Abstract
Here we provide the first report of protection against a vaginal challenge with a highly virulent simian immunodeficiency virus (SIV) by using a vaccine vector. New poliovirus vectors based on Sabin 1 and 2 vaccine strain viruses were constructed, and these vectors were used to generate a series of new viruses containing SIV gag, pol, env, nef, and tat in overlapping fragments. Two cocktails of 20 transgenic polioviruses (SabRV1-SIV and SabRV2-SIV) were inoculated into seven cynomolgus macaques. All monkeys produced substantial anti-SIV serum and mucosal antibody responses. SIV-specific cytotoxic T-lymphocyte responses were detected in three of seven monkeys after vaccination. All 7 vaccinated macaques, as well as 12 control macaques, were challenged vaginally with pathogenic SIVmac251. Strikingly, four of the seven vaccinated animals exhibited substantial protection against the vaginal SIV challenge. All 12 control monkeys became SIV positive. In two of the seven SabRV-SIV-vaccinated monkeys we found no virological evidence of infection following challenge, indicating that these two monkeys were completely protected. Two additional SabRV-SIV-vaccinated monkeys exhibited a pronounced reduction in postacute viremia to <103 copies/ml, suggesting that the vaccine elicited an effective cellular immune response. Three of six control animals developed clinical AIDS by 48 weeks postchallenge. In contrast, all seven vaccinated monkeys remained healthy as judged by all clinical parameters. These results demonstrate the efficacy of SabRV as a potential human vaccine vector, and they show that the use of a vaccine vector cocktail expressing an array of defined antigenic sequences can be an effective vaccination strategy in an outbred population.
The current human immunodeficiency virus (HIV) pandemic has affected a cumulative total approaching 40 million people, and the search for an AIDS vaccine continues. Live viral vectors are leading candidates in the hunt for a potential vaccine. Several viral vectors have showed promise in simian immunodeficiency virus (SIV) protection experiments in monkeys (11, 18, 53, 58), and numerous other viral vector systems are in earlier testing phases of vaccine development (8, 12, 14, 71, 73).
Poliovirus is an attractive live viral vector for several reasons. The Sabin live poliovirus vaccine is one of the best human vaccines in the world. It produces long-lasting immunity (59, 75) and herd immunity (75); it is very safe and easy to experimentally manipulate (47); it has a proven safety and efficacy record in over 1 billion vaccinees (75); it is inexpensive to produce and to distribute in developing countries (29); and, most importantly, it produces a potent mucosal immune response (51, 56, 79). The capacity of poliovirus to generate a strong mucosal immune response is particularly important given that more than 90% of HIV type 1 (HIV-1) infections worldwide have occurred via sexual transmission (77). Any strategy to control the AIDS pandemic must include a vaccine that prevents sexual transmission of HIV-1.
With the exception of live-attenuated viruses (which are generally considered too pathogenic for use in humans [7, 68]), no candidate AIDS vaccine has been demonstrated to consistently provide protection against mucosal challenge with a highly virulent simian immunodeficiency virus (SIV). Direct inoculation of a subunit vaccine into the iliac lymph nodes of macaques did provide protection against a rectal mucosal challenge with a virulent SIV (34), although that subunit vaccine was unable to consistently protect against infection after a vaginal challenge with the highly virulent SIVmac251 (40). Those experiments suggest that generating local mucosal immunity may be as important as other characteristics of the anti-SIV immune response generated by candidate vaccines.
More vaginal challenge experiments need to be done, because an AIDS vaccine needs to protect against vaginal-penile sexual transmission of HIV. At this point in time, there have been relatively few SIV vaginal-challenge experiments, and no vaccine vector has been demonstrated to provide any protection against a vaginal challenge.
We have previously reported the development of a recombinant poliovirus live viral vector system in which we inserted an immunogenic gene fragment of interest at the junction between the genes encoding the capsid proteins and the nonstructural proteins (the P1/P2 junction) in the poliovirus polyprotein reading frame (76). The gene fragment is expressed with the rest of the poliovirus genome as part of the polyprotein and is cleaved away from the polyprotein via the activity of poliovirus-encoded protease 2Apro, which cleaves at engineered proteolytic sites flanking the insert (76). That recombinant poliovirus live viral vector was tested in mice susceptible to poliovirus infection and was demonstrated to elicit strong antibody (76) and cytotoxic-T-lymphocyte (42, 72) responses.
In a study in which we immunized four cynomolgus macaques with two recombinant polioviruses expressing SIV antigens, we further demonstrated that poliovirus vectors are immunogenic in primates. Significant humoral, mucosal, and cellular anti-SIV immune responses were elicited (15). Notably, all macaques generated a mucosal anti-SIV immunoglobulin A (IgA) antibody response in rectal secretions, and strong anti-SIV serum IgG antibody responses lasting for at least 1 year were detected in two of the four monkeys (15).
Here we report the development of Sabin vaccine-based vectors that we used to produce a defined series of SabRV1- and SabRV2-SIV viruses containing SIV gag, pol, env, nef, and tat in overlapping fragments. We then evaluated the safety, immunogenicity, and protective efficacy of the SabRV1-SIV–SabRV2-SIV candidate SIV vaccine. Strikingly, four of seven vaccinated monkeys showed substantial protection against SIV viremia after a vaginal challenge with the highly virulent SIVmac251. Two of those vaccinated animals appear to have been completely protected by the SabRV1-SabRV2-SIV vaccine; all seven of the vaccinated monkeys have remained healthy for over 48 weeks postchallenge, while three of six control monkeys developed clinical AIDS. These results suggest that a Sabin-based viral vector may be a promising approach for developing a vaccine for the prevention of mucosal transmission of HIV.
MATERIALS AND METHODS
Plasmids.
The Sabin 1 plasmid (which we call pS1 for simplicity) was kindly provided by A. Nomoto [construct pVS(1)IC-0 (25, 33, 57)]. The entire Sabin 1 cDNA in pS1 was sequenced in our laboratory by the fluorescent dye terminator method, using an ABI 310 machine (Perkin-Elmer Applied Biosystems, Foster City, Calif.). The accuracy of the genome sequence as published (54) was confirmed. To construct pSabRV1, first the EcoRI and XhoI sites upstream of the T7 promoter of pS1 were eliminated by inserting a SalI linker (oligonucleotides C and D) at that position to create plasmid pS1XT. Then the 747-bp BstEII fragment of pMoV2.11—containing the duplicated 2Apro cleavage site, the 5-glycine spacer, and the EcoRI, NotI, and XhoI cloning sites—was swapped into pS1XT to create pSabRV1. The accuracy of the pSabRV1 construct was confirmed by restriction digestion and sequencing of the DNA. This DNA swap between pMoV2.11 and Sabin 1 results in Sabin 1 gaining three wild-type (wt) coding changes in 2A and one in 2B. None of the changes is associated with neurovirulence or other wt phenotypes. All pSabRV1 plasmids contain an Ampr selectable marker. Plasmids pS1, pS1XT, and pSabRV1 were electroporated into SURE cells (Stratagene, La Jolla, Calif.), which were plated on Luria-Bertani (LB) plus ampicillin agar plates and incubated for 20 to 24 h at 37°C. Single colonies were then inoculated into 50-ml cultures of Luria broth plus ampicillin (50 μg/ml) and grown at 30°C for 16 to 18 h. (Note that growth conditions for the Sabin 1 derivative plasmids [pS1, pS1XT, and pSabRV1] are specific because rearrangements of the plasmids and very low plasmid yields are frequently seen otherwise.) Plasmid DNA was isolated from cells by the QiaFilter Midiprep technique (Qiagen, Santa Clarita, Calif.).
For pSabRV1-SIV clones, SIVmac239 plasmids p239SpSp5′, p239SpE3′, and pSIV239opennef (obtained from the AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases, courtesy of Ronald Desrosiers [32]) were used as the PCR template to generate SIV inserts. Inserts were amplified by using Pfu Turbo high-fidelity DNA polymerase under the conditions recommended by the manufacturer (Stratagene). A complete table of the 40 oligonucleotides used for these reactions is available on request. PCR fragments were purified on Qiaquick spin columns; digested with the DpnI restriction enzyme (to eliminate any input SIV plasmid carried over), EcoRI, and XhoI; and then purified on a Qiaquick spin column a second time. Plasmid vector pSabRV1 was cut with EcoRI and XhoI, Qiaquick spin column purified, and then quantified by agarose gel electrophoresis. Gel purification of the vector was generally avoided. SIV inserts were ligated into pSabRV1 overnight at 16°C by using T4 DNA ligase (New England Biolabs, Beverly, Mass.) in a reaction mixture containing 25 ng of pSabRV1 and 20 ng of SIV insert DNA. Ligation products were dialyzed on 13-mm-diameter, 0.025-μm-pore-size VSWP membranes (Millipore, Bedford, Mass.) against 50 ml of deionized H2O for 10 min. Then 1 μl of the ligation product was electroporated into 25 μl of SURE cells in a 0.1-cm-light-path cuvette (BTX ElectroCell 600; 129 Ω, 1,400 V, 5-ms pulse). One milliliter of Luria broth was immediately added to the cuvette, and 20- to 200-μl volumes of electroporated SURE cells were plated on LB plus ampicillin plates and incubated at 37°C overnight. Further culturing and DNA isolation were performed as described above. All plasmid clones were analyzed by restriction digestion, and all inserts were DNA sequenced in their entirety to confirm that the appropriate clone had been obtained and was not mutated.
Sabin 2 early-passage virus (SO + 3) was kindly provided by K. Chumakov. HeLa cells were infected with Sabin 2 at a multiplicity of infection (MOI) of >1 and incubated at 37°C. Cells were harvested at 9 h postinfection; RNA was extracted from the cells by using an RNeasy kit (Qiagen), and cDNA was synthesized by using randomly primed Superscript II reagent (Life Technologies, Gaithersburg, Md.). Using XL polymerase (Perkin-Elmer Cetus, Emeryville, Calif.), 2 mM magnesium acetate, and 500 μM each deoxyribonucleoside triphosphate, full-length Sabin 2 was PCR amplified with primers SAB21 and SAB24 for 30 cycles consisting of 3 min at 94°C and 8 min at 65°C. The full-length Sabin 2 genome was then Qiaquick spin column purified, digested with SalI and HindIII, and ligated into SalI- and HindIII-digested pUC18. Ligations were introduced into chemically competent Escherichia coli DH5α cells as recommended by the manufacturer (Life Technologies). Plasmid minipreps of clones were analyzed by restriction digestion and tested for the ability to produce infectious virus. The three plasmid clones that produced virus (pS2-2, pS2-3, and pS2-10) were sequenced, and their genome sequences were compared with the Sabin 2 consensus sequence generated by Pollard et al. (63). Two coding mutations in pS2-10 were identified, one in Vp2 and one in 3C. The latter was corrected by swapping the 374-bp BsiWI-NcoI DNA fragment from a clone (pS2-3) with no 3C mutation into pS2-10 to create pS2-10F. We have since fixed the other coding mutation in pSabRV2 (nucleotide [nt] 1492, amino acid 249, F to L in Vp2) by site-directed mutagenesis and checked the resulting plasmid, pSabRV2.2, by DNA sequencing. Viruses derived from pS2-10, pS2-10F, pSabRV2, and pSabRV2.2 all grow in a manner identical to that of Sabin 2 as determined by plaque assay.
To generate pSabRV2, the 60-bp cloning site—which contains a 5-glycine spacer and AvrII and NotI restriction sites flanked by 2Apro cleavage sites (only the 5′ [or N-terminal] cleavage site is counted in the 60 bp, since it contains modified codon usage [76] and the 3′ [or C-terminal] cleavage site is endogenous and essential)—was cloned into pS2-10F. A BstEII-SnaBI fragment containing the unique SabRV2 cloning sites was generated by overlapping PCR of DNA fragments B and S and subsequent digestion with BstEII and SnaBI. DNA fragment B was achieved by PCR (Pfu Turbo; Stratagene) with oligonucleotides B1 and B2. Similarly, DNA fragment S was generated by PCR with oligonucleotides S1 (63 nt long, 45 nt of which overlap with oligonucleotide B2, with S1 and B2 together containing the full pSabRV2 cloning site) and S2. Both PCR fragments were gel purified with a Qiagen Qiaquick spin column and used as a template, together with oligonucleotides B1 and S2, in an overlapping PCR to generate a 1,635-bp fragment containing the 60-bp SabRV2 cloning site flanked by the BstEII and SnaBI restriction sites. The digested BstEII-SnaBI fragment was ligated into BstEII- and SnaBI-digested pS2-10F to create pSabRV2. Viruses derived from pS2-10, pS2-10F, pSabRV2, and pSabRV2.2 all grow identically to Sabin 2 as determined by plaque assay (Fig. 1C and data not shown).
FIG. 1.
(A) Recombinant Sabin poliovirus vector plasmid clones. Grey boxes indicate 2A proteolytic cleavage sites (GLTTY/GFGH). In both the pSabRV1 and pSabRV2 vectors, the first proteolytic-cleavage-site coding sequence is followed by a 5-glycine spacer (not marked) and, immediately prior to the second proteolytic cleavage site, the in-frame cloning sites (white boxes). In total, an additional 60 to 70 nt are added to the viral genome to create the vector. (B) Plaque assay of cloned Sabin 1 virus (pS1 derived) and the Sabin 1 recombinant virus vector (SabRV1) at 32°C. (C) Plaque assay of cloned Sabin 2 (pS2–10F derived) and the Sabin 2 recombinant virus vector (SabRV2) at 37°C. (D) SabRV1-SIV plaque assays. The titers of all SabRV1-SIV viruses made were determined by plaque assay. Growth of several representative viruses at 32°C is shown here. SabRV1 without an insert is shown as a control. (E) SabRV2-SIV plaque assays. The titers of all SabRV2-SIV virus constructs were determined by plaque assay. Growth of several representative viruses at 37°C is shown here. SabRV2 without an insert is shown as a control. (F) Library schematic. Map of SIV antigens used in SabRV1-SIV and SabRV2-SIV vaccine cocktails. Each of the numerically labeled fragments corresponds to the different SabRV-SIV constructs defined in Table 1.
For pSabRV2-SIV cloning, SIV PCR fragments were generated as described above (using similar oligonucleotides; a complete list of all 42 oligonucleotides is available on request), and cloning was done in a manner similar to that used for pSabRV1-SIVs, except that AvrII-NotI digestions were employed and XL1-Blue cells (Stratagene) were used for transformations. Stocks of pSabRV2-SIV plasmids were made by inoculating single colonies of transformed XL1-Blue cells (grown overnight on LB plus ampicillin plates) into 5-ml cultures of Luria broth plus ampicillin (50 μg/ml) and grown at 37°C for 8 to 14 h. Plasmid DNA was isolated from cells by the Qiafilter Miniprep technique (Qiagen). All clones were analyzed by restriction digestion, and all inserts were DNA sequenced in their entirety to confirm that the appropriate clone had been obtained and was not mutated.
All vectors and plasmids are readily available to any interested investigator.
Oligonucleotides.
The oligonucleotides used in this study were as follows: A, GGTGGGGGAGGTGAATTCATGGTGAGCAAGGGCGAGGAG; E, GTGGTCAGATCCTCGAGCTTGTACAGCTCGTCCATGCCG; C, AATTGGTTCCTGGTCGACCGATGATCCGCG; D, TCGACGCGGATCATCGGTCGACCAGGAACC; B1, ACATATTCGAGATTTGAC; B2, TGCGGCCGCTGCCCTAGGCCCTCCGCCACCTCCATGACCGAAACCGTATGTGGTCAGACCCTTTTCTGG; S1, GGTTTCGGTCATGGAGGTGGCGGAGGGCCTAGGGCAGCGGCCGCAGGATTAACGACTTATGGA; S2, GCTCAATACGGTGCTTGC; SAB21, AAAAGGTCGACTAATACGACTCACTATAGGTTAAAACAGCTCTGGGGTTG; SAB24, GGGGGAAGCTTAGGCCTTTTTTTTTTTTTTTTTTTTCCTCCGAATTAAAGAAAAAT; S1-3240F, CCTCCAAAATCAGAGTGTATC; S1-3580R, GCCCTGGGCTCTTGATTCTGT; S2-3151F, GAAGGCGATTCGTTGTAC; and S2-3518R, CTTGATTCAGCCACTAAG.
Transcriptions and electroporations.
Transcriptions were generally performed with T7 RNA polymerase (150 U; New England Biolabs), using the supplied transcription buffer supplemented with 40 U of RNasin (Promega, Madison, Wis.) and 1.25 mM each nucleoside triphosphate. Plasmid templates (1 to 3 μg) were first linearized with ClaI (pS1 or pSabRV1 vector) or HindIII (pS2-10F or pSabRV2) for 1 h at 37°C in a 20-μl volume. The restriction enzyme was then inactivated for 10 min at 65°C. Once linearized, plasmid template was added to the complete transcription mixture (total volume, 200 μl), and transcription was allowed to proceed for 60 to 90 min at 37°C before the reaction was terminated by freezing the mixture at −80°C. The RNA's quality and quantity were assessed by agarose gel electrophoresis before its use in subsequent experiments. RNA from transcription reactions was used directly, without purification, in electroporations.
Electroporations were performed with 40 to 75% confluent HeLa S3 cells (plated the previous day), which were then trypsinized, centrifuged, and resuspended at a concentration of 3 × 106/ml in Ca2+- and Mg2+-free phosphate-buffered saline (on some occasions, 293 cells were used in an identical manner). Cells (800 μl) were added to a cold 0.4-cm-path-length electroporation cuvette (Bio-Rad, Richmond, Calif., or BTX, San Diego, Calif.), 10 to 40 μg of RNA was added to the cells, and the cuvette was agitated multiple times to resuspend any cells that had settled; electroporation was immediately performed in a BTX electroporator with settings of 950 μF, 24 Ω, and 300 V. The entire contents of the cuvette were then added to a 6-cm-diameter dish (10-cm dishes were used for SabRV2 viruses) with 3 ml of warm Dulbecco's modified Eagle medium (DMEM)-F12 medium (50:50) plus 10% fetal calf serum (FCS) (see reference 23 for related details). These electroporation conditions consistently give a 50 to 80% electroporation efficiency (data not shown), resulting in first-generation (P0) virus stocks. Sabin 1 and SabRV1 recombinants were grown at 32°C because Sabin 1 has a tendency to acquire wt characteristics when passaged multiple times at temperatures higher than 34°C (65). Sabin 2 (S2-10F) and SabRV2 recombinants were grown at 37°C. Plates were incubated until complete cytopathic effect (CPE) was observed (frequently 24 to 36 h for SabRV1 and SabRV2 recombinants).
HeLa S3 cells obtained from the American Type Culture Collection (ATCC) (ATCC stock passaged 5 to 30 times) were grown in DMEM-F12 medium (Gibco/Life Technologies) supplemented with 10% FCS (Gibco/Life Technologies), penicillin-streptomycin, and l-glutamine. Adherent cell cultures were maintained at 10 to 80% confluence by incubation at 37°C in a 6% CO2 atmosphere. 293 cells were grown under the same conditions but were sometimes allowed to reach 100% confluence.
Viral stocks, passages, and plaque assays.
P0 viral stocks were harvested from electroporated cells exhibiting full CPE by taking the cells and supernatant and freezing-thawing three times, using a dry ice-ethanol bath and a 37°C water bath. Cellular debris was then pelleted by a 5-min, 300 × g centrifugation, and P0 viral stock supernatant was transferred to a fresh tube. The titers of some of the MoV2.11, SabRV1, and SabRV2 recombinant viral stocks appeared to be reduced by multiple freeze-thaw cycles. (This was not observed with normal wt poliovirus.) Therefore, viral stocks were stored in constant-temperature −30°C or −80°C freezers.
Concentration of several viruses was achieved with Centriprep concentration filters units with a molecular-mass cutoff of 50 kDa (Millipore). Low-titer SabRV1-SIV or SabRV2-SIV viral stocks (12 to 15 ml each) were spun in Centriprep filter units for 30 min at 3,000 × g. This resulted in a 5- to 15-fold concentration of virus. The titers of the concentrated stocks were then determined by plaque assay.
Equal amounts of nine P0 SabRV2-SIV viruses were mixed and passaged five times, at an MOI of 0.1, at both 32°C and 37°C (only the data from the 37°C incubation are shown in Fig. 2; identical data were obtained for passages at 32°C and are not shown). Passaging of SabRV2-SIV viruses was done by infecting 3 × 106 HeLa cells in 10-cm-diameter plates with the P0 viral cocktail stock at an MOI of 0.1. Cells were incubated at 32°C or 37°C in 3 ml of DMEM-F12 medium supplemented with 10% FCS, and P1 viral stock was harvested when complete CPE was observed (24 to 36 h postinfection). The same procedure was followed when carrying out passages P2 through P5. Each passage at an MOI of 0.1 represents approximately two generations of viral replication. In total, P5 viruses had gone through 10 to 12 generations of viral replication; this is represented as generation 10 in Fig. 2. P1 virus is conservatively represented as generation 2 in Fig. 2 for simplicity. The passaged cocktail viruses were tested for the presence of the SIV inserts by reverse transcription (RT)-PCR with primers in the poliovirus sequence flanking all of the SIV inserts.
FIG. 2.
Propagation of vaccine viruses. (A) Stability of SabRV2 recombinant viruses passaged as a cocktail. Nine SabRV2-SIV viruses were mixed in equal amounts and passaged five times at an MOI of 0.1, for a total of at least 10 generations of viral replication. The P1 virus is conservatively estimated as generation 2. Cocktail stocks were tested for the presence of the SIV inserts by RT-PCR using primers corresponding to the poliovirus sequence flanking the SIV inserts. The lane containing the SabRV2 empty-vector RT-PCR product is indicated by a V. The size of the SabRV2 band (427 bp) is the size of the virus containing no insert. Inserts were fully retained throughout all five passages. (B) Composition of SabRV2 cocktail over a series of passages. The passaged cocktail stocks were checked for the presence of the individual viruses by RT-PCR with primers specific for each SIV insert. Generation 1 indicates the original cocktail in which the nine P0 viral stocks obtained directly from high-efficiency transfections were mixed in equal proportions. The generation 1 stock contained all nine viruses, as determined by RT-PCR. The middle and right panels show the presence of all nine SabRV2-SIV viruses both at generation 6 and at generation 10. The small bands present in Env15C and Env17 are minor deletion products representing less than 1% of the virus population (data not shown).
All plaque assays were done as previously described (15, 16). Plates for plaque assays involving SabRV1 recombinants were incubated at 32°C for 5 days postinfection, while those for plaque assays involving SabRV2 recombinants were incubated at 37°C for 4 days postinfection.
Viruses used in the SabRV1-SIV and SabRV2-SIV vaccines are listed in Table 1. In the SabRV1-SIV cocktail, viruses were mixed together such that each virus (of the 20) was present at 2.5 × 106 PFU/ml, giving a final virus concentration of 5 × 107 PFU/ml. The SabRV2-SIV cocktail was mixed such that each virus (of the 20) was present at 5 × 104 PFU/ml, giving a final virus concentration of 106 PFU/ml. The cocktails were made with pure P0 viral stocks.
TABLE 1.
SabRV1/2-SIV vaccine library cocktails
| Virus
|
Amino acid coverage | |
|---|---|---|
| Sabin 1 | Sabin 2 | |
| SabRV1-Gag1 | SabRV2-Gag1 | Gag 2–134 (p17) |
| SabRV1-Gag2 | SabRV2-Gag2 | Gag 92–263 (p17/p24) |
| SabRV2-Gag3N | Gag 133–299 | |
| SabRV2-Gag3C | Gag 266–432 | |
| SabRV1-Gag4 | SabRV2-Gag4 | Gag 362–509 (p24/p9) |
| SabRV1-Pol6 | Pol −29–146 (protease) | |
| SabRV1-Pol7 | SabRV2-Pol7 | Pol 97–266 |
| SabRV1-Pol8 | SabRV2-Pol8 | Pol 218–330 |
| SabRV1-Pol9 | SabRV2-Pol9 | Pol 290–472 |
| SabRV1-Pol10 | SabRV2-Pol10 | Pol 397–530 |
| SabRV1-Pol11 | SabRV2-Pol11 | Pol 490–631 |
| SabRV1-Pol12 | Pol 597–767 | |
| SabRV1-Pol14 | SabRV2-Pol14 | Pol 828–981 |
| SabRV1-Env15 | Env 18–164 (gp120) | |
| SabRV2-Env15C | Env 71–211 (gp120) | |
| SabRV1-Env16M | SabRV2-Env16M | Env 148–249 (gp120) |
| SabRV1-Env17 | SabRV2-Env17 | Env 237–380 (gp120) |
| SabRV1-Env18 | SabRV2-Env18 | Env 335–498 (gp120) |
| SabRV1-Env20 | SabRV2-Env20 | Env 486–632 (gp120/gp41) |
| SabRV1-Env21L | SabRV2-Env21L | Env 526–698 (gp41) |
| SabRV1-Env22 | Env 712–879 (gp41) | |
| SabRV1-Nef23 | SabRV2-Nef23 | Nef 1–145 |
| SabRV1-Nef24 | SabRV2-Nef24 | Nef 126–262 |
| SabRV2-Tat25 | Tat 1–130 | |
The SIVmac251 stock used for challenge was obtained in May 1998 and had not been previously published. The SIVmac251 (5/98) stock has a titer of >105 50% tissue culture infective doses (TCID50) per ml.
RT-PCR of recombinant polioviruses.
HeLa cells (2 × 105 to 5 × 105) in six-well dishes were infected with the appropriate virus at an MOI of 0.5 to 10 (an MOI of 10 was used if possible). Cells were incubated at 37°C in 1 ml of DMEM-F12 plus 10% FCS for 6 to 8 h and then harvested by scraping or trypsinization. RNA was collected by using an RNeasy kit, and cDNA was synthesized by using randomly primed Superscript II (Life Technologies) reactions. PCR was done using rTth (Perkin-Elmer XL polymerase) and primers S1-3240F and S1-3580R (MoV2.11, S1, and SabRV1 recombinants) or primers S2-3151F and S2-3518R (S2-10F and SabRV2 recombinants). Conditions were as follows: 0.5 μl of cDNA, 2.2 mM magnesium acetate, 0.5 μl of XL polymerase, and manufacturer-recommended buffer and primer concentrations in a 50-μl reaction mixture, with 30 cycles consisting of 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min. Generally 1 to 5 μl of the final product was loaded on a 1.5% agarose gel for analysis.
Animals.
All animals used in this study were mature, cycling, female cynomolgus macaques from the California Regional Primate Research Center. The animals were housed in accordance with American Association for Accreditation of Laboratory Animal Care standards. When necessary, animals were immobilized by intramuscular injection of 10 mg of ketamine HCl (Parke-Davis, Morris Plains, N.J.) per kg of body weight. The investigators adhered to the recommendations set forth in the Guide for the Care and Use of Laboratory Animals (14a). Prior to use, animals were negative for antibodies to HIV-2, SIV, type D retrovirus, and simian T-cell leukemia virus type 1.
Intranasal inoculations of SabRV1-SIV and SabRV2-SIV were done with a total volume of 1 ml. The animals were anesthetized and placed in dorsal recumbancy with their heads tilted backward. One-half milliliter of virus preparation was instilled dropwise into each nostril. The animals were kept in this position for 10 min and then placed in lateral recumbancy until they had recovered from the anesthesia (30). Seven animals were inoculated intranasally with 1 ml (5 × 107 PFU) of SabRV1-SIV on days 1, 3, 14, and 16, for a total of four immunizations. Nineteen weeks after the first series of inoculations, these same seven animals were given boosters of two intranasal inoculations of 1 ml (106 PFU/ml) of SabRV2-SIV, one at week 19 and a second at week 21. Intranasal inoculations were done because cynomolgus macaques can be consistently infected with poliovirus by this route (15) and also because macaque experiments using the model antigen cholera toxin indicate that intranasal immunization is better at eliciting vaginal immune responses than is oral immunization (30).
The animals were challenged with 105 TCID50 of SIVmac251 intravaginally, using the SIVmac251 (5/98) stock (see above). Two intravaginal SIV inoculations were given to each monkey in a single day, with a 4-h rest period between the inoculation procedures. The procedure and technique used were previously described (48).
Serum and vaginal- and rectal-lavage antibody responses.
Anti-SIV IgG and IgA responses in vaginal and rectal washes and serum were measured at weekly time points during the study. Vaginal and rectal wash samples were collected and analyzed as previously described (38, 40, 48). Briefly, vaginal washes were collected by infusing 2 ml of sterile phosphate-buffered saline into the vaginal canal and aspirating the instilled volume. Rectal washes were collected in a comparable manner. Samples were immediately snap-frozen on dry ice and stored at −80°C until analysis. To account for the presence of IgG interfering with and reducing the detection of IgA, serum was first depleted of IgG by using protein G-Sepharose beads (Pharmacia Biotech, Uppsala, Sweden) prior to its use in the IgA enzyme-linked immunosorbent assay (ELISA). To deplete IgG, 25 μl of serum sample was incubated with 100 μl of protein G-Sepharose beads for 1 h at 37°C and then at 4°C overnight; subsequently, the protein G-Sepharose was pelleted and the supernatant was collected. During this process, samples were diluted 1:3. The change in optical density (ΔOD) between test and control wells was defined as the difference between the mean OD of sample tested in two antigen-coated wells and the mean OD of the sample tested in two antigen-free control wells. The negative-control OD value was determined from 12 uninfected monkey serum samples and defined stringently as the average OD plus 3 standard deviations. Endpoint titers were determined if the ΔOD of the test sample exceeded the negative-control value by a factor of 2. To then quantify anti-SIV antibody titers, serial fourfold dilutions of duplicate samples of serum, vaginal wash, or rectal wash were tested by an ELISA using whole pelleted SIVmac251 (Advanced Biologics Inc., Columbia, Md.). Antibody binding was detected with peroxidase-conjugated goat anti-monkey-IgG (Fc) or -IgA (Fc) (Nordic Laboratories, San Juan Capistrano, Calif.) and developed with o-phenylenediamine dihydrochloride (Sigma). The endpoint titer was defined as the reciprocal of the last dilution giving a ΔOD greater than 0.1 (15).
Neutralizing-antibody responses.
Neutralizing-antibody assays were performed as previously described (11, 50). A neutralizing-antibody titer was defined as the dilution resulting in 50% inhibition of cell killing by lab-adapted SIVmac251.
SIV isolation and serum viral RNA load determination.
Virus was isolated from heparinized whole blood obtained from the SIV-inoculated cynomolgus macaques. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient separation (Lymphocyte Separation Medium; Organon Teknika, West Chester, Pa.) and cocultured with CEMx174 cells (28) (provided by James A. Hoxie, University of Pennsylvania, Philadelphia) as previously described (37). PBMCs (5 × 106) were cocultivated with CEMx174 cells (2 × 106 to 3 × 106). Aliquots of the culture medium were regularly obtained and assayed for the presence of the SIV major core protein (p27) by antigen capture ELISA (37). Cultures were considered positive if they were antigen positive at two consecutive time points. A detailed description of the technique and criteria used to determine whether the culture medium was antigen positive has been published (43). All cultures were maintained for 8 weeks and tested for SIV p27 by ELISA before being scored as virus negative. Blood samples for virus isolation were collected at the times indicated in Table 2. SIV RNA loads (see Fig. 9) were determined by a modification (J. D. Lifson, unpublished data) of a real-time RT-PCR assay on monkey plasma samples, performed essentially as previously described (74). The assay has a threshold sensitivity of 100 copy equivalents/ml of plasma and an interassay coefficient of variation of <25%.
TABLE 2.
SIV isolation
| Monkeya | Result at
postimmunization week:
|
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 6 | 8 | 12 | 16 | |
| 27244 | − | − | − | − | − | − | − |
| 27270 | − | − | + | − | − | − | − |
| 25231 | + | + | + | + | + | + | + |
| 27250 | + | + | + | + | + | + | + |
| 28508 | + | + | + | + | + | + | − |
| 27253 | + | + | + | + | + | + | + |
| 27273 | + | + | + | + | + | + | + |
| 26385 | + | + | + | + | + | + | + |
| 28118 | + | + | + | + | + | + | + |
| 26560 | + | + | + | + | + | − | + |
| 26383 | − | + | + | + | + | + | + |
| 26405 | − | + | + | + | + | + | + |
| 23414 | − | + | + | + | + | + | + |
The upper seven monkeys were vaccinated; the lower six were control monkeys.
FIG. 9.
Serum anti-SIV IgG antibody responses postchallenge. Antibody titers are shown for all animals postchallenge. Vaccinated monkeys (right panel) are indicated by the symbols used in previous figures: 25231, ▪; 27244, ●; 27250, ▴; 27253, ♦; 27270, □; 27273, ○; and 28508, ▵. Control monkeys (left panel) are indicated by symbols as follows: 26383, ★; 26385, ✖; 23414, ; 26405, ⋄; 26560, ▿; and 28118, ◂.
SIV provirus PCR analysis.
Nested PCR was carried out on PBMC genomic DNA in a DNA thermal cycler (Perkin-Elmer) as previously described (48). Briefly, cryopreserved PBMCs isolated from whole blood of each monkey in the experiment were washed three times in Tris buffer at 4°C and resuspended at a density of 107 cells/ml. Ten microliters of the cell suspension was added to 10 μl of PCR lysis buffer (50 mM Tris-HCl [pH 8.3], 0.45% NP-40, 0.45% Tween 20) with 200 μg of proteinase K/ml. The cells were incubated for 3 h at 55°C and then for 10 min at 96°C. Two 30-cycle rounds of amplification were performed on aliquots of plasmid DNA containing the complete genome of SIVmac1A11 (positive control) or aliquots of cell lysates, using conditions described elsewhere (48). The primers used specifically amplify SIV Gag. DNA from uninfected CEMx174 cells was amplified as a negative control in all assays to monitor potential reagent contamination. β-Actin DNA sequences were amplified from all PBMC lysates by two rounds of PCR (30 cycles/round) to detect potential inhibitors of Taq polymerase. Following the second round of amplification, a 10-μl aliquot of the reaction product was removed and electrophoresed on a 1.5% agarose gel. Amplified products in the gel were visualized by ethidium bromide staining. Blood samples for PCR analysis were collected at the times indicated in Table 3.
TABLE 3.
SIV proviral DNA PCRa
| Monkeyb | Result at
week
|
|||
|---|---|---|---|---|
| 2 | 4 | 8 | 12 | |
| 27244 | − | − | − | − |
| 27270 | − | − | − | − |
| 25231 | + | − | + | + |
| 27250 | − | + | + | + |
| 28508 | − | + | + | + |
| 27253 | − | + | + | + |
| 27273 | − | + | + | + |
| 26385 | − | − | + | + |
| 28118 | − | − | + | + |
| 26560 | + | + | + | + |
| 26383 | + | − | + | + |
| 26405 | − | − | + | + |
| 23414 | NDc | + | + | + |
Nested PCR used gag-specific primers. At week 1, results for all monkeys were negative.
The upper seven monkeys were vaccinated; the lower six were control monkeys.
ND, not done.
Western blot analysis of serum antibody responses.
HeLa cells (2 × 106) infected with wt poliovirus were incubated for 7 h at 37°C. Cells were harvested and lysed on ice for 1 min (the lysis buffer consisted of 10 mM Tris [pH 7.5], 140 mM NaCl, 5 mM KCl, and 1% IGEPAL [Sigma, St. Louis, Mo.]), and the nuclei were removed by centrifugation. Poliovirus-infected whole-cell lysate (20 μl) and sucrose-gradient-purified SIVmac251 (ABI, Columbia, Md.; 22 μg) were electrophoresed in parallel lanes through a sodium dodecyl sulfate–10 or 12% polyacrylamide gel and analyzed by immunoblotting. The anti-SIV serum used as a positive control for SIV proteins was pooled serum from SIV-infected rhesus macaques. The antipoliovirus serum used as a positive control for poliovirus proteins was obtained from a poliovirus-immune human. Antisera from all vaccinated cynomolgus macaques were used as primary antibodies (serum collected on the day of challenge was used for monkeys 27270, 27244, 28508, 27273, and 27253, while serum collected 1 month prior to challenge was used for monkeys 25231 and 27250). Also, preimmune serum from monkey 27250 was used. The secondary antibody (horseradish peroxidase-conjugated rabbit anti-human IgG) was obtained from DAKO (Carpinteria, Calif.) and used for monkeys 27270, 27244, 28508, 27273, 27253, and 27250 (preimmune). A horseradish peroxidase-conjugated rabbit anti-rhesus monkey IgG (Sigma, St. Louis, Mo.) was used for monkeys 25231 and 27250, employing serum obtained 1 month prechallenge. Blots were probed with 1:100-diluted monkey serum in TBST (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.15% Tween 20) with 10% fat-free dry milk (Bio-Rad, Hercules, Calif.), washed twice in TBST containing 0.15% Tween 20 and once in TBST containing 0.5% Tween 20, probed with the secondary antibody (1:2,000 dilution), and then detected by enhanced chemiluminescence (ECL; Amersham, Arlington Heights, Ill.) as specified by the manufacturer. Rhesus monkey serum was used at a dilution of 1:200, and poliovirus-immune human serum was used at a dilution of 1:25. Films were digitally scanned and exported to Photoshop 5.5 (Adobe, San Jose, Calif.).
Statistical methods.
SIV viremia levels were analyzed by Student's t test by comparison of the 24- to 32-week average log viral load of each animal in the two groups (vaccinated:control) with a two-tailed distribution. Weight gain (or loss) was analyzed by the Mann-Whitney rank test of the 33- to 44-week average weight change (from day of challenge) of each animal in the two groups (vaccinated versus control) with a two-tailed distribution. Mortality differences between the two groups at week 48 were analyzed by Fisher's exact test.
Lymphocyte proliferative responses to SIV antigens.
Antigen-specific proliferation was tested as described elsewhere, using PBMCs from fresh blood samples (46). The cells were suspended at a density of 2 × 106 per ml in RPMI 1640 medium supplemented with 10% FCS and plated in triplicate at 50 μl per well in 96-well round-bottom microtiter plates. Antigen dilutions or control reagents were plated at 50 μl per well. Fresh medium (100-μl volumes) was added after 48 h, and the plates were incubated for 7 days in a CO2 incubator. The wells were pulsed with [3H]thymidine (1 μCi per well; NEN-DuPont Co., Wilmington, Del.) overnight prior to harvest. The plates were aspirated onto fiberglass filters and washed with a cell harvester (Inotech Biosystems International, Lansing, Mich.). The filters were saturated with scintillation cocktail, and counts in the 3H window were measured with a 96-well scintillation counter (Microbeta 1450; Wallac Biosystems, Gaithersburg, Md.). The SIV antigen was whole inactivated SIVmac239 (kindly provided by Larry Arthur). Concanavalin A (ConA) was tested as a positive-control antigen. Medium alone was used as a control for spontaneous proliferation. The antigens were tested at 0.1, 1.0, and 10 μg/ml in every assay. This assay was used in our previous study (15). Lymphocyte proliferation assays were performed before immunization and at regular intervals after immunization (data not shown), using PBMCs. Because of an unusually high level of spontaneous proliferation (∼10,000 to 100,000 cpm/well) in negative-control wells (PBMCs plus medium alone) at all time points tested over a 28-week period, it was difficult to assess SIV-specific CD4+ T-cell responses in the immunized animals, since minimal additional stimulation was seen in the presence of antigen or ConA.
SIV-specific CTL responses.
The presence of SIV-specific cytotoxic T lymphocytes (CTLs) in cynomolgus PBMCs was assessed as previously reported (15). Briefly, PBMCs from immunized monkeys were stimulated with ConA (Sigma) at a concentration of 10 μg/ml and cultured for 14 days in complete RPMI 1640 medium supplemented with 5% human-lymphocyte-conditioned medium (human interleukin-2; Hemagen Diagnostics, Waltham, Mass.). Autologous B cells were transformed by herpesvirus papio (595Sx1055 producer cell line, provided by M. Sharp, Southwest Foundation for Biomedical Research, San Antonio, Tex.) and infected overnight at an MOI of 30 with wt vaccinia virus (vvWR) or a recombinant vaccinia virus expressing the p55gag (vv-gag) or gp160env (vv-env) protein of SIVmac239 (provided by L. Giavedoni and T. Yilma, University of California, Davis). The level of vaccinia virus infection of target cells was estimated by an indirect-immunofluorescence technique using a monkey anti-vaccinia virus antibody followed by fluoresceinated goat anti-human IgG (Vector Laboratories, Burlingame, Calif.). The level of vaccinia virus infection of target cells in this series of experiments was estimated to fall between 5 and 15%. Target cells were labeled with 50 μCi of 51Cr (Na2CrO4; Amersham, Arlington Heights, Ill.) per 106 cells. Effector and target cells were added together at multiple effector/target ratios in a 4-h chromium release assay. Assays were considered reliable if specific lysis was >10% and at least twice the level of spontaneous lysis of vvWR-infected cells. At many time points, the data from the CTL assays could not be meaningfully interpreted because of a high level of spontaneous lysis of the cynomolgus macaque transformed B-cell targets. Lysis was not due to NK cell activity, since no lysis was seen when the NK target cell line K562 was substituted as a negative control (data not shown). Numerous variations of the CTL assay were attempted in an effort to generate consistently reliable chromium release assay data for immunized or infected cynomolgus macaques. Cold-target inhibition, a variety of stimulation procedures, and enrichment of CD8+ cells by using anti-CD8 bead purification failed to consistently resolve this problem.
RESULTS
Sabin-based vaccine vector construction and production.
Given the excellent safety record of Sabin vaccine strain polioviruses in humans (75), we wished to do all future experiments, in primates and humans, with only Sabin-based viruses produced from molecularly defined constructs. Hence, we engineered new plasmid clones of Sabin 1- and Sabin 2-derived vectors (pSabRV1 and pSabRV2, respectively) (Fig. 1A to C). We then constructed a collection of 20 SabRV1 viruses, expressing SIV gag, pol, env, and nef, which represent nearly the entire SIV genome (Fig. 1F and Table 1). These viruses grew well, as assessed by plaque assay (Fig. 1D). Twenty SabRV2-SIV viruses were produced in a comparable manner, being selected to represent a similar coverage of the major SIV genes plus Tat (Fig. 1F and Table 1). These viruses also grew well, as assessed by plaque assay (Fig. 1E). In some cases, there were difficulties in producing genetically pure stocks by the use of recombinant polioviruses, since viruses with deletions in their insert sequences can accumulate as recombinant polioviruses replicate through a number of generations (15, 52, 76). Therefore, we took great care to check the viral stocks for deletions. We did so by using a sensitive RT-PCR assay capable of detecting deleted virus comprising as little as 0.1% of the stock (data not shown). The SabRV1-SIV vaccine stocks were more than 99.9% pure in total (data not shown), as were the SabRV2-SIV stocks (Fig. 2B). To further test the viability and stability of the recombinant viruses, a cocktail of nine of the SabRV2-SIV viruses was then passaged repeatedly and assessed by RT-PCR (Fig. 2). The vaccine cocktail as a whole maintained the SIV sequences for at least 10 generations (Fig. 2A), and all of the individual component viruses maintained their inserts and viability (Fig. 2B).
The 20 SabRV1-SIV virus stocks were then mixed to create a defined vaccine cocktail of 5 × 107 PFU/ml for use in the primate vaccinations described below. The 20 SabRV2-SIV virus stocks were mixed to make a defined vaccine cocktail of 106 PFU/ml.
Monkey immunizations.
Producing a candidate SIV vaccine in two different serotypes of poliovirus (type 1 and type 2 strains) was a strategy employed in our last macaque study to create a better opportunity for an effective booster immunization with the second vector serotype, since there is no significant cross-neutralization or cross-protection between these two serotypes. That approach resulted in anemnestic booster responses in the immunized animals (15) and led us to utilize the same strategy in the study reported here.
Cynomolgus macaques are used in our studies because they are susceptible to poliovirus administered either orally or intranasally. Because the goal of our studies is to test poliovirus vectors as potential mucosal AIDS vaccines, we used a route of inoculation that would elicit a vaginal immune response. An intranasal route of inoculation was chosen for these experiments because previous experiments using SIV subunits plus cholera toxin have demonstrated that the intranasal route of inoculation elicits a better vaginal mucosal immune response than does oral or rectal immunization (30).
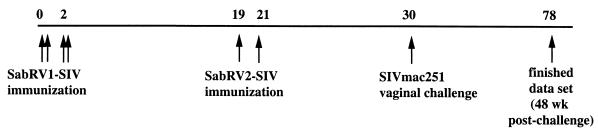
In this study, seven cynomolgus macaques were immunized intranasally with SabRV1-SIV (5 × 107 PFU) at weeks 0 and 2 (Fig. 3). Then, at weeks 19 and 21, these animals were booster immunized intranasally with SabRV2-SIV (106 PFU). These doses are comparable to normal Sabin oral poliovirus vaccine doses used in children (2).
FIG. 3.
Time line of vaccination and challenge. The numbers above the line indicate the weeks at which the various steps were performed.
Immunization induces strong serum anti-SIV IgG and IgA responses.
The results of ELISAs for serum IgG and IgA responses against SIV are shown in Fig. 4. All seven monkeys exhibited a rapid and strong anti-SIV IgG response after immunization with SabRV1-SIV (Fig. 4A). Three of the seven monkeys showed serum anti-SIV IgA responses, and in two of those animals the SabRV1-SIV-elicited IgA responses persisted for at least 19 weeks (Fig. 4B).
FIG. 4.
Serum anti-SIV antibodies. (A) Anti-SIV IgG titers in the sera of monkeys immunized with SabRV1-SIV and SabRV2-SIV. Seven cynomolgus macaques were inoculated intranasally with SabRV1-SIV at weeks 0 and 2 (indicated by ▵ below) and given intranasal boosters at weeks 19 and 21 with SabRV2-SIV (indicated by ▴ below). Monkeys are labeled as follows: 25231, ▪; 27244, ●; 27250, ▴; 27253, ♦; 27270, □; 27273, ○; and 28508, ▵. Titers indicated are reciprocal dilutions. A titer of 1 is stringently defined as an ELISA optical density reading 3 standard deviations above the average optical density for a group of negative-control monkeys (see Materials and Methods). (B) Anti-SIV IgA titers in the sera of SabRV1-SIV- and SabRV2-SIV-immunized monkeys. Symbols are as noted above. A clear anemnestic IgA response is evident for all seven macaques after SabRV2-SIV immunization at week 19. Symbols are as for panel A.
The SabRV2-SIV booster immunization at 19 weeks resulted in a 20- to 80-fold increase in anti-SIV IgG antibody titers in all monkeys within 7 days, a classic anamnestic antibody response (Fig. 4A). Additionally, all seven monkeys were positive for anti-SIV serum IgA at 1 week postboost, with a more than 10-fold titer increase in all monkeys, confirming the presence of an anamnestic IgA response in all vaccinated animals (Fig. 4B).
All seven monkeys demonstrated comparable serum IgG anti-SIV antibody titers, and generally the monkeys had comparable serum IgA anti-SIV antibody titers after the SabRV2-SIV booster immunization. Individual variability in the immune response to the vaccine was seen; monkey 27270 exhibited the strongest anti-SIV serum IgG and IgA response after both the SabRV1-SIV and SabRV2-SIV immunizations (Fig. 4).
Immunization induces vaginal and rectal anti-SIV antibody responses.
We found in our previous study (15) that recombinant polioviruses expressing SIV antigens could induce anti-SIV vaginal and rectal antibody responses after intranasal inoculation. In this study, we again analyzed antibody samples taken from the surfaces of the vaginal and rectal mucosae. It was recently shown that macaque vaginal antibody secretions are affected by the menstrual cycle (38), and therefore in this study we took mucosal antibody samples on a weekly basis to assess the antibody levels more accurately.
In our previous study we observed that four of four monkeys had rectal anti-SIV IgA antibody responses. In this study, we again observed that 100% of the immunized monkeys exhibited rectal mucosal anti-SIV antibody responses. After the SabRV1-SIV immunization, all seven monkeys demonstrated at least transient anti-SIV rectal IgA responses, even though only three had detectable levels of anti-SIV IgA in their sera (Fig. 4B and Fig. 5B). These results were consistent with our previous study in which one monkey had a mucosal IgA anti-SIV response even though it showed no detectable serum anti-SIV IgG or IgA titers at any time point (15). Conversely, neither of the two monkeys that had long-lasting serum IgA responses (27270 and 28508 [Fig. 4B]) after SabRV1-SIV immunization in the present study exhibited detectable levels of rectal IgA antibodies for longer than 2 weeks.
FIG. 5.
Rectal anti-SIV antibodies. (A) Anti-SIV IgG titers in the rectal washes of monkeys immunized with SabRV1-SIV and SabRV2-SIV. Vaccinated monkeys were divided into two groups (upper and lower panels) for easier viewing of data. Titers indicated are reciprocal dilutions. A titer of 1 is stringently defined as an ELISA optical density reading 3 standard deviations above the average optical density for a group of negative-control monkeys (see Materials and Methods). (B) Anti-SIV IgA titers in the rectal washes of monkeys immunized with SabRV1-SIV and SabRV2-SIV. Vaccinated monkeys were divided into the same two groups (upper and lower panels) as in part A, for easier viewing of data. Monkeys are labeled as described in the legend to Fig. 4.
We detected anti-SIV IgG in rectal secretions from all seven monkeys after the SabRV2-SIV immunization (Fig. 5A). It is uncommon to observe IgG in rectal secretions (38, 55, 78). Monkeys 27253 and 27270 exhibited particularly strong, 50- to 100-fold anamnestic rectal IgG responses after the SabRV2-SIV immunization. Additionally, we were intrigued that the SabRV2-SIV immunization appeared to reduce rather than boost the rectal IgA anti-SIV response in the monkeys (Fig. 5B). Four monkeys that had a transient rectal IgA responses after the SabRV1-SIV immunization (25231, 28508, 27244, and 27270) had no detectable anti-SIV rectal IgA after the SabRV2-SIV immunization (Fig. 5B), even though all four monkeys showed a substantial increase in serum anti-SIV IgA titer (Fig. 4B).
All of the monkeys demonstrated vaginal IgG anti-SIV responses after SabRV1-SIV–SabRV2-SIV immunization, and six of seven monkeys had vaginal IgA anti-SIV responses (Fig. 6). Interestingly, the monkey with the most substantial vaginal IgA antibody response (27250) after SabRV1-SIV immunization (Fig. 6B) did not exhibit a concurrent serum IgA response (Fig. 4B). This animal also had a robust rectal IgA response (Fig. 5B), providing more evidence that the immune responses to the vaccine in some animals are compartmentalized.
FIG. 6.
Vaginal anti-SIV antibodies. (A) Anti-SIV IgG titers in the vaginal secretions of monkeys immunized with SabRV1-SIV and SabRV2-SIV. Vaccinated monkeys are divided into two groups (upper and lower panels) for easier viewing of data, as was done in Fig. 4 and 5. (B) Anti-SIV IgA titers in the vaginal secretions of monkeys immunized with SabRV1-SIV and SabRV2-SIV. Monkeys are labeled as described for Fig. 4.
As with rectal antibodies, the strongest vaginal IgG antibody responses occurred after the SabRV2-SIV booster immunization. A 200- to 1,000-fold increase in vaginal anti-SIV IgG was seen in monkeys 27270 and 25231 after the booster immunization. A 100-fold increase in vaginal IgA anti-SIV antibodies was seen in the same two monkeys. For each individual monkey, the patterns of vaginal IgA and IgG anti-SIV responses were generally similar (Fig. 6).
Taken together, although all monkeys exhibited similar serum IgG anti-SIV antibody responses and similar serum IgA responses after the booster immunization, there were substantial differences in the mucosal antibody responses of different monkeys. In animals with a strong initial rectal IgA response (monkeys 27253 and 27273 [Fig. 5B]), vaginal IgA responses were weak or absent. SabRV2 appeared to elicit rectal IgG antibody responses but not rectal IgA responses, as noted above. The three monkeys that showed anti-SIV serum IgA responses after SabRV1-SIV immunization had the strongest rectal IgG responses after the SabRV2-SIV booster immunization, but the significance of this is unclear. The results of these experiments clearly demonstrate that serum antibody titers are not a good indicator of mucosal antibody responses, consistent with compartmentalization of the immune response.
Diversity of antigens recognized in monkeys immunized with SabRV1-SIV–SabRV2-SIV.
We are unaware of any precedent for immunization of primates with a viral vector (or any vector) expressing a defined library of antigens. Therefore, it was important to determine whether the measured antibody responses were against a single antigen (expressed by a single SabRV-SIV virus) or multiple antigens (expressed by different SabRV-SIV viruses). To explore this issue, we examined the anti-SIV and antipoliovirus specificities of the serum antibodies in immunized animals by Western blotting. All seven monkeys seroconverted to poliovirus antigen positivity, generally with a strong response against capsid protein VP1 and weaker responses against two to four other poliovirus proteins (Fig. 7). All seven monkeys also seroconverted to SIV antigen positivity, as determined by Western blot analysis, confirming the SIV ELISA results shown in Fig. 4. Importantly, a majority of monkeys demonstrated substantial antibody responses to multiple SIV proteins (Fig. 7). Antibody responses against reverse transcriptase (p51/65; all seven monkeys), Gag (p55; six monkeys [27244, 28508, 27270, 27273, 27253, and 27250]), p17 (monkeys 27270 and 27253), p27 (monkeys 27244 and 27250), Env gp41 (monkeys 27270 and 27253), and Env gp120 (monkeys 27244, 28508, 27270, 25231, and 27253) were all apparent (Fig. 7). Antibodies against Nef and Tat (also represented in the SabRV1-SIV and/or SabRV2-SIV cocktail) were not assayed in this experiment because they are not packaged in SIV virions, which is the target material for the immunoblot analyses. At least five different SabRV1-SIV or SabRV2-SIV viruses, and possibly many more, were immunogenic and elicited antibody responses, since the responses against SIV p27, reverse transcriptase, p17, gp41, and gp120 must have been elicited from different SabRV-SIVs. In summary, a majority of monkeys responded to multiple poliovirus and SIV proteins, indicating that the library vaccine approach is successful at eliciting responses to multiple expressed proteins, even in a complex cocktail of 20 different viruses.
FIG. 7.
Western blotting. All seven monkeys had anti-SIV and anti-poliovirus antibody responses that were detectable by Western blotting. Each serum was immunoblotted against purified SIV virion-infected (left lanes) and poliovirus-infected (PV; right lanes) HeLa cell extracts. Positive controls used were SIV-positive rhesus serum (SIV+) and human poliovirus-immune serum (Polio+). Preimmune serum from monkey 27250 was used as a negative control (preimm.). SIV antigens recognized by each monkey are indicated by symbols on the left of the blot as follows: reverse transcriptase (RT), ▹; Gag, –; gp120, ●; and gp41, ○. Poliovirus VP1, recognized by all monkeys, is indicated by the leftward-pointing black triangles to the right of each blot. Bands do not necessarily line up precisely because several of the blots were done at different times, but SIV and poliovirus positive controls were always run as markers.
Cellular immune responses.
Poliovirus vectors can elicit potent CTL responses in both mice (42, 72) and primates (15). In the present study, we were able to detect SIV Env-specific CTLs in three of seven monkeys (25231, 27244, and 27250) after SabRV1-SIV vaccination by a standard bulk PBMC cytolytic assay (Fig. 8A). After the SabRV2-SIV vaccinations, we detected SIV Gag- and Env-specific CTLs in monkey 25231 (Fig. 8B). Cellular immune responses are technically difficult to assess in cynomolgus macaques (see Materials and Methods), and we frequently experienced difficulties because of a high level of background lysis. This technical complication prevented accurate assessment of CTL activity at additional time points.
FIG. 8.
SIV-specific CTLs. (A) SIV-specific CTLs after immunization with SabRV1-SIV. SIV-specific CTLs were detected using bulk PBMCs collected 2 weeks after immunization with SabRV1-SIV. Monkeys 25231, 27244, and 27250 tested positive for SIV Env-specific CTLs. □, negative-control target cells; ●, Env-expressing target cells. (B) SIV-specific CTLs after immunization with SabRV2-SIV. SIV-specific CTLs were detected using bulk PBMCs collected 6 weeks after immunization with SabRV2-SIV. Monkey 25231 tested positive for SIV Gag-specific CTLs and possibly Env-specific CTLs. □, negative-control target cells; ▴, Gag-expressing target cells; ●, Env-expressing target cells. E:T, effector/target ratio.
The three monkeys that tested positive for SIV-specific CTLs after SabRV-SIV vaccination (25231, 27244, and 27250) also tested positive for SIV-specific lymphoproliferative responses (stimulation indices of 3.3, 4.1, and 2.7, respectively).
Virologic outcome of vaginal challenge with SIVmac251.
All 7 vaccinated monkeys and a total of 12 control monkeys were challenged with a vaginal inoculum of SIVmac251. SIVmac251 is an uncloned and highly virulent virus that has proven to be extremely difficult to protect against (i.e., prevention of infection is not easily achieved) or control with vaccine-induced immune responses (14, 17, 22, 25, 39, 40, 69). The vaginal challenge route was chosen because our primary interest in the SabRV vector is as a vaccine capable of protecting against sexually transmitted HIV.
Six control cynomolgus macaques were first challenged intravaginally with 105 TCID50 of SIVmac251 twice in 1 day (this dose had previously infected 15 of 15 rhesus macaques intravaginally [C. J. Miller, unpublished data]). All six of those control cynomolgus macaques became infected, as judged by positive SIV virus isolation, positive SIV provirus PCR, and seroconversion to SIV antigen positivity (data not shown).
At week 30, 9 weeks after the last immunization, we challenged all seven of the SabRV-SIV-vaccinated animals, as well as six additional concurrently tested control cynomolgus macaques, with two vaginal inoculations of 105 TCID50 of SIVmac251 in 1 day (Fig. 3). All 6 concurrently tested control monkeys became SIV positive by virus isolation (Table 2), SIV provirus PCR (Table 3), and seroconversion to SIV positivity (tested by using neutralizing antibodies [Table 4] and by ELISA [Fig. 9]), bringing the total to 12 of 12 control cynomolgus macaques infected after vaginal inoculation with the challenge dose. In the group of SabRV-SIV-immunized monkeys, two of the monkeys appeared to be fully protected. SIV was never isolated from the PBMCs of one animal (27244), while the other animal (27270) was SIV virus isolation positive at a single time point, 4 weeks postchallenge (Table 2). We were unable to detect SIV Gag in PBMC samples from either animal by PCR (Table 3). Neither animal had an anamnestic serum antibody response to SIV antigens after the challenge exposure (Fig. 9), again indicating that they were fully protected from SIV infection.
TABLE 4.
Neutralizing antibody titers
| Monkeya | Antibody titer at week 38b |
|---|---|
| 27244 | − |
| 27270 | − |
| 25231 | 7,673 |
| 27250 | 1,130 |
| 28508 | 6,386 |
| 27253 | 421 |
| 27273 | 8,348 |
| 26385 | 613 |
| 28118 | 1,215 |
| 26560 | 1,994 |
| 26383 | 512 |
| 26405 | 1,764 |
| 23414 | 1,301 |
The upper seven monkeys were vaccinated; the lower six were control monkeys.
Neutralizing antibodies were undetectable in all monkeys, on the day of initial SabRV1-SIV immunization, on the day of challenge (week 30), and 2 weeks after challenge (week 32) (see Materials and Methods). Titers indicated are reciprocal dilutions.
All seven vaccinated monkeys and the six new control monkeys were assayed for SIV-neutralizing antibody titers. No neutralizing antibodies were detected before the vaginal SIV challenge in any animal (Table 4). No neutralizing antibodies were evident postchallenge in vaccinated monkeys 27270 and 27244, again consistent with the SIV load data, indicating that these two monkeys were fully protected from infection. Among the remaining monkeys, fourfold-higher neutralizing-antibody titers were seen in the vaccinated monkeys than in the control monkeys postchallenge.
To quantify the SIV viremia in the challenged animals, a sensitive quantitative RT-PCR assay was used that has been used in several macaque SIV studies (36, 53, 67). All six concurrently challenged control monkeys had significant SIV loads, peaking between weeks 2 and 4 and reaching postacute geometric means of 9.3 × 105 copies/ml by weeks 24 to 32 (Fig. 10).
FIG. 10.
SIV RNA loads. SIV RNA levels in plasma were measured postchallenge. All seven vaccinated monkeys and six control monkeys were challenged with an intravaginal inoculation of highly virulent SIVmac251 at week 30 of the experiment (challenge was at week 0). Vaccinated monkeys (right panel) are indicated by the symbols used in previous figures: 25231, ▪; 27244, ●; 27250, ▴; 27253, ♦; 27270, □; 27273, ○; and 28508, ▵. Control monkeys (left panel) are indicated by symbols as follows: 26383, ★; 26385, ✖; 23414, ; 26405, ⋄; 26560, ▿; and 28118, ◂. Vaccinated monkeys 27270 and 27244 were never positive for SIV RNA and appeared to be completely protected. The threshold sensitivity of the assay (indicated by a dashed line) is 100 RNA copy equivalents/ml. Data points below the threshold value are shown at 100. †, animal died between weeks 32 and 44 postchallenge.
SabRV-SIV-vaccinated animals 27244 and 27270 had no detectable SIV RNA in their plasma at any time point, confirming that these two animals were solidly protected (Fig. 10). Compared with the control monkeys, the seven monkeys vaccinated with SabRV1-SIV–SabRV2-SIV exhibited a 3.0 log10 reduction in postacute geometric-mean viral load (P < 0.01). Control of postacute viremia was particularly obvious in two vaccinated monkeys: vaccinated monkey 28508 exhibited stable long-term control of viremia to 103 copies/ml, and vaccinated monkey 25231 reduced its SIVmac251 viremia by more than 106-fold during the postacute phase, indicative of a strong vaccine-elicited cellular immune response (Fig. 10).
Clinical outcome of vaginal challenge with SIVmac251.
The clinical outcome of SIV infection was much worse in the control animals than in the SabRV-SIV-immunized animals. Five of six control animals had marked decreases in CD4+ T-lymphocyte counts (Fig. 11A) and body weight (Fig. 11B) over the 48-week postchallenge observation period. Three of the six control animals were euthanatized, at 34, 35, and 44 weeks postchallenge, because of severe clinical AIDS (Fig. 11C). At necropsy, two of the animals (23414 and 26560) had lymphoma and the other animal (28118) had severe nonresponsive enteritis and wasting.
FIG. 11.
Clinical outcomes of vaginal challenge with SIVmac251. (A) Postchallenge CD4+ T-lymphocyte counts. CD3+ CD4+ T-lymphocyte counts in the peripheral blood of naive control cynomolgus macaques (left) and SabRV1-SIV- and SabRV2-SIV-vaccinated macaques (right) were determined on the day of challenge and through 36 weeks postchallenge. Symbols are as defined in the legend to Fig. 10. (B) Body weight. Weights of control macaques (left) and SabRV1-SIV- and SabRV2-SIV-vaccinated macaques (right) were measured on the day of challenge and through 44 weeks postchallenge. Weight is indicated as a percentage of the body weight measured on the day of challenge. Body weight changes of vaccinated animals were significantly better (P < 0.003) than those of the control monkeys. Symbols used are the same as for panel A. (C) Mortality curve. SabRV-SIV-vaccinated animals, ▪; control animals, ●.
In sharp contrast, all seven of the vaccinated monkeys were alive (P < 0.07) and healthy (significantly higher body weight, P < 0.003 [Fig. 11B]) at 48 weeks postchallenge. Although CD4+ T-cell counts declined initially after challenge, the counts stabilized at about 16 weeks postchallenge for five of the seven vaccinated animals (Fig. 11A). Over the course of the study, the SabRV-SIV-vaccinated animals had higher average CD4+ counts than the control animals. At 36 weeks postchallenge, the average CD4+ cell count of vaccinated animals was 840/μl, while five of six control monkeys had CD4+ counts below 150 cells/μl. Two vaccinated animals (27250 and 27273) had depressed CD4+ T-cell counts after challenge, consistent with their higher SIV viremia levels, but their body weights have remained stable (Fig. 11B) and they appear clinically normal. The other five animals have gained weight steadily since the vaccine challenge (Fig. 11B). These results demonstrate that the SabRV-SIV vaccine protects monkeys from SIV-related disease progression.
DISCUSSION
The primary goals of this study were to construct SabRV-SIV candidate SIV vaccine viruses and assess their immunogenicity and efficacy in preventing vaginal transmission of SIV. A vaginal challenge was chosen because our primary interest in the SabRV vector is as a vaccine vector capable of protecting against sexually transmitted HIV. Given that more than 90% of HIV-1 infections worldwide occur via sexual transmission, any strategy to truly control the AIDS pandemic must include a vaccine that prevents sexual transmission of HIV-1. SIVmac251 is an uncloned and highly virulent virus that has proven to be extremely difficult to protect against in rhesus and cynomolgus macaque challenge experiments (14, 17, 22, 25, 39, 69). We decided to use SIVmac251 as the challenge virus because the use of a pathogenic, uncloned, difficult-to-neutralize virus models “real-world” HIV transmission.
The SabRV1-SIV–SabRV2-SIV vaccine provided protection from SIV infection in two of seven vaccinated monkeys and rendered protection from disease progression in all vaccinated monkeys. SIV replication was controlled (650 copies/ml and <100 copies/ml at week 32) in two monkeys. Thus, there was a clear difference between the clinical courses of the postchallenge SIV infection in the control and vaccinated animals. Half of the control animals developed end-stage clinical AIDS, while all of the vaccinated monkeys were protected from AIDS through the 48-week postchallenge observation period (P < 0.07). Although not all vaccinated monkeys were protected from infection, the SabRV-SIV vaccine protected all of the immunized monkeys from SIV-related disease progression, in terms of both viral load (P < 0.01) and general health (body weight; P < 0.003). This is the first report of a vaccine vector providing protection against a vaginal challenge with a highly virulent SIV. Indeed, although experiments with cynomolgus macaques and rhesus macaques are not necessarily directly comparable, the results reported here appear equivalent to or better than the highest levels of mucosal protection afforded by any published subunit vaccine (40), DNA vaccine (25), or viral vector system (3, 11, 14, 24) in macaques challenged with a highly virulent uncloned SIV. The significant levels of protection observed in this study indicate that SabRV has considerable potential as a human vaccine vector.
Immunogenicity.
We previously showed that live poliovirus-based vaccine vectors elicit humoral, mucosal, and cellular immune responses in primates (15). In the present study, we observed better serum and mucosal antibody responses than we observed with the previous poliovirus vector system, in terms of the proportion of monkeys responding, maximum antibody titers, and the consistency of the immune responses. We believe that these enhanced responses are due to higher-quality virus stocks, the use of vectors based on Sabin 1 and Sabin 2 molecular clones, and the use of a cocktail vaccine approach that allowed the expression of a 10-fold-larger number of SIV antigens. This was our first use of pSabRV2- or pSabRV1-derived viruses, and the results demonstrate that Sabin 1- and Sabin 2-based constructs are immunogenic as viral vectors. Also, these data show the effectiveness of a prime-and-boost strategy using two serotypes of the same viral vector.
A consistent feature in both of our live poliovirus vector primate experiments to date is the compartmentalization of the IgA immune responses in some monkeys. We found that all seven monkeys exhibited at least transient anti-SIV rectal IgA responses after SabRV1-SIV immunization, even though only three had detectable levels of anti-SIV IgA in their sera (Fig. 4B and 5B). Neither of the two monkeys that had long-lasting serum IgA responses after SabRV1-SIV immunization (27270 and 28508 [Fig. 4B]) had a detectable level of rectal IgA antibodies for more than 2 weeks. The results of these experiments are consistent with the conclusion that serum IgA and rectal IgA are from different sources (local versus systemic) and demonstrate that serum antibody levels (IgG or IgA) cannot be used as an indicator of mucosal antibody levels.
Poliovirus vectors can elicit potent CTL responses in both mice (41, 42, 72) and primates (15). In this study, we were able to detect SabRV-SIV-elicited, SIV-specific CTL responses in three of seven monkeys. Monkey 25231 had the strongest cellular immune responses, generating an SIV-antigen-specific lymphoproliferative response and having CTLs specific for both SIV Gag and Env antigens. SIV-specific CD4+ cells were likely present in all seven monkeys after the SabRV-SIV immunizations reported here, because Ig class switching to IgA and IgG is T helper dependent (1).
Correlates of protection.
Correlates of protective immunity have not been clearly determined in any HIV or SIV vaccine study. In vaccinia virus vector studies, vaccine-elicited anti-SIV envelope antibodies strongly correlated with protection against intravenous or rectal infection with a moderately virulent uncloned SIV isolate (61, 62). Recent experiments have shown that passive immunization with large quantities of HIV-neutralizing antibodies can protect macaques from an intravaginal challenge with a highly virulent lentivirus (simian-human immunodeficiency virus 89.6PD) (6, 44, 66). Those results are encouraging, because antibodies are the correlate of protection for all currently licensed human vaccines for which a correlate of protection is known (60). However, even the most robust antibody responses in HIV-infected patients do not appear to provide protection from disease progression, and thus the role of vaccine-induced antibodies in eliciting protection from SIV infection and disease remains unclear.
Likewise, the role of vaccine-induced cellular immune responses in protecting against an SIV infection is unclear. It is generally accepted that cellular immune responses play a major role in controlling (i.e., reducing the viral load of) primate lentivirus infections (9, 35, 70). Although fully protective anti-SIV cellular immunity has not been directly demonstrated in any vaccine challenge experiment (9, 21, 25, 80), experiments utilizing a live-attenuated SIV vaccine implicate cellular immune responses in protection (31).
Successful viral-vector vaccine challenges with uncloned, highly virulent SIVs such as SIVmac251 (11) and SIVsmE660 (18) have not identified clear vaccine-induced correlates of protection, and no arm of the adaptive immune system can be ruled out as a critical component of an AIDS vaccine. The idea that multiple antigens and a range of immune responses are needed for protective immunity against a retrovirus is supported by studies using the Friend mouse retrovirus system. In that system, a combination of antigen-specific B-cell, CD4+ cell, and CD8+ cell responses is necessary for full protection (19); no single arm of the adaptive immune system is sufficient.
No clear and consistent correlate of immunity was observed in the SabRV-SIV vaccination and vaginal challenge experiment reported here, but several vaccine-elicited immune responses appear to be associated with protection. Of the five monkeys with anti-Env gp120 antibody responses, four animals exhibited substantial protection against the SIV challenge. The monkey with the strongest anti-gp120 antibody response (27270), as measured in serum and vaginal secretions, was one of the two fully protected monkeys. The other fully protected monkey (27244) had the strongest anti-Env response by Western blot analysis (Fig. 7). It is therefore reasonable to propose that the SabRV-SIV vaccine-induced anti-SIV envelope antibody responses in the four protected monkeys may have played a role in the observed protection. It is intriguing to consider that protective anti-HIV or -SIV mucosal antibodies may not need to be classical neutralizing antibodies (as determined by in vitro neutralization assays). Direct neutralization is not the only effector function of antibodies. Binding of antibody to envelope protein on whole SIV virions may efficiently trap virus in the thick mucus layer, preventing the virus from reaching its target cell type. Alternatively, mucosal antibodies may activate complement-mediated destruction of virions or prevent transcytosis of virions (55, 66). These additional mechanisms of antibody action warrant further investigation in vaccine experiments designed to prevent mucosal transmission.
Regarding possible cellular correlates of immunity, monkey 25231 had the strongest vaccine-elicited CTL responses, and the CTL response may explain the striking >106-fold reduction in postacute viremia in that monkey. One of the fully protected monkeys (27244) also had SIV-specific CTLs and lymphoproliferative responses after SabRV-SIV vaccination, and those cellular responses may have played an important role in the observed protection. In summary, a variety of mechanisms may play roles in the observed protection.
RNA virus vectors.
This is the first report of a successful primate protection experiment using a live RNA virus vector. In addition, recent data by Davis et al. showed successful protection of some vaccinated animals against a highly virulent SIV by using Venezuelan equine encephalitis virus propagation-defective replicon vectors (18). Together these results prove that RNA viruses can be effective vaccine vectors. It would be prudent to pursue the development of multiple RNA virus vaccine vector systems in the interest of developing novel human and animal vaccines (4, 5, 10, 13, 18, 27, 45).
This was the first SIV challenge experiment using live poliovirus SIV vaccine vectors. It is likely that the efficacy of the SabRV-SIV vaccine can be improved. It is possible to elicit neutralizing antibodies against SIV with a vaccine (14, 26, 58), and we plan to examine the abilities of various new SabRV-Env viruses to elicit SIV-neutralizing antibodies in a mouse model system. We also plan to explore which SabRV-SIV viruses in the cocktail are required for protection by using smaller cocktails in future challenge experiments (for example, inoculating one group of monkeys with SabRV-Gag/Pol/Tat and a second group with SabRV-Env). Additionally, combining SabRV with other vaccine strategies may improve the efficacy of the vaccine. Priming macaques first with a DNA vaccine, or boosting SabRV1-SIV–SabRV2-SIV-vaccinated macaques with gp41/gp120 protein or a second viral vaccine vector, may drive the anti-SIV immune response and provide more consistent protection from challenge.
The SabRV doses used in our SIV challenge experiment are comparable to normal Sabin oral poliovirus vaccine doses used in infants, children, and adults (2). We believe that SabRV would be substantially more efficacious in humans than it was in monkeys because Sabin viruses are several orders of magnitude more infectious in people (50% infectious dose [ID50] = 50 PFU) (49) than in cynomolgus macaques (ID100= 106 PFU) (15). Thus, Sabin virus-based vectors are also likely to replicate more efficiently in people than they do in monkeys. The enhanced replication of the vectors would be expected to generate a significantly stronger immune response to SabRV-expressed antigens in people than in monkeys.
There has been some belief that the polio eradication effort of the World Health Organization makes a vaccine vector based on poliovirus a moot line of study. We strongly disagree with that notion. There are several reasons to pursue the use of SabRV as a human vaccine vector. The World Health Organization wild poliovirus eradication effort has been wonderfully successful, and we are very hopeful that wild poliovirus infections can be eliminated. However, we and others have expressed reservations about the ability to eliminate the Sabin live poliovirus vaccine viruses at any time in the near future (15, 20, 64). We believe that the experiments with recombinant poliovirus vectors reported here and previously (5, 15, 42, 72, 81) demonstrate that the Sabin strains have real potential as human vaccine vectors. Finally, to our knowledge, this is the first report of a successful primate protection experiment using a defined library cocktail vaccination approach. This indicates that vaccination with an array of defined antigenic sequences could be an effective strategy that could be pursued with other vectors. Furthermore, because of the great antigenic variability of HIV, it is possible that a similar strategy using a cocktail of multiple HIV antigens can be used to protect against diverse HIV strains.
ACKNOWLEDGMENTS
We thank Kristen Bost, Steve Joye, and Dave Bennett for technical assistance. We thank T. Parks and M. Piatak for performing the plasma viral load analysis. We thank David Montefiori for the neutralizing-antibody assays.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. NO1-CO-56000. This work was supported by Public Health Service grants AI36178 (to R.A.) and AI33434 (to C.J.M.). S.C. is a Howard Hughes Medical Institute doctoral fellow.
REFERENCES
- 1.Abbas A K, Lichtman A H, Pober J S. Cellular and molecular immunology. 4th ed. Philadelphia, Pa: W. B. Saunders; 2000. [Google Scholar]
- 2.AFHS. AFHS drug information. Bethesda, Md: SilverPlatter International; 1998. [Google Scholar]
- 3.Ahmad S, Lohman B, Marthas M, Giavedoni L, el-Amad Z, Haigwood N L, Scandella C J, Gardner M B, Luciw P A, Yilma T. Reduced virus load in rhesus macaques immunized with recombinant gp160 and challenged with simian immunodeficiency virus. AIDS Res Hum Retrovir. 1994;10:195–204. doi: 10.1089/aid.1994.10.195. [DOI] [PubMed] [Google Scholar]
- 4.Altmeyer R, Girard M, van der Werf S, Mimic V, Seigneur L, Saron M-F. Attenuated Mengo virus: a new vector for live recombinant vaccines. J Virol. 1995;69:3193–3196. doi: 10.1093/benz/9780199773787.article.b00034516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andino R, Silvera D, Suggett S D, Achacoso P L, Miller C J, Baltimore D, Feinberg M B. Engineering poliovirus as a vaccine vector for the expression of diverse antigens. Science. 1994;265:1448–1451. doi: 10.1126/science.8073288. [DOI] [PubMed] [Google Scholar]
- 6.Baba T W, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini L A, Posner M R, Katinger H, Stiegler G, Bernacky B J, Rizvi T A, Schmidt R, Hill L R, Keeling M E, Lu Y, Wright J E, Chou T C, Ruprecht R M. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 7.Baba T W, Liska V, Khimani A H, Ray N B, Dailey P J, Penninck D, Bronson R, Greene M F, McClure H M, Martin L N, Ruprecht R M. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5:194–203. doi: 10.1038/5557. . (Erratum, 5:590.) [DOI] [PubMed] [Google Scholar]
- 8.Barnett S W, Klinger J M, Doe B, Walker C M, Hansen L, Duliège A M, Sinangil F M. Prime-boost immunization strategies against HIV. AIDS Res Hum Retrovir. 1998;14(Suppl. 3):S299–S309. [PubMed] [Google Scholar]
- 9.Barouch D H, Santra S, Schmitz J E, Kuroda M J, Fu T M, Wagner W, Bilska M, Craiu A, Zheng X X, Krivulka G R, Beaudry K, Lifton M A, Nickerson C E, Trigona W L, Punt K, Freed D C, Guan L, Dubey S, Casimiro D, Simon A, Davies M E, Chastain M, Strom T B, Gelman R S, Montefiori D C, Lewis M G. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 10.Beard M R, Cohen L, Lemon S M, Martin A. Characterization of recombinant hepatitis A virus genomes containing exogenous sequences at the 2A/2B junction. J Virol. 2001;75:1414–1426. doi: 10.1128/JVI.75.3.1414-1426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benson J, Chougnet C, Robert-Guroff M, Montefiori D, Markham P, Shearer G, Gallo R C, Cranage M, Paoletti E, Limbach K, Venzon D, Tartaglia J, Franchini G. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J Virol. 1998;72:4170–4182. doi: 10.1128/jvi.72.5.4170-4182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berglund P, Quesada-Rolander M, Putkonen P, Biberfeld G, Thorstensson R, Liljestrom P. Outcome of immunization of cynomolgus monkeys with recombinant Semliki Forest virus encoding human immunodeficiency virus type 1 envelope protein and challenge with a high dose of SHIV-4 virus. AIDS Res Hum Retrovir. 1997;13:1487–1495. doi: 10.1089/aid.1997.13.1487. [DOI] [PubMed] [Google Scholar]
- 13.Bledsoe A W, Jackson C A, McPherson S, Morrow C D. Cytokine production in motor neurons by poliovirus replicon vector gene delivery. Nat Biotechnol. 2000;18:964–969. doi: 10.1038/79455. [DOI] [PubMed] [Google Scholar]
- 14.Buge S L, Richardson E, Alipanah S, Markham P, Cheng S, Kalyan N, Miller C J, Lubeck M, Udem S, Eldridge J, Robert-Guroff M. An adenovirus-simian immunodeficiency virus envvaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J Virol. 1997;71:8531–8541. doi: 10.1128/jvi.71.11.8531-8541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Committee on Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. Institute of Laboratory Resources, National Resource Council, National Academy Press; 1996. [Google Scholar]
- 15.Crotty S, Lohman B L, Lü F X-S, Tang S, Miller C J, Andino R. Mucosal immunization of cynomolgus macaques with two serotypes of live poliovirus vectors expressing simian immunodeficiency virus antigens: stimulation of humoral, mucosal, and cellular immunity. J Virol. 1999;73:9485–9495. doi: 10.1128/jvi.73.11.9485-9495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crotty S, Maag D, Arnold J J, Zhong W, Lau J Y, Hong Z, Andino R, Cameron C E. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat Med. 2000;6:1375–1379. doi: 10.1038/82191. [DOI] [PubMed] [Google Scholar]
- 17.Daniel M D, Mazzara G P, Simon M A, Sehgal P K, Kodama T, Panicali D L, Desrosiers R C. High-titer immune responses elicited by recombinant vaccinia virus priming and particle boosting are ineffective in preventing virulent SIV infection. AIDS Res Hum Retrovir. 1994;10:839–851. doi: 10.1089/aid.1994.10.839. [DOI] [PubMed] [Google Scholar]
- 18.Davis N L, Caley I J, Brown K W, Betts M R, Irlbeck D M, McGrath K M, Connell M J, Montefiori D C, Frelinger J A, Swanstrom R, Johnson P R, Johnston R E. Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J Virol. 2000;74:371–378. doi: 10.1128/jvi.74.1.371-378.2000. . (Erratum, 74:3430.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dittmer U, Brooks D M, Hasenkrug K J. Requirement for multiple lymphocyte subsets in protection by a live attenuated vaccine against retroviral infection. Nat Med. 1999;5:189–193. doi: 10.1038/5550. [DOI] [PubMed] [Google Scholar]
- 20.Dove A W, Racaniello V R. The polio eradication effort: should vaccine eradication be next? Science. 1997;277:779–780. doi: 10.1126/science.277.5327.779. [DOI] [PubMed] [Google Scholar]
- 21.Emini E A, Schleif W A, Quintero J C, Conard P G, Eichberg J W, Vlasuk G P, Lehman E D, Polokoff M A, Schaeffer T F, Schultz L D, et al. Yeast-expressed p55 precursor core protein of human immunodeficiency virus type 1 does not elicit protective immunity in chimpanzees. AIDS Res Hum Retrovir. 1990;6:1247–1250. doi: 10.1089/aid.1990.6.1247. [DOI] [PubMed] [Google Scholar]
- 22.Giavedoni L D, Planelles V, Haigwood N L, Ahmad S, Kluge J D, Marthas M L, Gardner M B, Luciw P A, Yilma T D. Immune response of rhesus macaques to recombinant simian immunodeficiency virus gp130 does not protect from challenge infection. J Virol. 1993;67:577–583. doi: 10.1128/jvi.67.1.577-583.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gohara D W, Crotty S, Arnold J J, Yoder J D, Andino R, Cameron C E. Poliovirus RNA-dependent RNA polymerase (3Dpol): structural, biochemical, and biological analysis of conserved structural motifs A and B. J Biol Chem. 2000;275:25523–25532. doi: 10.1074/jbc.M002671200. [DOI] [PubMed] [Google Scholar]
- 24.Hanke T, Neumann V C, Blanchard T J, Sweeney P, Hill A V, Smith G L, McMichael A. Effective induction of HIV-specific CTL by multi-epitope using gene gun in a combined vaccination regime. Vaccine. 1999;17:589–596. doi: 10.1016/s0264-410x(98)00238-2. [DOI] [PubMed] [Google Scholar]
- 25.Hanke T, Samuel R V, Blanchard T J, Neumann V C, Allen T M, Boyson J E, Sharpe S A, Cook N, Smith G L, Watkins D I, Cranage M P, McMichael A J. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J Virol. 1999;73:7524–7532. doi: 10.1128/jvi.73.9.7524-7532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch V M, Fuerst T R, Sutter G, Carroll M W, Yang L C, Goldstein S, Piatak M, Jr, Elkins W R, Alvord W G, Montefiori D C, Moss B, Lifson J D. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Höfling K, Tracy S, Chapman N, Kim K-S, Leser J S. Expression of an antigenic adenovirus epitope in a group B coxsackievirus. J Virol. 2000;74:4570–4578. doi: 10.1128/jvi.74.10.4570-4578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoxie J A, Haggarty B S, Bonser S E, Rackowski J L, Shan H, Kanki P J. Biological characterization of a simian immunodeficiency virus-like retrovirus (HTLV-IV): evidence for CD4-associated molecules required for infection. J Virol. 1988;62:2557–2568. doi: 10.1128/jvi.62.8.2557-2568.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hull H F, Ward N A, Hull B P, Milstien J B, de Quadros C. Paralytic poliomyelitis: seasoned strategies, disappearing disease. Lancet. 1994;343:1331–1337. doi: 10.1016/s0140-6736(94)92472-4. [DOI] [PubMed] [Google Scholar]
- 30.Imaoka K, Miller C J, Kubota M, McChesney M B, Lohman B, Yamamoto M, Fugihashi K, Someya K, Honda M, McGhee J R, Kiyono H. Nasal immunization of nonhuman primates with simian immunodeficiency virus p55gagand cholera toxin adjuvant induces Th1/Th2 help for virus specific immune responses in reproductive tissues. J Immunol. 1998;161:5952–5958. [PubMed] [Google Scholar]
- 31.Johnson R P, Desrosiers R C. Protective immunity induced by live attenuated simian immunodeficiency virus. Curr Opin Immunol. 1998;10:436–443. doi: 10.1016/s0952-7915(98)80118-0. [DOI] [PubMed] [Google Scholar]
- 32.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, Desrosiers R. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 33.Kohara M, Abe S, Kuge S, Semler B L, Komatsu T, Arita M, Itoh H, Nomoto A. An infectious cDNA clone of the poliovirus Sabin strain could be used as a stable repository and inoculum for the oral polio live vaccine. Virology. 1986;151:21–30. doi: 10.1016/0042-6822(86)90100-5. [DOI] [PubMed] [Google Scholar]
- 34.Lehner T, Wang Y, Cranage M, Bergmeier L A, Mitchell E, Tao L, Hall G, Dennis M, Cook N, Brookes R, Klavinskis L, Jones I, Doyle C, Ward R. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat Med. 1996;2:767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 35.Letvin N L, Schmitz J E, Jordan H L, Seth A, Hirsch V M, Reimann K A, Kuroda M J. Cytotoxic T lymphocytes specific for the simian immunodeficiency virus. Immunol Rev. 1999;170:127–134. doi: 10.1111/j.1600-065x.1999.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 36.Lifson J D, Rossio J L, Arnaout R, Li L, Parks T L, Schneider D K, Kiser R F, Coalter V J, Walsh G, Imming R J, Fisher B, Flynn B M, Bischofberger N, Piatak M, Jr, Hirsch V M, Nowak M A, Wodarz D. Containment of simian immunodeficiency virus infection: cellular immune responses and protection from rechallenge following transient postinoculation antiretroviral treatment. J Virol. 2000;74:2584–2593. doi: 10.1128/jvi.74.6.2584-2593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohman B L, Higgins J, Marthas M L, Marx P A, Pedersen N C. Development of simian immunodeficiency virus isolation, titration, and neutralization assays which use whole blood from rhesus monkeys and an antigen capture enzyme-linked immunosorbent assay. J Clin Microbiol. 1991;29:2187–2192. doi: 10.1128/jcm.29.10.2187-2192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lü F X, Ma Z, Rourke T, Srinivasan S, McChesney M, Miller C J. Immunoglobulin concentrations and antigen-specific antibody levels in cervicovaginal lavages of rhesus macaques are influenced by the stage of the menstrual cycle. Infect Immun. 1999;67:6321–6328. doi: 10.1128/iai.67.12.6321-6328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu S, Arthos J, Montefiori D C, Yasutomi Y, Manson K, Mustafa F, Johnson E, Santoro J C, Wissink J, Mullins J I, Haynes J R, Letvin N L, Wyand M, Robinson H L. Simian immunodeficiency virus DNA vaccine trial in macaques. J Virol. 1996;70:3978–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lü X, Kiyono H, Lu D, Kawabata S, Torten J, Srinivasan S, Dailey P J, McGhee J R, Lehner T, Miller C J. Targeted lymph-node immunization with whole inactivated simian immunodeficiency virus (SIV) or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251. AIDS. 1997;12:1–10. doi: 10.1097/00002030-199801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandl S, Hix L, Andino R. Preexisting immunity to poliovirus does not impair the efficacy of recombinant poliovirus vaccine vectors. J Virol. 2001;75:622–627. doi: 10.1128/JVI.75.2.622-627.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandl S, Sigal L J, Rock K L, Andino R. Poliovirus vaccine vectors elicit antigen-specific cytotoxic T cells and protect mice against lethal challenge with malignant melanoma cells expressing a model antigen. Proc Natl Acad Sci USA. 1998;95:8216–8221. doi: 10.1073/pnas.95.14.8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marthas M L, Ramos R A, Lohman B L, Van Rompay K K A, Unger R E, Miller C J, Banapour B, Pedersen N C, Luciw P A. Viral determinants of simian immunodeficiency virus (SIV) virulence in rhesus macaques assessed by using attenuated and pathogenic molecular clones of SIVmac. J Virol. 1993;67:6047–6055. doi: 10.1128/jvi.67.10.6047-6055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mascola J R, Stiegler G, VanCott T C, Katinger H, Carpenter C B, Hanson C E, Beary H, Hayes D, Frankel S S, Birx D L, Lewis M G. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 45.McAllister A, Arbetman A E, Mandl S, Peña-Rossi C, Andino R. Recombinant yellow fever viruses are effective therapeutic vaccines for treatment of murine experimental solid tumors and pulmonary metastases. J Virol. 2000;74:9197–9205. doi: 10.1128/jvi.74.19.9197-9205.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McChesney M B, Collins J R, Lu D, Lu X, Torten J, Ashley R L, Cloyd M W, Miller C J. Occult systemic infection and persistent simian immunodeficiency virus (SIV)-specific CD4+-T-cell proliferative responses in rhesus macaques that were transiently viremic after intravaginal inoculation of SIV. J Virol. 1998;72:10029–10035. doi: 10.1128/jvi.72.12.10029-10035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melnick J L. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 655–712. [Google Scholar]
- 48.Miller C J, McChesney M B, Lü X, Dailey P J, Chutkowski C, Lu D, Brosio P, Roberts B, Lu Y. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J Virol. 1997;71:1911–1921. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minor P D. Poliovirus. In: Nathanson N, Ahmed R, editors. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven Publishers; 1997. pp. 555–574. [Google Scholar]
- 50.Montefiori D C, Baba T W, Li A, Bilska M, Ruprecht R M. Neutralizing and infection-enhancing antibody responses do not correlate with the differential pathogenicity of SIVmac239Δ3 in adult and infant rhesus monkeys. J Immunol. 1996;157:5528–5535. [PubMed] [Google Scholar]
- 51.Morrow C D, Novak M J, Ansardi D C, Porter D C, Moldoveanu Z. Recombinant viruses as vectors for mucosal immunity. Curr Top Microbiol Immunol. 1999;236:255–273. doi: 10.1007/978-3-642-59951-4_13. [DOI] [PubMed] [Google Scholar]
- 52.Mueller S, Wimmer E. Expression of foreign proteins by poliovirus polyprotein fusion: analysis of genetic stability reveals rapid deletions and formation of cardioviruslike open reading frames. J Virol. 1998;72:20–31. doi: 10.1128/jvi.72.1.20-31.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy C G, Lucas W T, Means R E, Czajak S, Hale C L, Lifson J D, Kaur A, Johnson R P, Knipe D M, Desrosiers R C. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J Virol. 2000;74:7745–7754. doi: 10.1128/jvi.74.17.7745-7754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nomoto A, Omata T, Toyoda H, Kuge S, Horie H, Kataoka Y, Genba Y, Nakano Y, Imura N. Complete nucleotide sequence of the attenuated poliovirus Sabin 1 strain genome. Proc Natl Acad Sci USA. 1982;79:5793–5797. doi: 10.1073/pnas.79.19.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogra P L. Mucosal immunology. 2nd ed. San Diego, Calif: Academic Press; 1999. [Google Scholar]
- 56.Ogra P L, Karon D T. Formation and function of poliovirus antibody in different tissues. Prog Med Virol. 1971;13:156–193. [Google Scholar]
- 57.Omata T, Kohara M, Sakai Y, Kameda A, Imura N, Nomoto A. Cloned infectious complementary DNA of the poliovirus Sabin 1 genome: biochemical and biological properties of the recovered virus. Gene. 1984;32:1–10. doi: 10.1016/0378-1119(84)90026-x. [DOI] [PubMed] [Google Scholar]
- 58.Ourmanov I, Brown C R, Moss B, Carroll M, Wyatt L, Pletneva L, Goldstein S, Venzon D, Hirsch V M. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J Virol. 2000;74:2740–2751. doi: 10.1128/jvi.74.6.2740-2751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paul J R, Riordan J T, Melnick J L. Antibodies to three different antigenic types of poliomyelitis virus in sera from North Alaskan eskimos. Am J Hyg. 1951;54:275–285. doi: 10.1093/oxfordjournals.aje.a119485. [DOI] [PubMed] [Google Scholar]
- 60.Plotkin S, Orenstein W, editors. Vaccines. 3rd ed. Philadelphia, Pa: W. B. Saunders; 1999. [Google Scholar]
- 61.Polacino P, Stallard V, Klaniecki J E, Montefiori D C, Langlois A J, Richardson B A, Overbaugh J, Morton W R, Benveniste R E, Hu S-L. Limited breadth of the protective immunity elicited by simian immunodeficiency virus SIVmne gp160 vaccines in a combination immunization regimen. J Virol. 1999;73:618–630. doi: 10.1128/jvi.73.1.618-630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Polacino P, Stallard V, Montefiori D C, Brown C R, Richardson B A, Morton W R, Benveniste R E, Hu S-L. Protection of macaques against intrarectal infection by a combination immunization regimen with recombinant simian immunodeficiency virus SIVmne gp160 vaccines. J Virol. 1999;73:3134–3146. doi: 10.1128/jvi.73.4.3134-3146.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pollard S R, Dunn G, Cammack N, Minor P D, Almond J W. Nucleotide sequence of a neurovirulent variant of the type 2 oral poliovirus vaccine. J Virol. 1989;63:4949–4951. doi: 10.1128/jvi.63.11.4949-4951.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Racaniello V R. It is too early to stop polio vaccination. Bull W H O. 2000;78:359–360. [PMC free article] [PubMed] [Google Scholar]
- 65.Rezapkin G V, Alexander W, Dragunsky E, Parker M, Pomeroy K, Asher D M, Chumakov K M. Genetic stability of Sabin 1 strain of poliovirus: implications for quality control of oral poliovirus vaccine. Virology. 1998;245:183–187. doi: 10.1006/viro.1998.9191. [DOI] [PubMed] [Google Scholar]
- 66.Robert-Guroff M. IgG surfaces as an important component in mucosal protection. Nat Med. 2000;6:129–130. doi: 10.1038/72206. [DOI] [PubMed] [Google Scholar]
- 67.Robinson H L, Montefiori D C, Johnson R P, Manson K H, Kalish M L, Lifson J D, Rizvi T A, Lu S, Hu S L, Mazzara G P, Panicali D L, Herndon J G, Glickman R, Candido M A, Lydy S L, Wyand M S, McClure H M. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat Med. 1999;5:526–534. doi: 10.1038/8406. [DOI] [PubMed] [Google Scholar]
- 68.Ruprecht R M. Live attenuated AIDS viruses as vaccines: promise or peril? Immunol Rev. 1999;170:135–149. doi: 10.1111/j.1600-065x.1999.tb01335.x. [DOI] [PubMed] [Google Scholar]
- 69.Schlienger K, Montefiori D C, Mancini M, Rivière Y, Tiollais P, Michel M-L. Vaccine-induced neutralizing antibodies directed in part to the simian immunodeficiency virus (SIV) V2 domain were unable to protect rhesus monkeys from SIV experimental challenge. J Virol. 1994;68:6578–6588. doi: 10.1128/jvi.68.10.6578-6588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 71.Schultz A. Using recombinant vectors as HIV vaccines. IAVI Rep. 1998;3:1–4. [Google Scholar]
- 72.Sigal L J, Crotty S, Andino R, Rock K L. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 73.Singh M, Cattaneo R, Billeter M A. A recombinant measles virus expressing hepatitis B virus surface antigen induces humoral immune responses in genetically modified mice. J Virol. 1999;73:4823–4828. doi: 10.1128/jvi.73.6.4823-4828.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suryanarayana K, Wiltrout T A, Vasquez G M, Hirsch V M, Lifson J D. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res Hum Retrovir. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 75.Sutter R W, Cochi S L, Melnick J L. Live attenuated poliovirus vaccines. In: Plotkin S, Orenstein W, editors. Vaccines. 3rd ed. Philadelphia, Pa: W. B. Saunders; 1999. pp. 364–408. [Google Scholar]
- 76.Tang S, van Rij R, Silvera D, Andino R. Toward a poliovirus-based simian immunodeficiency virus vaccine: correlation between genetic stability and immunogenicity. J Virol. 1997;71:7841–7850. doi: 10.1128/jvi.71.10.7841-7850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.UNAIDS and World Health Organization. Report on the global HIV/AIDS epidemic. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 78.Wang S-W, Kozlowski P A, Schmelz G, Manson K, Wyand M S, Glickman R, Montefiori D, Lifson J D, Johnson R P, Neutra M R, Aldovini A. Effective induction of simian immunodeficiency virus-specific systemic and mucosal immune responses in primates by vaccination with proviral DNA producing intact but noninfectious virions. J Virol. 2000;74:10514–10522. doi: 10.1128/jvi.74.22.10514-10522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weeks-Levy C, Ogra P L. Polioviruses and mucosal vaccines. In: Kiyono H, Ogra P, McGhee J, editors. Mucosal vaccines. New York, N.Y: Academic Press; 1996. pp. 283–294. [Google Scholar]
- 80.Yasutomi Y, Koenig S, Woods R M, Madsen J, Wassef N M, Alving C R, Klein H J, Nolan T E, Boots L J, Kessler J A, Emini E A, Conley A J, Letvin N L. A vaccine-elicited, single viral epitope-specific cytotoxic T lymphocyte response does not protect against intravenous, cell-free simian immunodeficiency virus challenge. J Virol. 1995;69:2279–2284. doi: 10.1128/jvi.69.4.2279-2284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yim T J, Tang S, Andino R. Poliovirus recombinants expressing hepatitis B virus antigens elicited a humoral immune response in susceptible mice. Virology. 1996;218:61–70. doi: 10.1006/viro.1996.0166. [DOI] [PubMed] [Google Scholar]