ABSTRACT
This study employs knowledge mapping and bibliometric techniques to analyze the research landscape of trained immunity over the past 20 years and to identify current research hotspots and future development directions. The literature related to trained immunity was searched from the Web of Science Core Collection database, spanning 2004 to 2023. VOSViewer, CiteSpace and Bibliometrix were used for the knowledge mapping analysis. The foremost research institutions are Radboud University Nijmegen, University of Bonn, and Harvard University. Professor Netea MG of Radboud University Nijmegen has published the greatest number of articles. The current research focus encompasses immune memory, nonspecific effects, epigenetics, metabolic reprogramming, BCG vaccine, and the development of trained immunity-based vaccines. It is likely that research on trained immunity-based vaccines will become a major focus in the development of new vaccines in the future. It would be advantageous to observe a greater number of prospective clinical studies with robust evidence.
KEYWORDS: Trained immunity, innate immune memories, Vosviewer, CiteSpace, Bibliometrix, visual analysis
Introduction
The human immune defense system responds to exogenous pathogens, such as bacteria, viruses, fungi, and parasites, and endogenous stimuli, including cytokines, the release of intracellular components, and stress responses. This response is mediated through two primary mechanisms: innate and adaptive immune responses.1 Innate immunity, which acts rapidly upon antigen exposure, is distinguished by its swift, nonspecific nature and a historical absence of associated immune memory.2 In contrast, adaptive immunity represents a particular immune response that emerges 4 to 5 days following antigen exposure. It is characterized by a memory component that enables it to recognize and respond with greater efficacy to antigens that have been encountered previously.3 The phenomenon of immune memory represents a significant distinguishing characteristic between the two principal forms of immunity: innate and adaptive.4,5 It was previously assumed that immune memory was exclusive to adaptive immunity and applicable only to vertebrate immune modifications.6 Nevertheless, recent research has revealed that a subset of innate immune cells, including monocytes, macrophages, and natural killer cells, also exhibit what is now defined as innate immune memory.7,8 This memory enables the cells to demonstrate augmented responsiveness in subsequent immune challenges. The phenomenon, which has been designated “trained immunity,” was initially proposed by Professor Netea MG in 2011.9 Trained immunity represents an innate immune memory that enables heightened inflammatory and antibacterial responses, thus improving nonspecific defenses against subsequent infections and enhancing host survival.10 It is important to note that while trained immunity is typically associated with a beneficial enhancement of the innate immune response, it can also have adverse effects. In certain instances, the memory responses of innate immunity may diminish or become suppressed, which can result in the development of immune tolerance.11 This study thus examines both the enhancing (training) and suppressing (tolerance) facets of innate immune memory, offering a comprehensive account of the dual roles these mechanisms play in host defense and disease. The mechanism of action of trained immunity is dependent on the interaction between innate immunity, epigenetic reprogramming and metabolic remodeling, which serve to either enhance or suppress the effects of trained immunity.12 The training activators of trained immunity encompass a range of compounds, including β-glucan, LPS, MMR or BCG, chitin, metabolites, and receptor agonists.13–20 The process of trained immunity is initiated by the activation of pathogen recognition receptors (PRRs) by inducers, including Toll-like receptors (TLRs), C-type lectin receptors (CLRs), NOD-like receptors (NLRs), and RIG-like helicases.21,22 These receptors are capable of specifically activating innate immune cells by recognizing conserved pathogen-associated molecular patterns (PAMPs) in a specific manner.23 Nevertheless, the exact mechanisms by which these activators influence the epigenetic landscape and metabolic processes within innate immune cells remain poorly understood. Further research is required to elucidate the specific pathways and molecular interactions involved.
Metabolic reprogramming and epigenetic modifications represent pivotal steps in the regulation of trained immunity, whereby various signaling pathways and transcription factors exert control.24 Metabolic reprogramming shifts immune cells from a resting to an activated state, enhancing their response to secondary stimuli. The main metabolic changes involved include adjustments in energy metabolism, glucose metabolism, and lipid metabolism.25 These changes affect not only cellular energy production and utilization but also signaling pathways and overall function, ultimately influencing immune cell activity and potency.26 Epigenetic modifications influence the development of immune responses by regulating the expression of crucial immune genes through alterations in DNA methylation, histone modifications, and chromatin remodeling.27 These modifications do not alter the DNA sequence but facilitate the expression of genes involved in inflammatory processes and immune cell development.28,29 Metabolic reprogramming provides the energy and substrates for epigenetic modifications, which interact in trained immunity to jointly regulate immune responses.A comprehensive understanding of the mechanisms underlying trained immunity is crucial for elucidating the regulation of the immune system and for developing novel immunotherapeutic strategies. However, the precise roles and interactions of distinct immune cell subsets in trained immunity remain largely unresolved, particularly with regard to how these cells integrate signals from innate and adaptive immune responses to maintain a sustained state of readiness.
The application of trained immunity has been demonstrated to have applications in infection prevention, immune disease treatment, and vaccine development, thereby enhancing immune defense. The potential therapeutic effects of trained immunity have been investigated in the context of cancer, neurological disorders, viral infections, and autoimmune diseases, with particular promise observed in the field of bladder cancer immunotherapy.17,30,31 The concept of trained immunity represents a novel approach in the field of vaccinology. vaccines based on trained immunity (TIbV) are designed to elicit broad and sustained nonspecific enhancement effects, thereby enhancing the body’s defense mechanisms.32,33 However, trained immunity also carries the potential for adverse effects, as it may promote chronic inflammation and autoimmune diseases.9 In particular, the sustained hyperreactivity of innate immune cells has the potential to result in adverse outcomes, including the development of atherosclerosis and Alzheimer’s disease.34,35 The phenomenon of trained immunity is subject to influence from a number of environmental and life experiences, with different geographical regions and lifestyles observed to affect its state and the regulation of the immune system.36–38 While its benefits are widely acknowledged, the mechanisms by which it may exacerbate certain inflammatory or autoimmune diseases remain unclear, which in turn limits its therapeutic applications.39,40 Globally, research on trained immunity is of great importance due to its significant potential in enhancing immune function, increasing resistance to infections, and advancing the development of vaccines and therapies. International collaborative research and the sharing of findings contribute to a deeper understanding of the universality and diversity of trained immunity.A multidimensional bibliometric analysis of published works on a research topic based on a scientific knowledge map can facilitate the recognition, understanding, and interpretation of complex relationships, such as networks, structures, intersections, evolutions, and derivations among knowledge units or knowledge groups of the topic through visualization. This approach permits the investigation of pivotal themes, research focal points, or frontiers that are extensively documented in the literature. It also offers insights into the contributions of the literature, authors, institutions, and other relevant entities, as well as the relationships within the knowledge collaboration network.41–43 The study of the mechanisms of trained immunity has emerged as a new research focus over the past two decades.44–46 Nevertheless, there is currently no systematic review of the literature on trained immunity. Accordingly, the objective of this study is to conduct a bibliometric analysis of the literature on trained immunity research published between 2004 and 2023, based on the knowledge map. This will facilitate an intuitive understanding of the progress of research related to trained immunity and enable the assessment of future research trends.
In the context of the current era of big data, bibliometric tool software can assist researchers in the rapid and comprehensive analysis and processing of vast quantities of literature information. It facilitates the visualization of internal references, collaborations, co-occurrences, and other relationships within the literature, thereby enhancing the efficiency of scientific research. Currently, there is a plethora of general-purpose bibliometric software tools, including CitNetExplorer, SATI, Vosviewer, Bibexcel, Sci2, Hiscite, Citespace, Ucinet, NetDraw, and Bibliometrix. Each of these tools possesses distinctive features and applications.47–49 Bibliometrix has good scalability and a rich computational visualization package, including the provision of relevant index evaluations such as the H-index for research topics. However, the usefulness of this approach depends on the availability of the necessary metadata.50 A unique feature of VOSviewer is its ability to effectively prevent overlapping visual labels using intelligent labeling algorithms. In addition, the software includes a lexicon building module that allows extraneous data to be removed at any stage of the analysis, making it easier to study the impact of specific topics. It should be noted that the functionality of the software is limited by the fact that the statistics are not applicable to datasets with fewer than 100 entries.51 CiteSpace is an appropriate tool for conducting dynamic, complex, and phased analysis, offering distinctive visualization capabilities that facilitate the examination of interconnections between journals, the evolution of scientific research frontiers, and the identification of citation bursts. It should be noted that the content analysis method is not without limitations. These include the restriction of the number of papers that can be analyzed and the potential subjectivity of the author’s opinions.52 Furthermore, these three tools are incapable of accounting for the multiplicity of affiliations of authors, which may result in the introduction of certain biases when analyzing countries and institutions. A visual bibliometric analysis of the literature on immuno-training research in the Web of Science core collection over the last 20 years was conducted using the unique capabilities of CiteSpace, VOSviewer, and Bibliometrix (the R language biblioshiny package). The analysis encompasses multiple dimensions, including publication date, geographic region, institution, journal of publication, as well as keywords and citation frequency of articles.
Methods
Data retrieval
The Web of Science Core Collection (WOS) was employed as the data source for the literature pertaining to immunization training. The search was conducted on 31 December 2023 using the basic search mode with the search field set to the “topic” (TS) field and the search topic defined as “immunisation training.” The search strategy, constructed as Topic=((train* near/1 immun*) or (innate near/0 immun* near/0 memor*)), was set to a time frame from 1 January 2004 to 31 December 2023. The initial search yielded a total of 1895 literature records. In the secondary screening of the results, the document types were filtered to articles and reviews, and the language was restricted to English. This resulted in a total of 1,587 literature records. The literature data was exported in three formats: The data were exported in three formats: plain text, BibTeX, and Excel. The exported data comprised full-text and referenced citations, which were prepared for subsequent processing and visualization analysis. Please direct your attention to Figure 1, which illustrates the precise data retrieval process.
Figure 1.
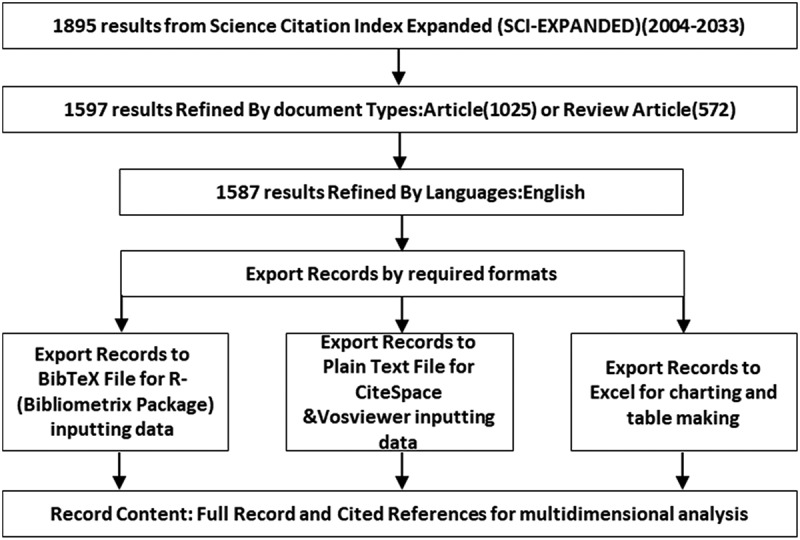
Flowchart of the data retrieval and export.
Data processing and visualization
In this study, we employed the Bibliometrix software package, version 4.3.0 in R, to conduct visual analyses of publication output, citation trends, global collaboration networks, as well as the H-index and G-index of prolific authors in the field under investigation. Additionally, version 1.6.20 of VOSviewer was employed to visualize analyses including country/region collaborations, journal citations, author collaborations, and distributions of keyword influence. Furthermore, version 6.2.R4 Advanced of CiteSpace was employed to conduct visual analyses on institutional distributions and collaborations, co-citation networks, citation bursts in references and keywords, keyword timeline graphs, and overlay of dual-map journals. Furthermore, Microsoft Office Excel 2021 (Microsoft, Redmond, WA, USA) was employed as an additional tool for the repair of data nodes. The combination of these tools enabled a more effective implementation of bibliometric analysis and the visualization of knowledge graphs.
Results
The growth and productivity trends
Figure 2 illustrates the annual growth rate of the number of papers on trained immunity/innate immune memory from 2004 to 2023. The growth is divided into two phases. The first phase (2004–2017) is characterized by a relatively slow rate of publications, while the second phase (2016–2023) is characterized by rapid progress in publications.
Figure 2.
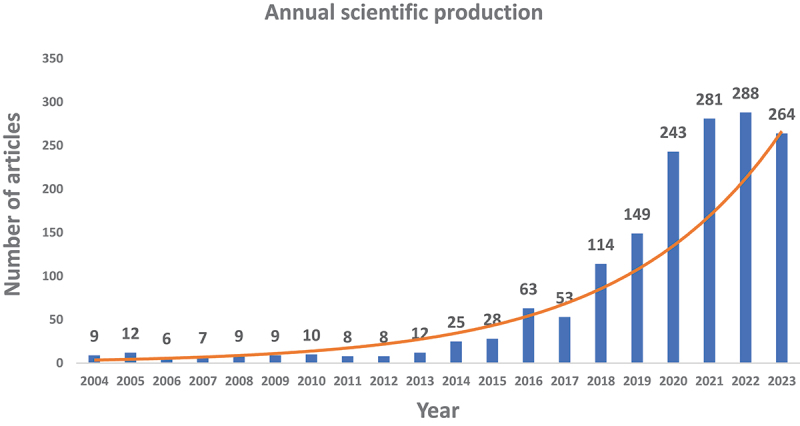
Trends of trained immunity/innate immune memory publications over the past 20 years. The orange curve in the figure represents the trend of annual scientific production.
Contribution of Countries/Regions and institutions
From 2004 to 2023, 108 countries or regions published studies related to trained immunity or innate immune memory. The United States had the greatest number of publications (348 articles), followed by the Netherlands (191 articles) and China (144 articles) (Figure S1a). Figure S1b demonstrates that Radboud University Nijmegen consistently exhibits the greatest degree of collaboration and research output, with the University of Bonn and Harvard University following in second and third place, respectively.
Influential journals
Table 1 presents a list of the 10 most prolific journals in this field, based on the number of published articles. The majority of papers in this field were published by Frontiers in Immunology (204 documents, 39 h-index), followed by the International Journal of Molecular Sciences (37 documents, 11 h-index) and Vaccine (30 documents, 11 h-index). With an impact factor of 8.8, Cell Reports was the most influential of the top 10 most prolific journals.
Table 1.
Top 10 active journals for research related to trained immunity/innate immune memory from 2004 to 2023.
| Rank | Journal | Documents | Citations | JIF | Quartile | H-index |
|---|---|---|---|---|---|---|
| 1 | Frontiers In Immunology | 204 | 4696 | 5.7 | Q1 | 39 |
| 2 | International Journal of Molecular Sciences | 37 | 429 | 4.9 | Q1 | 11 |
| 3 | Vaccine | 30 | 453 | 4.5 | Q2 | 12 |
| 4 | Vaccines | 22 | 130 | 5.2 | Q1 | 7 |
| 5 | Cell Reports | 17 | 1008 | 7.5 | Q1 | 12 |
| 6 | Journal Of Immunology | 17 | 361 | 3.6 | Q2 | 11 |
| 7 | Developmental And Comparative Immunology | 17 | 352 | 2.7 | Q3 | 10 |
| 8 | Plos One | 16 | 237 | 2.9 | Q1 | 10 |
| 9 | Seminars In Immunology | 15 | 896 | 7.4 | Q1 | 13 |
| 10 | Human Vaccines & Immunotherapeutics | 15 | 183 | 4.1 | Q2 | 8 |
Analysis of authors and references
A total of 8,724 researchers have been involved in immunology-related research, as indicated by VOSviewer. The ten most active authors are presented in Table 2. Netea MG has published the greatest number of articles (157), followed by Joosten LAB (81) and Van CREVEL R (46). Figure 3 presents the top 25 references with the most substantial citation bursts. The references include articles published by Netea et al.9and Aaby et al.53indicate that the research focus from 2012 to 2016 was centered on innate immune memory and the nonspecific protective effects of BCG vaccination. The articles by Saeed et al.54and Cheng et al.20emphasise the sustained interest in the mechanisms of epigenetic and metabolic reprogramming in trained immunity, which is likely to remain a pivotal research area in this field. The articles by Giamarellos-Bourboulis et al.55and Noval et al.56indicate that current research may focus on the relationship between BCG vaccination and infection risk, particularly in the context of the ongoing pandemic of the novel coronavirus (2019-nCoV), which could become a significant area of investigation in the field of trained immunity.
Table 2.
The top 10 active authors for research related to trained immunity/innate immune memory from 2004 to 2023.
| Rank | Author | Articles | ACPP | H index | Gindex | Country | Institution |
|---|---|---|---|---|---|---|---|
| 1 | NETEA MG | 157 | 117 | 58 | 135 | Netherlands | Radboud Univ |
| 2 | JOOSTEN LAB | 81 | 122 | 39 | 81 | Netherlands | Radboud Univ |
| 3 | VAN CREVEL R | 46 | 114 | 31 | 46 | Netherlands | Radboud Univ |
| 4 | RIKSEN NP | 34 | 106 | 22 | 34 | Netherlands | Radboud Univ |
| 5 | MOORLAG SJCFM | 33 | 85 | 19 | 33 | Netherlands | Radboud Univ |
| 6 | ARTS RJW | 27 | 189 | 20 | 27 | Netherlands | Radboud Univ |
| 7 | DOMINGUEZ-ANDRES J | 27 | 73 | 16 | 27 | Netherlands | Radboud Univ |
| 8 | WANG H | 26 | 30 | 14 | 26 | USA | Temple Univ |
| 9 | LI Y | 26 | 112 | 13 | 26 | Netherlands | Radboud Univ |
| 10 | BEKKERING S | 25 | 102 | 17 | 25 | Netherlands | Radboud Univ |
Figure 3.
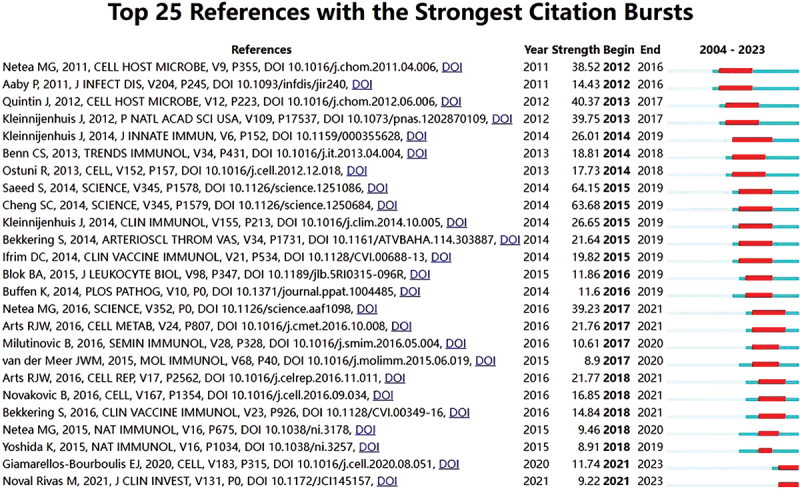
The top 25 references with the strongest citation bursts in he co-citation network. The blue line serves as the timeline, with the red segments indicating the beginning year, ending year, and duration of the burst.
Analysis of keyword co-occurrence and impact view
In order to elucidate the evolving dynamics and interconnections within the field of trained immunity, this study analyzed the co-occurrence and citation bursts of keywords derived from a comprehensive dataset. This study conducted an in-depth analysis of 6,727 keywords within the field of trained immunity. Table 3 presents the co-occurrence frequency and corresponding total link strength of the 20 most frequently occurring keywords in the field of trained immunity research. In this research domain, keywords such as trained immunity, innate immunity, and nonspecific protection are of central importance. Furthermore, other keywords such as epigenetics and NK cells also exert a significant influence on trained immunity. It is notable that there is a strong co-occurrence relationship among keywords such as trained immunity, innate immunity, epigenetics, and macrophages, which serves to underscore the close interconnection of these concepts in the context of trained immunity research. Moreover, the emergence of new keywords, such as “Covid-19” and “Sars-cov-2,” indicates the most recent research areas of interest within this field. As illustrated in Figure 4a, the keyword co-occurrence visualization network identifies the research hotspots within the field of trained immunity, with the involved keywords serving as key indicators. The keywords primarily encompass various aspects of trained immunity, ranging from the fundamental mechanisms of immune responses to immunological issues associated with specific diseases, and extending to the interplay between the immune system and other physiological processes. It is noteworthy that this includes key terms related to immune regulation, such as “inflammation,” “macrophages,” “expression,” and “innate immune memory,” which are associated with factors such as metabolism, exercise, and the activation of cell types. This cluster primarily illustrates the manner in which the immune system interacts with other physiological and environmental factors to modulate immune responses and inflammatory processes. Furthermore, immune responses associated with vaccines, immune memory, and specific diseases such as tuberculosis and coronavirus disease 2019 (Covid-19) represent pivotal aspects. The following keywords are particularly relevant: “trained immunity,” “BCG” (Bacillus Calmette-Guerin vaccine), “vaccination” and “Covid-19.” The research within this cluster is focused on the following areas: vaccination, training of immune memory, and immune responses to different diseases. Furthermore, research into innate defense mechanisms and immune cell types represents a significant area of investigation. The keywords associated with this cluster include “innate immunity,” “nonspecific protection,” “dendritic cells,” “T-cells,” and “NK cells.” This cluster is closely associated with the defense mechanisms related to innate immunity. In addition, there are keywords that pertain to the immune system’s responses, memory, and protective mechanisms against infections. The following keywords are also included: infection, protection, memory, responses, reinfection and induction. This cluster is concerned with the processes by which the immune system recognizes and responds to pathogens, as well as the formation of protective memory against future infections. A citation burst can be defined as a significant change in the frequency of citations of specific keywords or literature within a relatively short period of time. The occurrence of a burst keyword indicates a notable change in the frequency of usage during a specific time frame, thereby revealing the developmental trajectory of the research field. They are of great importance in the delineation of the growth patterns of frontier theories and thematic content in research. Citations bursts are indicative of substantial shifts in the frequency of a specific keyword being cited within a relatively brief period. They offer insight into the research priorities, frontiers, and future trends within a given field over defined time periods, thereby elucidating the evolution trajectory of that field. They are of great importance in illustrating the growth patterns of frontier theories and thematic content in research. The top 25 keywords with the strongest citation bursts in Figure 4b illustrate some of the research hotspots and frontiers in the field of trained immunity. Of these, the term “dendritic cells” demonstrated the greatest relative citation burst intensity between 2017 and 2018. Dendritic cells play a pivotal role in various aspects of trained immunity research, including antigen presentation, establishment of immune memory, induction of trained immunity, and immunomodulation. It seems reasonable to posit that dendritic cells will remain a research focus in the future. Furthermore, from a temporal perspective, the predominant citation burst keywords before 2019 were primarily focused on ‘adaptive immunity’ and “natural killer cells,” whereas those appearing after 2019 were predominantly concerned with “BCG vaccine,” “vaccine,” and “tolerance.” This indicates that recent research hotspots and frontiers in the field of trained immunity have centered on topics such as immune cell types, immune responses, vaccines, and diseases (such as SARS). It seems likely that future research will increasingly involve aspects such as immune cell functions, gene expression, antigen presentation, and TIbV.
Table 3.
Top 20 keywords in terms of frequency of occurrence and the corresponding total link strength.
| Rank | Keyword | Occurrences | Total link strength |
|---|---|---|---|
| 1 | trained immunity | 678 | 2982 |
| 2 | innate immunity | 264 | 1270 |
| 3 | infection | 245 | 1232 |
| 4 | inflammation | 201 | 899 |
| 5 | protection | 167 | 895 |
| 6 | activation | 147 | 674 |
| 7 | nonspecific protection | 146 | 841 |
| 8 | macrophages | 146 | 738 |
| 9 | memory | 145 | 743 |
| 10 | expression | 144 | 522 |
| 11 | innate immune memory | 143 | 634 |
| 12 | bcg vaccination | 133 | 756 |
| 13 | cells | 125 | 569 |
| 14 | covid-19 | 121 | 565 |
| 15 | dendritic cells | 117 | 492 |
| 16 | monocytes | 113 | 602 |
| 17 | bcg | 112 | 690 |
| 18 | vaccination | 104 | 532 |
| 19 | responses | 100 | 463 |
| 20 | reinfection | 94 | 558 |
Figure 4.
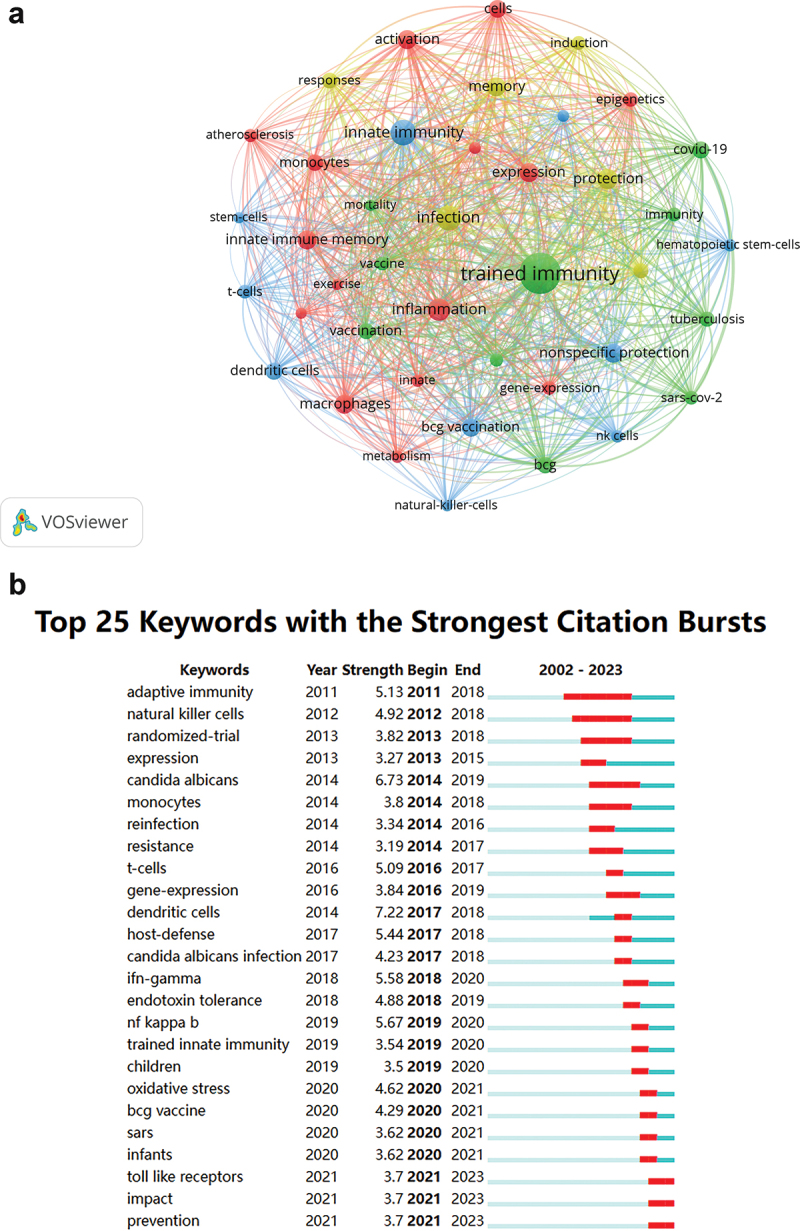
The network visualization of keywords. a. Visualization network of keyword influence coverage. The minimum occurrence threshold for keywords is set to 50, resulting in a total of 41 matched keywords. Keywords nodes are clustered into four colors: red, green, blue, and yellow, representing four main research aspects of trained immunity. The size of the node circle indicates the co-occurrence frequency of the keyword, while the width of the lines between nodes represents the degree of linkage between keywords. b. The top 25 keywords with the strongest citation bursts. A blue line serves as the timeline, with red segments indicating the starting year, ending year, and duration of the burst.
Discussion
Overall distribution
Over the two-decade period from 2004 to 2023, we conducted an in-depth bibliometric analysis of 1,587 articles related to trained immunity/innate immune memory. The articles in question involved a total of 8,724 authors, representing 2,286 different institutions across 103 countries worldwide, and were published in 607 academic journals. The exponential growth in the number of publications is an indisputable indication of a burgeoning interest in the field of trained immunity. The concept of trained immunity was first proposed in 2011, and since that time, the volume of research in this area has experienced a marked increase. By 2023, the number of published articles had increased twentyfold in comparison to the number published in 2013. This trend reflects the growing body of knowledge and research into trained immunity among the scientific community. It is noteworthy that the annual average citation count per article reached its peak between 2010 and 2014. This suggests that the research outcomes generated during this period have become pivotal foundations for trained immunity research. The significance and value of these studies have been widely acknowledged and referenced over the past decade.
The journal “Frontiers in Immunology” has demonstrated both an exceptional publication volume and a significantly elevated citation frequency, placing it at the pinnacle of its field and surpassing other journals. It is noteworthy that, despite the relatively lower publication volume of “Cell Reports,” it achieves the highest average citation frequency and impact factor.
Professor Netea MG is the most prolific author in the field of trained immunity, as evidenced by his first-place ranking in publication volume, total citation frequency, and co-citation frequency. Professor Netea MG occupies significant roles at Radboud University Nijmegen in the Netherlands and the University of Bonn in Germany. In 2011, Professor Netea MG and colleagues published an article in the journal Cell Host Microbe, entitled ‘Trained immunity: a memory for innate host defense,’ in which they introduced the concept of trained immunity for the first time. This concept describes a form of nonspecific immune memory that confers cross-protection against a range of pathogens. This groundbreaking discovery offers researchers a novel perspective through which to comprehend the functioning of the immune system. Furthermore, in 2016, Professor Netea MG and colleagues published an article entitled “Trained immunity: A program of innate immune memory in health and disease, published in the journal Science, explores the mechanisms, regulatory factors, and importance of trained immunity in regulating immune system function under both healthy and diseased conditions. These two articles are the second and third most frequently cited in the field, respectively, and have laid essential foundations for subsequent research on trained immunity.
Hotspot and future perspectives
Current research focuses on the identification of different activators of trained immunity and their interactions with corresponding receptors. Activated signaling pathways and the release of induced cytokines are being investigated, together with mechanisms of metabolic reprogramming, including enhanced glycolysis and oxidative phosphorylation. In addition, epigenetic modifications such as histone methylation and acetylation are being studied. These studies hold great promise for disease intervention and treatment, particularly in antiviral, antitumor and immunomodulatory contexts, and are likely to shape future directions in the study of adaptive immunity.
The emergence of high-frequency keywords such as BCG, vaccines, immune cells and tolerance suggests that future research in trained immunity may focus on the interaction mechanisms between different immune cell types, immune responses, pathogens, TIbV and diseases such as chronic inflammation and the ongoing SARS-CoV-2 pandemic. Further investigations may address aspects of immune cell function, gene expression, antigen presentation and TIbV development. In addition, studies examining the relationship between BCG vaccination and susceptibility to infection, particularly in the context of the pandemic, are expected to become a prominent area of investigation in the field of trained immunity.
Mechanisms of action of training immunity
The process of trained immunity is initiated by the activation of pathogen recognition receptors (PRRs) by various inducers, which detect conserved pathogen-associated molecular patterns. This detection results in the selective reprogramming of metabolism and epigenetic modifications in innate immune cells, which in turn give rise to enduring alterations in their metabolic status and gene expression. The following section provides an overview of the known activators and their roles in inducing specific molecular mechanisms of metabolic and epigenetic reprogramming within immune cells. While the pathways and effects of these activators remain a topic of active investigation, forthcoming studies are likely to identify additional inducers of trained immunity and elucidate their mechanisms of action. Figure 5 summarizes the mechanism of action of training immunity.
Figure 5.
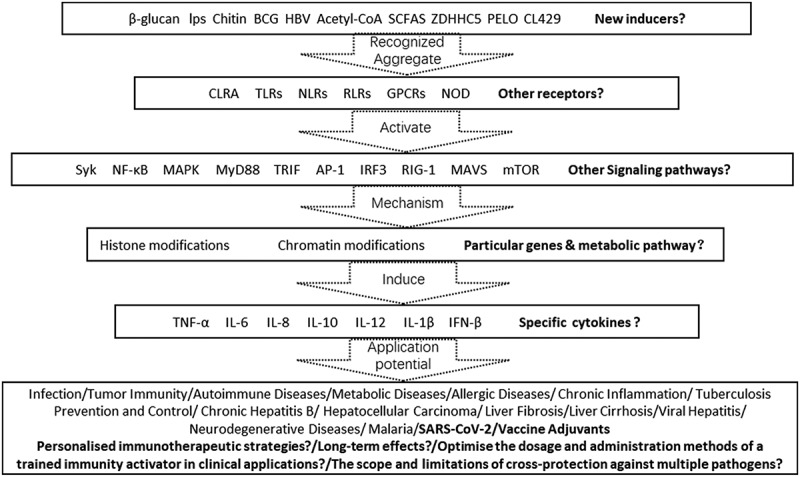
Summary of mechanisms of trained immunity.
β-glucan functions as a potent immunological activator, stimulating macrophages and natural killer cells, which subsequently enhance glycolysis and accumulate metabolic intermediates, including fumarate and succinate, in the tricarboxylic acid cycle. These metabolic alterations form the foundation of trained immunity. Moreover, β-glucan exerts an influence on the integration of immune and metabolic pathways through its impact on epigenetic reprogramming. A crucial mechanism in this process is the inhibition of KDM5 histone demethylase, which plays a pivotal role in enhancing epigenetic reconfiguration in monocytes, thereby enhancing the efficacy of trained immunity29 Further study indicates that β-glucan-induced trained immunity in the context of tumor resistance is associated with alterations in the transcriptome and the epigenetics of granulopoiesis. This reprogramming may result in the generation of granulocytes with anti-tumor activities.16 Moreover, β-glucan has been shown to induce inflammatory responses, thereby enhancing the rapid reactivity of the innate immune system to pathogenic threats.57
Lipopolysaccharide (LPS) is a major component of the outer membrane of Gram-negative bacteria and acts as a potent immunostimulant, capable of activating a range of immune cells, including B cells, monocytes, macrophages, and mast cells. LPS plays a complex role in trained immunity, whereby its effects are contingent upon the level of exposure. Prolonged exposure to LPS or high doses of LPS may induce a form of innate immune memory, designated as LPS tolerance.58,59 This phenomenon results in a reduction in the immune system’s response to unrelated pathogens, representing a detrimental consequence of chronic stimulation of bone marrow-derived cells by LPS. This contributes to immune training. Nevertheless, the trained immunity induced by extremely low doses of LPS can elicit physiological immune responses rather than pathological inflammation, thus preventing tissue damage and autoimmune diseases resulting from excessive immune responses. For example, LPS has been demonstrated to induce a transformation of microglial cells into a neuroprotective phenotype, thereby preventing neuronal dysfunction.60 Some research data indicate that lipopolysaccharide (LPS) induces hematopoietic stem cells (HSCs) to produce sustained long-term changes via Toll-like receptors (TLRs) or indirectly through receptors that sense elevated levels of cytokines during infection. This activation results in the enhancement of long-term protective responses against secondary bacterial infections, such as those caused by Pseudomonas aeruginosa.15
The primary component of fungal cell walls, chitin, exerts a profound influence on the immune system, both through the induction of trained immunity and through modulation of the host’s response to pathogens. Specifically, chitin activation has been demonstrated to result in the enhanced production of pro-inflammatory cytokines, including TNF-α and IL-6, by trained monocytes during subsequent encounters with pathogens.61 It seems probable that this training effect is mediated by alterations in specific immune circuits, which could have a significant impact on the adaptability of mammals to environments rich in chitin.62
The Bacillus Calmette-Guérin (BCG) vaccine is renowned for its ability to modulate the immune system, exerting effects that extend beyond the prevention of tuberculosis. It has been demonstrated to elicit a broad stimulation of the human immune system. This stimulation enhances the functionality of innate immune cells, thereby augmenting the body’s capacity to combat a variety of new infections.63 The trained immunity induced by BCG involves interactions with hematopoietic stem cells (HSCs), which result in their reprogramming or training toward a modified immune set point. This reprogramming enables them to effectively respond to subsequent pathogen challenges and provides long-term protection against pathogen invasion. Upon re-stimulation with BCG, trained immunity induces the immune system to produce elevated levels of pro-inflammatory factors.64 Furthermore, BCG interacts with the NOD2 receptor, resulting in epigenetic and metabolic reprogramming of innate immune cells. This encompasses alterations such as DNA methylation and histone modifications, in addition to the upregulation of glycolysis and fatty acid oxidation. The interplay between epigenetic reprogramming and metabolic alterations plays a pivotal role in reinforcing these adaptations, ultimately leading to the establishment of trained immunity in the host.65,66
The hepatitis B virus (HBV) is a DNA virus that causes a viral infection with the potential to induce persistent chronic infection, leading to both acute and chronic diseases such as hepatic inflammation and cancer. During the course of an HBV infection, the virus is capable of exploiting mononuclear myeloid-derived suppressor cells in order to suppress the development of immature HBV-specific CD8+ T cells within the thymus. This process ultimately establishes an immune tolerance mechanism against the virus. This suppression indicates that the immune response to HBV is partially compromised, thereby reducing the host’s efficacy in clearing the virus.67 The latest research indicates that intrauterine exposure to HBV may result in the induction of trained immunity, characterized by the activation of innate immune cells and an increase in the development of Th1 cells (a subtype of immune cells). Such alterations may augment the reactivity of umbilical cord blood immune cells to bacterial infections.19,68 However, further research is necessary to confirm the presence of HBV-induced trained immunity and to understand the broader implications of intrauterine HBV exposure on the immune system.
A variety of metabolites exert influence over the functionality of immune cells via a number of distinct mechanisms. It is noteworthy that insulin and metabolites of the tricarboxylic acid cycle, such as fumarate and acetyl-CoA, modulate the activity of epigenetic regulatory enzymes within cells. These include histone demethylases, such as lysine-specific demethylase 5 (KDM5), and histone acetyltransferases.69,70 The regulation of these enzymes’ activities gives rise to specific epigenetic changes, including histone methylation and gene acetylation. These modifications subsequently affect the functionality and immune responses of the cells, thereby demonstrating the critical role of metabolites in immune regulation.
Upon activation, various receptors are capable of recognizing pathogen-associated molecular patterns, which then modulate the immune system and enhance nonspecific antimicrobial responses. It is noteworthy that following stimulation by extracellular pathogens, the ZDHHC5 protein mediates the NOD1 and NOD2 receptors and undergoes a palmitoylation modification. This modification enables NOD1 and NOD2 to localize themselves at the cell membrane in a functional manner, thereby promoting bacterial phagocytosis.71 Furthermore, PELO functions as an interacting factor for all cytoplasmic NOD-like receptor (NLR) proteins, effectively activating the ATPase activity of NLR proteins. Furthermore, it regulates the oligomerisation, assembly and activation process of NLR proteins, thereby participating in the control of multiple immune-inflammatory responses mediated by the NLR family of proteins.72 Moreover, CL429, a Toll-like receptor 2 nucleotide-binding oligomerization domain-containing protein 2 agonist, has been observed to imitate the regulatory effect of lactobacilli, thereby providing relief from acute Treponema pallidum infections that have been induced by innate immunity.73 As research progresses, it is anticipated that a greater number of receptor agonists that mediate trained immunity will be identified, thereby enhancing our comprehension of receptor-based immune modulation.
Advances in the treatment of diseases for trained immunity
Trained immunity has been successfully employed in the context of therapeutic interventions for a range of diseases, including inflammatory disorders, neurological disorders, the treatment of patients with the novel coronavirus SARS-CoV-2, and cancer. In the context of inflammatory diseases, trained immunity can prompt macrophages and other immune cells to produce anti-inflammatory factors, thereby modulating inflammatory responses.74 In the context of neurodegenerative diseases, inflammatory stimuli have been demonstrated to induce long-term training of microglia, the brain’s resident macrophages. This process of reprogramming has the potential to alter disease progression. In the case of viral infections such as SARS-CoV-2, vaccines such as BCG, MMR, and OPV have been shown to induce trained immunity. This induction enhances the reactivity of B cells and T cells to mRNA and adenovirus-based vaccines, thereby providing a broader immunological defense against SARS-CoV-2.75 In the context of cancer, trained immunity has demonstrated significant potential by inducing epigenetic reprogramming of myeloid progenitor cells, which in turn can inhibit tumor growth. This multifaceted approach highlights the versatility of trained immunity in addressing a diverse spectrum of pathological conditions.
It is of paramount importance to recognize the dual nature of trained immunity in the context of therapeutic interventions for diseases. As an aspect of innate immune memory, it has the capacity to trigger rapid immune responses, which can result in enhanced and protective effects. However, in certain circumstances, it may also result in the attenuation or suppression of immune responses, ultimately leading to immune tolerance and adverse outcomes. Furthermore, trained immunity has the potential to induce and perpetuate autoimmune and autoinflammatory disorders, as well as to facilitate tumor growth and metastasis. This complexity highlights the necessity for careful consideration in the utilization of trained immunity in medical treatments and interventions.Limitations
It must be acknowledged that this study is not without limitations. Firstly, the potential absence of data sources presents constraints. The data used in this study is drawn exclusively from the WOS database, and only English-language publications are included. Secondly, there are limitations due to the influence of subjective factors. (1) Inconsistencies in the data may occur, for example, an institution may be listed under different names at different times. (2) It is possible that recent literature, despite its relevance, may have lower citation counts and thus may not have been adequately represented in the analysis. Finally, the limitations inherent in the analytical scope of bibliometric tools may result in biased analytical outcomes. Professor Netea MG holds dual affiliations as Professor of Experimental Medicine in Internal Medicine at Radboud University Medical Center, Nijmegen, the Netherlands (since 2007), and as Professor of Immunometabolism at the University of Bonn, Germany (since 2017). This dual affiliation situation may result in an overrepresentation of Germany’s influence in the field of trained immunity and its close collaboration with the Netherlands.Conclusions
This study employs bibliometric analysis to examine the trends, salient topics, and emerging frontiers in research on trained immunity over the past two decades. A systematic literature review reveals that this is the inaugural comprehensive bibliometric analysis within the domain of trained immunity. The considerable increase in the number of publications is indicative of a growing global interest in the study of trained immunity. The analysis has identified several key areas of focus, including the interplay between mechanisms in innate immunity, epigenetics, and metabolic reprogramming. Radboud University Nijmegen in the Netherlands has been identified as the most influential institution in this field. Notable journals such as Frontiers in Immunology, Cell, and the Journal of Immunology have played a pivotal role in disseminating influential research. Professor Netea MG is acknowledged as a preeminent figure in this field. The current research focus is on various immune cell types, immune responses, epigenetic modifications, metabolic reprogramming, and the development of TIbV. Potential future research directions include the relationship between trained immunity and conditions such as chronic inflammation and microorganism-associated immunopathologies. This study offers valuable insights into the mechanisms of trained immunity, current research trends, and potential future directions. The aim is to assist researchers in better understanding the overall development and focal points within this dynamic field.
Supplementary Material
Biographies
Guoqian Jiang is currently an Associate Professor with the School of Electrical Engineering, Yanshan University. He received the B.S. degree in measurement control technology and instrumentation and the Ph.D. degree in control science and engineering from Yanshan University, Qinhuangdao, China, in 2011 and 2018, respectively. He was a joint Ph.D. Student with the Department of Electrical, Computer, and Biomedical Engineering, University of Rhode Island, Kingston, RI, USA, from 2015 to 2017. His research interests include intelligent information processing and big data analytics.
Jianlei Hao Ph.D., Associate Professor, Biomedical Translational Research Institute, Jinan University, Guangzhou, China. He received B.Sc. (2007) and Ph.D. (2012) at Nankai University in Tianjin, China. He then went to work as a postdoc in Northwestern University Feinberg School of Medicine in Chicago, IL, USA (2012-2013) and Jinan University (2013-2017). During the postdoc period, he worked in Yale University School of Medicine in New Haven, CT, USA as a lab associate (2014-2016). From 2017, he served as an associate professor and PI in Jinan University. His research mainly focuses on the differentiation and function of gamma delta T cells. His ORCID: https://orcid.org/0000-0003-4600-3082.
Lijun Fang is an Attending Physician at the Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences. She received the Ph.D. degree in Medicine, Peking Union Medical College, China. As the first author or co-first author, she has published several journal papers in Nature Communications, Cancer Letters, and Mediators of Inflammation, as well as articles in core Chinese journals. Her research mainly focuses on immunological mechanisms of primary thrombocytopenia and hematological malignancies and integrated traditional Chinese and Western medicine treatments.
Funding Statement
This work was supported by the National Natural Science Foundation of China [31970830], the National Natural Science Foundation of China [32370921].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
Jianlei Hao designed this study. Jiacheng He performed the search and collected data.Guoqian Jiang and Hongxia Cui re-checked data. Lijun Fang,Jianlei Hao,Guoqian Jiang,Hongxia Cui and Jiacheng He performed statistical analysis. All authors contributed to this article and approved the submitted version.
Data availability atatement
All the data involved in this study can be found in online repositories. Details about these repositories can be found in the manuscript.
Ethics approval statement
Ethics approval does not apply to this study.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2415823
References
- 1.Deng J, Yu XQ, Wang PH.. Inflammasome activation and th17 responses. Mol Immunol. 2019;107:142–13. doi: 10.1016/j.molimm.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet. 2010;11(1):47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Netea MG, Schlitzer A, Placek K, Joosten LAB, Schultze JL. Innate and adaptive immune memory: an evolutionary continuum in the host’s response to pathogens. Cell Host Microbe. 2019;25(1):13–26. doi: 10.1016/j.chom.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Gourbal B, Pinaud S, Beckers G, Van Der Meer JWM, Conrath U, Netea MG. Innate immune memory: an evolutionary perspective. Immunol Rev. 2018;283(1):21–40. doi: 10.1111/imr.12647. [DOI] [PubMed] [Google Scholar]
- 7.Strutt TM, Mckinstry KK, Swain SL. Control of innate immunity by memory cd4 t cells. Adv Exp Med Biol. 2011;780:57–68. doi: 10.1007/978-1-4419-5632-3_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narni-Mancinelli E, Ugolini S, Vivier E. Natural killer cells: adaptation and memory in innate immunity. Med Sci (Paris). 2013;29(4):389–395. doi: 10.1051/medsci/2013294012. [DOI] [PubMed] [Google Scholar]
- 9.Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Netea MG, Joosten LA, Latz E, Mills KHG, Natoli G, Stunnenberg HG, O’Neill LAJ, Xavier RJ. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boraschi D, Italiani P. Innate immune memory: time for adopting a correct terminology. Front Immunol. 2018;9:799. doi: 10.3389/fimmu.2018.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zubair K, You C, Kwon G, Kang K. Two faces of macrophages: training and tolerance. Biomedicines. 2021;9(11):1596. doi: 10.3390/biomedicines9111596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Röring RJ, Debisarun PA, Botey-Bataller J, Suen TK, Bulut Ö, Kilic G, Koeken VACM, Sarlea A, Bahrar H, Dijkstra H, et al. Mmr vaccination induces trained immunity via functional and metabolic reprogramming of γδ t cells. J Clin Invest. 2024;134(7). doi: 10.1172/JCI170848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moerings B, de Graaff P, Furber M, Witkamp RF, Debets R, Mes JJ, van Bergenhenegouwen J, Govers C. Continuous exposure to non-soluble β-glucans induces trained immunity in m-csf-differentiated macrophages. Front Immunol. 2021;12:672796. doi: 10.3389/fimmu.2021.672796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Laval B, Maurizio J, Kandalla PK, Brisou G, Simonnet L, Huber C, Gimenez G, Matcovitch-Natan O, Reinhardt S, David E, et al. C/ebpβ-dependent epigenetic memory induces trained immunity in hematopoietic stem cells. Cell STEM Cell. 2020;26(5):657–674.e8. doi: 10.1016/j.stem.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Kalafati L, Kourtzelis I, Schulte-Schrepping J, Li X, Hatzioannou A, Grinenko T, Hagag E, Sinha A, Has C, Dietz S, et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell. 2020;183(3):771–785.e12. doi: 10.1016/j.cell.2020.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Netea MG, Giamarellos-Bourboulis EJ, Domínguez-Andrés J, Curtis N, van Crevel R, van de Veerdonk FL, Bonten M. Trained immunity: a tool for reducing susceptibility to and the severity of sars-cov-2 infection. Cell. 2020;181(5):969–977. doi: 10.1016/j.cell.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang TT, Shao TY, Ang W, Kinder JM, Turner LH, Pham G, Whitt J, Alenghat T, Way SS. Commensal fungi recapitulate the protective benefits of intestinal bacteria. Cell Host Microbe. 2017;22(6):809–816.e4. doi: 10.1016/j.chom.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong M, Bertoletti A. Tolerance and immunity to pathogens in early life: insights from hbv infection. Semin Immunopathol. 2017;39(6):643–652. doi: 10.1007/s00281-017-0641-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JHA, Rao NA, Aghajanirefah A, et al. mTOR- and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345(6204):1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mourits VP, Arts R, Novakovic B, Matzaraki V, de Bree LCJ, Koeken VACM, Moorlag SJCFM, van Puffelen JH, Groh L, van der Heijden CDCC, et al. The role of toll-like receptor 10 in modulation of trained immunity. Immunology. 2020;159(3):289–297. doi: 10.1111/imm.13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardenas-Tueme M, Montalvo-Martínez L, Maldonado-Ruiz R, Camacho-Morales A, Reséndez-Pérez D. Neurodegenerative susceptibility during maternal nutritional programming: are central and peripheral innate immune training relevant? Front Neurosci. 2020;14. doi: 10.3389/fnins.2020.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumagai Y, Akira S. Identification and functions of pattern-recognition receptors. J Allergy Clin Immunol. 2010;125(5):985–992. doi: 10.1016/j.jaci.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 24.Koeken V, van Crevel R, Netea MG, Li Y. Resolving trained immunity with systems biology. Eur J Immunol. 2021;51(4):773–784. doi: 10.1002/eji.202048882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fanucchi S, Domínguez-Andrés J, Joosten L, Netea MG, Mhlanga MM. The intersection of epigenetics and metabolism in trained immunity. Immunity. 2021;54(1):32–43. doi: 10.1016/j.immuni.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38(4):633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleinnijenhuis J, van Crevel R, Netea MG. Trained immunity: consequences for the heterologous effects of bcg vaccination. Trans R Soc Trop Med Hyg. 2015;109(1):29–35. doi: 10.1093/trstmh/tru168. [DOI] [PubMed] [Google Scholar]
- 28.Acevedo OA, Berrios RV, Rodríguez-Guilarte L, Lillo-Dapremont B, Kalergis AM. Molecular and cellular mechanisms modulating trained immunity by various cell types in response to pathogen encounter. Front Immunol. 2021;12:745332. doi: 10.3389/fimmu.2021.745332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arts RJ, Novakovic B, Ter Horst R, Carvalho A, Bekkering S, Lachmandas E, Rodrigues F, Silvestre R, Cheng S-C, Wang S-Y, et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016;24(6):807–819. doi: 10.1016/j.cmet.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bekkering S, Domínguez-Andrés J, Joosten L, Riksen NP, Netea MG. Trained immunity: reprogramming innate immunity in health and disease. Annu Rev Immunol. 2021;39(1):667–693. doi: 10.1146/annurev-immunol-102119-073855. [DOI] [PubMed] [Google Scholar]
- 31.Larsen ES, Joensen UN, Poulsen AM, Goletti D, Johansen IS. Bacillus Calmette–Guérin immunotherapy for bladder cancer: a review of immunological aspects, clinical effects and BCG infections. APMIS. 2020;128(2):92–103. doi: 10.1111/apm.13011. [DOI] [PubMed] [Google Scholar]
- 32.Martín-Cruz L, Sevilla-Ortega C, Angelina A, Domínguez‐Andrés J, Netea MG, Subiza JL, Palomares O. From trained immunity in allergy to trained immunity-based allergen vaccines. Clin Exp Allergy. 2023;53(2):145–155. doi: 10.1111/cea.14261. [DOI] [PubMed] [Google Scholar]
- 33.Sánchez-Ramón S, Conejero L, Netea MG, Sancho D, Palomares Ó, Subiza JL. Trained immunity-based vaccines: a new paradigm for the development of broad-spectrum anti-infectious formulations. Front Immunol. 2018;9:2936. doi: 10.3389/fimmu.2018.02936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tercan H, Riksen NP, Joosten L, Netea MG, Bekkering S. Trained immunity: long-term adaptation in innate immune responses. Arterioscler Thromb Vasc Biol. 2021;41(1):55–61. doi: 10.1161/ATVBAHA.120.314212. [DOI] [PubMed] [Google Scholar]
- 35.Włodarczyk M, Druszczyńska M, Fol M. Trained innate immunity not always amicable. Int J Mol Sci. 2019;20(10):2565. doi: 10.3390/ijms20102565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christ A, Lauterbach M, Latz E. Western diet and the immune system: an inflammatory connection. Immunity. 2019;51(5):794–811. doi: 10.1016/j.immuni.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Burgess SL, Petri WJ. The intestinal bacterial microbiome and e. Histolytica infection. Curr Trop Med Rep. 2016;3(3):71–74. doi: 10.1007/s40475-016-0083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta R, Misra A. Covid19 in south asians/asian Indians: heterogeneity of data and implications for pathophysiology and research. Diabetes Res Clin Pract. 2020;165:108267. doi: 10.1016/j.diabres.2020.108267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ampofo WK, Baylor N, Cobey S, Cox NJ, Daves S, Edwards S, Ferguson N, Grohmann G, Hay A, Katz J, et al. Improving influenza vaccine virus selectionReport of a WHO informal consultation held at WHO headquarters, Geneva, Switzerland, 14–16 June 2010. Influenza Resp Viruses. 2012;6(2):142–152, e1–e5. doi: 10.1111/j.1750-2659.2011.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ampofo WK, Azziz-Baumgartner E, Bashir U, Cox NJ, Fasce R, Giovanni M, Grohmann G, Huang S, Katz J, Mironenko A, et al. Strengthening the influenza vaccine virus selection and development process: report of the 3rd who informal consultation for improving influenza vaccine virus selection held at WHO headquarters, Geneva, Switzerland, 1-3 April 2014. Vaccine. 2015;33(36):4368–4382. doi: 10.1016/j.vaccine.2015.06.090. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Goerlandt F, Reniers G. An overview of scientometric mapping for the safety science community: methods, tools, and framework. Saf Sci. 2021;134:105093. doi: 10.1016/j.ssci.2020.105093. [DOI] [Google Scholar]
- 42.Abu-Salih B. Domain-specific knowledge graphs: a survey. J Netw Comput Appl. 2021;185:103076. doi: 10.1016/j.jnca.2021.103076. [DOI] [Google Scholar]
- 43.Ji S, Pan S, Cambria E, Marttinen P, Philip SY. A survey on knowledge graphs: representation, acquisition, and applications. IEEE Trans Neural Netw Learn Syst. 2022;33(2):494–514. doi: 10.1109/TNNLS.2021.3070843. [DOI] [PubMed] [Google Scholar]
- 44.Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, van der Meer JWM, Mhlanga MM, Mulder WJM, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao Y, Saredy J, Yang W, Sun Y, Lu Y, Saaoud F, Drummer C, Johnson C, Xu K, Jiang X, et al. Vascular endothelial cells and innate immunity. Arterioscler Thromb Vasc Biol. 2020;40(6):138–152. doi: 10.1161/ATVBAHA.120.314330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arts R, Moorlag S, Novakovic B, Li Y, Wang S-Y, Oosting M, Kumar V, Xavier RJ, Wijmenga C, Joosten LAB, et al. Bcg vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1):89. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Moral-Muñoz JA, Herrera-Viedma E, Santisteban-Espejo A, Cobo MJ. Software tools for conducting bibliometric analysis in science: an up-to-date review. Prof Inf. 2020;29(1). doi: 10.3145/epi.2020.ene.03. [DOI] [Google Scholar]
- 48.Pan XL, Yan EJ, Cui M, Hua W. Examining the usage, citation, and diffusion patterns of bibliometric mapping software: a comparative study of three tools. J Informetr. 2018;12(2):481–493. doi: 10.1016/j.joi.2018.03.005. [DOI] [Google Scholar]
- 49.Li J. Principles and applications of mapping knowledge domains: a beginner’s guide to vosviewer and citnetexplorer. Beijing. 2018;2018:53. [Google Scholar]
- 50.Aria M, Cuccurullo C. Bibliometrix: an r-tool for comprehensive science mapping analysis. J Informetr. 2017;11(4):959–975. doi: 10.1016/j.joi.2017.08.007. [DOI] [Google Scholar]
- 51.Van Eck NJ, Waltman L. Vos: a new method for visualizing similarities between objects. Berlin (Heidelberg): Springer; 2007. p. 299–306. [Google Scholar]
- 52.Chen C, Chen C. Mapping scientific frontiers. Berlin (Heidelberg): Springer; 2003. [Google Scholar]
- 53.Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, Stensballe L, Diness BR, Lausch KR, Lund N, et al. Randomized trial of bcg vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204(2):245–252. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 54.Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F, Cheng S-C, Ratter J, Berentsen K, van der Ent MA, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345(6204):1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, Antonakos N, Kotsaki A, Domínguez-Andrés J, Kyriazopoulou E, Gkavogianni T, Adami M-E, Damoraki G, et al. Activate: randomized clinical trial of bcg vaccination against infection in the elderly. Cell. 2020;183(2):315–323.e9. doi: 10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rivas MN, Ebinger JE, Wu M, Sun N, Braun J, Sobhani K, Van Eyk JE, Cheng S, Arditi M. Bcg vaccination history associates with decreased sars-cov-2 seroprevalence across a diverse cohort of health care workers. J Clin Invest. 2021;131(2). doi: 10.1172/JCI145157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen J, Raymond K. Beta-glucans in the treatment of diabetes and associated cardiovascular risks. Vasc Health Risk Manag. 2008;4(6):1265–1272. doi: 10.2147/vhrm.s3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Zuani M, Dal Secco C, Tonon S, Arzese A, Pucillo CEM, Frossi B. Lps guides distinct patterns of training and tolerance in mast cells. Front Immunol. 2022;13:835348. doi: 10.3389/fimmu.2022.835348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seeley JJ, Ghosh S. Molecular mechanisms of innate memory and tolerance to lps. J Leukoc Biol. 2017;101(1):107–119. doi: 10.1189/jlb.3MR0316-118RR. [DOI] [PubMed] [Google Scholar]
- 60.Mizobuchi H, Soma GI. Low-dose lipopolysaccharide as an immune regulator for homeostasis maintenance in the central nervous system through transformation to neuroprotective microglia. Neural Regen Res. 2021;16(10):1928–1934. doi: 10.4103/1673-5374.308067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rizzetto L, Ifrim DC, Moretti S, Tocci N, Cheng S-C, Quintin J, Renga G, Oikonomou V, De Filippo C, Weil T, et al. Fungal chitin induces trained immunity in human monocytes during cross-talk of the host with saccharomyces cerevisiae. J Biol Chem. 2016;291(15):7961–7972. doi: 10.1074/jbc.M115.699645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim DH, Wang Y, Jung H, Field RL, Zhang X, Liu T-C, Ma C, Fraser JS, Brestoff JR, Van Dyken SJ, et al. A type 2 immune circuit in the stomach controls mammalian adaptation to dietary chitin. Science. 2023;381(6662):1092–1098. doi: 10.1126/science.add5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moorlag S, Arts R, van Crevel R, Netea MG. Non-specific effects of bcg vaccine on viral infections. Clin Microbiol Infect. 2019;25(12):1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 64.Cirovic B, de Bree L, Groh L, Blok BA, Chan J, van der Velden WJFM, Bremmers MEJ, van Crevel R, Händler K, Picelli S, et al. Bcg vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe. 2020;28(2):322–334.e5. doi: 10.1016/j.chom.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, et al. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA. 2012;109(43):17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arts R, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, Kleinnijenhuis J, Lachmandas E, Gonçalves LG, Belinha A, et al. Immunometabolic pathways in bcg-induced trained immunity. Cell Rep. 2016;17(10):2562–2571. doi: 10.1016/j.celrep.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang Z, Zhang Y, Zhu Z, Wang C, Hu Y, Peng X, Zhang D, Zhao J, Shi B, Shen Z, et al. Monocytic mdscs homing to thymus contribute to age-related cd8+ t cell tolerance of hbv. J Exp Med. 2022;219(4). doi: 10.1084/jem.20211838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hong M, Sandalova E, Low D, Gehring AJ, Fieni S, Amadei B, Urbani S, Chong Y-S, Guccione E, Bertoletti A, et al. Trained immunity in newborn infants of hbv-infected mothers. Nat Commun. 2015;6(1):6588. doi: 10.1038/ncomms7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferreira AV, Alarcon-Barrera JC, Domínguez-Andrés J, Bulut Ö, Kilic G, Debisarun PA, Röring RJ, Özhan HN, Terschlüsen E, Ziogas A, et al. Fatty acid desaturation and lipoxygenase pathways support trained immunity. Nat Commun. 2023;14(1):7385. doi: 10.1038/s41467-023-43315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ieronymaki E, Theodorakis EM, Lyroni K, Vergadi E, Lagoudaki E, Al-Qahtani A, Aznaourova M, Neofotistou-Themeli E, Eliopoulos AG, Vaporidi K, et al. Insulin resistance in macrophages alters their metabolism and promotes an m2-like phenotype. J Immunol. 2019;202(6):1786–1797. doi: 10.4049/jimmunol.1800065. [DOI] [PubMed] [Google Scholar]
- 71.Lu Y, Zheng Y, Coyaud É, Zhang C, Selvabaskaran A, Yu Y, Xu Z, Weng X, Chen JS, Meng Y, et al. Palmitoylation of nod1 and nod2 is required for bacterial sensing. Science. 2019;366(6464):460–467. doi: 10.1126/science.aau6391. [DOI] [PubMed] [Google Scholar]
- 72.Wu X, Yang ZH, Wu J, Han J. Ribosome-rescuer pelo catalyzes the oligomeric assembly of nod-like receptor family proteins via activating their ATPase enzymatic activity. Immunity. 2023;56(5):926–943.e7. doi: 10.1016/j.immuni.2023.02.014. [DOI] [PubMed] [Google Scholar]
- 73.Santecchia I, Vernel-Pauillac F, Rasid O, Quintin J, Gomes-Solecki M, Boneca IG, Werts C. Innate immune memory through tlr2 and nod2 contributes to the control of Leptospira interrogans infection. PLOS Pathog. 2019;15(5):e1007811. doi: 10.1371/journal.ppat.1007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Municio C, Criado G. Therapies targeting trained immune cells in inflammatory and autoimmune diseases. Front Immunol. 2020;11:631743. doi: 10.3389/fimmu.2020.631743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Netea MG, Ziogas A, Benn CS, Giamarellos-Bourboulis EJ, Joosten LAB, Arditi M, Chumakov K, van Crevel R, Gallo R, Aaby P, et al. The role of trained immunity in covid-19: lessons for the next pandemic. Cell Host Microbe. 2023;31(6):890–901. doi: 10.1016/j.chom.2023.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data involved in this study can be found in online repositories. Details about these repositories can be found in the manuscript.


