Abstract
Aim: Tumor markers often remain elevated after intended curative resection of medullary thyroid carcinoma (MTC). The aim of this study was to determine the expression of αvβ3, a promising theranostics target, in MTC and its metastases.
Materials & methods: Avβ3 expression was analyzed in 104 patients using a tissue microarray and correlated with clinicopathological variables and survival.
Results: Cytoplasmic αvβ3 positivity was seen in 70 patients and was associated with lymph node metastases at time of initial surgery. Membranous positivity was considered positive in 30 patients and was associated with sporadic MTC.
Conclusion: Avβ3 was expressed in the cytoplasm of 67% of MTC patients. Membranous expression, which is presumably most relevant for the theranostic use of αvβ3, was seen in 29%.
Keywords: : medullary thyroid carcinoma, nuclear imaging, nuclear therapy, tissue microarray, αvβ3
Plain language summary
Article highlights.
After intended curative resection of medullary thyroid carcinoma (MTC), calcitonin levels often remain elevated indicating remnant disease or metastasis.
There is a demand for new imaging and therapeutic options for patients with MTC.
Avβ3, an integrin expressed in many tumors, is a promising target for nuclear imaging and treatment using radiolabeled arginine-glycine-aspartic acid (RGD).
Cytoplasmic and membranous αvβ3 expression was analyzed using a tissue microarray including primary tumors and lymph node metastases of 104 and 27 patients, respectively.
Cytoplasmic expression was considered positive in 67% of patients and was associated with lymph node metastases at the time of initial surgery.
Membranous expression, which is thought to be most relevant for the theranostic use of αvβ, was seen in 29% of patients and was associated with sporadic MTC.
Survival analysis showed no prognostic value of αvβ3.
The correlation of immunohistochemical αvβ3 expression and uptake of radiolabeled RGD should be further assessed in patients with membranous αvβ3 expression.
1. Background
Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor, derived from the calcitonin-producing parafollicular c-cells of the thyroid. Although MTC accounts for only 1–2% of thyroid carcinomas, it is accountable for 13% of thyroid cancer related deaths [1,2]. In 75% of cases, MTC occurs sporadically, while it can also occur as part of the hereditary tumor syndrome Multiple Endocrine Neoplasia type 2 (MEN2) [3]. Treatment with curative intent consists of total thyroidectomy and dissection of the central lymph node compartment. However, despite treatment, over half of patients continue to exhibit elevated calcitonin levels, indicating persistent disease. Conventional imaging modalities are inadequate for detecting low tumor marker levels in these cases. Imaging modalities are not sufficient in these patients with low tumor markers. Moreover, possibilities for adjuvant therapy are limited. Consequently, survival rates have not increased significantly in the last decades [1]. Therefore, there is a demand for new imaging and therapeutic options that also target lymph node metastases, which will enable better treatment of patients who present with metastases or rapidly progress.
Neuroendocrine tumors are highly vascularized and angiogenesis plays a major role in the development of thyroid tumors. Most current adjuvant treatments, such as tyrosine kinase inhibitors, target angiogenesis pathways. Avβ3 is a target for nuclear imaging and treatment (theranostics), which is also strongly involved in the regulation of angiogenesis [4,5]. It is largely expressed in neovasculature and tumor cells of various malignancies including melanoma, glioma, breast, pancreas, prostate, lung, head and neck, and gastric cancer [6–14]. Also, αvβ3 integrin affects tumor growth, local invasion and development of metastases [15]. Arginine-glycine-aspartate (RGD) peptides have high affinity and specificity for the extracellular domain of αvβ3 integrin [4]. Therefore, radiolabeled RGD can be used for imaging of malignancies as well as for subsequent treatment with peptide receptor radionuclide therapy (PRRT).
The aim of this study was to determine αvβ3 integrin expression in MTC and its lymph node metastases to assess its suitabilitiy as a nuclear target. Correlation of αvβ3 with clinicopathologic variables and survival was assessed.
2. Materials & methods
The same cohort, database and TMA were used as described in our previous research [16,17].
2.1. Patients
Patients who underwent surgery between 1988 and 2014 for MTC were identified from the pathology databases of five Dutch tertiary referral centers: Leiden University Medical Center (LUMC), Amsterdam University Medical Center (AUMC), Radboud University Medical Center (RUMC), University Medical Center Groningen (UMCG) and University Medical Center Utrecht (UMCU). Formalin fixed paraffin embedded (FFPE) tissues were retrieved from pathology archives. Primary tumor tissue was available from 104 patient for inclusion in the tissue microarray (TMA). Additionally, tissue of lymph node metastases from 27 patients from theLUMC and UMCU was available.
Clinical and pathological data was obtained from patient records. Germline mutation analysis of the RET gene was performed to confirm all MEN2 diagnoses. Sporadic patients either had a negative germline mutation analysis or a negative family history. Microscopically detected positive resection margins were not included as a separate variable but incorporated into the T-stage classification. Disease status was based on postoperative calcitonin and CEA serum values. Given the range of assays used across five centers over nearly three decades, no exact values or doubling times were used. CEA or calcitonin level above the, at that time applicable, reference range was considered indicative of persistent disease, while values within normal range was interpreted as cured. Only postoperative CEA and calcitonin values measured more than 6 months after surgery were considered. Necrosis, angioinvasion and desmoplasia were scored on whole slides, on the same FFPE blocks that were used for the construction of the TMA. Necrosis and angioinvasion were scored as absent or present. Desmoplasia was scored as negative, some, moderate or severe.
This study was performed according to national guidelines with respect to the use of leftover tissue and approval for this study, including the use of patient data, was obtained from the Institutional Review Board of the UMCU.
2.2. Construction of the tissue microarray
An automated machine (TMA grand master, 3D Histech, Budapest, Hungary) was used to create the TMA. Three cores of 0.6 mm were punched from each FFPE block of primary tumor and available lymph node metastases. To ensure that cores were punched from tumor regions, a pathologist (PJvD) identified and marked cell-rich areas on H&E slides. These slides were then scanned and the marked areas were manually circled using TMA software (3D Histech).
2.3. Immunohistochemistry
TMA blocks and whole slides were cut at 4 μm and mounted on coated slides. Staining for αvβ3 was carried out manually following protocol: after baking the slides at 60°C for 10 min, slides were deparaffinized in xylene for 10 min and hydrated in a series of 100% ethanol, 70% ethanol and rinsed with demi-water. Hereafter, slides were washed with PBS twice. Endogenous peroxidase was blocked using 3% H2O2 in PBS for 15 min. Antigen retrieval was performed in Tris-EDTA buffer (pH 9) by boiling. Slides were washed with PBS-Tween two-times, then were then incubated with Pierce protein- free T20 (PBS) blocking buffer (PIER37573, Thermo Scientific) and incubated at room temperature in a dark place for 15 min. The primary αvβ3 antibody (1:25 ab7166 mouse monoclonal [BV3], Abcam) was incubated overnight in a dark place at 4°C. Slides were washed with PBS-Tween three-times. Then, a 2 step detection system was used (VWRKC-DPVB110HRP, Immunologic). First, a post-blocking step was performed for 15 min and slides were washed with PBS-tween three-times. Secondly, poly-HRP-anti-mouse/rabbit HRP was added for 30 min; both incubations took place in the dark at room temperature. Slides were washed with PBS-Tween three-times. Bright DAB (VWRKBS04-110, Immunologic) was added and the slides were incubated for 8 min in the dark at room temperature. Slides were washed with tap water, counterstained with 3x diluted Mayer's hemalum solution (1.09249.0500, Sigma-Aldrich), washed with tap water and coverslipped. Tissue of renal cell carcinoma and hemangioma was used as positive controle. As a negative controle, the staining was performed on tissue of renal cell carcinoma and MTC without addition of the primary antibody.
2.4. Scoring of the immunohistochemistry
The cores included in the TMA and whole slides were scored by an experienced pathologist (PJvD) and researcher (LHdV) for cytoplasmic and membranous staining, both blinded to clinicopathologic characteristics. Any dDisagreements were resolved through discussion, when necessary with help of a third reviewer (LL). The intensity of cytoplasmic staining was scored as absent (0), weak (1), moderate (2) or strong (3). Membranous staining was scored as present or absent. Staining was considered homogenous if the intensity across various cores was consistent. Figure 1 shows representative scores of all immunostainings. Data on hypoxia inducible factor-1 alpha (HIF-1α), VEGF, glucose transporter 1 (Glut-1), carbonic anhydrase IX (CAIX), microvessel density (MVD) and somatostatin receptor 2A (SSTR2A) was available from previous studies [16,17].
Figure 1.
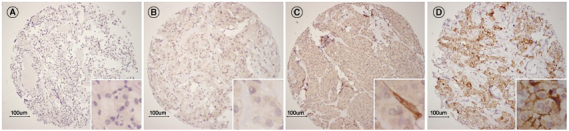
Representative examples of immunohistochemical staining for αvβ3 expression in TMA of MTC. (A) Absent αvβ3 staining. (B) Avβ3 staining with intensity 1. (C) Avβ3 staining with intensity 2 with positively stained vasculature. (D) Avβ3 staining with intensity 3 with positively stained membranes.
2.5. Statistical analysis
Categorical data were summarized using frequencies and percentages, while continuous data were summarized using medians and ranges. To enhance the statistical power, categorical data were recoded into dichotomous variables. Grade of desmoplasia was recoded into none-some vs. moderate-severe. Stage was recoded into stage I–III and stage IV. Hereditability was recoded as either sporadic disease or MEN2 syndrome. Avβ3 scorings were transformed into a dichotomous variable, considered positive in case of average intensity of cytoplasmic staining in the scored cores >1 or if membranous staining was present in ≥1 of the scored cores. Overall survival (OS) was defined as time to death from any cause. Progression-free survival (PFS) was defined as time to development of distant metastases or death. Univariate Cox regression survival analysis was performed. Furthermore, Kaplan-Meier survival curves were plotted and significance was calculated using log rank test. All reported p-values were two sided. Analysis was performed using SPSS software, version 25.0 (IBM, Armonk, NY, USA).
3. Results
3.1. Clinicopathological variables
Baseline characteristics are shown in Table 1. One-hundred-and-four patients were included. Patients were aged 10 to 82 years (mean 45.8, SD 16.3). Half of patients were male. The majority of patients had sporadic disease (56.8%), 38.9% MEN2A and 4.2% MEN2B. Patients presented with stage I, II, III and IV in 13.5%, 24.0%, 16.7% and 45.8%, respectively. Tumor size ranged from 4 to 70 mm (mean 25.6 mm, SD 14.8). At time of initial surgery, 63.4% of patients had developed lymph node metastases.
Table 1.
Baseline characteristics.
| N (%) | 104 (100) |
|---|---|
| Mean age in years (SD) | 46 (16.3) |
| Gender | |
| Male (%) | 50 (49.5) |
| Female (%) | 51 (50.5) |
| Heritability | |
| Sporadic (%) | 54 (56.8) |
| MEN2a/b (%) | 41 (43.2) |
| Stage | |
| I–III (%) | 51 (53.7) |
| IV (%) | 44 (46.3) |
| Mean size in mm (SD) | |
| <20 mm (%) | 36 (39.6) |
| ≥20 mm (%) | 55 (60.4) |
| Lymph node metastasis | |
| No (%) | 37 (36.6) |
| Yes (%) | 64 (61.5) |
| Overall survival | |
| Did not die (%) | 87 (89.7) |
| Died (%) | 10 (10.3) |
| Progression free survival | |
| No progression/death (%) | 32 (33.0) |
| Progression/death (%) | 65 (67.0) |
| Cytoplasmic αvβ3 | |
| Negative | 34 (32.7) |
| Positive | 70 (67.3) |
| Membranous αvβ3 | |
| Negative | 74 (71.2) |
| Positive | 30 (28.8) |
3.2. Avβ3 expression in primary tumor
The mean intensity of αvβ3 in all cores containing primary tumor was 1.6 (SD 0.58). Only two patients showed no cytoplasmic αvβ3 expression in one or more cores. The intensity of the scored cores was 0, 1, 2 and 3 in 0.8%, 42.8%, 52.4%% and 4.0%, respectively. Among the 91 patients with multiple cores available for analysis, 71.4% exhibited homogeneous expression throughout the primary tumor. Membranous staining was seen in 28.8% patients. In 75.8% of patients with multiple cores available for analysis, membranous staining was consequently present or absent in all cores.
3.3. Avβ3 expression in primary tumor vs. lymph node metastases
The average expression in primary tumor and lymph node metastases for these individual patients is demonstrated in Figure 2. Tissue of lymph node metastases of 27 patients was available in the TMA. Twenty-three patients had cytoplasmic αvβ3 positive primary tumors. These 23 patients had 29 lymph nodes available for analysis, of which six had negative and 23 had αvβ positive cytoplasm. Two of the four patients with αvβ3 negative cytoplasm in the primary tumor had positive cytoplasm in the lymph node metastases. Eleven of the 27 patients had αvβ3 positive membranes in the primary tumor, of which two patients also showed membranous expression in the lymph node metastases. Four patients had negative membranes in the primary tumor but positive membranes in the lymph node metastases.
Figure 2.
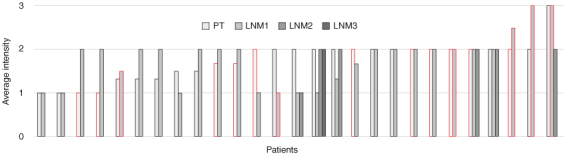
Average intensity of αvβ3 expression in primary tumor (PT) and their subsequent lymph node metastases (LNM). The vertical axis shows the average intensity of the staining, the horizontal axis shows the tissues of 27 patients. Red contour shows presence of membranous staining.
3.4. Association between αvβ3 expression in primary tumor & clinicopathological variables
Table 2 shows αvβ3 expression in comparison with clinicopathological variables. Avβ3 positive membranes were seen significantly (p = 0.01) more often in patients with sporadic MTC compared with patients with MEN2 (77.8 vs. 22.2%, respectively). For membranous positivity no other significant variables were found. Patients with lymph node metastases at time of initial surgery had significantly (p = 0.02) more often αvβ3 positive cytoplasm compared with patients without lymph node metastases (71.0 vs. 29.0%, respectively). Avβ3 positive membranes were seen significantly (p = 0.01) more often in patients with sporadic MTC compared with patients with MEN2 (77.8 vs. 22.2%, respectively).
Table 2.
Clinicopathological characteristics of MTC patients stratified by αvβ3 positivity.
| Cytoplasmic | Membranous | |||||
|---|---|---|---|---|---|---|
| αvβ3 negative | αvβ3 positive | αvβ3 negative | αvβ3 positive | |||
| N (%) | 34 (32.7) | 70 (67.3) | p-value | 74 (71.2) | 30 (28.8) | p-value |
| Mean age in years (SD) | 43.4 (16.1) | 46.9 (16.3) | 42.0 (15.1) | 55.5 (15.4) | ||
| Gender | 0.22 | 0.47 | ||||
| Male (%) | 13 (40.6) | 37 (53.6) | 34 (47.2) | 16 (55.2) | ||
| Female (%) | 19 (59.4) | 32 (46.4) | 38 (52.8) | 13 (44.8) | ||
| Heritability | 0.07 | 0.01 a | ||||
| Sporadic (%) | 14 (43.8) | 40 (63.5) | 33 (48.5) | 21 (77.8) | ||
| MEN2a/b (%) | 18 (56.3) | 23 (36.5) | 35 (51.5) | 6 (22.2) | ||
| Stage | 0.10 | 0.50 | ||||
| I–III (%) | 21 (65.6) | 30 (47.6) | 38 (55.9) | 13 (48.1) | ||
| IV (%) | 11 (34.4) | 33 (52.4) | 30 (44.1) | 14 (51.9) | ||
| Mean size in mm (SD) | 26.0 (15.3) | 25.5 (14.6) | 0.77 | 25.5 (15.5) | 25.9 (13.0) | 0.89 |
| <20 mm (%) | 12 (33.3) | 24 (66.7) | 26 (72.2) | 10 (27.8) | ||
| ≥20 mm (%) | 20 (36.4) | 35 (63.6) | 39 (70.9) | 16 (29.1) | ||
| Lymph node metastasis | 0.02 a | 0.23 | ||||
| No (%) | 17 (53.1) | 20 (29.0) | 29 (40.3) | 8 (27.6) | ||
| Yes (%) | 15 (46.9) | 49 (71.0) | 43 (59.7) | 21 (72.4) | ||
| Overall survival | 1.00 | 0.48 | ||||
| Did not die (%) | 25 (89.3) | 53 (86.9) | 58 (89.2) | 20 (83.3) | ||
| Died (%) | 3 (10.7) | 8 (13.1) | 7 (10.8) | 4 (16.7) | ||
| Progression free survival | 0.36 | 0.45 | ||||
| No progression/death (%) | 25 (80.6) | 46 (71.9) | 53 (76.8) | 18 (69.2) | ||
| Progression/death (%) | 6 (19.4) | 18 (28.1) | 16 (23.2) | 8 (30.8) | ||
| Disease status | 0.11 | 0.28 | ||||
| Normal CEA/calcitonin (%) | 15 (50.0) | 20 (32.8) | 28 (41.8) | 7 (29.2) | ||
| Elevated CEA/calcitonin (%) | 15 (50.0) | 41 (67.2) | 39 (58.2) | 17 (70.8) | ||
| Necrosis | 0.71 | 0.71 | ||||
| Absent (%) | 29 (93.5) | 54 (88.5) | 60 (90.9) | 23 (88.5) | ||
| Present (%) | 2 (6.5) | 7 (11.5) | 6 (9.1) | 3 (11.5) | ||
| Angioinvasion | 1.00 | 1.00 | ||||
| Absent (%) | 28 (90.3) | 54 (88.5) | 59 (89.4) | 23 (88.5) | ||
| Present (%) | 3 (9.7) | 7 (11.5) | 7 (10.6) | 3 (11.5) | ||
| Desmoplasia | 0.61 | 0.90 | ||||
| None-some (%) | 17 (54.8) | 30 (49.2) | 34 (51.5) | 13 (50.0) | ||
| Moderate-severe (%) | 14 (45.2) | 31 (50.8) | 32 (48.5) | 13 (50.0) | ||
| HIF-1α | 0.82 | 0.28 | ||||
| Negative (%) | 14 (43.8) | 26 (41.3) | 31 (45.6) | 9 (33.3) | ||
| Positive (%) | 18 (56.3) | 37 (58.7) | 37 (54.4) | 18 (66.7) | ||
| CAIX | 0.61 | 0.28 | ||||
| Negative (%) | 17 (53.1) | 30 (47.6) | 36 (52.9) | 11 (40.7) | ||
| Positive (%) | 15 (46.9) | 33 (52.4) | 32 (47.1) | 16 (59.3) | ||
| Glut-1 | 0.66 | 1.00 | ||||
| Negative (%) | 31 (96.6) | 59 (93.7) | 64 (94.1) | 26 (96.3) | ||
| Positive (%) | 1 (3.1) | 4 (6.3) | 4 (5.9) | 1 (3.7) | ||
| MVD (SD) | 12.6 (5.3) | 15.0 (8.8) | 0.71 | 14.1 (7.0) | 14.4 (9.9) | 0.67 |
| <14.3 vessels/core (%) | 17 (53.1) | 36 (57.1) | 37 (54.4) | 16 (59.3) | ||
| ≥14.3 vessels/core (%) | 15 (46.9) | 27 (42.9) | 31 (45.6) | 11 (40.7) | ||
| VEGF | 0.56 | 0.18 | ||||
| Negative (%) | 10 (33.3) | 25 (39.7) | 22 (33.3) | 13 (48.1) | ||
| Positive (%) | 20 (66.7) | 38 (60.3) | 44 (66.7) | 14 (51.9) | ||
| SSTR2a | 0.99 | 0.75 | ||||
| Negative (%) | 15 (44.1) | 31 (44.3) | 32 (43.2) | 31 (46.7) | ||
| Positive (%) | 19 (55.9) | 39 (55.7) | 42 (56.8) | 16 (53.3) | ||
Bold terms represent the significant results.
3.5. Prognostic value
Univariate survival analysis for cytoplasmic and membranous αvβ expression was not significant for PFS or OS as is outlined in Table 3. In Supplementary Figure S1, Kaplan-Meier survival curves are shown. For cytoplasmic αvβ3 positive vs. negative MTC, 10-year survival rates were 84 and 81% for PFS, and 70 and 64% for OS, respectively. For membranous αvβ3 positivity and negativity, PFS was 70 and 52%, and OS was 84 and 75% after 10 years, respectively.
Table 3.
Univariate Cox regression survival analysis on progression free and overall survival.
| PFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|
| N = 95 | PFS (N) | PFS (%) | p-value | N = 89 | OS (N) | OS (%) | p-value | |
| Cytoplasmic αvβ3 | 0.45 | 0.72 | ||||||
| Negative | 31 | 25 | 80.6 | 28 | 25 | 89.3 | ||
| Positive | 64 | 46 | 71.9 | 61 | 53 | 86.9 | ||
| Membranous αvβ3 | 0.18 | 0.41 | ||||||
| Negative | 69 | 53 | 76.8 | 65 | 58 | 89.2 | ||
| Positive | 26 | 18 | 69.2 | 24 | 20 | 83.3 | ||
OS: Overall survival; PFS: Progression free survival.
4. Discussion
This study shows that the theranostic target αvβ3 was expressed in cytoplasm in the majority and on the membrane in a minority of MTC. In most cases, αvβ3 positive tumors exhibited homogeneous expression throughout the primary tumor. Survival analysis showed no prognostic value of αvβ3.
While Cheng et al. examined αvβ3 expression in three PTC cell lines using immunofluorescence and showed moderate to high expression on the cell surface (p = 0.05), immunohistochemical staining of αvβ3 has not been evaluated on thyroid tumors in other series [18]. In pancreas carcinoma, predominantly cytoplasmic staining is observed [9]. Gastric cancer shows mainly membranous staining. In case of strong membranous staining, also some cytoplasmic staining is seen [10]. Brain metastases of lung carcinoma exhibit prominent membranous staining [11]. Prostate cancer displays some cytoplasmic staining, but lacks membranous staining [12]. Our immunohistochemistry results show that αvβ3 is largely expressed in the cytoplasm of MTC rather than in the membrane. Only three cores in the TMA did not express any cytoplasmic αvβ3 while 67.3% of patients were deemed αvβ3 positive using our cut off value. Membranous staining was seen in 28.8% patients.
Avβ3 expression and imaging with radiolabeled RGD has not yet been investigated in MTC, nor has treatment with 177Lu-labeled RGD. However, imaging and treatment with radiolabeled RGD has been investigated in differentiated thyroid carcinoma (DTC). Zhao et al. described uptake of radioactive iodine (RAI) refractory metastatic lesions in ten DTC patients on 99mTc-3PRGD2 SPECT imaging [19]. Vatsa et al. presented a case of RAI and 18F-FDG non avid papillary thyroid carcinoma (PTC), in which 68Ga-DOTA-RGD2 was able to depict cervical lymph node metastases [20]. Parihar et al. compared 68Ga-DOTA-RGD2 to 18F-FDG PET/CT in 44 patients with RAI-refractory DTC and found a similar sensitivity but a significantly higher specificity of 68Ga-DOTA-RGD2, especially for lymph node metastases [21]. Furthermore, they have reported results suggesting response to 177Lu-DOTA-RGD2 treatment with a follow-up time of four months in a single DTC patient with uptake in the thyroid remnant, cervical and mediastinal lymph nodes, bone lesions and lung nodules on 68Ga-DOTA-RGD2 PET/CT [22].
In our analysis, a distinction was made between patients with cytoplasmic and membranous expression. RGD binds to the extracellular domain of the αvβ3 integrin [4]. Therefore, membranous expressions is interesting for theranostic purposes and should be the expression to focus on in further research. Patients with sporadic MTC had significantly more often αvβ3 positive membranes. Hence, this subgroup of patients, though small, may benefit more from imaging with radiolabeled RGD and may be more eligible for PRRT, especially when curative surgery is no longer possible. It is plausible that patients with more abundant membranous αvβ3 expression show more uptake on RGD imaging. However, this has not been studied in thyroid cancer or other tumors. Further research on the relation between immunohistochemical αvβ3 expression and uptake of radiolabeled RGD is therefore needed.
Avβ3 integrin has a strong effect on angiogenesis and is associated with tumor growth, tumor invasion and development of metastases in various malignancies, which are all prognostically relevant [4,7,9,23–25]. Our results show a correlation between cytoplasmic expression and having lymph node metastases at time of the primary surgery, which is in line with results on pancreas cancer [9]. Furthermore, the expression of αvβ3 was correlated with bone metastases in prostate and breast carcinoma [8,26–28]. Further research is needed to investigate whether αvβ3 is also correlated with distant metastases in MTC. A correlation with tumor size was not seen in our study, contrary to results of studies describing tumor growth and proliferation in ovarian cancer [29]. In cervical cancer, αvβ3 is significantly correlated with decreased survival [12]. This in contrast with the findings of Böger et al., which showed a significantly increased survival for patients with αvβ3 positive gastric cancer [10]. In our study, survival analysis showed no significant results.
A strength of this study is the relatively large sample size of 104 patients, considering the rarity of MTC. Another strength is the long follow-up time (mean 68.9 months, range 0–318 months), which is essential since MTC has low proliferative activity and low event rates. Furthermore, for the first time immunohistochemical αvβ3 data was combined with clinical end points such as the development of distant metastases and death. Most limitations of this study are a result of the retrospective design and the low incidence of MTC. To assess a substantial amount of data, patients were included from five tertiary referral centers comprising almost thirty years. As a consequence, variables which were consistent over time and between centers had to be used in our analysis and our follow-up ranges widely. Over the years, surgical guidelines have changed and surgical techniques may have differed between centers. A subanalysis of progressive patients would have been of added value, but was not possible due to the sample size. For future research involving a larger cohort, it would be interesting to use a more extensive IHC scoring system such as the immunoreactive score (IRS).
5. Conclusion
To conclude, αvβ3 seems to be frequently expressed in the cytoplasm and less often on the membranes of MTC cells. For future research, implementing a more extensive IHC scoring system such as the IRS would be advisable. Also, the correlation of immunohistochemical αvβ3 expression and uptake of radiolabeled RGD should be further assessed in patients with Membranous αvβ3 expression.
Supplementary Material
Acknowledgments
We would like to thank H Morreau for his time to select the MTC tissue of the LUMC, D Castiliego for his contribution in the construction of the TMA, D Lobeek for sharing her knowledge about αvβ3 staining and the αvβ3 staining protocol used in the RUMC, and U Flucke for her contribution to the establishment and assessment of the αvβ3 stained TMA.
Supplemental material
Supplemental data for this article can be accessed at https://doi.org/10.1080/14796694.2024.2376511
Author contributions
All authors contributed to the article and approved the submitted version.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
All procedures performed in this study were in accordance with ethical standards of the Institutional Review Board of the UMC Utrecht and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
Papers of special note have been highlighted as: • of interest
- 1.Wells SA, Asa SL, Dralle H, et al. Revised American Thyroid Association Guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567–610. doi: 10.1089/thy.2014.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kebebew E, Ituarte PHG, Siperstein AE, Duh Q-Y, Clark OH. Medullary thyroid carcinoma. clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer. 2000;88:1139–1148. doi: [DOI] [PubMed] [Google Scholar]
- 3.Fagin JA, Wells SA. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016;375:1054–1067. doi: 10.1056/NEJMra1501993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danhier F, Breton A Le, Préat V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol Pharm. 2012;9:2961–2973. doi: 10.1021/mp3002733 [DOI] [PubMed] [Google Scholar]; • This is of interest as it describes the theranostic potential of αvβ3.
- 5.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347);298–307. doi: 10.1038/nature10144 [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is of interest as it describes the theranostic potential of αvβ3.
- 6.Neto DS, Pantaleão L, De Sá BCS, Landman G. Alpha-v-beta3 integrin expression in melanocytic nevi and cutaneous melanoma. J Cutan Pathol. 2007;34:851–856. doi: 10.1111/j.1600-0560.2007.00730.x [DOI] [PubMed] [Google Scholar]
- 7.Schnell O, Krebs B, Wagner E, et al. Expression of integrin αvβ3 in gliomas correlates with tumor grade and is not restricted to tumor vasculature. Brain Pathol. 2008;18:378–386. doi: 10.1111/j.1750-3639.2008.00137.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takayama S, Ishii S, Ikeda T, Masamura S, Doi M, Kitajima M. The relationship between bone metastasis from human breast cancer and integrin αvβ3 expression. Anticancer Res. 2005;25:79–83. [PubMed] [Google Scholar]
- 9.Hosotani R, Kawaguchi M, Masui T, et al. Expression of integrin αvβ3 in pancreatic carcinoma: relation to MMP-2 activation and lymph node metastasis. Lippincott Williams & Wilkins. 2002;25:30–35. doi: 10.1097/00006676-200208000-00021 [DOI] [PubMed] [Google Scholar]
- 10.Böger C, Warneke VS, Behrens HM, et al. Integrins αvβ3 and αvβ5 as prognostic, diagnostic, and therapeutic targets in gastric cancer. Gastric Cancer. 2015;18:784–795. doi: 10.1007/s10120-014-0435-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berghoff AS, Kovanda AK, Melchardt T, et al. avb3, avb5 and avb6 integrins in brain metastases of lung cancer. Clin Exp Metastasis. 2014;31(7):841–851. doi: 10.1007/s10585-014-9675-0 [DOI] [PubMed] [Google Scholar]
- 12.Gruber G, Hess J, Stiefel C, et al. Correlation between the tumoral expression of β3-integrin and outcome in cervical cancer patients who had undergone radiotherapy. Br J Cancer. 2005;92:41–46. doi: 10.1038/sj.bjc.6602278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heß K, Böger C, Behrens HM, Röcken C. Correlation between the expression of integrins in prostate cancer and clinical outcome in 1284 patients. Ann Diagn Pathol. 2014;18:343–350. doi: 10.1016/j.anndiagpath.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 14.Fabricius EM, Wildner GP, Kruse-Boitschenko U, Hoffmeister B, Goodman SL, Raguse JD. Immunohistochemical analysis of integrins αvβ3, αvβ5 and α5β1, and their ligands, fibrinogen, fibronectin, osteopontin and vitronectin, in frozen sections of human oral head and neck squamous cell carcinomas. Exp Ther Med. 2011;2:9–19. doi: 10.3892/etm.2010.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727 [DOI] [PubMed] [Google Scholar]
- 16.Lodewijk L, van Diest P, van der Groep P, et al. Expression of HIF-1α in medullary thyroid cancer identifies a subgroup with poor prognosis. Oncotarget. 2017;8:28650–28659. doi: 10.18632/oncotarget.15622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vries LH, Lodewijk L, Willems SM, et al. SSTR2A expression in medullary thyroid carcinoma is correlated with longer survival. Endocrine. 2018;62(3):639–647. doi: 10.1007/s12020-018-1706-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng W, Feng F, Ma C, Wang H. The effect of antagonizing RGD-binding integrin activity in papillary thyroid cancer cell lines. Onco Targets Ther. 2016;9:1415–1423. doi: 10.2147/OTT.S99166 [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is of interest as it describes αvβ3 in thyroid cancer.
- 19.Zhao D, Jin X, Li F, Liang J, Lin Y. Integrin αvβ3 imaging of radioactive iodine-refractory thyroid cancer using 99mTc-3PRGD2. J Nucl Med. 2012;53:1872–1877. doi: 10.2967/jnumed.112.107821 [DOI] [PubMed] [Google Scholar]
- 20.Vatsa R, Shykla J, Mittal BR, et al. Usefulness of 68Ga-DOTA-RGD (αvβ3) PET/CT imaging in thyroglobulin elevation with negative iodine scintigraphy. Clin Nucl Med. 2017;42:471–472. doi: 10.1097/RLU.0000000000001645 [DOI] [PubMed] [Google Scholar]
- 21.Parihar AS, Mittal BR, Kumar R, Shukla J, Bhattacharya A. 68Ga-DOTA-RGD2 positron emission tomography/computed tomography in radioiodine refractory thyroid cancer: prospective comparison of diagnostic accuracy with 18F-FDG positron emission tomography/computed tomography and evaluation toward potential theranost. Thyroid. 2020;30:557–567. doi: 10.1089/thy.2019.0450 [DOI] [PubMed] [Google Scholar]; • This is of interest as it describes αvβ3 in thyroid cancer.
- 22.Parihar AS, Sood A, Kumar R, Bhusari P, Shukla J, Mittal BR. Novel use of 177Lu-DOTA-RGD2 in treatment of 68Ga-DOTA-RGD2-avid lesions in papillary thyroid cancer with TENIS. Eur J Nucl Med Mol Imaging. 2018;1836–1837. doi: 10.1007/s00259-018-4036-x [DOI] [PubMed] [Google Scholar]; • This is of interest as it describes αvβ3 in thyroid cancer.
- 23.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities Jay. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748 [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is of interest as it describes the theranostic potential of αvβ3.
- 24.Liu S, Hsieh WY, Jiang Y, et al. Evaluation of a 99mTc-labeled cyclic RGD tetramer for noninvasive imaging integrin αvβ3-positive breast cancer. Bioconjug Chem. 2007;18:438–446. doi: 10.1021/bc0603081 [DOI] [PubMed] [Google Scholar]
- 25.Ludwig BS, Kessler H, Kossatz S, Reuning U. Rgd-binding integrins revisited: how recently discovered functions and novel synthetic ligands (re-)shape an ever-evolving field. Cancers. 2021;13(7):1711. doi: 10.3390/cancers13071711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin αvβ3 modulates bone metastatic growth and tissue remodeling. 2007;26:6238–6243. doi: 10.1038/sj.onc.1210429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sloan EK, Pouliot N, Stanley KL, et al. Tumor-specific expression of αvβ3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:1–14. doi: 10.1186/bcr1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felding-Habermann B, O'Toole TE, Smith JW, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci USA. 2001;98:1853–1858. doi: 10.1073/pnas.98.4.1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landen CN, Kim TJ, Lin YG, et al. Tumor-selective response to antibody-mediated targeting of αvβ3 integrin in ovarian cancer. Neoplasia. 2008;10:1259–1267. doi: 10.1593/neo.08740 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


