Abstract
Effects of seven alkaloids, geissoschizine methyl ether (GM), hirsutine, hirsuteine, rhynchophylline, isorhynchophylline, corynoxeine and isocorynoxeine, in Uncaria hook, a constituent of the kampo medicine yokukansan, on serotonin7 (5-HT7) receptor were investigated using Chinese hamster ovary (CHO) cell membranes and human embryonic kidney 293 (HEK293) cells stably expressing the human recombinant 5-HT7 receptor. A competitive binding assay using CHO membranes showed that GM (IC50 = 0.034 μM) more strongly inhibited the binding of the radioligand [3H] LSD to 5-HT7 receptor than the other alkaloids, suggesting that GM is bound to 5-HT7 receptor. Agonistic/antagonistic effects of GM (1–50 μM) on the receptor were evaluated by measuring intracellular cAMP levels in HEK239 cells. GM (IC50 = 6.0 μM) inhibited 5-HT-induced cAMP production in a concentration-dependent manner, as well as the specific 5-HT7 receptor antagonist SB-269970 (0.1–1 μM). However, GM did not induce intracellular cAMP production as 5-HT did. These results suggest that GM has an antagonistic effect on 5-HT7 receptor.
Keywords: Geissoschizine methyl ether, 5-HT7 receptor, cAMP, CHO cells, HEK293 cells
Introduction
Yokukansan is one of the traditional Japanese medicines called kampo medicines in Japan. It has been approved by the Ministry of Health, Labour, and Welfare of Japan as a remedy for neurosis, insomnia, and irritability in children. Yokukansan was reported to improve behavioral and psychologic symptoms such as hallucinations, agitation, and aggressiveness in patients with Alzheimer’s disease, dementia with Lewy bodies, and other forms of senile dementia (Iwasaki et al. 2005a, b; Mizukami et al. 2009; Monji et al. 2009).
Recently, various basic studies have been performed to clarify the effects of yokukansan. We demonstrated that yokukansan ameliorated aggressive and antisocial behaviors in rats treated with the serotonergic neurotoxin para-chloroamphetamine (Kanno et al. 2009) or socially isolated mice (Nishi et al. 2012), and that the ameliorative effect was counteracted by the co-administration of the serotonin1A (5-HT1A) receptor agonist WAY-100635. Further in vitro receptor binding assays demonstrated that yokukansan and a constituent herb, Uncaria hook (UH), bound to 5-HT1A receptors as a partial agonist (Terawaki et al. 2010). From these results, we hypothesized that the psychotropic effect of yokukansan might be due to UH functioning as a 5-HT1A receptor partial agonist, and these results also suggest that UH must contain the active components. More recently, we demonstrated by an in vitro binding assay that geissoschizine methyl ether (GM), which is one of the UH-derived alkaloids, had a potency toward 5-HT1A receptors (Nishi et al. 2012) that is similar to yokukansan and UH. GM ameliorated the isolation-induced aggressive and antisocial behaviors in mice, and the ameliorative effect was counteracted by a 5-HT1A antagonist, suggesting that GM might be one of the active components of the pharmacological effect of yokukansan (Nishi et al. 2012).
GM is an indole alkaloid that includes an indole structure similar to that of the neurotransmitter 5-HT. Recently, Ueda et al. (2009, 2011) developed an in vitro calcium imaging assay which demonstrated that GM behaved as a potent antagonist toward 5-HT7 receptor, in addition to having a partial agonistic effect toward 5-HT1A receptors. The 5-HT7 receptor is a G protein-coupled receptor linked to Gαs which activates adenylate cyclase and increases second messenger cAMP (Sunahara et al. 1996). This receptor does not link to an intracellular calcium mobilization ([Ca2+]i) system. That is, it is different from Gαq-linked G protein-coupled receptors like 5-HT2 receptors which activate inositol trisphosphate and then induce [Ca2+]i mobilization (Exton 1996). Thus, although it is generally impossible to evaluate intrinsic activity of 5-HT7 receptor by changes of [Ca2+]i mobilization, the developed method enabled it by transfection of Gα15 (Gα15 integrates into the downstream calcium flux) in human embryonic kidney (HEK) 293T cells which express human recombinant 5-HT7 receptor (Ueda et al. 2009, 2011). However, the receptor binding rate of the test substance is not clear in this method, and the measurement of the cAMP level is the most appropriate for the G protein-coupled receptors linked to Gαs and Gαi for direct and absolute evaluation.
Moreover, UH contains various indole and oxyindole alkaloids such as hirsuteine (HTE), hirsutine (HIT), rhynchophylline (RP), isorhynchophylline (IRP), corynoxeine (CX), and isocorynoxeine (ICX) besides GM. However, the effects of these alkaloids on 5-HT7 receptor, to date, remain still unclear.
The purpose of the present study was to clarify the effects of UH alkaloids on 5-HT7 receptor. To resolve the issue, competitive binding of the alkaloids to 5-HT7 receptor was first assayed using Chinese hamster ovary cell membranes (CHO-h5-HT7 cell membranes) stably expressing human recombinant 5-HT7 receptor. Next, the agonistic and antagonistic effects of these test substances on 5-HT7 receptor were directly or absolutely evaluated by the measurement of intracellular cAMP levels in HEK293 cells stably expressing human recombinant 5-HT7 receptor.
Materials and Methods
Drugs and Reagents
UH Alkaloids
GM, HTE, HIT, RP, IRP, CX, and ICX were supplied by the Botanical Raw Materials Research Department of Tsumura & Co. (Ibaraki, Japan).
Reagents for Competitive Binding Assay
CHO-h5-HT7 cell membranes were purchased from PerkinElmer (Waltham, MA, USA). Radioligand [3H]lysergic acid diethylamide (LSD) (NET638 80 Ci/mmol) was also purchased from PerkinElmer. 5-HT was purchased from Sigma-Aldrich (St. Louis, MO, USA). Other reagents used for the binding analysis were purchased from commercial sources.
Reagents for the Production of HEK293-h5-HT7 Cells Lines
A full-length 5-HT7 receptor plasmid (ID:IOH45473) in an Ultimate Human ORF clone, expression vector (pcDNA6.2/cTC-Tag-DEST), Dulbecco’s modified Eagle’s medium (DMEM), Lipofectamin2000 Transfection Reagent, and Blasticidin S were purchased from Life Technologies (Carlsbad, CA, USA). HEK293 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). Fetal bovine serum (FBS) was purchased from JRH Bioscience (Lenexa, KS, USA).
Reagents for Intracellular cAMP Assay
A cAMP-Glo assay kit was purchased from Promega (Madison, WI, USA). DMEM and dialyzed FBS were purchased from Life Technologies. The 96-well tissue culture plates were purchased from BD Bioscience (San Jose, CA, USA). Isobutyl-1-methylxanthine (IBMX), 4-(3-butoxy-4-methoxy-benzyl) imidazolidone (Ro 20-1724), 5-HT, forskolin, and SB-269970 were purchased from Sigma-Aldrich.
Competitive Binding Assay to 5-HT7 Receptor
Various concentrations of test compounds (GM, HTE, HTI, RP, IRP, CX, and ICX) were prepared by dissolving each in 1.0 % dimethyl sulfoxide (DMSO). A competitive binding assay for 5-HT7 receptor was carried out by the method previously described (Shen et al. 1993; Roth et al. 1994). In brief, 2 μl of the test compound solution or vehicle was incubated in duplicate with 200 μl of membrane solution (130 μg protein/ml) of CHO-h5-HT7 cells and 20 μl of 40 nM radioligand [3H] LSD (NET638 80 Ci/mmol) in the incubation buffer (50 mM Tris–HCl, pH 7.4, 0.5 mM ethylenediaminetetraacetic acid (EDTA), and 10 mM MgCl2) for 120 min at 25 °C. Non-specific binding was determined by adding 10 μM 5-HT. After incubation, 5-HT7 receptor-ligand complexes were isolated by rapid filtration through a Whatman GF/C filter using a cell harvester (FilterMate Universal Harvester, PerkinElmer). The trapped radioactive complexes were rinsed four times with ice-cold 50 mM Tris-HCl buffer and dried. Radioactivity (count per minute, cpm) trapped on the dried filter was measured using a liquid scintillation counter (Top Count NXT, PerkinElmer). The specific binding was determined by subtracting non-specific binding from total binding, and expressed as the percentage inhibition by the following formula: Inhibition (%) = [1 − (c − a)/(b − a)] × 100, where a is the average cpm of non-specific binding, b is the average cpm of total binding, and c is average cpm in the presence of the test substance. IC50 (50 % inhibition concentration of the specific binding) values of test substances were determined by a non-linear least squares regression analysis by means of MathIQTM (ID Business Solutions Ltd., Guildford, UK).
Construction of HEK293-h5-HT7 Cell Llines
The full-length human 5-HT7 receptor plasmid was subcloned into a mammalian expression vector, pcDNA6.2/cTC-Tag-DEST, using Gateway Technology (Marsischky and LaBaer 2004; Liang et al. 2004). This 5-HT7 receptor expression plasmid was transfected into HEK293 cells that were cultured in DMEM supplemented with 10 % FBS using Lipofectamin 2000 Transfection Reagent. Twenty-four hours later, the transfected cells were seeded in 10-cm diameter dishes containing 8 μg/ml Blasticidin S and then cultured for 2 weeks. The colonies formed by Blasticidin S-resistant cells were isolated and expanded and then 5-HT-mediated cAMP response cells were further screened using a cAMP-Glo Assay Kit. The HEK293-h5-HT7 cells obtained by the above procedure were maintained by culturing in the presence of 8 μg/ml Blasticidin S and were used to evaluate agonistic and antagonistic effects of test compounds to 5-HT7 receptor.
Agonist/Antagonist Assay for Test Compounds to 5-HT7 Receptor
HEK293-h5-HT7 cells were seeded in 96-well tissue culture plates at a density of 4,000 cells per well in DMEM supplemented with 0.5 % dialyzed FBS. The next day, agonist/antagonist assays of test compounds on 5-HT7 receptor were evaluated by measuring the intracellular cAMP level using a cAMP-Glo assay kit according to the manufacturer’s instructions (Shultz et al. 2008). Briefly, in the agonist assay, the culture medium was removed and 20 μL of induction buffer [1× phosphate-buffered saline (PBS)] including 500 μM IBMX and 100 μM Ro 20-1724, which are phosphodiesterase inhibitors to inhibit cAMP hydrolysis, and various concentrations of test compounds were added and incubated for 30 min at room temperature. After incubation, 20 μl of cAMP-Glo lysis buffer and 40 μl of cAMP detection solution containing protein kinase A (PKA) were added and incubated for 20 min at room temperature. After 80 μl of Kinase-Glo Reagent consisting of luciferin and luciferase was added, the mixture was further incubated for 10 min at room temperature. The luminescence was measured using an Infinite M200 plate reader (Tecan, Grödig, Austria).
Antagonism of the test substances was assayed by evaluating the inhibition of 5-HT-induced cAMP production, i.e., in the procedure described above, the HEK293-h5-HT7 cells were incubated with a mixture of the test compound and 100 nM 5-HT. If a test compound had an antagonistic effect, the 5-HT-induced increase in the intracellular cAMP level was inhibited.
In this assay, cAMP activates PKA, and the activated PKA phosphorylates the PKA substrate and reduces ATP. The luminescence produced by luciferin/luciferase activity is dependent on the amount of ATP. Therefore, cAMP production decreases the luminescence, creating an inverse relationship between luminescence and the amount of cAMP. If a test compound has an agonistic effect, the luminescence decreases due to an increase in the cAMP level. In contrast, if a test compound has an antagonistic effect, the luminescence increases due to inhibition of 5-HT-induced increase in cAMP level.
Statistical Analysis
Data on competitive binding assays were expressed as the mean value of duplicate determinations. Other data are presented as the mean ± SEM. The statistical significance of differences between groups was assessed by one-way analysis of variance (ANOVA), followed by Scheffé’s post hoc test. The significance level in each statistical analysis was accepted at P < 0.0001.
Results
Binding of Seven UH Components to 5-HT7 Receptor
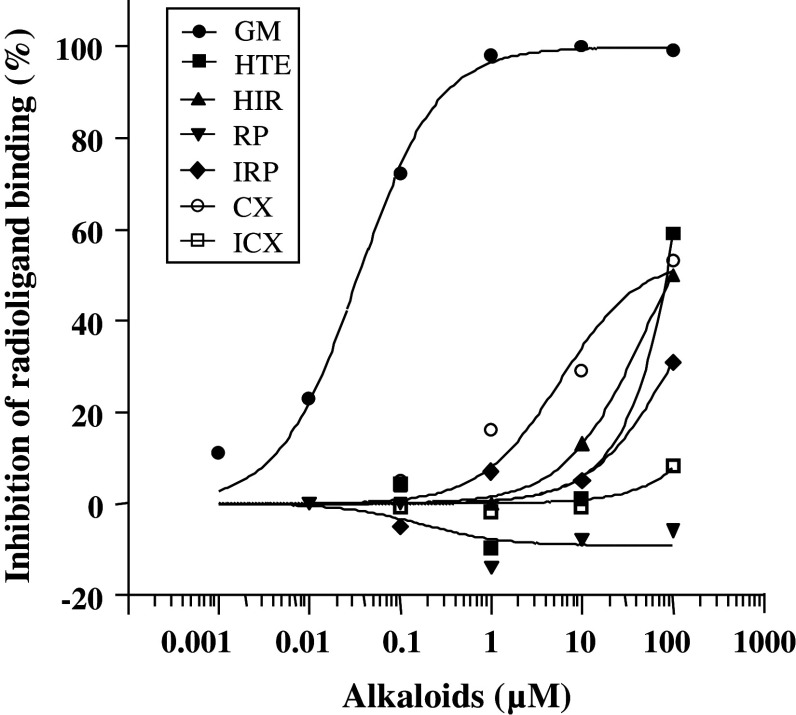
The concentration–response curves in the competitive binding assays of seven UH components against radioligand [3H] LSD binding to 5-HT7 receptor are shown in Fig. 1, and their IC50 values are shown in Table 1. Except for RP, IRP, and ICX, the alkaloids inhibited the radioligand binding to 5-HT7 receptor in a concentration-dependent manner. GM (0.034 μM) showed the highest affinity (IC50), followed by CX, HTE, and HTI. However, the potencies of the latter components (CX: 75.6 μM, HTE: 84.7 μM, HIT: 99.0 μM) were markedly lower than that in GM. On the other hand, RP, IRP, and ICX did not inhibit the radioligand binding to 5-HT7 receptor, i.e., the IC50 values of these constituents were not measurable at a concentration of 100 μM.
Fig. 1.
In vitro competitive binding assays of seven UH alkaloids to 5-HT7 receptor. Competitive binding of each compound was evaluated by the inhibition of [3H] LSD binding to 5-HT7 receptor in CHO cell membranes. Each data point represents the mean of duplicate determinations
Table 1.
IC50 values of seven UH components
| UH alkaloid | IC50 (μM) |
|---|---|
| GM | 0.034 |
| HTE | 84.7 |
| HIT | 99.0 |
| RP | – |
| IRP | – |
| CX | 75.6 |
| ICX | – |
GM geissoschizine methyl ether, HTE hirsuteine, HIT hirsutine, RP rhynchophylline, IRP isorhynchophylline, CX corynoxeine, ICX isocorynoxeine
–, IC50 values of these components were not able measurable at the concentration of 100 μM
Effects of 5-HT and Forskolin on cAMP Production in HEK293-h5-HT7 Cells
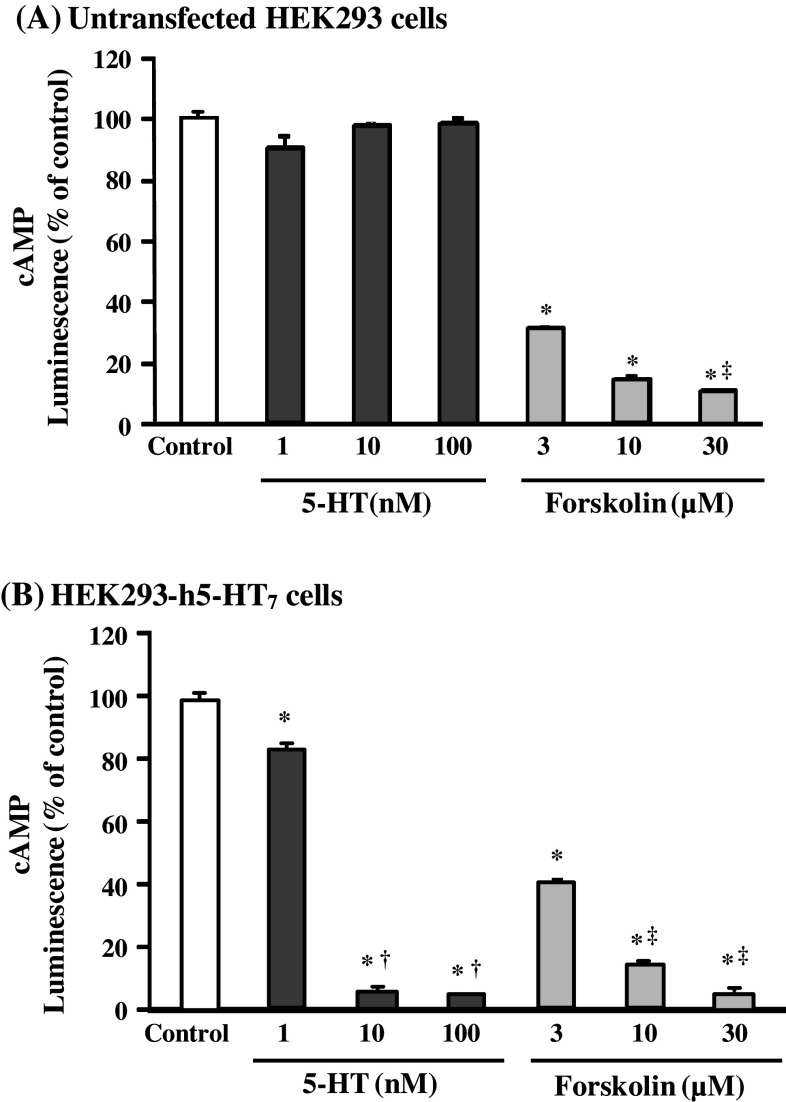
In order to clarify the agonistic/antagonistic effects of the seven UH components on 5-HT7 receptor, HEK293-h5-HT7 cells were used. Figure 2 shows the effects of 5-HT (1, 10, and 100 nM) and forskolin (3, 10, and 30 μM), which directly activates adenylyl cyclase and raises cAMP levels in a wide variety of cell types (Insel and Ostrom 2003), on the luminescence, which is decreased by cAMP production, in both untransfected HEK293 cells (non-receptor-expressing cells) and HEK293-h5-HT7 cells (receptor-expressing cells). In untransfected HEK293 cells (Fig. 2a), a significant change in luminescence was not observed after addition of 5-HT, but an intensity decrease (i.e., cAMP production) was observed after addition of forskolin. In receptor-expressing cells (Fig. 2b), the intensity decrease after the addition of 5-HT was observed in a concentration-dependent manner similar to that after the addition of forskolin.
Fig. 2.
Effects of 5-HT and forskolin on intracellular cAMP levels in untransfected cells (a) and 5-HT7 receptor-transfected HEK293 cells (HEK293-h5-HT7 cells) (b). In this assay, cAMP phosphorylates the PKA substrate and reduces ATP. Because the luminescence produced by luciferin/luciferase activity is dependent on the amount of ATP, cAMP production decreases the luminescence. Each value represents the mean ± SEM (n = 3). *P < 0.0001 versus control, † P < 0.0001 versus 1 nM 5-HT, ‡ P < 0.0001 versus 3 nM forskolin: one-way ANOVA + Scheffé’s test
Agonist/Antagonist Effects of Seven UH Alkaloids on 5-HT7 Receptor
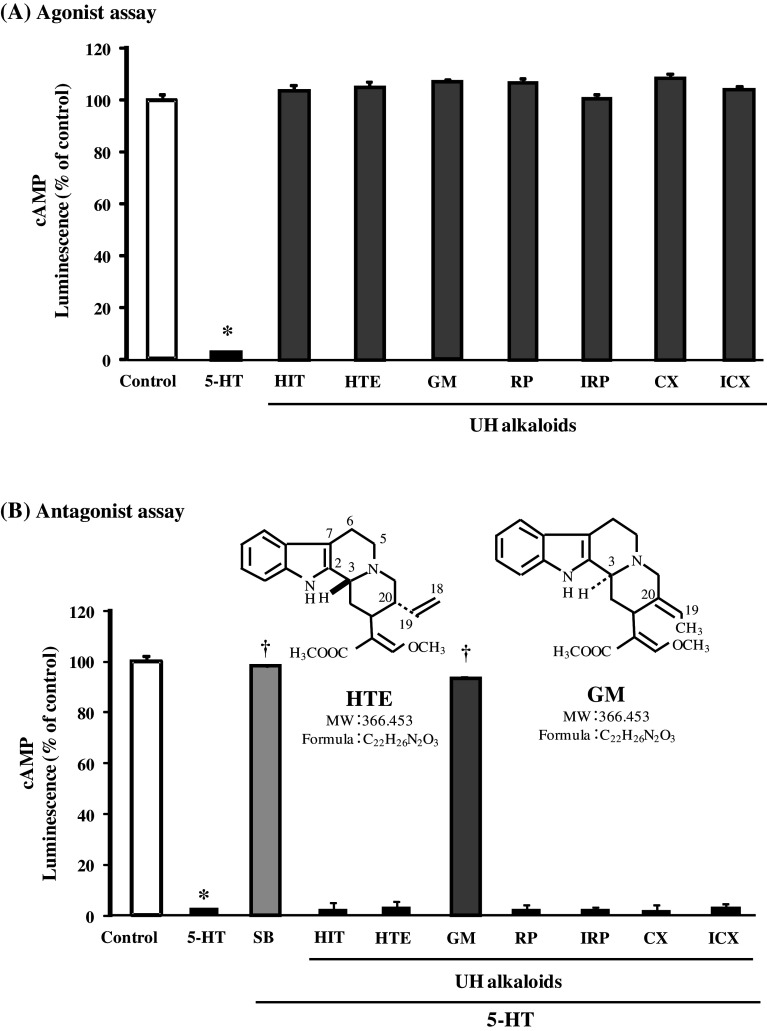
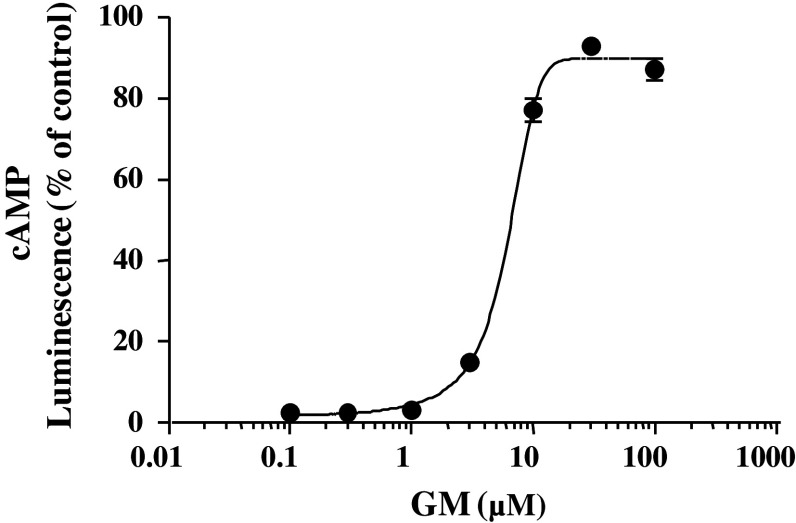
Figure 3 shows agonistic/antagonistic effects of seven UH alkaloids at a fixed concentration of 50 μM on 5-HT7 receptor in HEK293-h5-HT7 cells. Only 5-HT (100 nM) significantly decreased the luminescence (i.e., agonized cAMP production), while the seven alkaloids did not affect the intensity (Fig. 3a). On the antagonistic effect, 5-HT (100 nM) significantly decreased the luminescence, and only GM significantly ameliorated this 5-HT-induced decrease in intensity as well as a specific 5-HT receptor antagonist SB-269970 (1 μM) did (Fig. 3b). GM (0.1–100 μM) increased it in a concentration-dependent manner (Fig. 4). That is, GM blocked 5-HT-induced cAMP production. The IC50 value of GM was 6.0 μM.
Fig. 3.
Agonistic (a) and antagonistic (b) effects of seven UH alkaloids on 5-HT7 receptor. These effects were examined by the measurement of intracellular cAMP levels in HEK293-h5-HT7 cells. In this assay, cAMP phosphorylates the PKA substrate and reduces ATP. Because the luminescence produced by luciferin/luciferase activity is dependent on the amount of ATP, cAMP production decreases the intensity. The effects of each UH compound were examined at a fixed concentration (50 μM). Abbreviations: SB-269970 (SB), geissoschizine methyl ether (GM), hirsutine (HIT), hirsuteine (HTE), rhynchophylline (RP), corynoxeine (CX), isorhynchophylline (IRP), and isocorynoxeine (ICX). Each value represents the mean ± SEM (n = 3). *P < 0.0001 versus control, † P < 0.0001 versus 5-HT: one-way ANOVA + Scheffé’s test
Fig. 4.
Concentration-dependent antagonistic responses of GM to 5-HT7 receptor. These responses were examined by the measurement of intracellular cAMP levels in HEK293-h5-HT7 cells. In this assay, cAMP phosphorylates the PKA substrate and reduces ATP. Because the luminescence produced by luciferin/luciferase activity is dependent on the amount of ATP, cAMP production decreases the luminescence. Each value represents the mean ± SEM (n = 3)
In untransfected HEK293 cells, the seven alkaloids did not affect the luminescence (data not shown).
Discussion
In the competitive binding study using CHO-h5-HT7 cells, GM strongly inhibited the radioligand binding to 5-HT7 receptor compared with the other six alkaloids, suggesting that this compound strongly binds to 5-HT7 receptor. This study initially showed the comparative potencies of UH alkaloids to the 5-HT7 receptor, a G protein-coupled receptor. This receptor is positively coupled to adenylate cyclase, preferentially via Gs (Vanhoenacker et al. 2000; Hedlund and Sutcliffe 2004). The pharmacological profile of binding to the 5-HT7 receptor is characterized by high affinity for the 5-HT1 agonists 5-carboxyamidotryptamine (5-CT), 5-HT, and 8-hydroxy-2-(di-n-propylamino) tetraline (8-OH-DPAT), and the 5-HT2 receptor antagonists ritanserine, metergoline, mesulergine, and risperidone (Heidmann et al. 1997; Krobert et al. 2001; Bonaventure et al. 2002, Pittala and Pittala 2011). More recently, we demonstrated that GM had an agonistic effect on 5HT1A receptors (Nishi et al. 2012). Ueda et al. (2011) reported that GM also had an antagonistic effect on 5-HT2A receptors. These findings also support the hypothesis that GM has a high affinity for 5-HT7 receptor.
To examine the agonistic/antagonistic effects of UH alkaloids including GM, these effects were directly evaluated by the measurement of intracellular cAMP levels in HEK293-h5-HT7 cells, which stably express human recombinant 5-HT7 receptor. As shown in Fig. 2a, forskolin induced intracellular cAMP production in untransfected cells, although 5-HT did not. Therefore, the present results suggest that HEK293 cells are primarily equipped with Gs-AC complexes to induce cAMP, though 5-HT7 receptor is not expressed. On the other hand, as shown in Fig. 2b, 5-HT induced intracellular cAMP production in the receptor-expressing cells, suggesting that our technique for transfecting 5-HT7 receptor, which linked to Gs–AC complexes in HEK239 cells, was appropriate.
In the 5-HT7 receptor-expressing cells, only GM among the seven UH alkaloids had an antagonistic effect. GM, HTE, and HTI are indole alkaloids. On the other hand, RP, IRP, CX, and ICX are oxyindole alkaloids, which have carbonyl group at the C12 position of the five-membered ring of the indole alkaloid structure. The oxyindole alkaloids showed no antagonistic activity at all. An interesting point is that GM showed strong antagonistic activity, but HTE had none at all though both components have the same chemical composition (C22H26N2O3). As shown in Fig. 3b, the conformations at C3 and the side chain at C20 (GM has a double bond in C19-20, and HTE has it in C18-19) are different. This steric difference might be related to the binding to and activity with 5-HT7 receptor.
The antagonistic effect of GM is suggested to be not a direct effect on AC like forskolin because no agonistic/antagonistic effects of GM were observed in untransfected cells. That is, the inhibition of 5-HT-induced cAMP production by GM is suggested to be due to the specific antagonistic binding to 5-HT7 receptor.
The physiologic functions of 5-HT7 receptor are not fully understood. However, several studies of several vascular preparations suggest an involvement in relaxation (Bard et al. 1993; Shen et al. 1993) and circadian rhythm control (Lovenberg et al. 1993; Gannon 2001). Hedlund and Sutcliffe (2004) also suggested important functional roles for the 5-HT7 receptor in thermoregulation, circadian rhythm, learning and memory, hippocampal signaling, and sleep. In addition, because atypical antipsychotics, such as clozapine and risperidone, and some antidepressants display high affinity for the 5-HT7 receptor as antagonists, this receptor blockade effect may be involved in antipsychotic or antidepressant actions (Eglen et al. 1997; Vanhoenacker et al. 2000; Bonaventure et al. 2002; Leopoldo et al. 2011).
Yokukansan and its components were also demonstrated to have a potent vasorelaxant effect in the isolated rat thoracic aorta (Yuzurihara et al. 2002) and an ameliorative effect in rapid eye movement sleep behavior disorder in humans (Shinno et al. 2008), which is related to circadian rhythm control. Yokukansan and its component GM have been demonstrated to ameliorate isolation-induced aggressiveness and sociality/anxiety in mice (Nishi et al. 2012). Such effects of yokukansan are interpreted to be instrumental in the 5-HT1A antagonistic effect to induce inhibition of the intracellular cAMP levels by Gi activation by GM. In this study, GM also decreased intracellular cAMP levels by the inhibition of 5-HT7 receptor-coupled Gs protein. These findings suggest a possibility that the 5-HT7 antagonistic effect of GM also is partly related to the pharmacological effect of yokukansan although the details require investigation in the future.
We recently detected GM in the plasma and brain of rats orally administered yokukansan and thus demonstrated that GM was able to cross the blood-brain barrier (BBB) in an in vitro BBB assay (Imamura et al. 2011). These in vivo and in vitro results suggest that GM in yokukansan administered orally is absorbed into the blood and then reaches the brain through the BBB, and further supports the hypothesis that GM is an active component in the psychotropic effect of yokukansan.
In conclusion, we directly demonstrated that GM had an antagonistic effect on 5-HT7 receptor using a combination of a competitive binding assay and measurement of the intracellular cAMP in human recombinant 5-HT7 receptor-expressing cells. Our present data support the previous results obtained by a calcium-imaging assay.
References
- Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshanl RL (1993) Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem 268:23422–23426 [PubMed] [Google Scholar]
- Bonaventure P, Nepomuceno D, Kwok A, Chai W, Langlois X, Hen R, Stark K, Carruthers N, Lovenberg TW (2002) Reconsideration of 5-hydroxytryptamine (5-HT)7 receptor distribution using [3H]5-carboxamidotryptamine and [3H]8-hydroxy-2-(di-n-propylamino) tetraline: analysis in brain of 5-HT1A knockout and 5-HT1A/1B double-knockout mice. J Pharmacol Exp Ther 302:240–248 [DOI] [PubMed] [Google Scholar]
- Eglen RM, Jasper JR, Chang DJ, Martin GR (1997) The 5-HT7 receptor: orphan found. Trends Pharmacol Sci 18:104–107 [DOI] [PubMed] [Google Scholar]
- Exton JH (1996) Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annu Rev Pharmacol Toxicol 36:481–509 [DOI] [PubMed] [Google Scholar]
- Gannon RL (2001) 5-HT7 receptors in the rodent suprachiasmatic necleus. J Biol Rhythms 16:19–24 [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Sutcliffe JG (2004) Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol Sci 25:481–486 [DOI] [PubMed] [Google Scholar]
- Heidmann DE, Metcalf MA, Kohen R, Hamblin MW (1997) Four 5-hydroxytryptamine7 (5-HT7) receptor isoforms in human and rat produced by alterative splicing species differences due to altered intron-exon organization. J Neurochem 68:1372–1381 [DOI] [PubMed] [Google Scholar]
- Imamura S, Tabuchi M, Kushida H, Nishi A, Kanno H, Yamaguchi T, Sekiguchi K, Ikarashi Y, Kase Y (2011) The blood-brain barrier permeability of geissoschizine methyl ether in Uncaria hook, a galenical constituent of the traditional Japanese medicine yokukansan. Cell Mol Neurobiol 31:787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel PA, Ostrom RS (2003) Forskolin as a tool for examining adenyalyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol 23:305–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K, Satoh-Nakagawa T, Maruyama M, Monma Y, Nemoto M, Tomita N, Tanji H, Fujiwara H, Seki T, Fujii M, Arai H, Sasaki H (2005a) A randomized, observer-blind, controlled trial of the traditional Chinese medicine Yi-Gan San for improvement of behavioral and psychological symptoms and activities of daily living in dementia patients. J Clin Psychiatry 66:248–252 [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Maruyama M, Tomita N, Furukawa K, Nemoto M, Fujiwara H, Seki T, Fujii M, Kodama M, Arai H (2005b) Effects of the traditional Chinese herbal medicine Yi-Gan San for cholinesterase inhibitor-resistant visual hallucinations and neuropsychiatric symptoms in patients with dementia with Lewy bodies. J Clin Psychiatry 66:1612–1613 [DOI] [PubMed] [Google Scholar]
- Kanno H, Sekiguchi K, Yamaguchi T, Terawaki K, Yuzurihara M, Kase Y, Ikarashi Y (2009) Effect of yokukansan, a traditional Japanese medicine, on social and aggressive behaviour of para-chloroamphetamine-injected rats. J Pharm Pharmacol 61:1249–1256 [DOI] [PubMed] [Google Scholar]
- Krobert KA, Bach T, Syversveen T, Kvingedal AM, Levy FO (2001) The cloned human 5-HT7 receptor splice variants: a comparative characterization of their pharmacology, function, and distribution. Naunyn-Schmiedeberg’s Arch Pharmacol 363:620–632 [DOI] [PubMed] [Google Scholar]
- Leopoldo M, Lacivita E, Berardi F, Perrone R, Hedlund PB (2011) Serotonin 5-HT7 receptor agents: structure–activity relationships and potential therapeutic applications in central nervous system disorders. Pharmacol Ther 129:120–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Udayakumar M, Parvizi B, Yen J, Duan D, Mirchandani J, Hashima S, Nguyen U, Ubil E, Loewenheim J, Yu X, Sipes S, Williams W, Wang L, Bennett R, Carrino J (2004) ORFDB: an information resource linking scientific content to a high-quality open reading frame (ORF) collection. Nucl Acids Res 32:D595–D599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW (1993) A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11:449–458 [DOI] [PubMed] [Google Scholar]
- Marsischky G, LaBaer J (2004) Many paths to many clones: a comparative look at high-throughput cloning methods. Genome Res 14:2020–2028 [DOI] [PubMed] [Google Scholar]
- Mizukami K, Asada T, Kinoshita T, Tanaka K, Sonohara K, Nakai R, Yamaguchi K, Hanyu H, Kanaya K, Takao T, Okada M, Kudo S, Kotoku H, Iwakiri M, Kurita H, Miyamura T, Kawasaki Y, Omori K, Shizaki K, Odawara T, Suzuki T, Yamada S, Nakamura Y, Toba K (2009) A randomized cross-over study of a traditional Japanese medicine (kampo), yokukansan, in the treatment of the behavioural and psychological symptoms of dementia. Int J Neuropsychopharmacol 12:191–199 [DOI] [PubMed] [Google Scholar]
- Monji A, Takita M, Samejima T, Takaishi T, Hashimoto K, Matsunaga H, Oda M, Sumida Y, Mizoguchi Y, Kato T (2009) Effect of yokukansan on the behavioral and psychological symptoms of dementia in elderly patients with Alzheimer’s disease. Prog Neuropharmacol Biol Psychiatry 33:308–311 [DOI] [PubMed] [Google Scholar]
- Nishi A, Yamaguchi T, Sekiguchi K, Imamura S, Tabuchi M, Kanno H, Nakai Y, Hashimoto K, Ikarashi Y, Kase Y (2012) Geissoschizine methyl ether, an alkaloid in Uncaria hook, is a potent serotonin1A receptor agonist and candidate for amelioration of aggressiveness and sociality by yokukansan. Neuroscience 207:124–136 [DOI] [PubMed] [Google Scholar]
- Pittala V, Pittala D (2011) Latest advances towards the discovery of 5-HT(7) receptor ligands. Mini Rev Med Chem 11:1108–1121 [DOI] [PubMed] [Google Scholar]
- Roth BL, Craigo SC, Choudhary MS, Uluer A, Monsma FJ Jr, Shen Y, Meltzer HY, Sibley DR (1994) Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J Pharmacol Exp Ther 268:1403–1410 [PubMed] [Google Scholar]
- Shen Y, Monsma FJ Jr, Metcalf MA, Jose PA, Hamblin MW, Sibley DR (1993) Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J Biol Chem 268:18200–18204 [PubMed] [Google Scholar]
- Shinno H, Kamei M, Nakamura Y, Inami Y, Horiguchi J (2008) Successful treatment with Yi-Gan San for rapid eye movement sleep behavior disorder. Prog Neuropsychopharmacol Biol Psychiatry 32:1749–1751 [DOI] [PubMed] [Google Scholar]
- Shultz S, Worzella T, Gallagher A, Shieh J, Goueli S, Hsiao K, Vidugiriene J (2008) Miniaturized GPCR signaling studies in 1536-well format. J Biomol Tech 19:267–274 [PMC free article] [PubMed] [Google Scholar]
- Sunahara RK, Dessauer CW, Gilman AG (1996) Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol 36:461–480 [DOI] [PubMed] [Google Scholar]
- Terawaki K, Ikarashi Y, Sekiguchi K, Nakai Y, Kase Y (2010) Partial agonistic effect of yokukansan on human recombinant serotonin 1A receptors expressed in the membranes of Chinese hamster ovary cells. J Ethnopharmacol 127:306–312 [DOI] [PubMed] [Google Scholar]
- Ueda T, Ugawa S, Ishida Y, Hondoh A, Shimada S (2009) Development of generic calcium imaging assay for monitoring Gi-coupled receptors and G-protein interaction. J Biomol Screen 14:781–788 [DOI] [PubMed] [Google Scholar]
- Ueda T, Ugawa S, Ishida Y, Shimada S (2011) Geissoschizine methyl ether has third-generation antipsychotic-like actions at the dopamine and serotonin receptors. Eur J Pharmacol 671:79–86 [DOI] [PubMed] [Google Scholar]
- Vanhoenacker P, Haegeman G, Leysen JE (2000) 5-HT7 receptors: current knowledge and future prospects. Trends Pharmacol Sci 21:70–77 [DOI] [PubMed] [Google Scholar]
- Yuzurihara M, Ikarashi Y, Goto K, Sakakibara I, Hayakawa T, Sasaki H (2002) Geissoschizine methyl ether, an indole alkaloid extracted from Uncaiae Ramulus et Uncus, is a potent vasorelaxant of isolated rat aorta. Eur J Pharmacol 444:183–189 [DOI] [PubMed] [Google Scholar]