spatial, and quality domains, with higher scores indicating improved perceived ability. Patient questionnaires demonstrated an improvement in both speech perception ability (F(3, 95) = 15.18, Bonferroni α = 0.0083, p<0.0001) and spatial hearing ability (F(3, 95) = 15.32, Bonferroni α = 0.0083, p<0.0001) at 6-months and was sustained at subsequent time points. With regards to patient-perceived quality of hearing, mean scores were not significantly different at the 6-month time points but were at the 12-month and most recent follow-ups (F(3, 95) = 6.978, Bonferroni α = 0.0083, p=0.0003). Interestingly, quality of hearing on the SSQ12 questionnaire did not significantly correlate to CNC outcomes at six (rp=0.1579, p=0.4410, n=26), twelve months (rp=0.1779, p=0.5222, n=15), or most recent (rp=0.0169, p=0.9306, n=29) time points.
FIG. 3.
Mean SSQ12 ratings for the pre-operative (black), 6-month (medium grey), 12-month (dark grey), and most recent (light grey) test intervals. Error bars represent one standard deviation. Asterisks note adjusted significant differences: ****p<0.0001, ***p<0.001, **p<0.01, and *p<0.05. SSQ: speech, spatial, and qualities of hearing scale.
Cochlear Implant Quality of Life
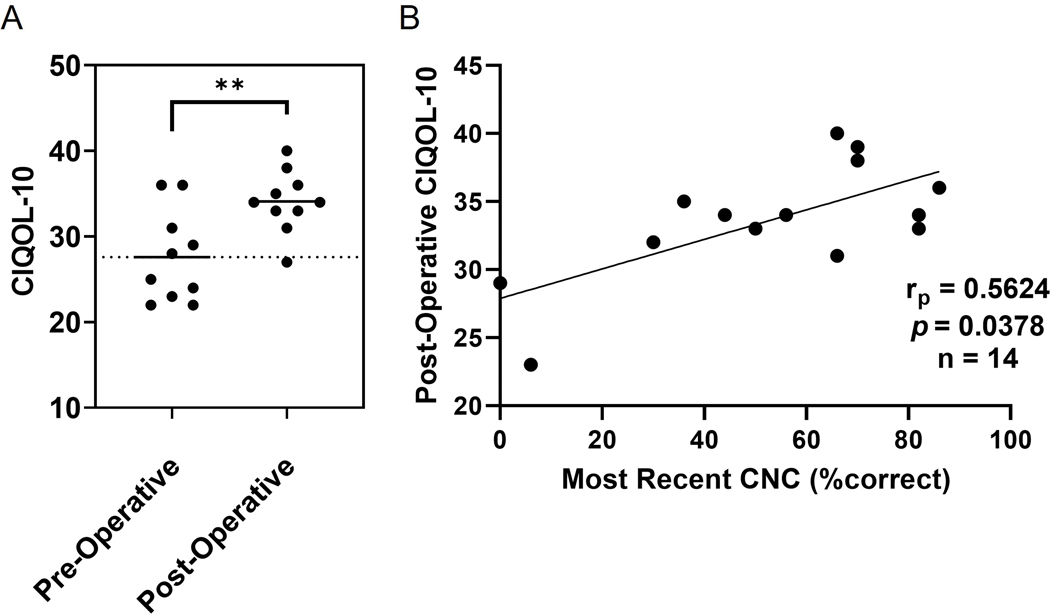
The CIQOL-10 questionnaire was introduced as part of our CI work-up in late 2019; a total of ten patients with SSD undergoing CI completed both pre- and post-implantation questionnaires (Figure 4A). The mean CIQOL-10 scores for these patients increased from 27.6 to 34.1 (t(18) = 3.183, p=0.0052), a difference of 6.5 points. Pearson correlational analysis demonstrated a moderate correlation between post-operative CNC speech recognition scores and post-operative CIQOL-10 scores (rp=0.5624, p=0.0378, n=14) as illustrated in Figure 4B.
FIG. 4.
A) Mean post-operative CIQOL-10 scores for these patients increased from a pre-operative score of 27.6 to 34.1, (p=0.0052), a difference of 6.5 points. B) Pearson correlational analysis demonstrated a moderate correlation between post-operative CNC speech recognition scores and post-operative CIQOL-10 scores. Asterisks note adjusted significant differences: **p<0.01.
Average Daily Usage
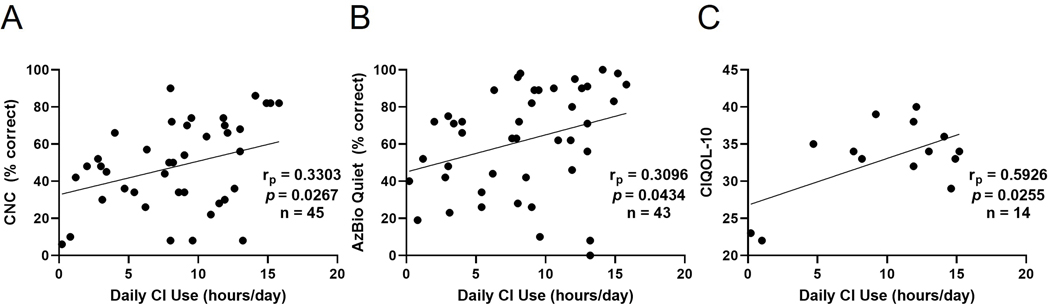
Average daily usage was normally-distributed (mean 8.0 hours, SD 4.6 hours) but less than large-volume cross-sectional analyses of adult and pediatric CI recipients such as Busch et al. (mean 10.74 hours, SD 3.45, n=1501).39 Pearson correlational analyses were performed to examine the relationship between average daily wear time (processor datalogging) and outcomes including speech recognition scores and CIQOL-10 (Figure 5). Patients who were non-users were not included in these analyses. A weak positive correlation was established for CI users between average hours of wear time per day and CNC word scores (rp=0.3303, p=0.0267, n=45) as well as AzBio sentence scores (rp=0.3096, p=0.0434, n=43). A moderate positive correlation was established between average hours of CI use per day and post-operative CIQOL-10 scores (rp=0.5926, p=0.0255, n=14).
FIG. 5.
A) Positive correlation between average hours of cochlear implant (CI) use per day and consonant nucleus-consonant (CNC) word scores, B) Positive correlation between average hours of CI use per day and AzBio sentence scores, C) Positive correlation between average hours of CI use per day and Cochlear Implant Quality of Life (CIQOL-10) scores.
Duration of Deafness and Patient Age
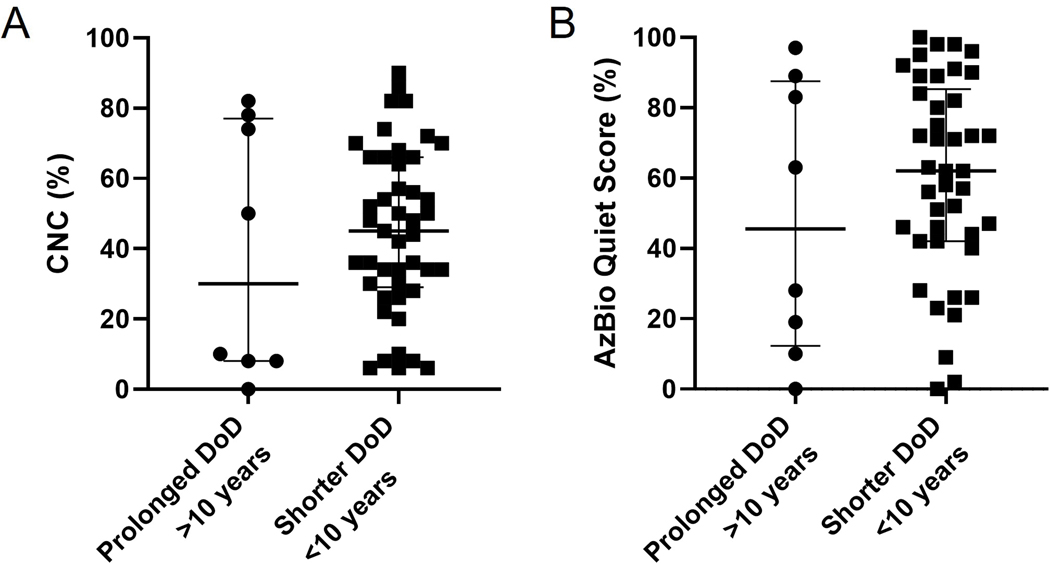
A total of eight users with a duration of deafness greater than 10 years were implanted for SSD (median 14.5 years, range 10–32 years). There was no statistical difference in CI-only CNC (p=0.6826) or AzBio (p=0.4619) scores at most recent assessment for CI users with DoD >10 years versus <10 years (Figure 6). Notably, two patients with DoD >10 years also had incomplete insertion of the CI electrodes due to cochlear fibrosis resulting in CNC/AzBio in Quiet scores of 0%/0% and 10%/19%, respectively. No correlation was demonstrated between patient age at implantation and speech recognition outcomes. It should be noted that our cohort was younger than traditional cohorts reported in the literature, with only one patient above the age of 70 years.
FIG. 6.
A) CI users with a duration of deafness greater than 10 years versus those with DoD less than 10 years. There was no statistical difference in CI-only CNC (Figure 6A) or AzBio (Figure 6B) scores at most recent assessment for CI users with DoD >10 years versus <10 years
DISCUSSION
FDA approval of CI for the treatment of SSD has brought changes to the contemporary counseling and management of this condition. These patients may be especially motivated by debilitating tinnitus, difficulties hearing in noise, poor sound localization, and/or issues communicating in social settings.7 In addition to a large cohort of CI-only speech recognition and tinnitus data, our data provide valuable insight into CI-specific quality of life measures, the role of duration of deafness (DoD), as well as the effect of daily device usage “datalogging” hours on performance and QoL.
Despite overall favorable outcomes, word recognition scores for our SSD CI recipients are variable, consistent with prior studies.40 Sladen and colleagues posit that average CNC word scores are lower in adult SSD CI recipients than those with bilateral hearing loss because individuals with SSD continue to rely heavily on their better-hearing ear after implantation, limiting their rehabilitation potential. SSD CI users in this study wore their devices about 2 hours less per day, on average, than traditional CI users. Other authors have reported similar differences between SSD and traditional CI users, and some have attributed this to selective, situational device utilization in SSD patients.7,39,41,42 At least three studies have reported less-than-average wear time in SSD populations while Rauch et al. reported equivalent average wear time.7,43–45 Increasingly, evidence shows that patients with CI for SSD may take longer to plateau in performance or may even continue to improve many years after implantation.31,46 This may be due to lower daily datalogging or the time necessary for neuroplasticity and integration of the electrical signal with the normal-hearing acoustic contralateral ear. As a result, we may be underestimating the benefit observed from longer periods of follow-up. Consistent with recent data from our institution, daily CI use (hours) was positively correlated with CNC and AzBio scores in the CI-only condition.41 Interestingly, post-operative CIQOL-10 scores displayed a moderate positive correlation with daily average wear time as well as speech recognition scores for patients with SSD. Whether specific clinician counseling to increase processor use significantly affects speech recognition scores, as demonstrated in non-SSD patients, remains an area of future investigation for SSD.47
Consistent with prior studies, 77% of CI users in this study experienced partial improvement or complete resolution of their tinnitus based on THI or other subjective measures.7,23,48–50 No patients experienced worsening tinnitus, and 23% reported no change. These findings support the notion that CI is an excellent treatment option for SSD patients with bothersome tinnitus.23 Nonetheless, 23% of our patients reported unchanged tinnitus after CI for SSD; further investigation of predictive factors will better inform patient selection and counseling.
Prior studies utilizing SSQ12 have demonstrated significant, long-term improvements in the all three subdomains after CI for SSD.14,16,51 The cohort herein displayed significant improvement in both speech and spatial hearing subdomains at 6 months. Quality of hearing was significantly improved only at 12 months and later. This gradual change in quality of perceived hearing for this cohort could be explained by baseline auditory function (due to normal contralateral hearing), disparity in sound quality between ears, and the lower daily processor wear time among SSD CI recipients.
Traditionally, QoL measures for CI recipients were validated for patients with mild-to-moderate hearing loss and/or hearing aids and were developed based on expert opinion rather than adult CI patient focus groups.52,53 More recently, the Cochlear Implant Quality of Life Development Consortium produced the patient-reported and psychometrically-sound CIQOL-10 Global and CIQOL-35 Profile measures.52,53 One approximation of the minimal clinical important difference (MCID) is half the SD from a measure’s normative data; for traditional (not SSD) CI candidates, the SD for CIQOL-10 is 10.9 points thus the MCID is approximately 5.5 points.54 Although further investigation and validation of these calculations are necessary for CIQOL-10 in SSD, extrapolation of these figures to our cohort suggest a potential statistically and clinically important improvement in CIQOL-10 scores.
Recent large multicenter and meta-analysis studies continue to support the notion that DoD and length of auditory deprivation is negatively correlated with speech outcomes for non-SSD CI patients.55–57 Unlike traditional CI candidates, patients with post-lingual SSD have the benefit of preserved, active central auditory processing pathways and DoD is often easier to define given the high incidence of sudden hearing loss in this population.7,46 In our study, a small cohort of patients (n=8) presented with DoD >10 years but demonstrated no difference in speech recognition scores as compared to CI users with DoD <10 years at any time point. This is despite incomplete electrode insertions for two users with DoD >10, which we feel is the most important factor contributing to their decreased performance. Similar to Nassiri and colleagues, who showed that DoD had no effect on speech understanding outcomes for adults with acquired SSD, we demonstrate that DoD >10 years is not a relative contraindication for CI in SSD for motivated and interested patients.58
Limitations of this study include its retrospective nature. Metrics including SSQ12, CIQOL-10, and THI were not uniformly collected at every follow-up visit; individual data sets are smaller than the total cohort. In addition, survey instruments were often collected at just one post-operative timepoint; further changes or improvement in outcomes may be obscured. Many known current users were implanted and activated at our institution but chose to be programmed and followed closer to their homes. We acknowledge a high percentage of non-users (14%) within this study population. Insight towards predictors for non-usage in SSD is useful for patient selection and counseling; we suspect there may be a lower threshold to discontinue routine processor use under adversity for some patients given their normal contralateral hearing. With hearing rehabilitation for SSD, it is prudent for clinicians to anticipate possible complications of CI including magnet retention issues (i.e. increased scalp thickness), poor wound healing or systemic factors leading to infection/extrusion, device artifact on imaging, and lack of perceived resolution of primary symptom (i.e. tinnitus). We did not compare outcomes among other hearing rehabilitation options for SSD including CROS-HA or BCHD. Lastly, we recognize that the testing method for speech recognition testing was not optimal and uniform. As a result, we report CI-only measures when recommended outcome measures include speech-in-noise testing with three spatial configurations as well as localization testing.31 All currently available testing methods including ‘plug-and-muff’, masking, and direct connect have limitations; direct connect is arguably the most consistent and accurate, but verification of the stimulus level and confirmation of calibration is limited or nonexistent for clinical patients.9 In the future, more uniform and protocolled data-collection based on the best available tools for testing patients with SSD will improve our understanding of patient outcomes.
CONCLUSION
In this large, single-institution cohort, CI users demonstrated improvement in CI-only speech recognition scores, tinnitus reduction, and improved quality of hearing metrics despite a broad range of etiologies for their SSD. Significant improvement in QoL after activation was noted on the more recent and increasingly utilized CIQOL-10 Global measure. Additional novel findings include positive correlations between daily device usage, speech recognition performance, and QoL outcomes for these patients. Furthermore, speech recognition testing suggests that patients with a duration of hearing loss greater than ten years do not fare any worse than patients with a shorter DoD. In the absence of factors that would make CI more difficult (i.e., cochlear fibrosis from trauma or infection), we advise a gradual, non-urgent approach to hearing rehabilitation for SSD given that many patients compensate well with non-surgical options and the timing of intervention is not urgent. Careful consideration of patient-related factors that may influence device non-usage is paramount. Nonetheless, insights beyond speech recognition testing cement CI as a superior rehabilitation option for SSD.
FINANCIAL MATERIAL & SUPPORT:
The work presented here was supported by the National Institutes of Health NIH/NIDCD R01 DC13117 and UL1 TR000445.
INSITUTIONAL REVIEW BOARD:
Vanderbilt University Medical Center IRB# 211355
Footnotes
CONFLICT(S) OF INTEREST TO DECLARE:
DSH is a consultant for Advanced Bionics Corp., Cochlear Corp., MED-EL GmbH, Stryker, Synthes, Grace Medical, and Oticon. RHG is a consultant for Advanced Bionics, Akouos, and Cochlear Americas, on the clinical advisory board for Frequency Therapeutics, and on the Board of Directors for the American Auditory Society. KOT has served as an advisory board member for GlaxoSmithKline.
MEETING INFORMATION: The work herein was presented as an oral presentation at the American Neurotology Society section of the Combined Otolaryngology Spring Meeting in Dallas, Texas, USA on April 30, 2022.
REFERENCES
- 1.Kay-Rivest E, Irace AL, Golub JS, Svirsky MA. Prevalence of Single-Sided Deafness in the United States. Laryngoscope. Published online November 10, 2021. doi: 10.1002/lary.29941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buss E, Dillon MT, Rooth MA, et al. Effects of Cochlear Implantation on Binaural Hearing in Adults With Unilateral Hearing Loss. Trends Hear. 2018;22:2331216518771173. doi: 10.1177/2331216518771173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen MR, Gantz BJ, Dunn C. Outcomes after cochlear implantation for patients with single-sided deafness, including those with recalcitrant Ménière’s disease. Otol Neurotol. 2013;34(9):1681–1687. doi: 10.1097/MAO.0000000000000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saroul N, Nicolas S, Akkari M, et al. Long-term benefit and sound localization in patients with single-sided deafness rehabilitated with an osseointegrated bone-conduction device. Otol Neurotol. 2013;34(1):111–114. doi: 10.1097/MAO.0b013e31827a2020 [DOI] [PubMed] [Google Scholar]
- 5.Huber AM, Strauchmann B, Caversaccio MD, et al. Multicenter Results With an Active Transcutaneous Bone Conduction Implant in Patients With Single-sided Deafness. Otol Neurotol. 2022;43(2):227–235. doi: 10.1097/MAO.0000000000003418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desmet JBJ, Wouters K, De Bodt M, Van de Heyning P. Comparison of 2 implantable bone conduction devices in patients with single-sided deafness using a daily alternating method. Otol Neurotol. 2012;33(6):1018–1026. doi: 10.1097/MAO.0b013e31825e79ba [DOI] [PubMed] [Google Scholar]
- 7.Deep NL, Spitzer ER, Shapiro WH, Waltzman SB, Roland JTJ, Friedmann DR. Cochlear Implantation in Adults With Single-sided Deafness: Outcomes and Device Use. Otology & Neurotology. 2021;42(3):414–423. doi: 10.1097/MAO.0000000000002955 [DOI] [PubMed] [Google Scholar]
- 8.Arndt S, Aschendorff A, Laszig R, et al. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2011;32(1):39–47. doi: 10.1097/MAO.0b013e3181fcf271 [DOI] [PubMed] [Google Scholar]
- 9.Galvin JJI, Fu QJ, Wilkinson EP, et al. Benefits of Cochlear Implantation for Single-Sided Deafness: Data From the House Clinic-University of Southern California-University of California, Los Angeles Clinical Trial. Ear and Hearing. 2019;40(4):766–781. doi: 10.1097/AUD.0000000000000671 [DOI] [PubMed] [Google Scholar]
- 10.Slattery WH, Middlebrooks JC. Monaural sound localization: Acute versus chronic unilateral impairment. Hearing Research. 1994;75(1):38–46. doi: 10.1016/0378-5955(94)90053-1 [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki S, Sano H, Nishio S, et al. Hearing Handicap in Adults With Unilateral Deafness and Bilateral Hearing Loss. Otology & Neurotology. 2013;34(4):644–649. doi: 10.1097/MAO.0b013e318287f1fe [DOI] [PubMed] [Google Scholar]
- 12.Punte AK, Vermeire K, Hofkens A, De Bodt M, De Ridder D, Van de Heyning P. Cochlear implantation as a durable tinnitus treatment in single-sided deafness. Cochlear Implants Int. 2011;12 Suppl 1:S26–29. doi: 10.1179/146701011X13001035752336 [DOI] [PubMed] [Google Scholar]
- 13.Van de Heyning P, Vermeire K, Diebl M, Nopp P, Anderson I, De Ridder D. Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Ann Otol Rhinol Laryngol. 2008;117(9):645–652. doi: 10.1177/000348940811700903 [DOI] [PubMed] [Google Scholar]
- 14.Vermeire K, de Heyning PV. Binaural Hearing after Cochlear Implantation in Subjects with Unilateral Sensorineural Deafness and Tinnitus. AUD. 2009;14(3):163–171. doi: 10.1159/000171478 [DOI] [PubMed] [Google Scholar]
- 15.Friedmann DR, Ahmed OH, McMenomey SO, Shapiro WH, Waltzman SB, Roland JTJ. Single-sided Deafness Cochlear Implantation: Candidacy, Evaluation, and Outcomes in Children and Adults. Otology & Neurotology. 2016;37(2):e154. doi: 10.1097/MAO.0000000000000951 [DOI] [PubMed] [Google Scholar]
- 16.Dillon MT, Buss E, Rooth MA, et al. Effect of Cochlear Implantation on Quality of Life in Adults with Unilateral Hearing Loss. AUD. 2017;22(4–5):259–271. doi: 10.1159/000484079 [DOI] [PubMed] [Google Scholar]
- 17.Sladen DP, Frisch CD, Carlson ML, Driscoll CLW, Torres JH, Zeitler DM. Cochlear implantation for single-sided deafness: A multicenter study. The Laryngoscope. 2017;127(1):223–228. doi: 10.1002/lary.26102 [DOI] [PubMed] [Google Scholar]
- 18.Távora-Vieira D, Rajan GP, Van de Heyning P, Mertens G. Evaluating the Long-Term Hearing Outcomes of Cochlear Implant Users With Single-Sided Deafness. Otol Neurotol. 2019;40(6):e575–e580. doi: 10.1097/MAO.0000000000002235 [DOI] [PubMed] [Google Scholar]
- 19.Prejban DA, Hamzavi JS, Arnoldner C, et al. Single Sided Deaf Cochlear Implant Users in the Difficult Listening Situation: Speech Perception and Subjective Benefit. Otol Neurotol. 2018;39(9):e803–e809. doi: 10.1097/MAO.0000000000001963 [DOI] [PubMed] [Google Scholar]
- 20.Kamal SM, Robinson AD, Diaz RC. Cochlear implantation in single-sided deafness for enhancement of sound localization and speech perception. Curr Opin Otolaryngol Head Neck Surg. 2012;20(5):393–397. doi: 10.1097/MOO.0b013e328357a613 [DOI] [PubMed] [Google Scholar]
- 21.Buss E, Dillon MT, Rooth MA, et al. Effects of Cochlear Implantation on Binaural Hearing in Adults With Unilateral Hearing Loss. Trends Hear. 2018;22:2331216518771173. doi: 10.1177/2331216518771173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arts RAGJ, George ELJ, Stokroos RJ, Vermeire K. Review: cochlear implants as a treatment of tinnitus in single-sided deafness. Curr Opin Otolaryngol Head Neck Surg. 2012;20(5):398–403. doi: 10.1097/MOO.0b013e3283577b66 [DOI] [PubMed] [Google Scholar]
- 23.Peter N, Liyanage N, Pfiffner F, Huber A, Kleinjung T. The Influence of Cochlear Implantation on Tinnitus in Patients with Single-Sided Deafness: A Systematic Review. Otolaryngol Head Neck Surg. 2019;161(4):576–588. doi: 10.1177/0194599819846084 [DOI] [PubMed] [Google Scholar]
- 24.Arndt S, Aschendorff A, Laszig R, et al. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol. 2011;32(1):39–47. doi: 10.1097/MAO.0b013e3181fcf271 [DOI] [PubMed] [Google Scholar]
- 25.Härkönen K, Kivekäs I, Rautiainen M, Kotti V, Sivonen V, Vasama JP. Single-Sided Deafness: The Effect of Cochlear Implantation on Quality of Life, Quality of Hearing, and Working Performance. ORL. 2015;77(6):339–345. doi: 10.1159/000439176 [DOI] [PubMed] [Google Scholar]
- 26.Louza J, Hempel JM, Krause E, Berghaus A, Müller J, Braun T. Patient benefit from Cochlear implantation in single-sided deafness: a 1-year follow-up. Eur Arch Otorhinolaryngol. 2017;274(6):2405–2409. doi: 10.1007/s00405-017-4511-1 [DOI] [PubMed] [Google Scholar]
- 27.Noble W, Jensen NS, Naylor G, Bhullar N, Akeroyd MA. A short form of the Speech, Spatial and Qualities of Hearing scale suitable for clinical use: The SSQ12. Int J Audiol. 2013;52(6):409–412. doi: 10.3109/14992027.2013.781278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marx M, Mosnier I, Venail F, et al. Cochlear Implantation and Other Treatments in Single-Sided Deafness and Asymmetric Hearing Loss: Results of a National Multicenter Study Including a Randomized Controlled Trial. AUD. 2021;26(6):414–424. doi: 10.1159/000514085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwa TP, Sturm JJ, Losenegger T, et al. Impact of Underlying Diagnosis on Speech and Quality of Life Outcomes After Cochlear Implantation for Single-Sided Deafness. Otol Neurotol. 2020;41(4):e432–e440. doi: 10.1097/MAO.0000000000002578 [DOI] [PubMed] [Google Scholar]
- 30.Bojrab D, Hong RS. The case for cochlear implantation in unilateral and asymmetric sensorineural hearing loss. Curr Opin Otolaryngol Head Neck Surg. 2020;28(5):329–334. doi: 10.1097/MOO.0000000000000656 [DOI] [PubMed] [Google Scholar]
- 31.Van de Heyning P, Távora-Vieira D, Mertens G, et al. Towards a Unified Testing Framework for Single-Sided Deafness Studies: A Consensus Paper. Audiol Neurootol. 2016;21(6):391–398. doi: 10.1159/000455058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143–148. doi: 10.1001/archotol.1996.01890140029007 [DOI] [PubMed] [Google Scholar]
- 33.Zeman F, Koller M, Figueiredo R, et al. Tinnitus handicap inventory for evaluating treatment effects: which changes are clinically relevant? Otolaryngol Head Neck Surg. 2011;145(2):282–287. doi: 10.1177/0194599811403882 [DOI] [PubMed] [Google Scholar]
- 34.Luxford WM. Minimum Speech Test Battery for Postlingually Deafened Adult Cochlear Implant Patients. Otolaryngol Head Neck Surg. 2001;124(2):125–126. doi: 10.1067/mhn.2001.113035 [DOI] [PubMed] [Google Scholar]
- 35.Spahr AJ, Dorman MF, Litvak LM, et al. Development and validation of the AzBio sentence lists. Ear Hear. 2012;33(1):112–117. doi: 10.1097/AUD.0b013e31822c2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord. 1962;27:62–70. doi: 10.1044/jshd.2701.62 [DOI] [PubMed] [Google Scholar]
- 37.Rodgers JL, Nicewander WA. Thirteen Ways to Look at the Correlation Coefficient. The American Statistician. 1988;42(1):59. doi: 10.2307/2685263 [DOI] [Google Scholar]
- 38.Thornton AR, Raffin MJ. Speech-discrimination scores modeled as a binomial variable. J Speech Hear Res. 1978;21(3):507–518. doi: 10.1044/jshr.2103.507 [DOI] [PubMed] [Google Scholar]
- 39.Busch T, Vanpoucke F, van Wieringen A. Auditory Environment Across the Life Span of Cochlear Implant Users: Insights From Data Logging. Journal of Speech Language and Hearing Research. 2017;60(5):1362. doi: 10.1044/2016_JSLHR-H-16-0162 [DOI] [PubMed] [Google Scholar]
- 40.Sladen DP, Frisch CD, Carlson ML, Driscoll CLW, Torres JH, Zeitler DM. Cochlear Implantation for Single-Sided Deafness: A Multicenter Study. Laryngoscope. 2016;EPub:1-6. doi: 10.1002/lary.26102 [DOI] [PubMed] [Google Scholar]
- 41.Holder JT, Dwyer NC, Gifford RH. Duration of processor use per day is significantly correlated with speech recognition abilities in adults with cochlear implants. Otol Neurotol. 2020;41(2):e227–e231. doi: 10.1097/MAO.0000000000002477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schvartz-Leyzac KC, Conrad CA, Zwolan TA. Datalogging Statistics and Speech Recognition During the First Year of Use in Adult Cochlear Implant Recipients. Otology & Neurotology. 2019;40(7):e686–e693. doi: 10.1097/MAO.0000000000002248 [DOI] [PubMed] [Google Scholar]
- 43.Polonenko MJ, Papsin BC, Gordon KA. Children With Single-Sided Deafness Use Their Cochlear Implant. Ear and hearing. 38(6):681–689. doi: 10.1097/AUD.0000000000000452 [DOI] [PubMed] [Google Scholar]
- 44.Ganek HV, Cushing SL, Papsin BC, Gordon KA. Cochlear Implant Use Remains Consistent Over Time in Children With Single-Sided Deafness. Ear Hear. 2020;41(3):678–685. doi: 10.1097/AUD.0000000000000797 [DOI] [PubMed] [Google Scholar]
- 45.Rauch AK, Kagermann S, Wesarg T, et al. Data Logging Evidence of Cochlear Implant Use in Single-Sided and Bilateral Deafness. Audiol Neurootol. 2019;24(4):206–216. doi: 10.1159/000502051 [DOI] [PubMed] [Google Scholar]
- 46.Sullivan CB, Al-Qurayshi Z, Zhu V, et al. Long-term audiologic outcomes after cochlear implantation for single-sided deafness. The Laryngoscope. 2020;130(7):1805–1811. doi: 10.1002/lary.28358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holder JT, Gifford RH. Effect of Increased Daily Cochlear Implant Use on Auditory Perception in Adults. J Speech Lang Hear Res. 2021;64(10):4044–4055. doi: 10.1044/2021_JSLHR-21-00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holder JT, O’Connell B, Hedley-Williams A, Wanna G. Cochlear implantation for single-sided deafness and tinnitus suppression. Am J Otolaryngol. 2017;38(2):226–229. doi: 10.1016/j.amjoto.2017.01.020 [DOI] [PubMed] [Google Scholar]
- 49.Ahmed MFM, Khater A. Tinnitus suppression after cochlear implantation in patients with single-sided deafness. Egypt J Otolaryngol. 2017;33(1):61–66. doi: 10.4103/1012-5574.199404 [DOI] [Google Scholar]
- 50.Levy DA, Lee JA, Nguyen SA, McRackan TR, Meyer TA, Lambert PR. Cochlear Implantation for Treatment of Tinnitus in Single-sided Deafness: A Systematic Review and Meta-analysis. Otol Neurotol. 2020;41(8):e1004–e1012. doi: 10.1097/MAO.0000000000002711 [DOI] [PubMed] [Google Scholar]
- 51.Távora-Vieira D, Marino R, Acharya A, Rajan GP. The Impact of Cochlear Implantation on Speech Understanding, Subjective Hearing Performance, and Tinnitus Perception in Patients with Unilateral Severe to Profound Hearing Loss. Otology & Neurotology. 2015;36(3):430–436. doi: 10.1097/MAO.0000000000000707 [DOI] [PubMed] [Google Scholar]
- 52.McRackan TR, Hand BN, Cochlear Implant Quality of Life Development Consortium, Velozo CA, Dubno JR. Cochlear Implant Quality of Life (CIQOL): Development of a Profile Instrument (CIQOL-35 Profile) and a Global Measure (CIQOL-10 Global). J Speech Lang Hear Res. 2019;62(9):3554–3563. doi: 10.1044/2019_JSLHR-H-19-0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McRackan TR, Hand BN, Velozo CA, Dubno JR, Cochlear Implant Quality of Life Consortium. Validity and reliability of the Cochlear Implant Quality of Life (CIQOL)-35 Profile and CIQOL-10 Global instruments in comparison to legacy instruments. Ear Hear. 2021;42(4):896–908. doi: 10.1097/AUD.0000000000001022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McRackan TR, Hand BN, Chidarala S, Velozo CA, Dubno JR, Cochlear Implant Quality of Life Consortium. Normative Cochlear Implant Quality of Life (CIQOL)-35 Profile and CIQOL-10 Global Scores for Experienced Cochlear Implant Users from a Multi-Institutional Study. Otol Neurotol. 2022;43(7):797–802. doi: 10.1097/MAO.0000000000003596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernhard N, Gauger U, Romo Ventura E, et al. Duration of deafness impacts auditory performance after cochlear implantation: A meta-analysis. Laryngoscope Investigative Otolaryngology. 2021;6(2):291–301. doi: 10.1002/lio2.528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao EE, Dornhoffer JR, Loftus C, et al. Association of Patient-Related Factors With Adult Cochlear Implant Speech Recognition Outcomes: A Meta-analysis. JAMA Otolaryngol Head Neck Surg. 2020;146(7):613–620. doi: 10.1001/jamaoto.2020.0662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goudey B, Plant K, Kiral I, et al. A MultiCenter Analysis of Factors Associated with Hearing Outcome for 2,735 Adults with Cochlear Implants. Trends Hear. 2021;25:23312165211037524. doi: 10.1177/23312165211037525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nassiri AM, Wallerius KP, Saoji AA, Neff BA, Driscoll CLW, Carlson ML. Impact of Duration of Deafness on Speech Perception in Single-Sided Deafness Cochlear Implantation in Adults. Otol Neurotol. 2022;43(1):e45–e49. doi: 10.1097/MAO.0000000000003357 [DOI] [PubMed] [Google Scholar]