Abstract
Previous study has shown that there is a functional link between the transient receptor potential vanilloid type 1 (TRPV1) receptor and protease-activated receptor-4 (PAR4) in modulation of inflammation and pain. Capsaicin activation of TRPV1 is involved in enhancement of the expression of TRPV1 in mRNA and protein in dorsal root ganglion (DRG) in vivo. Whether capsaicin could influence expression of PAR4 in primary sensory neurons remains unknown. In the present study, expression of PAR4 in cultured rat DRG neurons was observed using immunofluorescence, real-time PCR and Western blots to examine whether increases in PAR4 mRNA and protein levels are induced by capsaicin treatment with or without pre-treatment of forskolin, a cyclic AMP/protein kinase A (cAMP/PKA) activator or PKA inhibitor fragment 14-22 (PKI14-22), a PKA inhibitor. Capsaicin treatment of cultured DRG neurons significantly increased the expression of PAR4 in mRNA and protein levels. The percentage of PAR4-, TRPV1-immunoreactive neurons and their co-localization in cultured DRG neurons increased significantly in the presence of capsaicin as compared with that in the absence of capsaicin. Compared with capsaicin-only group, pre-incubation with forskolin strongly enhanced the capsaicin-induced increase of PAR4 in mRNA and protein levels. Consistent with the involvement of PKA in the modulation of PAR4 expression, this evoked expression both at mRNA and protein levels was significantly inhibited after PKA was inhibited by pre-incubation with PKI14-22. Taken together, these results provide evidence that TRPV1 activation significantly increases the expression of PAR4 mRNA and protein levels in primary cultures of DRG neurons after capsaicin incubation. Effects of capsaicin on PAR4 expression appear to be mediated by cAMP/PKA signal pathways in DRG neurons.
Keywords: Protease-activated receptor-4, Transient receptor potential vanilloid type 1, Cultured primary afferent neurons, Protein kinase A, Real-time PCR, Rat
Introduction
Protease-activated receptors (PARs), a family of G-protein coupled receptors, are activated by proteases released by cell damage or blood clotting thought to be a potent modulator of inflammation and nociceptive responses (Ramachandran and Hollenberg 2008; García et al. 2010). Until now, four family members of PARs have been cloned and PAR4 is the last to be discovered. These receptors are activated by cleaving the extracellular N-terminal domain to expose a tethered peptide ligand (Julius and Basbaum 2001; Ramachandran and Hollenberg 2008). Several studies have described that PAR4 is expressed on numerous cell types, such as endothelial cells, macrophages, mast cells, sensory neurons, and primary sensory terminals (Vergnolle et al. 2002; Hollenberg et al. 2004; Asfaha et al. 2007; Zhao et al. 2012).
It is believed that PAR4 play important roles in the regulation of nociceptive processing underlying intracellular signal mechanisms (Augé et al. 2009; Karanjia et al. 2009; Vellani et al. 2010). PAR4 protein and mRNA are expressed in small nociceptive dorsal root ganglion (DRG) neurons and the majority of these cells are peptidergic that are marked by substance P and calcitonin gene-related peptide (CGRP) neurons (Zhu et al. 2005; Asfaha et al. 2007). Activation of PAR4 has been shown to induce neuropathic pain in primary sensory neurons of DRG (McDougall et al. 2009; Russell et al. 2010). PAR4 activation in vitro results in transient receptor potential vanilloid type 1 (TRPV1) receptor sensitization, whereas its activation in vivo results in painful behavior (Vellani et al. 2010), suggesting a functional link between TRPV1 and PAR4.
TRPV1 is an ion channels thought to be pain-transducers, the capsaicin receptor that responds to noxious heat (Dong et al. 2012; Han et al. 2012). Studies have provided evidence that capsaicin activation of TRPV1 is involved in noxious responses and in induction of the expression of TRPV1 and CGRP in mRNA and protein levels in DRG neurons (Xu et al. 2009). However, the role of capsaicin on expression of PAR4 in DRG is less clear.
Activation of protein kinase A (PKA) plays critical roles in cell signaling (Aley et al. 2001). Accumulating evidence suggests that cyclic AMP (cAMP)/PKA has a key role in the pain process induced by TRPV1 activating (Schnizler et al. 2008). Studies in cultured DRG neurons have shown that PKA potentiates heat, proton, capsaicin, and pro-inflammatory substance responses (Bhave et al. 2002; Mohapatra and Nau 2005; Vellani et al. 2008). PKA in primary sensory neurons plays a prominent role in the modulation of hypersensitivity to noxious stimuli after TRPV1 activation (Mohapatra and Nau 2005; Wang et al. 2012). Capsaicin and some other pain mediators stimulate PKA to induce phosphorylation of TRPV1 (Bhave et al. 2002; Lee et al. 2012). Previous study has showed PKA play critical roles in the cell signaling of TRPV1 induced by PARs activation (Amadesi et al. 2006; Zhang et al. 2011; Nishimura et al. 2010). Recently, PAR2 activation was described as a critical to induction of nerve injury-induced neuronal hyperexcitability and cAMP/PKA activation (Huang et al. 2012). Although some reports show that PAR4 sensitizes TRPV1, we are interested in the role that TRPV1 activation and cAMP/PKA might have synergistic effects on PAR4 expression as the drive for maintaining nociceptor sensitization.
In this study, we used immunofluorescence, real-time PCR, and Western blots to examine whether capsaicin regulated the expression of PAR4 in cultured rat DRG neurons, and PKA activator or inhibitor to identify whether their activation affects the expression of PAR4 in mRNA and protein levels.
Materials and Methods
Culture of Dissociated DRG Neurons
All procedures utilizing animals were approved by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Two-week-old male Wistar rats were decapitated after anesthesia. Bilateral DRGs was removed and placed in a 35-mm culture dish containing D-Hanks solution, and digested with 0.25 % collagenase (Sigma, St. Louis, MO) in D-Hanks solution at 37 °C for 15 min. After removing the collagenase and washing with fetal bovine serum (FBS), 0.25 % trypsin (Sigma) was added and digested for 10 min. Digestion was inhibited by FBS, cells were centrifuged and resuspended in culture medium (DMEM containing 10 % FBS, 1 % penicillin/streptomycin solution, and 1 % l-glutamine, GIBCO by Invitrogen), 10 μM cytosine arabinoside (Sigma, St. Louis, MO), and 50 ng/ml nerve growth factor (Promega). Cells were plated in 6-well plates (Costar, Corning, NY) and incubated at 37 °C in a humidified 95 % air/5 % CO2 incubator.
Drug Administration
Dissociated DRG neurons were cultured in 6-well plates overnight. Cultured neurons were continuously stimulated with capsaicin (a TRPV1 agonist) at the concentration of 1 μM for 1 h at 37 °C. To assess the possible underlying signaling pathway for regulation of capsaicin in PAR4 expression, 1 μM forskolin (a cAMP/PKA activator) or 3 μM myristoylated PKA inhibitor fragment 14–22 (PKI14–22, a PKA inhibitor) was added to 6-well plates 30 min before capsaicin incubation. Experience started just after the washout of the capsaicin-containing medium and continued from 30 to 60 min beyond washout of capsaicin. All drugs were from Sigma-Aldrich Chemicals. Cells with culture medium (DMEM) without any drugs treatment were used as control.
Immunocytochemistry for Cultured DRG Neurons
Dissociated DRG neurons were cultured on poly-l-lysine-coated coverslips in 6-well plates for 3 days. After incubation with capsaicin (1 μM, 37 °C) for 1 h, cultured neurons were washed in phosphate buffered saline (PBS) containing 1 % bovine serum albumin (BSA) and then incubated for 20 min in 4 % paraformaldehyde. Double-labeling immunoreactive procedure with PAR4 and TRPV1 on coverslip cultured neurons was performed. Coverslips were first blocked with 1 % BSA, 0.5 % Triton X-100, and 10 % normal donkey serum then incubated overnight with rabbit polyclonal anti-PAR4 antibody (1:200; Alomone Laboratories, Israel) and goat polyclonal anti-TRPV1 antibody (1:200; Santa Cruz Biotechnology Inc., USA). Coverslips were then incubated with a 1:1 mixture of the matching secondary antibodies. Immunoreactivity was detected using FITC conjugated donkey anti-rabbit secondary antibody (1:200, Jackson ImmunoResearch, West Grove, PA), and Cy3 conjugated donkey anti-goat secondary antibody (1:200, Jackson ImmunoResearch, West Grove, PA). Coverslips were mounted onto slides. As controls for antibody specificity, primary anti-serums were pre-incubated with cognate peptides used for immunization (10 μM) for 24 h at 4 °C before staining.
Labeled coverslips were examined and processed by confocal laser scanning microscopy (Nikon A1) for staining of PAR4- and TRPV1-immunofluorescence. Two fluorescence filters (FITC for green and Cy3 for red) were used to separate individual wave lengths for staining. A sequential scanning method was used to avoid bleed-through. Thus, digitized images were obtained of two different colors for PAR4 and TRPV1, respectively. Analysis of co-staining was done by matching of corresponding regions. A neuron was considered as positively double labeled if it showed two color codes overlapping in space. For quantification, the number of single- and double-labeled neurons with different labeling per coverslip was counted and the percentage of positive neurons relative to the total number of neurons visualized in each coverslip was calculated and averaged; ten coverslips were used for each condition.
Immunohistochemistry for DRG Sections
Adult male Wistar rats weighing 200–250 g were killed by decapitation; bilateral DRGs was removed and fixed in 4 % paraformaldehyde for 30 min. After an overnight cryoprotection in 20 % buffered sucrose, cryostat sections. 10 μm thick from DRG were mounted on poly-l-lysine-coated slides. Sections were preincubated with 1 % BSA, 0.5 % Triton X-100, and 10 % normal donkey serum for 1 h at room temperature. Double-labeling immunofluorescence staining for rabbit polyclonal anti-PAR4 antibody (1:200) and goat polyclonal anti-TRPV1 antibody (1:200) was performed. Sections were incubated with a mixture of two primary antibodies overnight at room temperature in a moist chamber. Then, sections were transferred to a secondary antibody solution containing FITC conjugated donkey anti-rabbit secondary antibody (1:200), and Cy3 conjugated donkey anti-goat secondary antibody (1:200) for 1 h at room temperature. Then slides were cover-slipped in a mixture of tris buffered saline (TBS) and glycerol (1:1, pH 8.6). For quantification, sections were taken from the fifth lumbar (L5) DRG. L5 DRG was chosen because it is the ganglion used most commonly for immunohistochemistry. 16 sections from four rats were used for each condition.
RNA Extraction and Quantitative Real-Time PCR
Total RNA was isolated from cultured DRG neurons using TRIzol reagent (Invitrogen). RNA concentration was determined spectrophotometrically. Following cDNA was synthesized using cDNA synthesis kit (Invitrogen Life Technologies, Grand Island, NY) according to the manufacturer’s instructions. The synthetic oligonucleotide primer sequences for PAR4 and β-actin were as follows: PAR4 5′-ACTGTTAGCTGAGTTGGGAAGG-3′ (sense) and 5′-ACTGTTAGCTGAGTTGGGAAGG-3′ (antisense). β-actin 5′-GAGACCTTCAACACCCCAGC-3′ (sense) and 5′-ATGTCACGCACGATTTCCCT-3′ (antisense). Following reverse transcription, quantitative real-time PCR was performed using a 7300 real-time PCR system (Applied Biosystems, Foster City, CA) according to the manufacture’s instructions. PCR were performed at 50 °C for 2 min, 94 °C for 15 min, followed by 40 cycles at 94 °C for 15 s, 58 °C for 30 s, and 72 °C for 30 s. Control reactions omitted reverse transcriptase. A comparative cycle of threshold fluorescence (ΔCt) method was used and the relative transcript amount of the target gene was normalized to that of β-actin using the 2−ΔΔCt method. The final results of real-time PCR were expressed as the ratio of mRNA of control.
Western Blot Analysis
The cells were lysed and protein was extracted. Protein lysate from each sample was separated electrophoretically in 12 % SDS–polyacrylamide gels, and transferred onto polyvinylidene lifluoride (PVDF) membranes. After blocking with 5 % non-fat, dried milk in TBST (20 mmol/L Tris–HCl, pH 7.5, 150 mmol/L NaCl, 0.05 % Tween 20), membranes were incubated with rabbit anti-PAR4 antibody (1:1000, Alomone Laboratories, Israel) in 5 % non-fat, dried milk in TBST overnight at 4 °C. After washes with TBST, membranes were incubated with horseradish peroxidase-labeled donkey anti-rabbit antibody (Jackson ImmunoResearch) diluted with 5 % non-fat dried milk in TBST and detected with enhanced chemiluminescence reagents (Amersham Biosciences). The blots were exposed to autoradiographic film and the intensity of immunoreactive bands of interest was quantified using densitometric-scanning analyses. β-actin immunoreactivity was used as a loading control. A single band for PAR4 in Western blots was expressed relative to the values for β-actin. Specificity of PAR4 antibody in immunoblot reaction was assessed by omission of primary antibody or replacement of primary antibody with normal serum.
Statistical Analysis
In each experiment, sister 6-well plates of DRG cultures were used to compare control neurons with neurons treated with capsaicin. Data are expressed as mean ± SEM. For comparison among groups, ANOVA followed by Bonferroni test was used. Significance was determined as P < 0.05.
Results
Specific Labeling of DRG Neurons by Anti-PAR4 and Anti-TRPV1
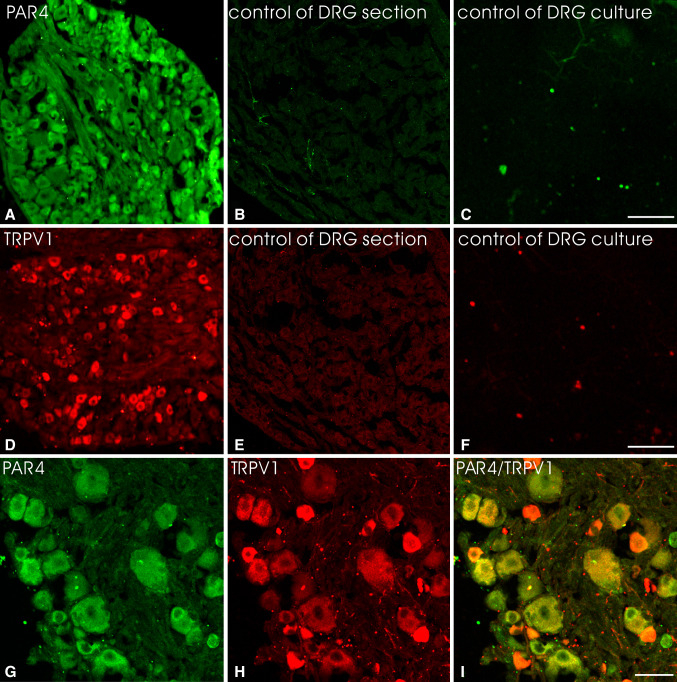
The immunoreactivity was readily discernible by the presence of an immunofluorescent labeling. Neuronal structures were considered to be immunopositive when their staining was stronger than that in the background. In DRG sections PAR4 or TRPV1-positive cell bodies were found in middle and small-size neuronal cells (Fig. 1a, d). There were no satellite cells or other elements in DRG sections expressing PAR4 and TRPV1, identical to those obtained by previous reports (Dattilio and Vizzard 2005; Augé et al. 2009; Xu et al. 2009). The immunoreactivity for PAR4 or TRPV1 was abolished in the DRG section (Fig. 1b, e) and DRG cultures (Fig. 1c, f) after preabsorption of antibody with the cognate peptide.
Fig. 1.
Immunofluorescent labeling of DRG neurons with rabbit anti-PAR4 or goat anti-TRPV1. Staining with anti-PAR4 (a) was blocked in the presence of PAR4 cognate peptide (HLRGQRWPFGEAA(S)R, 10 μM) in the DRG section (b) or DRG cultures (c). Similarly, staining with anti-TRPV1 (d) was blocked in the presence of TRPV1 cognate peptide (YTGSLKPEDAEVFKDSMVPGEK, 10 μM) in the DRG section (e) or DRG cultures (f). The DRG section co-stained with PAR4 and TRPV1 antibodies showing extensive co-localization of this two receptors in DRG neurons (g–i). There were no satellite cells in the DRG section expressing PAR4 and TRPV1. Scale bar (a–f) 100 μm; (g–i) 25 μm
PAR4 Expression and its Co-localization with TRPV1 in DRG Sections
Immunohistochemistry with an anti-PAR4 antibody demonstrated that the vast majority of neurons in DRG sections displayed PAR4 immunoreactivity and most of those co-expressed with TRPV1 (Fig. 1g–i). PAR4 staining neuronal cells were mostly of middle and small size (diameter <25 μm). Over 16 sections from four rats, 3,449 neurons were counted and 1,176 (34.1 %) expressed PAR4, 1,114 (32.3 %) displayed TRPV1 immunoreactivity, and 1,042 (30.2 %) expressed both PAR4 and TRPV1. About 88.6 % of PAR4 immnoreactive neurons expressed TRPV1 in DRG sections.
Immunofluorescence Staining of PAR4 and TRPV1 in Cultured DRG Neurons in Response to Capsaicin
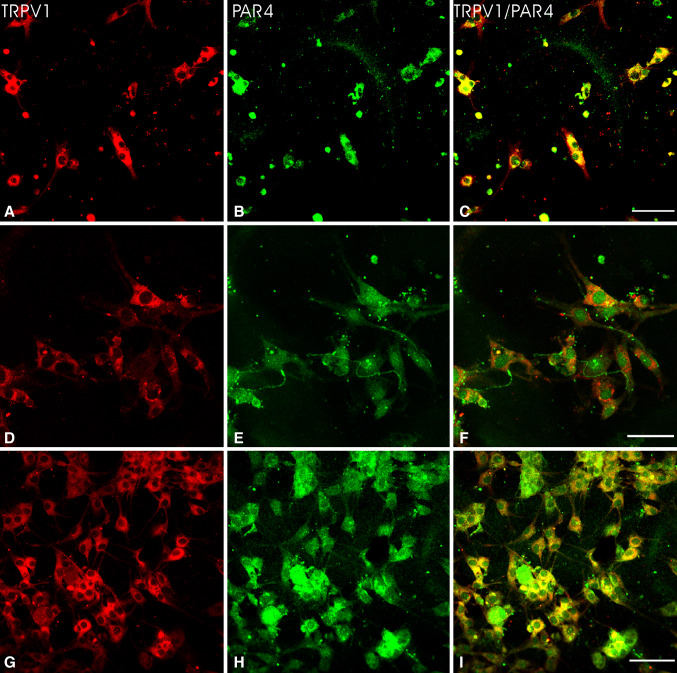
The expression of PAR4 or TRPV1 in cultured DRG neurons was determined by immunofluorescence at 3 days after plating. The most of neuronal cell bodies in DRG cultures exhibited PAR4- or TRPV1-immunoreactivity (Fig. 2). Neurons stained for these two receptors were mostly of small size. PAR4- and TRPV1-immunoreactive neurons were counted from 10 coverslips in the absence of capsaicin groups. About 33.2 ± 2.8 or 31.3 ± 3.1 % of cultured DRG neurons expressed PAR4 or TRPV1, consistent with those we observed in DRG sections.
Fig. 2.
Double immunofluorescent labeling of TRPV1 and PAR4 of cultured DRG neurons. PAR4 expression and TRPV1 staining were extensive overlap in cultured DRG neurons in the control group (a–f). Noted TRPV1 and PAR4 double-labeling neurons exhibited a long neurite that displayed PAR4 immunoreactivity lacked TRPV1 staining (d–f). The proportion of TRPV1 positive neurons with PAR4 labeling was most obvious in cultured DRG neurons after capsaicin treatment (g–i). Scale bar 40 μm
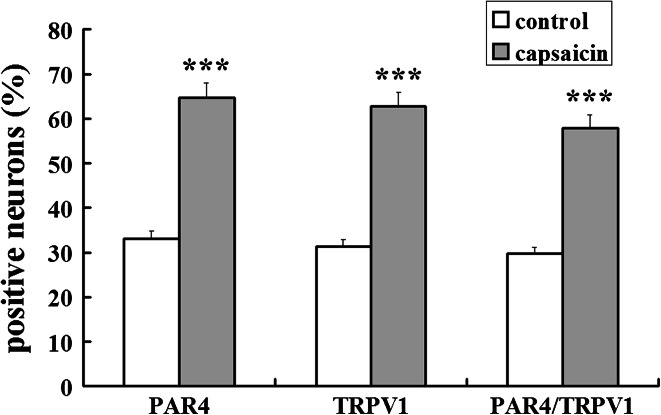
The proportion of PAR4- and TRPV1-immunofluorescence stained neurons in DRG cultures were examined at 60 min after capsaicin treatment, respectively. There were substantial increases in proportions of two receptor positive neurons seen at 60 min after capsaicin incubation compared to controls (see TRPV1, Fig. 2a, d vs. g; PAR4, Fig. 2b, e vs. h). Quantitative analysis of percentages of PAR4 and TRPV1 positive neurons and their double staining in DRG cultures is summarized in Fig. 3. There were significant increases in proportions of PAR4- and TRPV1-positive neurons in DRG cultures after capsaicin treatment compared to controls. The PAR4 and TRPV1 were detected in 64.7 ± 3.9 and 62.8 ± 3.6 % of cultured DRG neurons per coverslip after capsaicin treatment, significantly higher than those in control groups (P < 0.001 and P < 0.001).
Fig. 3.
Data summarizing changes in the percentage of TRPV1 and PAR4 positive neurons, and their co-localization in DRG cultures at 60 min after capsaicin incubation. The Y axis shows the percentage of stained neurons per coverslip. All values were mean ± SEM (n = 10 coverslips per group), *** P < 0.001 compared with control
Double-Labeling Neurons for TRPV1 and PAR4 in DRG Cultures in Response to Capsaicin
Double labeling showed that the PAR4 immunoreactivity was extensive co-localized with TRPV1 staining in cultured DRG neurons (Fig. 2a–f). Some PAR4/TRPV1 double-labeling neurons also exhibited a long neurite that was TRPV1 negative but PAR4 positive. An example showing such neurons is provided in Fig. 2d–f.
The proportion of PAR4 positive neurons with staining for TRPV1 was increased in DRG cultures at 60 min after capsaicin incubation as compared with control (see Fig. 2c, f vs. i). There was a significant increase in the percentage of PAR4 positive neurons with TRPV1 staining in DRG cultures after capsaicin treatment compared to control (Fig. 3). An average of 57.9 ± 2.6 % of cultured DRG neurons were PAR4 positive neurons with TRPV1 staining per coverslip after capsaicin treatment, significantly higher than percentage in the control group (29.7 % ± 1.7; P < 0.001).
Capsaicin Increased Expression of PAR4 mRNA and Protein in Cultured DRG Neurons
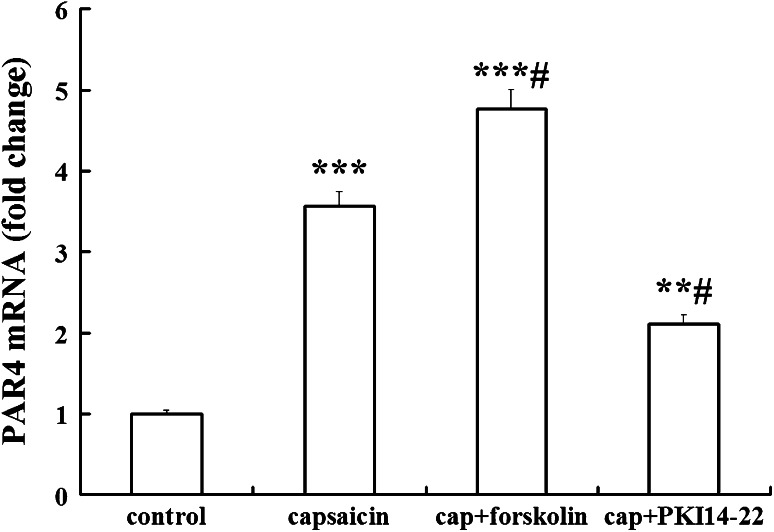
Effects of capsaicin on PAR4 expression in DRG cultures were assessed by measurement of PAR4 mRNA expression using real-time PCR. β-actin mRNA was used as internal control. PAR4 mRNA level was significantly increased in cultured DRG neurons after capsaicin treatment for 60 min. As shown in Fig. 4, compared with the control group, normalized level of PAR4 mRNA is higher in capsaicin-treated DRG neurons (3.57 ± 0.36 folds vs. control; n = 4; P < 0.001).
Fig. 4.
Real-timePCR analysis for PAR4 mRNA expression in DRG neurons following treatment of capsaicin (cap).The results were calculated by normalizing to β-actin in the same sample with ΔCt method. Changes in relative level of PAR4 mRNA expressed as fold of control. All values were mean ± SEM (n = 4). ** P < 0.01, *** P < 0.001 vs. control; # P < 0.05 vs. capsaicin-only groups
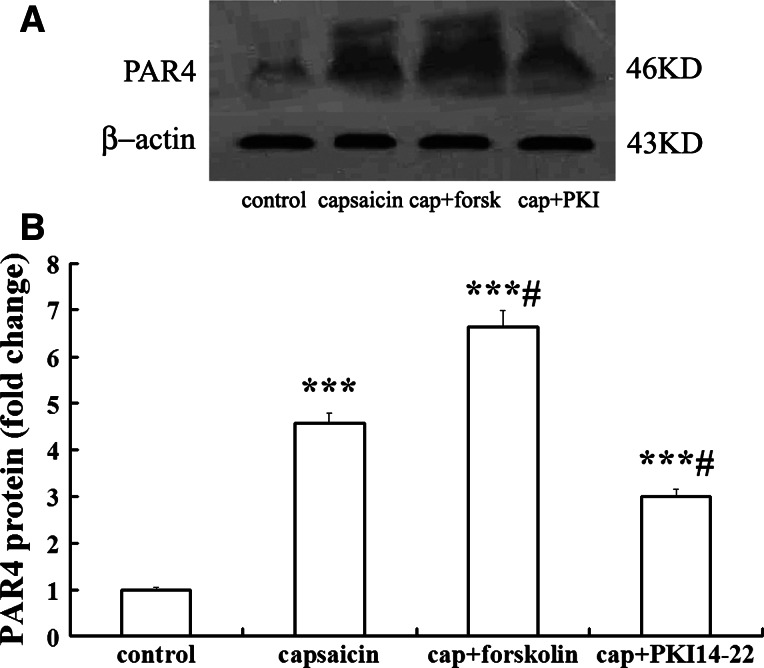
Protein level of PAR4 in cultured DRG neurons detected by Western blots showed change consistent with that of mRNA expression. As shown in Fig. 5, a main ~46 kD band was discovered with the PAR4 antibody in all tested samples, identical to the predicted size of PAR4 (Dattilio and Vizzard 2005), identifying the expression of PAR4 protein in those cultured DRG cells. No immunoblots was detected by omission of primary antibody or its replacement with normal serum at the same concentration. Figure 5 shows the relative density of immunoblots of PAR4 protein from DRG cultures after capsaicin treatment. The relative density of this receptor was significantly increased at 60 min after capsaicin treatment as compared with control (4.56 ± 0.41 folds vs. control; n = 4; P < 0.001).
Fig. 5.
Western blot analysis for PAR4 protein expression in DRG neurons following treatment of capsaicin. a Western blot showed the example of PAR4 protein bands at 46 kDa, following treatment of DRG cultures with control, capsaicin (cap), and pre-addition with forskolin (forsk) or PKI14-22 (PKI). b Statistical analysis of the relative amount of PAR4 protein. Mean optic density of PAR4 protein was calculated by normalizing to β-actin. All values were mean ± SEM (n = 4). *** P < 0.001, vs. control; #P < 0.05 vs. capsaicin-only groups
Effects of PKA on PAR4 Expression Induced by Capsaicin in Cultured DRG Neurons
Since the enhanced expression of PAR4 mRNA was seen after capsaicin treatment, the expression of PAR4 mRNA in cultured DRG neurons was examined after treatment of capsaicin with pre-incubation of cAMP/PKA activator or PKA inhibitor for 30 min. As shown in Fig. 4, forskolin, the activator of cAMP/PKA pre-treatment significantly increased PAR4 mRNA level in capsaicin-treated DRG neurons (1.34 ± 0.08 fold vs. capsaicin-only group; n = 4; P < 0.05). Consistent with the involvement of PKA in the modulation of PAR4, we observed that the PKA inhibitor, PKI14-22 pre-treatment significantly inhibited the effect of capsaicin on PAR4 mRNA expression (0.59 ± 0.05 fold vs. capsaicin-only group; n = 4; P < 0.05).
The change of PAR4 protein level in cultured DRG neurons was examined after treatment of capsaicin with pre-incubation of cAMP/PKA activator or PKA inhibitor for 30 min. As shown in Fig. 5, the relative density of immunoblots of PAR4 after capsaicin treatment with forskolin or PKI14-22 pretreatment was compared with those in capsaicin treatment only. Results showed that activator of PKA strongly enhanced the increase of PAR4 protein induced by capsaicin after pre-incubation with forskolin (1.46 ± 0.11 fold vs. capsaicin-only group; n = 4; P < 0.05). When PKA was inhibited by pretreatment with PKI14-22, the capsaicin-induced enhancement of expression of PAR4 protein was also significantly inhibited (0.66 ± 0.09 fold vs. capsaicin-only group; n = 4; P < 0.05).
Persistence of Capsaicin Effects on Expression of PAR4 mRNA and Protein Levels
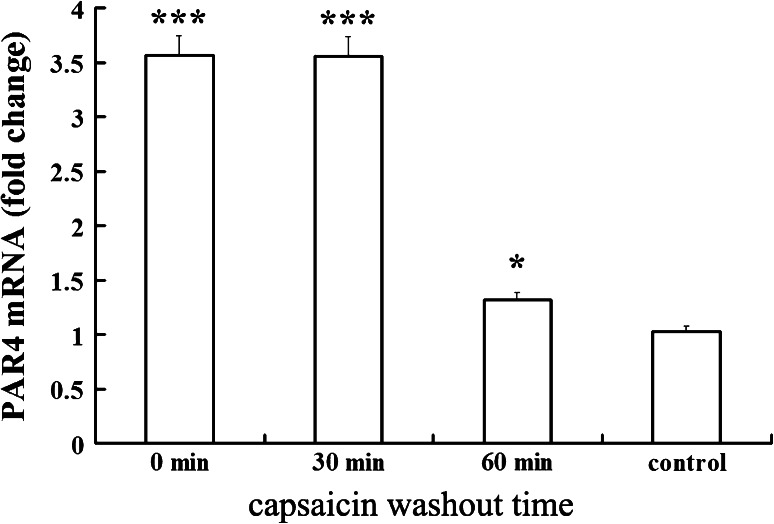
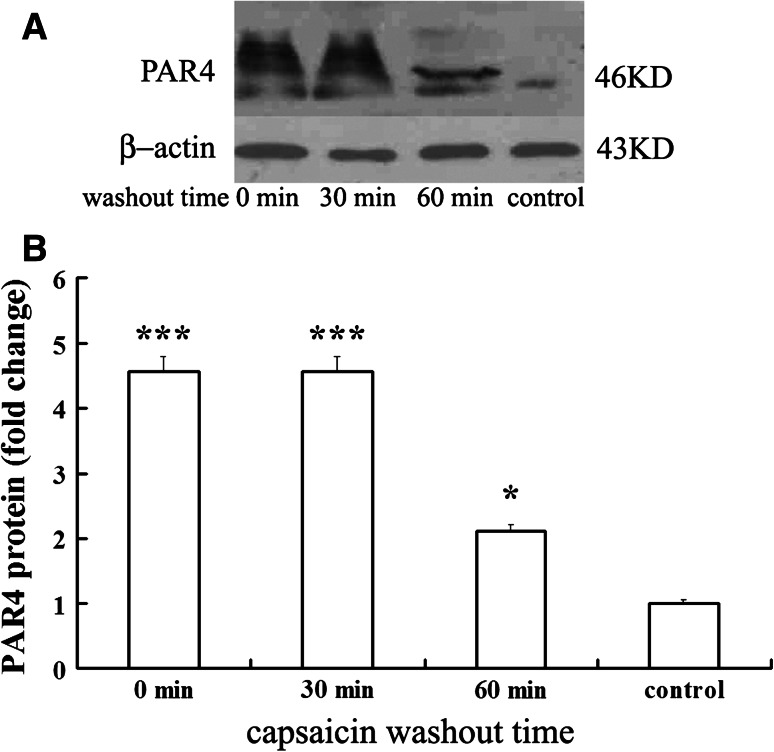
Upregulation of PAR4 mRNA and protein synthesis were detected at 30 and 60 min beyond washout of capsaicin after 1 h exposure to 1 μM capsaicin. Figure 6 shows that the PAR4 mRNA remained at a high level to 30 min after capsaicin washout and decreased significantly at 60 min beyond washout of capsaicin (0.37 ± 0.03 fold vs. capsaicin group, P < 0.05). PAR4 mRNA expression was coupled to PAR4 protein synthesis as shown by Western blot experiments (Fig. 7) in which the enhanced expression of PAR4 protein remained at a high level to 30 min after capsaicin washout and declined significantly at 60 min beyond washout of capsaicin (0.46 ± 0.04 fold vs. capsaicin group; P < 0.05).
Fig. 6.
Time course of PAR4 mRNA expression beyond washout of capsaicin. The relative level of PAR4 mRNA in DRG cultures were measured by real-time PCR at 30 and 60 min beyond washout of capsaicin, and the results were calculated by normalizing to β-actin in the same sample with ΔCt method. Changes in relative level of PAR4 mRNA expressed as fold of control. All values were mean ± SEM (n = 4). * P < 0.05, *** P < 0.001 vs. control
Fig. 7.
Time course of PAR4 protein level beyond washout of capsaicin. a Example of Western blot analysis of PAR4 protein from cultured DRG neurons at 30 and 60 min beyond washout of capsaicin. b Histogram for analysis of the relative amount of PAR4 protein measured with optical density. All values were mean ± SEM (n = 4). * P < 0.05, *** P < 0.001 vs. control
Discussion
There are two new major findings of this study. First, capsaicin activation of TRPV1 significantly increases the expression of PAR4 in mRNA and protein levels in primary cultures of rat DRG neurons after capsaicin incubation. Second, the effect of capsaicin on PAR4 expression appears to be mediated by the cAMP/PKA signal pathway. A novel mechanism by which capsaicin that activates TRPV1 receptors causes primary afferent neurons in the DRG to enhance the expression of TRPV1 and CGRP in mRNA and protein levels (Xu et al. 2009). The present study detected another feature of capsaicin, which was up-regulated expression of PAR4 in cultured DRG neurons.
TRPV1 is widely localized in the primary nociceptive afferent neurons (Dong et al. 2012). We demonstrated that the extensive overlap of PAR4 immunoreactivity with TRPV1 expression in primary cultured sensory neurons. PAR4 expression in cultured DRG neurons we observed was consistent with the existence of PAR4 mRNA in DRG neurons obtained from in situ hybridization (Zhu et al. 2005; Vellani et al. 2010). In the present study, we observed the capsaicin that activates TRPV1 receptors evoked increases in the expression of PAR4 in mRNA and protein levels and in the proportion of PAR4-, TRPV1-immunoreactive neurons and their co-localization in cultured DRG neurons. The cultured DRG neurons can share characteristics with nociceptors in vivo (Passmore 2005). A variety of chemical and environmental stimuli that might cause pain in vivo also cause stimulation on cultured DRG neurons (Senba et al. 2004). In the present study, primary cultured DRG neurons were used as an experimental model for detecting the expression of PAR4 induced by capsaicin, indicating that the PAR4 expression was also used as an indicator that reflects nociceptive effects of TRPV1 on DRG neurons in inflammation and pain processes.
Recent studies have found PAR4 plays an important role in neural pathways signaling nociceptive responses in primary afferent neurons (Russell et al. 2010; Vellani et al. 2010). PAR4 activation could lead to sensitization of TRPV1 receptor in DRG neurons (Vellani et al. 2010). Several reports have found that cytokine and other inflammatory mediator up-regulated PAR4 mRNA expression in various cell types (Hamilton et al. 2001; Ritchie et al. 2007), similar to those in cultured DRG neurons induced by capsaicin we observed. Although there are reports that PAR4 activating peptide increases sensitization of TRPV1 through PAR4 (Vellani et al. 2010), our results suggest that they can reciprocally regulate one another. The interactive relationship of these two types of receptors will be the target of further investigation since the present study suggests TRPV1 activation is inducing increase in PAR4 mRNA and protein levels.
Our results showed that application of cAMP/PKA activator enhanced capsaicin-induced increase of PAR4 mRNA and protein in cultured DRG neurons, while a PKA inhibitor did not. There was a rapid increase in the expression of PAR4 in mRNA and protein levels. So far there is little evidence that rapid changes in mRNA and protein levels of PAR4 or other nociceptive receptors were evoked by capsaicin, even though there is a report that the expression of CGRP and TRPV1 in mRNA and protein levels peaked at 30 min after intradermal injection of capsaicin (Xu et al. 2009). In another report, CGRP mRNA level peaked at 60 min after adjuvant injection (Bulling et al. 2001). However, this rapid up-regulation after capsaicin stimulation remains to be further investigated. Activation of PKA is one of the earliest events in a cascade that involves a variety of cellular sequential responses (Aley et al. 2001). Because selective activator of PKA enhanced PAR4 expression in mRNA and protein levels induced by capsaicin, it is likely that activation of this kinase mediated the action of TRPV1. We therefore hypothesized that a cAMP/PKA transcriptional and translational effect is involved in the expression of PAR4 induced by TRPV1 activation in DRG neurons. Consistent with this hypothesis, further real-time RT-PCR and Western blotting assays demonstrated that PAR4 mRNA and protein levels remained at high levels until 30 min beyond washout of capsaicin. Since there is such a close correlation between PAR4 mRNA levels and protein levels, it seems more likely that the effect of TRPV1 on PAR4 expression results from a change in transcription.
The activation of cAMP/PKA is involved in the pain process induced by TRPV1 activating (Bhave et al. 2002). TRPV1 contains phosphorylation site in its amino acid sequence (at Ser116) for PKA (De Petrocellis et al. 2001; Bhave et al. 2002; Rathee et al. 2002). The presence of phosphorylation site in TRPV1 implies possible regulatory actions by this kinase (Bhave et al. 2002). Activation of TRPV1 also involved a triggering of the transcriptional activity of cAMP response element-binding protein (CREB) in DRG neurons, which in turn stimulated gene promoter activity and up-regulated mRNA levels (Nakanishi et al. 2010). Following TRPV1 activation, PKA therefore probably involved in the upregulation of PAR4 mRNA expression via CREB-dependent transcription. Recent study proposed that the functions of PARs in nociception of sensory neurons were mediated by cAMP/PKA signal pathways (Amadesi et al. 2006; Huang et al. 2012). The activation of cAMP/PKA has been shown to mediate phosphorylation of PARs in sensory neurons, leading to changes in nociceptive response sensitivity (Amadesi et al. 2006; Huang et al. 2012). Present study gives preliminary evidence that TRPV1 activation up-regulates PAR4 mRNA and protein. The enhanced PAR4 mRNA and protein synthesis after capsaicin treatment of the cultured DRG neurons provided a molecular mechanism for expression of PAR4. However, more evidence is needed and related interesting questions, for example, interaction or cross-talk with other signaling pathways, are worthy of further investigation.
In summary, we have used primary cultures of rat DRG neurons to show, for the first time, that capsaicin can significantly increase PAR4 expression in mRNA and protein levels. Furthermore, these data indicate that the effects of capsaicin on PAR4 expression in mRNA and protein levels are mediated by cAMP/PKA signal pathway. Thus, these results are suggestive of a direct relationship between TRPV1 activation and change in primary sensory neurons PAR4 expression in mRNA and protein levels. The affect of capsaicin on PAR4 expression in sensory neurons may play a role in neurogenic inflammation or nociception.
Acknowledgments
This study was supported by the National Nature Science Foundation of China (81070898) and Nature Science Foundation of Shandong Province (2009ZRB01209).
References
- Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO (2001) Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci 21:6933–6939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW (2006) Protease-activated receptor 2 sensitizes TRPV1 by protein kinase C epsilon- and A-dependent mechanisms in rats and mice. J Physiol 575:555–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asfaha S, Cenac N, Houle S, Altier C, Papez MD, Nguyen C, Steinhoff M, Chapman K, Zamponi GW, Vergnolle N (2007) Protease-activated receptor-4: a novel mechanism of inflammatory pain modulation. Br J Pharmacol 150:176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augé C, Balz-Hara D, Steinhoff M, Vergnolle N, Cenac N (2009) Protease-activated receptor-4 (PAR 4): a role as inhibitor of visceral pain and hypersensitivity. Neurogastroenterol Motil 21:1189–e107 [DOI] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW 4th (2002) cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 35:721–731 [DOI] [PubMed] [Google Scholar]
- Bulling DGS, Kelly D, Bond S, McQueen DS, Seckl JR (2001) Adjuvant-induced joint inflammation causes very rapid transcription of β-preprotachykinin and a-CGRP genes in innervating sensory ganglia. J Neurochem 77:373–382 [DOI] [PubMed] [Google Scholar]
- Dattilio A, Vizzard MA (2005) Up-regulation of protease activated receptors in bladder after cyclophosphamide induced cystitis and colocalization with capsaicin receptor (VR1) in bladder nerve fibers. J Urol 173:635–639 [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Harrison S, Bisogno T, Tognetto M, Brandi I, Smith GD, Creminon C, Davis JB, Geppetti P, Di Marzo V (2001) The vanilloid receptor (VR1)-mediated effects of anandamide are potently enhanced by the cAMP-dependent protein kinase. J Neurochem 77:1660–1663 [DOI] [PubMed] [Google Scholar]
- Dong F, Du YR, Xie W, Strong JA, He XJ, Zhang JM (2012) Increased function of the TRPV1 channel in small sensory neurons after local inflammation or in vitro exposure to the pro-inflammatory cytokine GRO/KC. Neurosci Bull 28:155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García PS, Gulati A, Levy JH (2010) The role of thrombin and protease-activated receptors in pain mechanisms. Thromb Haemost 103:1145–1151 [DOI] [PubMed] [Google Scholar]
- Hamilton JR, Frauman AG, Cocks TM (2001) Increased expression of protease-activated receptor-2 (PAR2) and PAR4 in human coronary artery by inflammatory stimuli unveils endothelium-dependent relaxations to PAR2 and PAR4 agonists. Circ Res 89:92–98 [DOI] [PubMed] [Google Scholar]
- Han Y, Li Y, Xiao X, Liu J, Meng XL, Liu FY, Xing GG, Wan Y (2012) Formaldehyde up-regulates TRPV1 through MAPK and PI3K signaling pathways in a rat model of bone cancer pain. Neurosci Bull 28:165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg MD, Saifeddine M, Sandhu S, Houle S, Vergnolle N (2004) Proteinase-activated receptor-4: evaluation of tethered ligand-derived peptides as probes for receptor function and as inflammatory agonists in vivo. Br J Pharmacol 143:443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Li HC, Cowan AA, Liu S, Zhang YK, Song XJ (2012) Chronic compression or acute dissociation of dorsal root ganglion induces cAMP-dependent neuronal hyperexcitability through activation of PAR2. Pain 153:1426–1437 [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI (2001) Molecular mechanisms of nociception. Nature 413:203–210 [DOI] [PubMed] [Google Scholar]
- Karanjia R, Spreadbury I, Bautista-Cruz F, Tsang ME, Vanner S (2009) Activation of protease activated receptor-4 inhibits the intrinsic excitability of colonic dorsal root ganglia neurons. Neurogastroenterol Motil 21:1218–1221 [DOI] [PubMed] [Google Scholar]
- Lee J, Chung MK, Ro JY (2012) Activation of NMDA receptors leads to phosphorylation of TRPV1 S800 by protein kinase C and A-Kinase anchoring protein 150 in rat trigeminal ganglia. Biochem Biophys Res Commun 424:358–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall JJ, Zhang C, Cellars L, Joubert E, Dixon CM, Vergnolle N (2009) Triggering of proteinase-activated receptor 4 leads to joint pain and inflammation in mice. Arthritis Rheum 60:728–737 [DOI] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C (2005) Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem 280:13424–13432 [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Hata K, Nagayama T, Sakurai T, Nishisho T, Wakabayashi H, Hiraga T, Ebisu S, Yoneda T (2010) Acid activation of Trpv1 leads to an up-regulation of calcitonin gene-related peptide expression in dorsal root ganglion neurons via the CaMK-CREB cascade: a potential mechanism of inflammatory pain. Mol Biol Cell 21:2568–2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Ishikura H, Matsunami M, Shinozaki Y, Sekiguchi F, Naruse M, Kitamura T, Akashi R, Matsumura K, Kawabata A (2010) The proteinase/proteinase-activated receptor-2/transient receptor potential vanilloid-1 cascade impacts pancreatic pain in mice. Life Sci 87:643–650 [DOI] [PubMed] [Google Scholar]
- Passmore GM (2005) Dorsal root ganglion neurons in culture: a model system for identifying novel analgesic targets. J Pharmacol Toxicol Methods 51:201–208 [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Hollenberg MD (2008) Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol 153:S263–S282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M (2002) PKA/AKAP/VR-1 module: a common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci 22:4740–4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie E, Saka M, Mackenzie C, Drummond R, Wheeler-Jones C, Kanke T, Plevin R (2007) Cytokine upregulation of proteinase-activated receptors 2 and 4 expression mediated by p38 MAP kinase and inhibitory kappa B kinase b in human endothelial cells. Br J Pharmacol 150:1044–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell FA, Veldhoen VE, Tchitchkan D, McDougall JJ (2010) Proteinase-activated receptor-4 (PAR4) activation leads to sensitization of rat joint primary afferents via a bradykinin B2 receptor-dependent mechanism. J Neurophysiol 103:155–163 [DOI] [PubMed] [Google Scholar]
- Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM (2008) Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci 28(19):4904–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senba E, Katanosaka K, Yajima H, Mizumura K (2004) The immunosuppressant FK506 activates capsaicin- and bradykinin-sensitive DRG neurons and cutaneous C-fibers. Neurosci Res 50:257–262 [DOI] [PubMed] [Google Scholar]
- Vellani V, Petrosino S, De Petrocellis L, Valenti M, Prandini M, Magherini PC, McNaughton PA (2008) Functional lipidomics. Calcium-independent activation of endocannabinoid/endovanilloid lipid signalling in sensory neurons by protein kinases C and A and thrombin. Neuropharmacology 55:1274–1279 [DOI] [PubMed] [Google Scholar]
- Vellani V, Kinsey AM, Prandini M, Hechtfischer SC, Reeh P, Magherini PC, Giacomoni C, McNaughton PA (2010) Protease activated receptors 1 and 4 sensitize TRPV1 in nociceptive neurons. Mol Pain 6:61–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N, Derian CK, D’Andrea MR, Steinhoff M, Andrade-Gordon P (2002) Characterization of thrombin-induced leukocyte rolling and adherence: a potential pro-inflammatory role for proteinase-activated receptor-4 (PAR-4). J Immunol 169:1467–1473 [DOI] [PubMed] [Google Scholar]
- Wang W, Cao X, Liu C, Liu L (2012) Cannabinoid WIN 55, 212–2 inhibits TRPV1 in trigeminal ganglion neurons via PKA and PKC pathways. Neurol Sci 33:79–85 [DOI] [PubMed] [Google Scholar]
- Xu X, Wang P, Zou X, Li D, Fang L, Lin Q (2009) Increases in transient receptor potential vanilloid-1 mRNA and protein in primary afferent neurons stimulated by protein kinase C and their possible role in neurogenic inflammation. J Neurosci Res 87:482–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Gao J, Zhao T, Wei L, Wu W, Bai Y, Zou D, Li Z (2011) Proteinase-activated receptor 2 mediates thermal hyperalgesia and is upregulated in a rat model of chronic pancreatitis. Pancreas 40:300–307 [DOI] [PubMed] [Google Scholar]
- Zhao JH, Dong L, Shi HT, Wang ZY, Shi HY, Ding H (2012) The expression of protease-activated receptor 2 and 4 in the colon of irritable bowel syndrome patients. Dig Dis Sci 57:58–64 [DOI] [PubMed] [Google Scholar]
- Zhu WJ, Yamanaka H, Obata K, Dai Y, Kobayashi K, Kozai T, Tokunaga A, Noguchi K (2005) Expression of mRNA for four subtypes of the proteinase-activated receptor in rat dorsal root ganglia. Brain Res 1041:205–211 [DOI] [PubMed] [Google Scholar]