Abstract
Hydrogen peroxide (H2O2), a major reactive oxygen species produced during oxidative stress, has been implicated in the pathophysiology of various neurodegenerative conditions. Cyperus rotundus is a traditional medicinal herb that has recently found applications in food and confectionary industries. In the current study, the neuroprotective effects of Cyperus rotundus rhizome extract (CRE) through its antioxidant and anti-apoptotic machinery to attenuate H2O2-induced cell damage on human neuroblastoma SH-SY5Y cells have been explored. The results obtained demonstrate that pretreatment of cells with CRE for 2 h before administration of H2O2 for 24 h ameliorates the cytotoxicity induced by H2O2 as evidenced by MTT and LDH assays. CRE exhibited potent antioxidant activity by regulating the enzymes/proteins levels such as SOD, CAT, GPx, GR, HSP-70, Caspase-3, and Bcl-2. The pretreatment restored H2O2-induced cellular, nuclear, and mitochondrial morphologies as well as increased the expression of Brain derived nerve growth factor (BDNF). The anti-oxidant and anti-apoptotic potentials of the plant extract may account for its high content of phenolics, flavonoids, and other active principles. Taken together, our findings suggest that CRE might be developed as an agent for neurodegeneration prevention or therapy.
Keywords: Hydrogen peroxide, Oxidative stress, Neuroprotection, Cyperus rotundus, Human neuronal cells, Apoptosis
Introduction
Brain is characterized by its high susceptibility to oxidative stress because of its high oxygen consumption rate, high levels of fatty acids, reduced capacity of cellular regeneration, and low levels of antioxidant enzymes as compared with other organs (Friedman 2011). Neuronal damage of the brain resulting from oxidative stress is believed to be implicated in the etiology and progression of many neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (Andersen 2004). The process of oxidative stress is mediated by reactive oxygen species (ROS) which are generated in biological system as the by-products of normal and irregular metabolic processes that utilize molecular oxygen. Among all the ROS, H2O2 is an important risk factor to oxidative stress particularly in neurodegeneration because it can easily pass through the biological membranes (Varadarajan et al. 2000; Finkel 2003). Of late, there is an increasing interest among the researchers focusing on traditional medicines with antioxidant and neuroprotective activities due to their lesser or no side effects.
Cyperus rotundus (CR) (Family: Cyperaceae) is a traditional medicinal herb which grows naturally in tropical, sub-tropical, and temperate regions and is widespread in India. Sonwa and Konig (2001) reported that major chemical components of this herb are essential oils, flavonoids, terpenoids, sesquiterpenes, sitosterol, cyperene, cyperol, ascorbic acid, and polyphenols. It is reported that the herb can be used as analgesic, nervine tonic, nootropic, sedative, antispasmodic, antimalarial, to relieve diarrhea and stomach disorders (Zhu et al. 1998; Uddin et al. 2006; Sharma and Gupta 2007; Kilani-Jaziri et al. 2009). Apart from the above mentioned applications, Umerie and Ezeuzo (2000) had reported the utilization of amylose-rich starch extracted from this tuberous root in preparation of noodles. The methanolic extract of CR rhizome has been proven to exhibit anti-inflammatory property (Seo et al. 2001), which was further evaluated recently by Tsoyi et al. 2011, who proved that nootkatone and valencene are the major components responsible for anti-inflammation through hemeoxygenase-1 pathway. Lemaure et al. (2007) reported that anti-obese activity of CR is due to stimulation of brown adipose tissue thermogenesis. In another elegant study, Lee et al. (2010) demonstrated the neuroprotective role of CR in an in vitro model of Parkinsonism. The neuroprotective potential of total oligomeric flavonoids of CR in the middle cerebral artery occluded and reperfused rats have recently been demonstrated by Sunil et al. (2011).
In continuation, the current study was planned to elucidate the mechanism of ROS scavenging and neuroprotective potential of CR extract in SH-SY5Y human neuronal cell line. This study is an attempt aimed at obtaining some clues to help us elucidate the underlying antioxidant defense-mediated neuroprotective mechanism of the plant extract.
Materials and Methods
Chemicals and Reagents
MTT, 2′,7′-DCFH2DA, rhodamine 123, RIPA buffer, protease and phosphate inhibitor cocktail, were obtained from Sigma (St Louis, MO, USA), while H2O2 was obtained from Merck (Bangalore, India).
Preparation of CR Extract
The roots of CR, obtained from the local market of Mysore, India, were shade dried and finely powdered. The 70 % ethanolic extract was prepared by keeping the dry powder and solvent in a ratio of 1:10 (w/v). The extraction was continued for 1 week at room temperature by changing the solvent on alternative day. The pooled extract was filtered through Whatman No. 1 filter paper, and the filtrate was concentrated using a rotary vacuum evaporator. The concentrate was finally freeze dried, and the yield was recorded. The powder was dissolved in dimethyl sulfoxide (DMSO), filtered through 0.2-μm membrane filter (Millipore, Bangalore, India), and used in the experiments.
Determination of Total Phenolic and Flavonoid Contents
The total phenolic (TPC) and flavonoid contents were determined according to the methods of Kujala et al. (2000) and Delcour and Varebeke (1985), respectively.
Cell Culture and Treatments
The human neuroblastoma cell line, SH-SY5Y, was obtained from the National Centre for Cell Sciences, Pune, India. Cells were seeded into plates, flasks, or dishes in 1:1, DMEM/F-12 supplemented with 10 % FBS, 2 mM l-glutamine, antibiotic and antimycotic solution Sigma (St Louis, MO, USA) in a humid atmosphere of 5 % CO2 and 95 % air at 37 °C. The media were changed on alternative days, and freshly prepared H2O2 was added for 24 h to the cells with or without pretreatment with CRE for 2 h before any experiment.
Analysis of Cell Viability
Cell viability was determined by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. The SH-SY5Y cells were seeded in 96-well plates at a density of 1 × 104 cells/well and incubated for 24 h before experimental treatments. The cells were then subjected to the treatments of interest. After 24-h incubation, MTT (0.5 mg/ml) was added to each well. Following additional 2-h incubation at 37 °C, 100 μl of DMSO was added to dissolve the formazan crystals. The absorbance was then measured at 540 nm using a VERSA max Hidex plate chameleon™ V (Finland). Wells without cells were used as blanks and were subtracted as background from each sample. Results were expressed as percentage of control.
Lactate Dehydrogenase (LDH) Release Assay
Cytotoxicity was quantified by measuring the amount of LDH released in the medium by means of a LDH-estimation kit (Agappe-11407002, Mysore, India) according to the manufacturer’s instructions. LDH activity was measured through the oxidation of lactate to pyruvate with simultaneous reduction of nicotinamide adenine dinucleotide (NAD+) at a wavelength of 340 nm. The rate of increase in enzyme activity due to the formation of reduced nicotinamide adenine dinucleotide (NADH) is directly proportional to the LDH activity in the sample. The SH-SY5Y cells were plated at a density of 5 × 104 cells/well in 24-well plates; after 24 h, the cells were subjected to the treatments of interest. After the treatment period, 10 μl of cell lysis solution (2 % Triton X-100) was added to the untreated cells, which were selected as the total LDH activity. The cells were separated by centrifugation at 2,500 rpm for 5 min at 4 °C, and the supernatant (100 μl) was mixed with 900 μl of kit reaction mixture. Cell damage was evaluated by measuring the leakage of intracellular LDH into the medium.
Observations of Morphological Changes
The cells were seeded in petri dishes (1 × 106 cells) and then treated with H2O2 for 24 h with or without pretreatment of CRE for 2 h. The cellular morphology was observed and photographed using a phase contrast microscope (Olympus, Japan) equipped with Cool SNAP® Pro color digital camera.
Single Cell Gel Electrophoresis (SCGE) Assay
Alkaline comet assay (Singh et al. 1988) was performed to evaluate the eukaryotic DNA damage preventing efficacy of CRE against H2O2-induced oxidative stress. The SH-SY5Y cells were collected (4 × 105 cells/ml) and treated with different concentrations of H2O2 (100, 500 μM, 1, and 10 mM). In another group, cells were pre-incubated with CRE (25 and 50 μg/assay) for 1 h and treated with 100 μM H2O2 for 3 h, and the cell suspensions mixed with 100 μl of 0.7 % (w/v) low-melting agarose (LMA) and loaded to the frosted slides pre-coated with 1.0 % (w/v) normal melting agarose. After solidification of agarose, the slides were covered with another 100 μl of 0.7 % (w/v) LMA and immersed in lysis buffer (2.5 M NaCl, 100 mM EDTA, 10 mM Tris–HCl buffer, 0.1 % SDS, and 1 % Triton X-100 and 10 % DMSO; pH 10.0) for 90 min. Later, the slides were transferred into an electrophoresis tank containing unwinding buffer (3 M NaOH, 10 mM EDTA; pH 13.0) and run with an electric current of 25 V/300 mA for 20 min to unwind the DNA. After electrophoresis, the slides were washed twice with neutralizing buffer (0.4 M Tris–HCl; pH 7.5) for 10 min and treated with ethanol for another 5 min before staining with 40 μl of ethidium bromide (20 μg/ml). The photographs were taken with fluorescence microscope (Olympus, Japan) equipped with Cool SNAP® Pro color digital camera, and measurements were made by Image Pro® plus software to determine the tail length. The results were expressed as percent inhibition of tail length.
Preparation of Cell Extract and Antioxidant Enzymes Estimation
The cells (1 × 107 cells) were seeded in 75 cm2 flasks and treated as mentioned earlier. The cells were collected by trypsinization, washed twice with PBS, and the cell pellets were resuspended in ice-cold 50 mM potassium phosphate buffer, pH 7.4, containing 2 mM EDTA and 0.1 % Triton X-100. The cells were sonicated, followed by centrifugation at 13,000×g for 10 min at 4 °C to remove cell debris. The resulting supernatants were collected, and the protein contents were measured by Bradford method (1976) with BSA as the standard. The antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), and glucose-6-phosphate dehydrogenase were estimated according to the kit supplier protocols (Randox, Cat no. SD. 125, RS 504, GR 2368, PD 410, Canada). The catalase (CAT) activity was estimated manually by measuring the decay of 6 mM H2O2 solution at 240 nm by the spectrophotometric degradation method (Cohen et al. 1970).
Measurement of Intracellular ROS and Lipid Peroxidation
The oxidation-sensitive dye DCFH-DA was used to determine the formation of intracellular ROS (Wang and Joseph 1999). The SH-SY5Y cells were seeded in 24-well plates at a concentration of 4.0 × 105 cells/ml and treated as mentioned earlier. After treatment, 5 mg/ml DCFH-DA was added to cells and incubated for 30 min. The cells were collected after washing twice with PBS, and the fluorescence was detected at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using Hidex plate chameleon™ V (Finland).
The lipid peroxidation was evaluated by measuring malondialdehyde by the method described by Ohkawa et al. (1979) with slight modifications. The SH-SY5Y cells were seeded in 75 cm2 flask at a concentration of 1.0 × 107 cells/ml and incubated at 37 °C. After reaching confluency, the cells were treated as mentioned earlier. The cells were harvested, washed with PBS, and sonicated in ice-cold 1.15 % KCl with 1 % Triton X-100. Then, 100 μl of the cell lysates were mixed with 0.2 ml of 8.1 % SDS, 1.5 ml of 20 % acetic acid (pH 3.5), and 1.5 ml of 0.8 % thiobarbituric acid. The volume was made up to 4.0 ml using distilled water and boiled for 90 min. After cooling, the contents were centrifuged at 1,500 rpm for 10 min, the supernatants were separated, and the absorbance was measured at 532 nm.
Measurement of Mitochondrial Membrane Potential (MMP)
MMP was determined using the fluorescent dye rhodamine 123. The cells were cultured in 24-well plates and on cover slips in Petri dishes coated with poly l-lysine for fluorimetric analysis and imaging, respectively. The cells were treated as mentioned earlier. Rhodamine 123 (10 μg/ml) was added to the cells and incubated for 60 min at 37 °C. Then the cells were collected after washing twice with PBS and the fluorescence was detected at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using Hidex plate chameleon™ V (Finland). The cells grown on cover slips were imaged by using fluorescence microscope (Olympus, Japan) equipped with Cool SNAP® Pro color digital camera.
Mitochondrial Morphology by Transmission Electron Microscope (TEM)
The cells were grown in 75 cm2 flasks till the confluency reached and treated as mentioned earlier. After treatments, the cells were trypsinized, washed with cold PBS and cell pellets were fixed in 2.5 % glutaraldehyde (prepared in 0.1 mol/l PBS; pH 7.4) for 24 h at 4 °C. The samples were post-fixed in 1 % OsO4 in PBS for 90 min at room temperature. Fixed samples were washed in several changes of PBS, dehydrated in graded alcohols and cleared by treating with propylene oxide for 15 min. The cells were left in 1:1 mixture of propylene oxide and embedding medium (TAAB araldite, England) for overnight on a rotator. The cells were, then, infiltrated by freshly prepared araldite (Frasca and Parks 1965; Johannessen 1973) and transferred to flat silicon embedding molds and kept for polymerization at 60 °C for 48 h. Polymerized blocks were sectioned by ultramicrotome (Leica, Germany) and semi-thin sections (1 mm thick) were heat-stained with toluidine blue and scanned under light microscope (Olympus, Japan). Then ultra-thin sections were made using ultramicrotome and mounted on formvar-coated nickel grids and stained with uranyl acetate followed by lead citrate. The grids were observed under a TEM (FEI TECANI G2 Spirit BioTWIN, Netherland).
Immunoblotting
The cells (1 × 107 cells) were seeded in 75 cm2 flasks and treated as mentioned earlier. After treatment, the cells were washed twice with PBS and lysed using ice-cold RIPA buffer with protease and phosphatase inhibitor cocktail. The cell lysates were centrifuged at 12,000 rpm for 10 min at 4 °C, and the protein contents were determined as mentioned earlier. Protein separation was done by 8–12 % SDS-PAGE followed by transfer to nitrocellulose membranes. The membranes were, then, blocked overnight at 4 °C with 5 % (v/v) non-fat dry milk in Tris-buffered saline with Tween-20 (TBS-T) (10 mM Tris–HCl, 150 mM NaCl, and 0.1 % Tween-20, pH 7.5) and incubated with primary antibodies namely α-Tubulin (sc-5286), HSP-70 (sc-66048), SOD (sc-8637), CAT (sc-34280), GPx (sc-22146), GR (sc-32408) and Bcl-2 (sc-7382) (Santa Cruz Biotechnology, CA, USA) and caspase-3 (C8487, Sigma, St. Louis, MO, USA) at 1:1,000 dilution for 3 h with shaking. The membranes were washed in TBS-T followed by incubation for 2 h at room temperature in dark with horseradish peroxidase conjugated rabbit anti-goat, goat anti-mouse and goat anti-rabbit secondary antibodies (DAKO, Denmark) at 1:10,000 dilutions. The membranes were washed again and the immunoreactivity was detected by using the enhanced chemiluminescence peroxidase substrate kit (CPS-160, Sigma, St. Louis, MO, USA).
RNA Isolation and Reverse Transcription
The cells (1 × 107 cells) were seeded in 75 cm2 flasks and treated as mentioned earlier. After treatment, the cells were trypsinized and washed twice with PBS. Total RNA was extracted using RNA isolation kit (Sigma, St Louis, MO, USA) following the protocol provided by the company. Two micrograms of total RNA was reverse transcribed using HS-RT PCR kit (Sigma, St Louis, MO, USA) as per the given instructions. The quality of cDNA was verified by PCR amplification of β-actin.
Polymerase Chain Reaction (PCR) and Product Analysis
The cDNA in the RT product was amplified using Taq DNA polymerase (Sigma, St Louis, MO, USA). The reaction was performed in 20-μl reaction volume using 10 pmol of the following primers—brain derived nerve growth factor (BDNF), F:5′-GAT GAC CAT CCT TTT CCT TAC TAT GG-3′ R:5′-CTA TCT TCC CCT TTT AAT GGT CAA T-3′; β-actin, F:5′- CCT CTA TGC CAA CAC AGT-3′ R:5′-AGC CAC CAA TCC ACA CAG-3′ (Choi et al. 2010). The PCR conditions were—denaturation at 94 °C for 30 s, annealing at 55.8 °C for BDNF and at 56 °C for β-actin, and polymerization at 72 °C for 30 s. The cyclic process was performed 35 times for BDNF and 30 times for β-actin. The PCR products were analyzed on 1.0 % agarose gel and visualized by ethidium bromide, and the stained intensity of individual bands were evaluated.
Results and Discussion
Cyperus rotundus, a member of the Cyperaceae family, is represented throughout the world from tropics to temperate regions. Initially, the starch extracted from its root was used in paper and textile industry because of its large granules which act as bridge resulting in increased strength. Later, Umerie and Ezeuzo (2000) reported that starch could have edible applications in oil, food and confectionary industries. Keeping in view its suitability as food ingredient, the current investigation explores its mechanism of anti-apoptotic action on H2O2-induced neuronal damage. The anti-apoptotic activity was correlated with its antioxidant activity.
TPC and Flavonoid Contents
The CRE was found to have high contents of TPC (254.5 ± 5.26 μg GAE/mg extract) and flavonoid (164.34 ± 3.75 μg CE/mg extract) which are the principal elements for its anti-oxidant and anti-apoptotic activities. Recent reports by Sunil et al. (2011) and Tsoyi et al. (2011) indicate that total oligomeric flavonoids, sesquiterpenes nootkatone, and valencene are the active principles present in CRE, which are responsible for its protective effect against ischemia via its anti-oxidant activity and anti-inflammatory activities.
Protective Effects of CRE Against H2O2-induced Cytotoxicity
The SH-SY5Y cells were treated with different concentrations of CRE (0, 1, 10, 25, 50, and 100 μg/ml) for 24 h and the cell viability was determined by MTT assay. The viability of the SH-SY5Y cells was found same as untreated control cells when exposed to CRE concentrations of 50 μg/ml and lower. In order to evaluate H2O2-induced neuronal cytotoxicity, the SH-SY5Y cells were treated with different concentrations of H2O2 (0–200 μM) for 24 h and a dose-dependent cell death was observed (Fig. 1A). About 40 % of the cells were survived after 100 μM H2O2 insult and the same concentration was used in subsequent experiments. In order to determine the protective effects of CRE against H2O2-induced cytotoxicity, the SH-SY5Y cells were pretreated with 50 μg/ml of CRE for a period of 2 h, followed by treatment with 100 μM H2O2 for 24 h. As shown in Fig. 1B, H2O2-induced cell death was significantly ameliorated by CRE pretreatment.
Fig. 1.
A Dose-dependent effects of H2O2 on SH-SY5Y cell viability. B Dose-dependent protective effect of CRE on H2O2-induced cytotoxicity in SH-SY5Y cells. C Effect of CRE on LDH release. D Effects of CRE on H2O2-induced morphological alterations in SH-SY5Y cells by phase-contrast microscopy. The data are represented as mean ± SD of three independent experiments. # P < 0.05 versus control group, *P < 0.05 versus H2O2-treated group, and ##non-significant versus control group
In order to investigate further the cytotoxicity of H2O2 and the protective effect of CRE, LDH assay was carried out. The assay is based on the principle that the leakage of cytosolic LDH increases as the number of dead cells increases. The SH-SY5Y cells were pretreated with 50 μg/ml of CRE for 2 h, followed by 100 μM H2O2 treatment for 24 h. Figure 1C shows that release of LDH was increased significantly up to 46.2 % of total enzyme after exposure to 100 μM H2O2, indicating that H2O2 caused cytotoxicity in the SH-SY5Y cells. In contrast, the CRE pretreated cells showed a lower release of LDH as compared with H2O2-exposed cell group. The protective effect of CRE on H2O2-induced cytotoxicity determined by LDH assay correlates to the MTT assay. Results clearly showed that CRE rescued the H2O2-induced neurotoxicity suggesting its neuroprotective efficacy.
The protective effect of CRE was confirmed using the morphologic analysis. H2O2-treated neurons exhibited disappearance of the cellular processes which were protected with CRE pretreatment as evidenced by bright field images (Fig. 1D).
Eukaryotic DNA Damage Inhibitory Activity of CRE
SCGE assay was performed to assess the protective effect of CRE on H2O2-induced DNA damage in the SH-SY5Y cells. The assay is based on the principle that undamaged DNA retains a highly organized association with protein matrix in the nucleus, and when damaged, this organization is disrupted. When the electric field is applied, undamaged DNA strands, being too large, do not leave the cavity whereas the disrupted and smaller fragments move faster and were visualized in the form of a comet. The tail length of comet is measured as an index of DNA damage in the cell.
The tail dispersion showed a dose-dependent increase with increasing concentration of H2O2 (Fig. 2A). The tail length of control cell was 10.0 ± 1.5 μm, while in case of 100 μM H2O2 it was 65.74 ± 6.0 μm which was further increased to 164.17 ± 7.9 μm in the presence of 10 mM H2O2. To study the DNA damage-preventing efficacy, 25 and 50 μg CRE/reaction was used along with 100 μM H2O2, and the results are presented in Fig. 2B. The extract showed 42.6 and 54.7 % inhibitory effects against cell damage, as measured in terms of tail length, at a concentration of 25 and 50 μg/reaction, respectively. The results clearly indicate that H2O2-induced DNA damage was successfully overcome/repaired by the active compounds present in CRE (Fig. 2A, a–g). In a previous study, Zhang et al. (2008) have also reported the ability of flavonoid, butin to ameliorate DNA damage caused by H2O2-induced oxidative stress.
Fig. 2.
A, B Effect of CRE on DNA damage induced by H2O2 in SH-SY5Y cells. Control cells without any treatment (a), Dose-dependent effect of H2O2 (100 μM, 1 mM, 10 mM, and 50 mM) on DNA damage (b–e), SH-SY5Y cells were pretreated with CRE for 2 h at 25 and 50 μg/assay and induced with 100 μM H2O2 (f, g). Tail length (μm): Bars Inhibitory effect of CRE on cell damage: dashed diamond
Effects of CRE on Antioxidant Enzymes
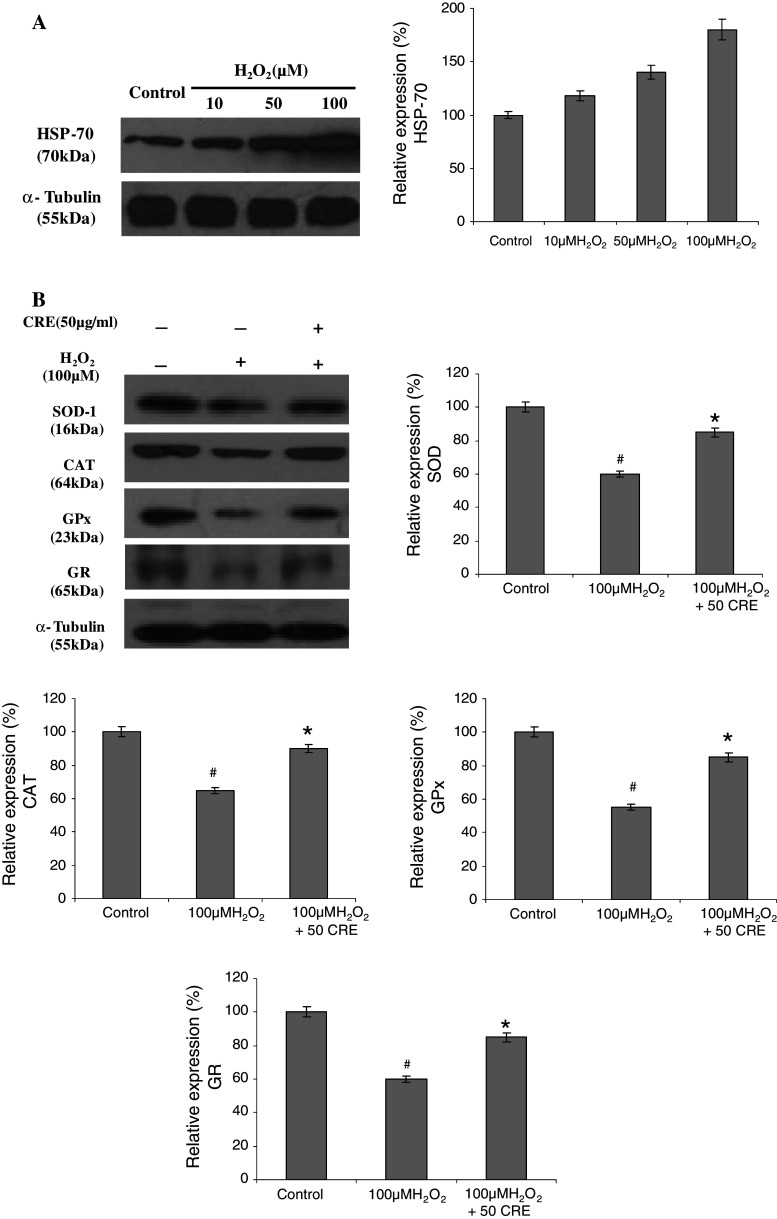
To investigate whether the radical scavenging activity of CRE was mediated by the activities of antioxidant enzymes, the activities of SOD, CAT, GPx, and GR in the SH-SY5Y cells were measured. H2O2-treated cells resulted in a decrease of activities of all enzymes, while CRE pretreatment restored their activities almost similar to that of control (Table 1). It is speculated that the higher level of antioxidant enzymes will attenuate the ROS-induced cell damage. Recently, a number of studies have correlated the enhanced production of ROS with H2O2 treatment, resulting in reduced antioxidant status (Lin et al. 2009; Choi et al. 2010). In an earlier study, Sunil et al. (2011) has proven that total oligomeric flavonoids of Cyperus extract ameliorate the symptoms of ischemic-reperfusion injury in rats by improving the antioxidant status.
Table 1.
Effects of CRE pretreatment on levels of SOD, catalase, GR, GPx, and G6-PDH in SH-SY5Y cells on H2O2 treatment
| Groups | SOD (U/mg protein) | CAT (U/mg protein) | GPx (U/mg protein) | GR (mU/mg protein) | G6-PDH (U/mg protein) |
|---|---|---|---|---|---|
| Control | 1.67 ± 0.05 | 2.62 ± 0.10 | 0.90 ± 0.04 | 17.36 ± 0.91 | 0.18 ± 0.05 |
| H2O2 | 1.02 ± 0.07# | 1.41 ± 0.09# | 0.37 ± 0.04# | 9.00 ± 0.38# | 0.09 ± 0.05# |
| H2O2 + CR50PE | 1.37 ± 0.08* | 2.29 ± 0.18* | 0.80 ± 0.05* | 16.01 ± 0.72* | 0.15 ± 0.05* |
The data are represented as mean ± SD of three independent experiments
# P < 0.05 versus control group, * P < 0.05 versus H2O2-treated group
CRE Inhibits H2O2-induced ROS Generation and Lipid Peroxidation
The pathological condition induced by H2O2 has been found to be associated with the increasing ROS generation (Andersen 2004). The non-ionic, non-polar dye, DCFH-DA, crosses the cell membranes and is hydrolyzed enzymatically by intracellular esterases to nonfluorescent DCFH. In the presence of ROS, DCFH is oxidized to highly fluorescent dichlorofluorescein (DCF) (Bass et al. 1910; LeBel et al. 1992). Therefore, the intracellular DCF fluorescence was used as an index to quantify the intracellular ROS. The emitted fluorescence is directly proportional to the concentration of H2O2. In human neuronal cells-treated with 100 μM H2O2, the fluorescence intensity was increased drastically by 50 % as compared with control group. In cells pretreated with CRE followed by H2O2 treatment for indicated time periods, the fluorescence intensity decreased to 112.81 ± 3.4 indicating its potent antioxidant potential (Fig. 3A). ROS can oxidize lipids to generate end-products, such as MDA that can bind to DNA to generate mutagenic adducts (Chaudhary et al. 1994). In the current study, it was observed that there was an enhanced ROS generation in H2O2-exposed cells which could be responsible for the observed increase in MDA levels. The pretreatment of cells with CRE significantly inhibited the ROS and MDA levels (Fig. 3B).
Fig. 3.
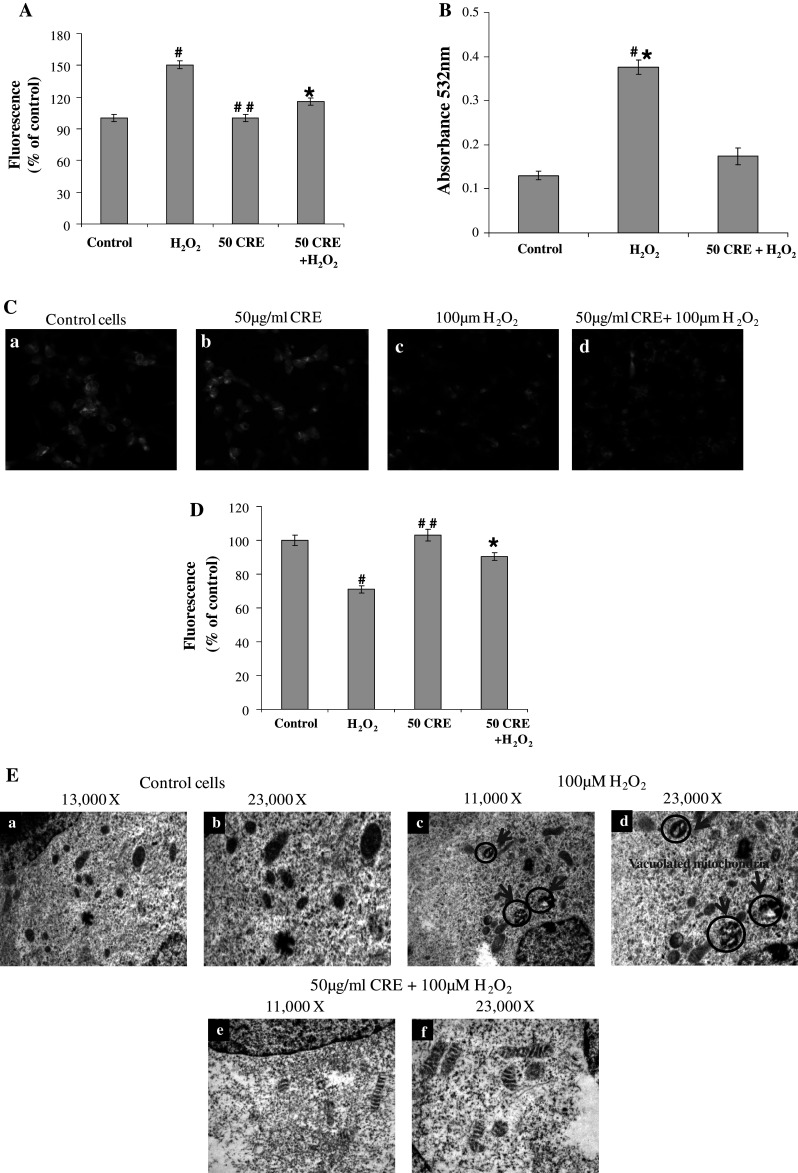
A Estimation of ROS production by 2′,7′,DCFH-DA using spectrofluorimeter. B Estimation of lipid peroxidation products by TBARS assay. C, D Effect of CRE on H2O2-induced decrease of MMP. The membrane potential was monitored by fluorescent microscope (Olympus, Japan) control cells without any treatment (a), 50 μg/ml CRE (b), 100 μM H2O2 (c), and cells pretreated with 50 μg/ml CRE for 30 min, followed by treatment with H2O2 (100 μM) (d). B The fluorescence intensity was determined using spectrofluorimeter. E Transmission electron-microscopic analysis of mitochondrial integrity of SH-SY5Y cells. The data are represented as mean ± SD of three independent experiments. # P < 0.05 versus control group, *P < 0.05 versus H2O2-treated group, and ##non-significant versus control group
Effects of CRE on H2O2-induced Reduction of the MMP
Rhodamine 123 is a cationic and lipophilic fluorescent dye, which partitions into active mitochondria based on the highly negative MMP. The diffusion and accumulation of rhodamine 123 in mitochondria are proportional to the degree of MMP (Ubl et al. 1996). The depolarization of MMP causes the loss of dye from mitochondria and a decrease in intracellular fluorescence. A collapse of MMP has been linked to several models of apoptosis (Prehn et al. 1996; Lin et al. 2009; Lee et al. 2010). To examine whether H2O2-induced apoptosis and its protection by CRE involves MMP pathway, measurement of MMP was carried out using rhodamine 123 in the SH-SY5Y human neuroblastoma cells. The corresponding changes of fluorescence intensity of rhodamine 123 were measured and presented in Fig. 3c, d. Data show that cells-treated with 100 μM H2O2 for 24 h, resulted in a marked decrease of MMP to 30 % compared to that of control indicating the depolarization of MMP. However, the cells pretreated with CRE before the addition of H2O2, showed a regain in the florescence intensity to 90.25 ± 2.3 %. These results showed that CRE extract potentially suppressed the H2O2-induced decrease of MMP. In support of our findings, Lee et al. (2010) have reported the restoration of MMP by Cyperus extract which was dissipated by 6-OHDA treatment. The current findings were further supported by the electron microscopic examination of the mitochondrial morphology.
Protective Effect of CRE on Mitochondrial Damage
Mitochondrial damage was evaluated by transmission electron microscopy. In control cells, the mitochondria appeared regular with respect to shape and structure Fig. 3E(a, b) 13,000 and 23,000× magnifications, respectively. H2O2 treatment dramatically altered the structure of mitochondria, and more than 70 % of the mitochondria were observed swollen and vacuolated, and showed destruction of cristae (Fig. 3E(c, d) 13,000 and 23,000× magnifications, respectively). However, pretreatment of CRE before H2O2 addition in the culture medium recovered the integrity of most of the mitochondria. Mitochondria appeared better preserved (Fig. 3E(e, f) 11,000 and 13,000× magnifications, respectively), compared with sole H2O2 treatment, showing a good conservation of the shape and of the cristae structure at higher magnifications. In a previous study, ALR protein (Alrp) was also found to improve the SH-SY5Y cells' survival in H2O2-induced apoptosis by maintaining mitochondrial membrane and cristae integrity (Lorenzo et al. 2009).
Mitochondrial metabolism is an important cellular activity for all cells in general and, brain cells in particular. It is well known that the physiological activity of brain mainly depends on the availability of glucose, oxygen, and mitochondrial-generated energy (Chetsawang et al. 2006). The study provides a clue that plant extract may help in better neuronal cell functioning by protecting mitochondrial integrity under stress.
Protective Effects of CRE on H2O2-induced HSP-70 Expression
HSP-70 is one of the most abundantly induced molecular chaperones under a variety of stress conditions Lachapelle et al. (2007). This protein plays a critical role during cell stress to prevent appearance of misfolded proteins. After treatment with H2O2, HSP-70 over-expressed consecutively in a dose-dependent manner compared with the control group, while the pretreatment of CRE down-regulated the H2O2-induced HSP expression (Fig. 4a, c). Many key components of survival and apoptotic pathways are regulated by interactions with HSPs. They are a class of proteins that interact with diverse protein substrates to assist in their folding and play a critical role during cell stress to prevent the appearance of folding intermediates that lead to misfolded or otherwise damaged molecules (Mayer and Bukau 2005). We obtained an increase in expression of HSP-70 after exposure to H2O2 for 3 h, suggesting that it acts as sensor and regulator of stress-induced apoptosis.
Fig. 4.
a Dose-dependent effect of H2O2 on HSP-70 expression. b The protective effect of CRE on H2O2-induced expression of oxidative stress marker proteins SOD, CAT, GPx, and GR. c The protective effect of CRE on H2O2-induced expression of oxidative stress marker protein HSP-70 and pro-apoptotic protein caspase-3, and anti-apoptotic protein Bcl-2 analyzed by immunoblotting. The band intensity is calculated by Image-J software. The data are represented as mean ± SD of three independent experiments. # P < 0.05 versus control group, *P < 0.05 versus H2O2-treated group
Effects of CRE on the Expression of Antioxidant Enzyme Levels in H2O2-induced SH-SY5Y Cells
Compelling evidence showed that oxidative stress is more in neuronal stress, particularly in Alzheimer’s and Parkinsion’s diseases (Prehn et al. 1996; Lee et al. 2010). To confirm the activation of SOD, CAT, GPx, and GR by CRE pretreatment in terms of protein expression, the Western blot analysis was carried out. As shown in Fig. 4b, the protein expressions of (Cu–Zn) SOD-1 (23 kDa), CAT (64 kDa), GPx (23 kDa), and GR (65 kDa) by CRE pretreatment were found to increase while H2O2-treated cells resulted in fewer expressions of all these enzymes. However, CRE pretreatment restored the H2O2-induced down-regulation of target proteins. The protein levels of all the enzymes were found consistent with their enzymatic activities (Table 1). It may be inferred that the enhancement in antioxidant enzyme activities by CRE pretreatment may be associated with the inhibition of the production of ROS. Previous reports also showed that neuroprotective effects of isolated polyphenols of spanish red wine, flavonoid butin, and total oligomeric flavonoid extract of CR rhizomes are through activation of antioxidant enzymes (Zhang et al. 2008; Martin et al. 2011; Sunil et al. 2011).
Effects of CRE on Caspase-3 (Pro-apoptotic) and Bcl-2 (Anti-apoptotic) Protein Expressions in H2O2-treated SH-SY5Y Cells
Caspases are the key mediators of cell death, and regulation of caspase activation is one of the key events in apoptosis. Caspase-3 is an executioner for the death program in response to various stressors. In the current study, we have examined whether H2O2-induced cell death was mediated through native caspase-3 cleavage. The 32-kDa native caspase-3 is cleaved to generate 17-kDa fragment which plays a critical role in apoptosis and is the final executor of apoptotic DNA damage. Figure 5 shows that the expression of 17-kDa cleaved caspase-3 protein was undetected in control cells but reported after H2O2 treatment. Furthermore, the effect of H2O2-induced stress could effectively be reversed by CRE pretreatment. Caspase-3 activation is widely used as a marker of apoptosis, and its inhibitors may have neuroprotective effects. In the H2O2-treated cells, the expression of Bcl-2 protein was down-regulated whereas the (Fig. 4c), apoptosis in SH-SY5Ycells is probably mediated by the mitochondrial pathway. However, pretreatment with CRE attenuated the change in Bcl-2 that was induced by H2O2, resulting in a decrease in the Bcl-2 expression.
Fig. 5.
Protective effect of CRE on H2O2-induced BDNF gene expression. The data are represented as mean ± SD of three independent experiments. # P < 0.05 versus control group, *P < 0.05 versus H2O2-treated group, and ##non-significant versus control group
The H2O2-induced oxidative stress leads to increased ROS generation which in turn depolarizes the mitochondrial membrane by stealing the electron. This results in decrease of MMP which triggers the apoptotic machinery of cell. The mitochondrial apoptotic pathway occurs by the activation of caspases which in turn induce cell death. The anti-apoptotic protein Bcl-2 plays an important role in mitochondrial-related apoptosis pathway by inhibiting cytochrome c release (Borner 2003). Decreased level of Bcl-2 causes an increase in mitochondrial permeability which results in the release of cytochrome c from the inter membrane space of mitochondria to cytosol (Chinnaiyan et al. 1996). The released cytochrome c triggers activation of caspase-9 which in turn activates caspase-3, and the activated caspase-3 induces cell death. Our results are in accordance with an earlier report on 6-OHDA-induced neuronal damage of PC12 cells leading to caspase-3 activation which is efficiently ameliorated by water extract of Cyperus rhizomes (Lee et al. 2010).
Effects of CRE on BDNF Expression
BDNF is a member of the nerve growth factor family, the most abundant neurotrophin in the brain which regulates neuronal cell survival, stress response, differentiation, synaptic strength, and morphology (Ghosh et al. 1994). It has been shown that the expression of this gene is reduced in Alzheimer’s, schizophrenia, and Huntington disease patients because of oxidative and apoptotic stress insults (Durany et al. 2001; Tan et al. 2005).
As BDNF regulates the proliferation, differentiation, and survival of dopaminergic neurons, we examined the effect of CRE on BDNF expression. As illustrated in Fig. 5, the mRNA levels of BDNF were drastically decreased by H2O2-treatment in the SH-SY5Y cells. However, pretreatment with CRE notably induced the expression of these enzymes and maintained the BDNF level even after H2O2 treatment.
Accumulating body of evidences shows that various molecules could regulate the expression of BDNF level in dopaminergic neurons. For example, the traditional food supplements and medicinal herbs, such as green tea component l-theanine, Rhus verniciflua Stokes, and Tripterygium regelii extracts, have been shown to increase the expression of BDNF in cultured cells and tissues and supported the survival and differentiation of dopamine neurons (Cho et al. 2008; Choi et al. 2010; Sapkota et al. 2011).
Conclusion
Herbal extracts have long been used in the traditional systems of medicine for treatment of neuronal stress because of their potent antioxidant activity and lesser or no side-effects. Recently, there is a surge of interest toward the search of natural substances with neuroprotective potential that can scavenge free radicals and protect cells from oxidative damage and apoptosis because of the adverse effects of conventional medicines. The current study shows that CRE can ameliorate the H2O2-induced oxidative stress by improving the antioxidant status, mitochondrial membrane integrity, regulating the apoptotic markers and maintaining the BDNF level. It can be concluded that regular intake of Cyperus extract along with regular diet will improve longevity, and neuronal- and age-associated disorders.
Acknowledgments
The authors are grateful to Dr. A. S. Bawa, Ex-Director, Defence Food Research Laboratory, Mysore, for providing constant encouragement during this investigation. The authors are highly thankful to Dr. N. Gayathri, Additional Professor and Mrs. B.N. Hemavathy, Neuropathology, NIMHANS, Bangalore, Karnataka for extending TEM facility.
References
- Andersen JK (2004) Oxidative stress in neurodegeneration: cause or consequence? Nat Med 5:S18–S25 [DOI] [PubMed] [Google Scholar]
- Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M (1910) Flow cytometry studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol 130:1910–1917 [PubMed] [Google Scholar]
- Borner C (2003) The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol 39:615–647 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 7:248–254 [DOI] [PubMed] [Google Scholar]
- Chaudhary AK, Nokubo M, Reddy GR, Yeola SN, Morrow JD, Blair IA, Marnett LJ (1994) Detection of endogenous malondialdehyde deoxyguanosine adducts in human liver. Science 265:1580–1582 [DOI] [PubMed] [Google Scholar]
- Chetsawang B, Putthaprasart C, Phansuwan-Pujito P, Govitrapong P (2006) Melatonin protects against hydrogen peroxide-induced cell death signaling in SH-SY5Y cultured cells: involvement of nuclear factor kappa B, Bax and Bcl-2. J Pineal Res 41:116–123 [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, Orth K, O’Rourke K, Duan H, Poirier GG, Dixit VM (1996) Molecular ordering of the cell death pathway-Bcl-2 and Bcl-x (L) function upstream of ced-3-like apoptotic proteases. J Biol Chem 1:4573–4576 [DOI] [PubMed] [Google Scholar]
- Cho HS, Kim S, Lee SY, Park JA, Kim SJ, Chun HS (2008) Protective effect of the green tea component l-theanine on environmental toxins-induced neuronal cell death. Neurotoxicology 29:656–662 [DOI] [PubMed] [Google Scholar]
- Choi BS, Sapkota K, Kim S, Lee HJ, Choi HS, Kim SJ (2010) Antioxidant activity and protective effects of Tripterygium regelii extract on hydrogen peroxide-induced injury in human dopaminergic cells, SH-SY5Y. Neurochem Res 35:1269–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G, Dembiec D, Marcus J (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34:30–38 [DOI] [PubMed] [Google Scholar]
- Delcour J, Varebeke DJ (1985) A new colorimetric assay for flavonoids in Pilsnerbeers. J Inst Brew 91:37–40 [Google Scholar]
- Durany N, Michel T, Zochling R, Boissl KW, Cruz-Sanchez FF, Riederer P (2001) Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res 52:79–86 [DOI] [PubMed] [Google Scholar]
- Finkel T (2003) Oxidant signals and oxidative stress. Curr Opin Cell Biol 15:247–254 [DOI] [PubMed] [Google Scholar]
- Frasca JM, Parks VR (1965) A routine technique for double staining ultra-thin sections using uranyl and lead salts. J Cell Biol 25:157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J (2011) Why is the nervous system vulnerable to oxidative stress? In: Gadoth N, Gobel HH (eds) Oxidative stress in applied basic research and clinical practice. The Humana Press, New York, pp 19–27
- Ghosh A, Carnahan J, Greenberg ME (1994) Requirement for BDNF in activity dependent survival of cortical neurons. Science 18:1618–1623 [DOI] [PubMed] [Google Scholar]
- Johannessen JV (1973) Rapid processing of kidney biopsies for electron microscopy. Kidney Int 3:46–50 [DOI] [PubMed] [Google Scholar]
- Kilani-Jaziri S, Neffati A, Limem I, Boubaker J, Skandrani I, Sghair MB, Bouhlel I, Bhouri W, Mariotte AM, Ghedira K, Dijoux Franca MG, Chekir-Ghedira L (2009) Relationship correlation of antioxidant and antiproliferative capacity of Cyperus rotundus products towards K562 erythroleukemia cells. Chem Biol Interact 181:85–94 [DOI] [PubMed] [Google Scholar]
- Kujala TS, Loponen JM, Klika KD, Pihlaja K (2000) Phenolic and betacyanins in red beetroot (Beta vulgaris) root: distribution and effects of cold storage on the content of total phenolics and three individual compounds. J Agri Food Chem 48:5338–5342 [DOI] [PubMed] [Google Scholar]
- Lachapelle G, Radicioni SM, Stankiewicz AR, Mosser DD (2007) Acute acidification or amiloride treatment suppresses the ability of Hsp70 to inhibit heat-induced apoptosis. Apoptosis 12:1479–1488 [DOI] [PubMed] [Google Scholar]
- LeBel CP, Ishiropoulos H, Bondy SC (1992) Evaluation of the probe 29,79-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231 [DOI] [PubMed] [Google Scholar]
- Lee CH, Hwang DS, Kim HG, Oh H, Park H, Cho JH, Lee JM, Jang JB, Lee KS, Oh MS (2010) Protective effect of Cyperi rhizoma against 6-hydroxydopamine-induced neuronal damage. J Med Food 13:564–571 [DOI] [PubMed] [Google Scholar]
- Lemaure B, Touche A, Zbinden I, Moulin J, Courtois D, Mace K, Darimont C (2007) Administration of Cyperus rotundus tubers extract prevents weight gain in obese Zucker rats. Phytother Res 21:724–730 [DOI] [PubMed] [Google Scholar]
- Lin YC, Huang YC, Che SC, Liaw CC, Kuo SC, Huang LJ, Gean PW (2009) Neuroprotective effects of ugonin K on hydrogen peroxide-induced cell death in human neuroblastoma SH-SY5Y cells. Neurochem Res 34:923–930 [DOI] [PubMed] [Google Scholar]
- Lorenzo P, Barbara P, Thomas L, Florenzo I, Leonardo R, Floriana G, Rosanna M, Maura B, Daniela S, Francesco V, Elisabetta MM, Antonio F (2009) Protective effect of augmenter of liver regeneration on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Free Radic Res 43:865–875 [DOI] [PubMed] [Google Scholar]
- Martin S, Gonzalez-Burgos E, Carretero ME, Gomez-Serranillos MP (2011) Neuroprotective properties of Spanish red wine and its isolated polyphenols on astrocytes. Food Chem 128:40–48 [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62:670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358 [DOI] [PubMed] [Google Scholar]
- Prehn JH, Bindokas VP, Jordan J, Galindo MF, Ghadge GD, Roos RP, Boise LH, Thompson CB, Krajewski S, Reed JC, Miller J (1996) Protective effect of transforming growth factor-beta 1 on beta-amyloid neurotoxicity in rat hippocampal neurons. Mol Pharmacol 49:319–328 [PubMed] [Google Scholar]
- Sapkota K, Kim S, Se-Eun P, Kim SJ (2011) Detoxified extract of Rhus verniciflua stokes inhibits rotenone-induced apoptosis in human dopaminergic cells, SH-SY5Y. Cell Mol Neurobiol 31:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo WS, Pae HO, Oh GS, Chai KY, Kwon TO, Yun YG, Kim NY, Chung HT (2001) Inhibitory effects of methanol extract of Cyperus rotundus rhizomes on nitric oxide and superoxide productions by murine macrophage cell line, RAW 264.7 cells. J Ethnopharmacol 76:59–64 [DOI] [PubMed] [Google Scholar]
- Sharma R, Gupta R (2007) Cyperus rotundus extract inhibits acetylcholinesterase activity from animal and plants as well as inhibits germination and seedling growth in wheat and tomato. Life Sci 80:2389–2392 [DOI] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191 [DOI] [PubMed] [Google Scholar]
- Sonwa MM, Konig WA (2001) Chemical study of essential oil of Cyperus rotundus. Phytochemistry 58:799–810 [DOI] [PubMed] [Google Scholar]
- Sunil AG, Kesavanarayanan KS, Kalaivani P, Sathiya S, Ranju V, Jyothi Priya R, Pramila B, Solomon Paul FD, Venkhatesh J, Saravana Babu C (2011) Total oligomeric flavonoids of Cyperus rotundus ameliorates neurological deficits, excitotoxicity and behavioral alterations induced by cerebral ischemic-reperfusion injury in rats. Brain Res Bull 84:394–405 [DOI] [PubMed] [Google Scholar]
- Tan YL, Zhou DF, Zhang XY (2005) Decreased plasma brain-derived neurotrophic factor levels in schizophrenic patients with tardive dyskinesia: association with dyskinetic movements. Schizophr Res 74:260–270 [DOI] [PubMed] [Google Scholar]
- Tsoyi K, Jang HJ, Lee YS, Kim YM, Kim HJ, Seo HG, Lee JH, Kwak JH, Lee DU, Chang KC (2011) (+)-Nootkatone and (+)-valencene from rhizomes of Cyperus rotundus increase survival rates in septic mice due to heme oxygenase-1 induction. J Ethnopharmacol 137:1311–1317 [DOI] [PubMed] [Google Scholar]
- Ubl JJ, Chatton JY, Chen S, Stucki JW (1996) A critical evaluation of in situ measurement of mitochondrial electrical potentials in single hepatocytes. Biochim Biophys Acta 1276:124–132 [DOI] [PubMed] [Google Scholar]
- Uddin SJ, Mondal K, Shilpi JA, Rahman MT (2006) Antidiarrhoeal activity of Cyperus rotundus. Fitoterapia 77:134–136 [DOI] [PubMed] [Google Scholar]
- Umerie SC, Ezeuzo HO (2000) Physicochemical characterization and utilization of Cyperus rotundus starch. Bioresour Technol 72:193–196 [Google Scholar]
- Varadarajan S, Yatin S, Aksenova M, Butterfield DA (2000) Alzheimer’s amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J Struct Biol 130:184–208 [DOI] [PubMed] [Google Scholar]
- Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616 [DOI] [PubMed] [Google Scholar]
- Zhang R, Chae S, Kang KA, Piao MJ, Ko DO, Wang ZH, Park DB, Park JW, You HJ, Hyun JW (2008) Protective effect of butin against hydrogen peroxide-induced apoptosis by scavenging reactive oxygen species and activating antioxidant enzymes. Mol Cell Biochem 318:33–42 [DOI] [PubMed] [Google Scholar]
- Zhu M, Luk HH, Fung HS, Luk CT (1998) Cytoprotective effects of Cyperus rotundus against ethanol induced gastric ulceration in rats. Phytother Res 11:392–394 [Google Scholar]