Abstract
Current reports on trace elements, oxidative stress, and the effect of antiepileptic drugs are poor and controversial. We aimed to review effects of most common used antiepileptics on antioxidant, trace element, calcium ion (Ca2+) influx, and oxidant systems in human and experimental animal models. Observations of lower blood or tissue antioxidant levels in epileptic patients and animals compared to controls in recent publications may commonly support the proposed crucial role of antioxidants in the pathogenesis of epilepsy. Effects of old and new antiepileptics on reactive oxygen species (ROS) production in epilepsy are controversial. The old antiepileptic drugs like valproic acid, phenytoin, and carbamazepine induced ROS overproduction, while new epileptic drugs (e.g., topiramate and zonisamide) induced scavenger effects on over production of ROS in human and animals. Antioxidant trace element levels such as selenium, copper, and zinc were generally low in the blood of epileptic patients, indicating trace element deficiencies in the pathogenesis of epilepsy. Recent papers indicate that selenium with/without topiramate administration in human and animals decreased seizure levels, although antioxidant values were increased. Recent studies also reported that sustained depolarization of mitochondrial membranes, enhanced ROS production and Ca2+ influx may be modulated by topiramate. In conclusion, there is a large number of recent studies about the role of antioxidants or neuroprotectants in clinical and experimental models of epilepsy. New antiepileptic drugs are more prone to restore antioxidant redox systems in brain and neurons.
Keywords: Antiepileptic drugs, Antioxidants, Oxidative stress, Trace element, Seizures, Ca2+ signaling
Introduction
Epilepsy is a common chronic neurological disorder with various etiological factors which affects about 2–3 % of the general population with approximately 50 million people worldwide (Azam et al. 2012). Epilepsy has been divided into idiopathic, symptomatic, and cryptogenic forms and the oxidative stress has important role on etiology of the epilepilectic forms (Hayashi 2009; Seven et al. 2012).
The brain is particularly susceptible to oxidative stress because it utilizes the highest amount of oxygen compared with other bodily organs. The brain also contains high concentrations of polyunsaturated fatty acids that are prone to lipid peroxidation, is rich in iron, which can catalyze hydroxyl radical formation, and is low in catalase activity (Shin et al. 2011; Nazıroğlu 2012). Additionally it produces high amount of reactive oxygen species (ROS) such as superoxide, hydrogen peroxide, and hydroxyl radical, owing to high aerobic metabolism. Eventually these products make the brain most sensitive to oxidative injury (Nazıroglu 2007; Ozmen et al. 2007).
A number of experimental and clinical reports suggest the involvement of oxidative stress in pathophysiology of epilepsy (Nazıroğlu et al. 2009; Rowley and Patel 2013). Increased free radicals in membrane lipid peroxidation and decreased glutathione (GSH) concentrations in the epileptic focus (Jesberger and Richardson 1991) were reported. Further, the involvement of free radicals in seizures is also supported by reports which indicate that exogenously administered antioxidant protects the brain against seizures (Gupta et al. 2003). Growing evidence indicates that long-term antiepileptic drug treatment leads to an increase in oxidative stress which is similar to that observed during epileptogenesis although the topics on the antiepileptic drugs are conflicting (Table 1). For example, valproic acid has been found to increase lipid peroxidation in patients receiving it (Martinez-Ballesteros et al. 2004). Contrary to this observation some anti-epileptic agents like phenytoin have been shown to decrease oxidative stress demonstrated by increase in glutathione reductase activity in patients receiving it (Stanton and Moskal 1991).
Table 1.
Effects of antiepileptic drugs on oxidative stress and trace elements in humans and animals
| Antiepileptic drug | Subject/material | Value/effect | References |
|---|---|---|---|
| Phenytoin | Rat/brain | MDA/increase | Reeta et al. (2009) |
| GSH/decrease | |||
| Human/blood | MDA/increase | Liu et al. (1997) | |
| Cu, Zn–SOD/increase | |||
| Cu/increase | |||
| GSH/decrease | |||
| Sodium valproate | Children/plasma | GSH-Px/increase | Kurekci et al. (1995) |
| Children/erythrocyte | GSH-Px/increase | Yuksel et al. (2001) | |
| SOD/increase | |||
| Adult/blood | Se–GSH-Px/increase | Hamed et al. (2004) | |
| Children/blood | GSH-Px/no effect | Ashrafi et al. (2007a) | |
| Adult/blood | Se–GSH-Px/no effect | Verrotti et al. (2002) | |
| Adult/erythrocyte | GSH-Px/no effect | Yis et al. (2009) | |
| SOD/increased | |||
| MDA/increased | |||
| Carbamazepine | Adult/plasma | Se–GSH-Px/no effect | Hamed et al. (2004) |
| Children/erythrocyte | GSH-Px/no effect | Ashrafi et al. (2007b) | |
| Adult/blood | Se–GSH-Px/no effect | Verrotti et al. (2002) | |
| Children/erythrocyte | MDA/increase | Yuksel et al. (2001) | |
| SOD/increase | |||
| Zonisamide | Mice brain/cultured neurons | GSH/increase | Asanuma et al. (2010) |
| PC12 cells | GSH-Px/increase | Yürekli and Naziroglu (2013) | |
| GSH/increase | |||
| MDA/decrease | |||
| Caspase-3/decrease | |||
| Cytosolic Ca2+/decrease | |||
| Topiramate | Rat brain/microsomes | GSH-Px/increase | Nazıroğlu et al. (2008) |
| Rat/erythrocytes | GSH-Px/increase | Nazıroğlu et al. (2009) | |
| Rat/hippocampus | GSH-Px/increase | Muriach et al. (2010) | |
| Rat/brain cortex | MDA/decrease | Nazıroğlu et al. (2008, 2009) | |
| Rat/erythrocyte and plasma | GSH-Px/increase | Nazıroğlu et al. (2009) | |
| GSH/increase | |||
| Vitamin C, vitamin E/increase | |||
| Mice/brain | GSH/decrease | Agarwal et al. (2011) | |
| MDA/increased | |||
| Human/blood | GSH, GSH-Px, vitamin A and vitamin C/increase | Yürekli and Naziroglu (2013) |
Se selenium, Cu copper, Se selenium, MDA malondialdehyde, GSH glutahhione, GSH-Px glutathione peroxidase, SOD superoxide dismutase
Main antioxidant trace elements are copper, zinc, and selenium. For example, selenium, an essential trace element in humans, has antioxidant properties; and prevents neuronal cell bodies from oxidation and keeps them biologically healthy. To date, approximately 30 types of selenoproteins have been identified. Some of these selenoproteins have vital enzymatic functions (Rayman 2000). The importance of selenium is because of the 21-amino-acid selenocysteine, one of the selenoproteins (Martinez-Ballesteros et al. 2004). Brain contains a high quantity of selenium, especially in gray matter (Nazıroğlu et al. 2009). The most important function of GSH-Px, a selenium-dependent enzyme, is reducing hydrogen and organic peroxides in the presence of reduced GSH (Weber et al. 1991). There is direct relationship between antioxidant trace elements and epilepsy. For example, correlation between selenium or GSH-Px deficiency and epilepsy has been shown (Schweizer et al. 2004).
The aim of this review paper is to investigate the role of antiepileptic drugs in relationship with oxidative stress, antioxidant redox systems, and trace elements in epilepsy.
Oxidative Stress and Epilepsy
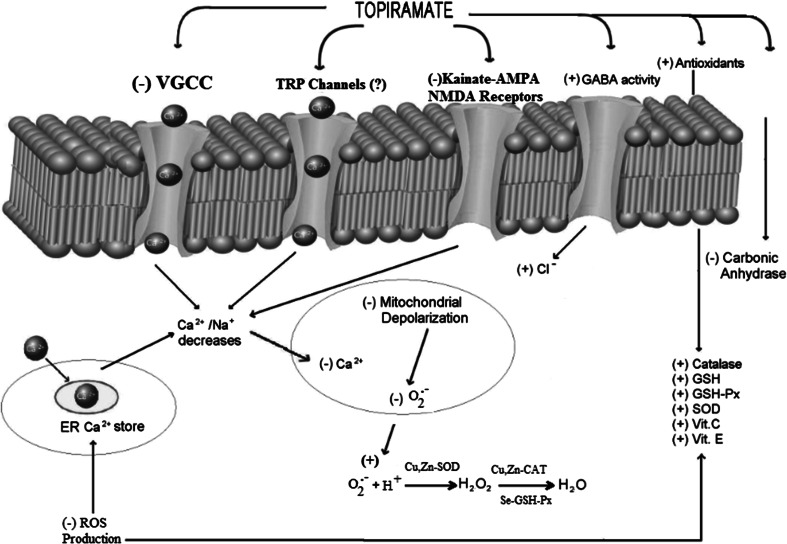
Initiation and progression of epilepsy are induced free oxygen radicals and, therapies aimed at reducing oxidative stress may ameliorate tissue damage and favorably alter the clinical course (Azam et al. 2012). At the cellular level, intense seizure activity typically initiates a massive influx of calcium ions via voltage-gated calcium channels and glutamate receptors, such as kainate, alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) and N-methyl-d-aspartate (NMDA)-dependent cation channels. Elevated intracellular Ca2+ leads to biochemical cascades which trigger acute hippocampal cell death after epilepsy (Nazıroğlu et al. 2009). Additionally, high levels of intracellular Ca2+ can induce generation of ROS, uncoupling of mitochondria and activation of a wide range catabolic enzymes that are capable of interfering with cell function (Pariente et al. 2001; Patel 2004; González et al. 2006, 2007). On the other hand, exposure of mitochondria to high cytosolic-free Ca2+ was shown to increase formation of ROS through depolarization of mitochondria (Fig. 1). Sustained depolarization of mitochondrial membranes and enhanced ROS formation could impair production of nicotinamide adenine dinucleotide phosphate (NADPH) and ATP. Indeed, rises in NAD(P)H auto-fluorescence associated with single seizure-like events in slices, decline with time during status epilepticus (Schuchmann et al. 1999). Moreover, in vivo studies suggested a failure of ATP production after prolonged status epilepticus (Gupta et al. 2001). Today, free radicals are known to be both the cause and the consequence of epileptic seizures (Patel 2004). Transient receptor potential (TRP) cation channels have six subfamilies and some TRP channels such as TRPM2 and TRPV1 are activated by oxidative stress (Naziroglu 2011; Nazıroğlu et al. 2012). These channels may play a role in the etiology of epilepsy due to their oxidative stress-dependent mechanisms. The subject should be clarified by future studies.
Fig. 1.
Free radical generation and Ca2+ uptake induce seizure activity by direct activation of Ca2+ channels although topiramate-induced modulator role in Ca2+ influx through voltage-gated Ca2+ channels (VGGC) and glutamate receptors namely AMPA, kainate, and N-methyl-d-aspartate (NMDA). During the physiological process, superoxide radicals (O2 −) produces and the radicals converted hydrogen peroxide (H2O2) by copper (Cu) and zinc (Zn) superoxide dismutase enzyme. The hydrogen peroxide (H2O2) is converted to water (H2O) by Cu- and Zn-dependent catalase (CAT) and selenium (Se)-dependent glutathione peroxidase enzyme (GSH-Px) enzymes. Mitochondria were reported to accumulate Ca2+ provided cytosolic Ca2+ rises or provided mitochondrial uptake exceeds mitochondrial Ca2+ extrusion, thereby leading to depolarization of mitochondrial membranes. On the other hand, exposure of mitochondria to high free Ca2+ was shown to increase formation of ROS. The sustained depolarization of mitochondrial membranes and enhanced ROS production may be modulated by topiramate. Transient potential (TRP) or TRP melastatin 2 (TRPM2) channel activity-induced Ca2+ influx increases may be modulated by topiramate. The molecular pathway may be a cause of epileptic seizures and represents a fruitful subject of topiramate for further study. − Decrease, + increase
Oxidative stress is defined as an imbalance between higher cellular levels and ROS such as superoxide radical, hydrogen peroxide, nitric oxide, and cellular antioxidant defense (Nazıroğlu 2012; Cardenas-Rodriguez et al. 2012; Espino et al. 2012). Neuronal cell death may be both a cause and consequence of epileptic seizures. Oxidative stress occurs when the productions of ROS exceed the removal capacity through the antioxidant redox system and results in excessive levels of free radical intermediates (Patel 2002). Liang and Patel (2004) have demonstrated oxidative damage to susceptible targets (protein, lipids, and DNA) caused by persistent seizures (status epilepticus). Neuronal cytosolic Ca2+ influx induces mitochondrial depolarization. Several studies have demonstrated an increase in mitochondrial oxidative stress and subsequent cell damage after persistent seizures (Weber et al. 1991; Pariente et al. 2001; González et al. 2006, 2007).
Antioxidant Trace Elements
There have been numerous reports on the association of trace elements and epilepsy. The cascade of neurotoxic events that lead to epileptic seizures is highly complex, but the main event involves the accumulation of ROS (Hayashi 2009). The ROS formation has been found to be both the cause and the result of epileptic seizures in human (Yuksel et al. 2001; Hamed et al. 2004). Selenium, zinc, and copper are three trace elements that are involved in the metabolism of ROS. For example, selenium is involved in the reduction of peroxide by participating in the structure of GSH-Px, which is a very important antioxidant enzyme (Hamed et al. 2004).
Selenium
The equilibrium of trace elements is essential for a healthy nervous system because most of them contribute to the activation of specific enzymes that play important roles in many pathways of the central nervous system. Antioxidant defense mechanisms are an important pathway involving trace elements. GSH-Px, which is a selenium-dependent enzyme that is involved in antioxidant defense mechanisms, controls the intracellular levels of hydrogen peroxide and hydroxyl radical (Nazıroğlu et al. 2009). It is well known that increased production of free radicals due to oxidative stress or the decreased functioning of antioxidant defense systems may lead to seizures or increase the risk of their recurrence (Savaskan et al. 2003; Hamed et al. 2004; Ashrafi et al. 2007a), because oxidative stress produces peroxidated membrane lipids and damages the cells (Hayashi 2009). Low levels of selenium and GSH-Px have been found in patients with epilepsy (Yuksel et al. 2001; Ashrafi et al. 2007b). Selenium-deficient rats have been found to be more susceptible to excitotoxicity (Savaskan et al. 2003).
The GSH-Px deficiency was reported in children with intractable epilepsy (Weber et al. 1991; Ramaekers et al. 1994). They found administration of selenium can help to treat the children following discontinuation of anticonvulsive drugs. Ashrafi et al. (2007a) reported that serum selenium level in intractable epilepsy patients was lower than that in healthy children, and they concluded that measurement of serum selenium in intractable epilepsy is helpful in recognizing the condition. In another study, Ashrafi et al. (2007b) found the patients affected by epilepsy have lower GSH-Px activity than the healthy children. Wirth et al. (2010) showed that cerebral selenium deficiency is associated with the incidence of seizure in mice through reduced activity of GSH-Px. Oztas et al. (2007) found that the damage to the blood–brain barrier in male rats due to seizure is increased if there is selenium and vitamin E deficiency; they concluded that management of seizure attacks with selenium has beneficial effects on reducing breakdown of the blood–brain barrier. Savaskan et al. (2003) observed a increased seizure rate in selenium-deficient rats due to greater susceptibility to kainate-induced excitotoxicity and the authors concluded that selenium has a fundamental role in neuronal susceptibility to excitotoxic lesions and seizure attacks. However, a study reported no significant change of serum selenium level in epilepsy patients as compared to healthy control patients during seizure attacks (Hamed et al. 2004). Mahyar et al. (2010) reported lower selenium level in children with simple febrile seizures than in febrile children without seizure. Recently, Seven et al. (2012) reported a significant decrease in selenium levels in patients with idiopathic intractable epilepsy. It has been suggested that antiepileptic drug therapies deplete total body selenium stores and failure to give appropriate selenium supplementation, especially to patients receiving valproic acid during pregnancy may increase the risk of neural tube defects or other free radical-mediated damage (Arakawa and Ito 2007).
Febrile seizures are the most common brain-related disease in children (Castano et al. 1997). Although the pathophysiology of febrile seizures is still unknown, several studies have indicated that multiple factors can be involved in the pathogenesis of febrile seizures, including elements such as iron and zinc (Tütüncüoğlu et al. 2001; Daoud et al. 2002). The role of other elements in developing febrile seizures and brain disorders, including selenium, has been reported in some studies (Tütüncüoğlu et al. 2001; Schweizer et al. 2004). Ramaekers et al. (1994) and Weber et al. (1991) investigated GSH-Px activity in children with intractable and they found administration of selenium can help to treat the children following discontinuation of anticonvulsive drugs. Nazıroğlu et al. (2008) reported on use of selenium for control or inhibition of seizure caused by excitotoxic agents.
Copper and Zinc
Zinc is an essential trace metal in humans and animals. Zinc deficiency results in defects of the central nervous system as well as peripheral neuropathy (Oki et al. 2012). Some evidence has indicated a relationship between zinc and seizure activity, but the detailed significance of zinc in convulsive activity is not clear (Seven et al. 2012). Clinical manifestations of zinc deficiency, such as memory deficits, learning disorders, and alterations in emotional behavior suggest hippocampal dysfunction (Oki et al. 2012). Changes in levels of trace elements have been proposed to underlie febrile seizures. Particularly, low zinc levels have been proposed as related factor of febrile seizure. The mechanism underlying the role of zinc levels in seizures has been examined in studies on mouse models and in vitro studies. Numerous reports have suggested that zinc modulates specific GABA receptors, and this mechanism is known to contribute to seizure inhibition (Andre et al. 2010; Amiri et al. 2010).
Amiri et al. (2010) reported decreased serum selenium, zinc, and copper levels in the children with febrile convulsion and in the control group. Ganesh et al. (2011) compared serum zinc levels in children (22 with epileptic seizures, 23 with simple febrile seizures and 22 controls) and they showed decreased serum zinc levels in children with febrile seizures than in those with epileptic seizures and normal children. Recently, Wojciak et al. (2013) assessed the serum zinc and copper concentrations in 23 children with initial recognition of epilepsy before beginning of pharmacological therapy in comparison with a healthy control group of 25 children. They demonstrated that epilepsy decreased zinc level although it increased copper levels in the patients.
On the other hand, Verrotti et al. (2002) assessed whether epileptic children have abnormal values of serum copper, zinc, selenium, GSH-Px and superoxide dismutase (SOD). They evaluated the effect of long-term therapy with sodium valproate and carbamazepine on these parameters in 36 epileptic patients before the beginning of therapy and after 1 year of therapy with sodium valproate or carbamazepine. After 1 year of therapy, patients treated with sodium valproate and carbamazepine continued to show normal values. They demonstrated that epilepsy per se and treatment with sodium valproate and carbamazepine do not affect levels of copper, zinc, and SOD values (Verrotti et al. 2002). Similarly Kurekci et al. (1995) investigated the effect of long-term antiepileptic drugs therapy on copper, zinc, manganese, magnesium, and SOD in the plasma in children with epilepsy. They reported plasma copper, zinc, manganese, and magnesium concentrations of patients were not different from those of control subjects during treatment with valproate or carbamazepine monotherapy. However, they observed serum sodium valproate levels were correlated with the increase of plasma zinc level in the patients.
Oxidative Stress and Antiepileptic Drugs
As it was mentioned above, free oxygen radicals are physiological products of the cellular metabolism. For examples, phagocytes are producing free oxygen radicals for killing ingested bacteria and virus. However, when the production of free radicals increases or defense mechanism of the body decreases, they cause cellular dysfunction by attacking at the polyunsaturated sites of the biological membranes leading to lipid peroxidation (Naziroglu 2007). As it was mentioned above, antioxidant enzymes are SOD, catalase, and GSH-Px in the brain. SOD dismutases superoxide radical to hydrogen peroxide. Catalase is an enzyme responsible for detoxification of the hydrogen peroxide formed by the action of SOD. The catalase activity in the rodent brain is very low. The involvement of free radicals in seizures is also supported by reports which indicate that exogenously administered antioxidant protects the brain against seizures (Gupta et al. 2003). Some papers indicate that long-term treatment with old antiepileptic drugs leads to an increase in oxidative stress which is similar to that observed during epileptogenesis (Chang and Abbott 2006). However, the idea was not further confirmed (Hamed et al. 2004; Ashrafi et al. 2007).
Hence, results on the subject are also conflicting. For example, sodium valproate has been found to increase lipid peroxidation in patients receiving it (Martinez-Ballesteros et al. 2004). Contrary to this observation some antiepileptic agents like phenytoin and topiramate have been shown to decrease oxidative stress, which were demonstrated to increase in glutathione reductase and GSH-Px activities in patients (Stanton and Moskal 1991; Nazıroğlu et al. 2009; Ganesh et al. 2011).
Topiramate
Topiramate, a sulfate-substituted monosaccharide, is a novel compound that has a broad spectrum against antiepileptic activity. Mechanisms that are likely to account for the anticonvulsant activity of topiramate include a negative modulatory effect on the a-amino-3-hydroxy-5-methyl-4-isoxazol propionic acid (AMPA)/kainate subtype of glutamate receptors, a positive modulatory effect on GABAA receptors, a use- and time-dependent blockade of voltage-activated Na+-channels, a negative modulatory effect on a neuronal L-type high voltage-activated Ca2+-channel and is also inhibitor of the carbonic anhydrases, particularly subtypes II and IV (White 2005). There are scarce report about interactions between oxidative stress and topiramate. Most of the papers indicated the antioxidant role of topiramate in brain and neurological cells. On the subject, Cardenas-Rodriguez et al. (2012) observed dose-dependent ROS scavenger effects of topiramate in different cell lines. The effect of introperitoneal topiramate was investigated by Kubera et al. (2004) (40 and 80 mg/kg) on the fully developed kainate (15 mg/kg)-induced status epilepticus in the rat. The topiramate at a dose of 80 mg/kg in frontal cortex of the rats reduced the kainate-induced lipid peroxidation (Kubera et al. 2004). Our group has also investigated the effects of selenium administration (0.3 mg/kg/day) on topiramate (50 mg/kg/day) and pentilentetrazol (60 mg/kg)-induced brain toxicity in rats. We have proved that topiramate administration with or without selenium caused decreased lipoperoxidation levels in the brain cortex (Nazıroğlu et al. 2008). Vitamin E (alpha tocopherol) is a lipid soluble strong antioxidant that interferes with the chain reaction of oxidative stress (Nazıroglu et al. 2004) although Vitamin C (ascorbic acid) is water soluble molecule that can scavenge several radicals (Ekmekcioğlu et al. 2008). Similarly, in another study of our group (Nazıroğlu et al. 2009) indicated that topiramate and vitamin E treatment caused a decrease in serum nitric oxide, erythrocyte and plasma lipoperoxidation levels and brain spike numbers, whereas GSH-Px, GSH, vitamin C and vitamin E levels and latency to the first spike of EEG were increased by the topiramate treatment.
Armagan et al. (2008) indicated that topiramate and vitamin E have protective effects on pentylenetetrazol-induced nephrotoxicity by inhibition of free radicals and support of the antioxidant redox system. In doses of 50 and 100 mg/kg/day topiramate and 150 mg/kg vitamin E caused an increase of kidney SOD and catalase enzyme activities in the same study.
We have previously investigated the effects of selenium and topiramate on pentylenetetrazole (PTZ)-induced blood toxicity in rats. We have found that selenium and topiramate induced protective effects on the PTZ-induced blood toxicity by inhibiting free radical supporting antioxidant redox system (Nazıroğlu et al. 2008).
Nuclear factor kappa B (NFkB) is known to respond to oxidative stress and to act as a regulator of apoptotic processes (Schreck et al. 1992). Muriach et al. (2010) investigated GSH, GSH-Px, and caspase 3 values for checking the influence of oxidative stress on NFkB response, and the possible induction of NFkB activation-related apoptosis in an experimental model of cocaine administration in rats. They concluded that topiramate had a modulatory role against necrosis NFkB activation in the frontal cortex and against NADPH positive cells in the hippocampus (Muriach et al. 2010).
Cardile et al. (2001) reported that topiramate (1–100 μg/ml) increased the oxidative stress in astrocytes. Agarwal et al. (2011) compared the effects of lamotrigine, oxcarbazepine, and topiramate on cognition during experimental epileptogenesis in mice. Topiramate administration (10 mg/kg) to kindled as well as non-kindled animals increased lipid peroxidation and malondialdehyde (MDA) production, and decreased GSH levels. It was reported that lamotrigine and oxcarbazepine did not show significant alteration in oxidative stress values (Agarwal et al. 2011).
Recently, our group investigated effects of topiramate and selenium supplementation on antioxidant and oxidant stems in patients with epilepsy and refractory epilepsy (Yürekli and Nazıroğlu 2013) and we observed a modulatory role of topiramate and selenium supplementation on GSH, GSH-Px, total antioxidant capacity, vitamins A and vitamin C in the blood of epileptic patients.
Sodium Valproate and Carbamazepine
Sodium valproate is an effective drug for treating simple and complex epileptic seizures as a monotherapy and as a component of polytherapy. The effects of sodium valproate on oxidant status are conflicting in different studies. Chang and Abbott (2006) showed that oxidative stress has a potential role on sodium valproate-induced hepatotoxicity. Solowiej and Sobaniec (2003) reported insignificant elevations of MDA concentrations in patients treated with sodium valproate and carbamazepine. Michoulas et al. (2006) also reported higher urinary levels of 15-F2T-isoprostane, a marker of oxidative stress in epileptic children treated with sodium valproate. On the other hand, Verrotti et al. (2002) found that sodium valproate therapy does not appear to cause oxidative stress in epileptic children who remained non-obese during treatment. Yis et al. (2009) found that GSH-Px activity does not change during treatment with sodium valproate and they found elevated levels of superoxide dismutase in patients with newly diagnosed idiopathic epilepsy. They also showed a positive correlation between duration of treatment and SOD activities (Yis et al. 2009).
In another study, Cengiz et al. (2000) evaluated the effects of sodium valproate and carbamazepine therapy on erythrocyte GSH, GSH-Px, SOD, and lipid peroxidation in epileptic children. They found that GSH levels were reduced and GSH-Px increased in the sodium valproate and carbamazepine groups (Cengiz et al. 2000). Reduction in the GSH amount may result in an increase of organic hydroperoxides. Yuksel et al. (2001) determined changes in the antioxidant system in epileptic children receiving long-term antiepileptic drugs. In their study 16 patients were treated with sodium valproate and 14 with carbamazepine; 13 months later these parameters were retested. Their results showed that SOD and lipid peroxidation levels were increased but the GSH-Px levels were decreased in epileptic children on sodium valproate therapy compared with the control group and the results before treatment. No significant differences of these parameters were reported in epileptic children undergoing carbamazepine therapy compared with the control group, although lipid peroxidation level was slightly higher in epileptic patients before treatment. They concluded that antioxidant systems in epileptic children on carbamazepine therapy are better regulated in comparison with epileptic children on sodium valproate therapy (Yuksel et al. 2001).
Levetiracetam
Levetiracetam, the S-enantiomer of a-ethyl-2-oxo-1-pyrrolidine acetamide, is an antiepileptic drug that has broad-spectrum effects on partial and generalized seizures in several models of epilepsy. The clinical effectiveness of LEV has been reported in patients with partial refractory epilepsy (Oliveira et al. 2007). The therapeutic mechanism of levetiracetam remains unclear, some studies considering that is unrelated to any modulation of neuronal voltage-gated Na+ or low-voltage-activated Ca2+ (T type) channels (Oliveira et al. 2007). Meanwhile, other in vitro and in vivo studies suggested a role of both calcium channels N-type and GABAergic in the activity of levetiracetam (Lukyanetz et al. 2002; Poulain and Margineanu 2002). Oliveira et al. (2007) study indicated that levetiracetam may alter pilocarpine-induced changes in catalase and reduced GSH levels, in lipid peroxidation level, and nitrite–nitrate formation in mice hippocampus and they observed that lipid peroxidation, nitrite/nitrate formation, and changes in antioxidant brain enzymes are involved in the pathophysiology of pilocarpine-induced seizures and status epilepticus (Oliveira et al. 2007). Except its antiepileptic potential, Stettner et al. (2011) indicated that levetiracetam may also act as a histone deacetylase inhibitor, suggesting that this drug exhibits both anti-inflammatory and anti-oxidative effects, and it may be potentially useful for treating oxidative stress and inflammation in the peripheral nerve (Stettner et al. 2011).
Zonisamide
Zonisamide is originally synthesized in Japan and has been used for over 10 years to treat intractable epilepsy. Zonisamide has significant effects on T type Ca2+ channels and oxidative stress (Murata 2004). To our knowledge, there is not enough study about the effects of zonisamide on oxidative stress, trace elements and epilepsy. Asanuma et al. (2010) investigated changes in GSH and GSH synthesis-related molecules, and the neuroprotective effects of zonisamide on dopaminergic neurodegeneration using 6-hydroxydopamine-injected hemiparkinsonian mice brain and cultured neurons or astrocytes. They observed protective effects of zonisamide on GSH levels in astroglial C6 cells by enhancing the astroglial cystine transport system and/or astroglial proliferation via S100beta production or secretion. Yurekli et al. (2012) investigated the effect of zonisamide on the oxidative stress, cell viability, Ca2+ signaling, and caspase activity that induced by the MPP+ model of Parkinson’s in neuronal PC12 cells. Lipid peroxidation and cytosolic-free Ca2+ concentrations were higher in the MPP+ group than in control, although their levels were lower in zonisamide and the zonisamide plus MPP+ groups than in control. Reduced GSH and glutathione GSH-Px were lower in the MPP+ group, although they were higher in the zonisamide and the zonisamide plus MPP+ groups than in control (Yurekli et al. 2012).
Phenytoin
Phenytoin was introduced nearly 60 years ago for use in epilepsy and is still widely prescribed for partial and generalized seizures. Similar to carbamazepine, it blocks voltage-dependent neuronal sodium channels (Yaari et al. 1986). Other effects of phenytoin include diminishing synaptic transmission, limiting fluctuation of neuronal ionic gradients via sodium–potassium ATPase, and affecting second messenger systems by inhibiting Ca2+-calmodulin protein phosphorylation (Delgado-Escueta and Horan 1980; Holland et al. 1993).
Phenytoin is effective in the treatment of both generalized tonic-colonic and focal onset seizures (Miller et al. 2004). However, phenytoin has a narrow margin of safety and its use in epileptic patients has occasionally been associated with disturbances in the blood antioxidant defense systems and increased lipid peroxidation (Herzog et al. 2005; Hirsch et al. 2008). Many authors have suggested that phenytoin initiates oxidative damage and cognitive impairment in experimental animals and epileptic patients taking phenytoin monotherapy or receiving multiple drugs (Reeta et al. 2009). Reeta et al. (2009) measured the levels of MDA and GSH in rat brain after phenytoin treatment. MDA levels in the rat brain were significantly increased and the GSH levels were significantly reduced in the phenytoin-treated rats (Reeta et al. 2009). Liu et al. (1997) measured the serum MDA, serum copper, serum zinc, copper/zinc SOD, and reduced GSH concentrations in 20 female epileptics with phenytoin monotherapy compared with 12 female epileptics without anticonvulsant therapy and 20 female healthy controls. For the female epileptics with phenytoin monotherapy, serum MDA concentration, copper/zinc SOD, and serum copper content in their study were increased whereas GSH level was significantly decreased. They observed also that the level of serum MDA was associated with the elevation of copper/zinc SOD activity and serum copper content in all the samples collected from epileptics and controls. They concluded that oxidative stress was enhanced in the female epileptics with phenytoin monotherapy (Liu et al. 1997).
Phenytoin is also known to deplete vital nutrients, such as calcium, folic acid, vitamin D, vitamin K, biotin, carnitine, copper, selenium, and zinc (Thaakur and Pushpakumari 2007). In the literature there is not enough information about selenium and phenytoin interaction. On the subject, Ozolins et al. (1996) suggested that selenium-dependent and -independent GSH-Px detoxifies hydrogen peroxide and lipid hydroperoxides may mediate the teratogenicity of phenytoin and related xenobiotics. They were the first to demonstrate selenium-dependent GSH-Px activities in embryonic tissues of CD-1 mice with dietary selenium-deprivation. Their results implicated ROS and lipid hydroperoxides in the mechanism of phenytoin teratogenicity and suggested that GSH-Px are important embryoprotective enzymes (Ozolins et al. 1996).
Lamotrigine
Lamotrigine is an antiepileptic drug, also known as a mood stabilizer, that inhibits presynaptic voltage-gated Na+ channels and reduces the presynaptic release of glutamate in pathological states (White 2005). Scarcely studies are in the literature about the effects of lamotrigine on oxidative stress. Arora et al. (2010) observed the effect of lamotrigine and carbamazepine on cognitive function and oxidative stress in brain during chemically induced epileptogenesis in rats. They indicated that lamotrigine treatment had no effect on oxidative stress parameters alone, while it significantly decreased oxidative stress in the PTZ-kindled group as compared to the PTZ-kindled carbamazepine-treated group (Arora et al. 2010). In a similar study as mentioned above Agarwal et al. (2011) assessed the effect of three anticonvulsants, lamotrigine, oxcarbazepine, and topiramate on cognitive function and oxidative stress during pentylenetetrazole kindling in mice. MDA, GSH levels, SOD, and catalase activity were measured as an indicator of oxidative stress. Lamotrigine and oxcarbazepine did not show significant alteration in oxidative stress parameters and cognitive functions tests (Agarwal et al. 2011). Neuroprotective effects of this drug have also been demonstrated in cerebral ischemia models (Tufan et al. 2008). Literature connecting lamotrigine and trace elements is scarce. In a traumatic brain injury model in rats, Hellmich et al. (2007) suggested that lamotrigine treatment inhibits presynaptic release of glutamate and reduces neurotoxic zinc levels after traumatic brain injury.
Conclusion and Future Directions
The existing knowledge about the impact of epilepsy and antiepileptic drugs on trace elements and free radical/antioxidant system is poor and controversial. There are four main future directions. (1) Information on trace element and new antiepileptic drugs, such as zonisamide and lamotrigine, is scarce. In addition, reports of old and new antiepileptic drugs are conflicting on antioxidant levels in human and animals relationships between old and new antiepileptic drugs. Hence, effects of the new drugs on the oxidant and antioxidant values such as MDA, GSH-Px, and SOD should be investigated by further experiments. (2) The second topic is calcium ion signaling and transient receptor potential (TRP) channels in epileptic hippocampal neurons. There is no report on the oxidative stress-dependent activation of TRP cation channels in epileptic patients and animals via over production of free oxygen radicals. Hence, these subjects should be clarified by future experiments.
Clinical reports suggest that both epilepsy and antiepileptic drugs, especially older generation antiepileptics, have negative effect on cognition which affects the life quality of epileptic patients. However, it is not clear whether it is epilepsy or oxidative stress or both which contribute to the decline of cognitive function. It is well known that older generation antiepileptic drugs cause cognitive impairment oxidative stress could be the triggering mechanism involved in cognitive impairment during experimental epileptogenesis as well as during drug treatment. Third, on new antiepileptic drugs, particularly topiramate and zonisamide, further, clinical and biochemical studies are required, to demonstrate a correlation between cognitive dysfunction and oxidative stress during epilepsy and antiepileptic drug therapy.
Fourth, in different studies low serum zinc levels were determined but there are poor evidences that zinc, copper, and selenium supplementations are able to reduce the incidence of febrile seizure, and further investigation is necessary. Present studies do not catch the full complexity trace elements deficiencies in epileptogenesis, and we believe that further studies will have a huge clinical contribution to in improving the life quality of of epileptic patients.
Acknowledgments
Conflict of interest
There is no conflict interest and financial support for the study.
Abbreviations
- AMPA
Alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid
- Cu
Copper
- GSH
Glutathione
- GSH-Px
Glutathione peroxidase
- MDA
Malondialdehyde
- NFkB
Nuclear factor kappa B
- NMDA
N-methyl-d-aspartate
- PTZ
Pentylenetetrazole
- ROS
Reactive oxygen species
- Se
Selenium
- SOD
Superoxide dismutase
References
- Agarwal NB, Agarwal NK, Mediratta PK, Sharma KK (2011) Effect of lamotrigine, oxcarbazepine and topiramate on cognitive functions and oxidative stress in PTZ-kindled mice. Seizure 20(3):257–262 [DOI] [PubMed] [Google Scholar]
- Amiri M, Farzin L, Moassesi ME, Sajadi F (2010) Serum trace element levels in febrile convulsion. Biol Trace Elem Res 135(1–3):38–44 [DOI] [PubMed] [Google Scholar]
- Andre VM, Cepeda C, Vinters HV, Huynh M, Mathern GW, Levine MS (2010) Interneurons, GABAA currents, and subunit composition of the GABAA receptor in type I and type II cortical dysplasia. Epilepsia 51(Suppl 3):166–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa M, Ito Y (2007) N-acetylcysteine and neurodegenerative diseases: basic and clinical pharmacology. Cerebellum 6(4):308–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armagan A, Kutluhan S, Yilmaz M, Yilmaz N, Bulbul M, Vural H, Soyupek S, Naziroglu M (2008) Topiramate and vitamin e modulate antioxidant enzyme activities, nitric oxide and lipid peroxidation levels in pentylenetetrazol-induced nephrotoxicity in rats. Basic Clin Pharmacol Toxicol 103(2):166–170 [DOI] [PubMed] [Google Scholar]
- Arora T, Mehta AK, Sharma KK, Mediratta PK, Banerjee BD, Garg GR, Sharma AK (2010) Effect of carbamazepine and lamotrigine on cognitive function and oxidative stress in brain during chemical epileptogenesis in rats. Basic Clin Pharmacol Toxicol 106:372–377 [DOI] [PubMed] [Google Scholar]
- Asanuma M, Miyazaki I, Diaz-Corrales FJ, Kimoto N, Kikkawa Y, Takeshima M, Miyoshi K, Murata M (2010) Neuroprotective effects of zonisamide target astrocyte. Ann Neurol 67(2):239–249 [DOI] [PubMed] [Google Scholar]
- Ashrafi MR, Shabanian R, Abbaskhanian A, Nasirian A, Ghofrani M, Mohammadi M, Zamani GR, Kayhanidoost Z, Ebrahimi S, Pourpak Z (2007a) Selenium and intractable epilepsy: is there any correlation? Pediatr Neurol 36(1):25–29 [DOI] [PubMed] [Google Scholar]
- Ashrafi MR, Shams S, Nouri M, Mohseni M, Shabanian R, Yekaninejad MS, Chegini N, Khodadad A, Safaralizadeh R (2007b) A probable causative factor for an old problem: selenium and glutathione peroxidase appear to play important roles in epilepsy pathogenesis. Epilepsia 48(9):1750–1755 [DOI] [PubMed] [Google Scholar]
- Azam F, Prasad MV, Thangavel N (2012) Targeting oxidative stress component in the therapeutics of epilepsy. Curr Top Med Chem 12:994–1007 [DOI] [PubMed] [Google Scholar]
- Cardenas-Rodriguez N, Coballase-Urrutia E, Huerta-Gertrudis B, Garcia-Cruz ME, Pedraza-Chaverri J, Coria-Jimenez R, Bandala C, Ruiz-Garcia M (2012) Antioxidant activity of topiramate: an antiepileptic agent. Neurol Sci. doi:10.1007/s10072-012-1127-5 [DOI] [PubMed] [Google Scholar]
- Cardile V, Pavone A, Renis M, Maci T, Perciavalle V (2001) Effects of Gabapentin and Topiramate in primary rat astrocyte cultures. NeuroReport 12(8):1705–1708 [DOI] [PubMed] [Google Scholar]
- Castano A, Ayala A, Rodriguez-Gomez JA, Herrera AJ, Cano J, Machado A (1997) Low selenium diet increases the dopamine turnover in prefrontal cortex of the rat. Neurochem Int 30(6):549–555 [DOI] [PubMed] [Google Scholar]
- Cengiz M, Yuksel A, Seven M (2000) The effects of carbamazepine and valproic acid on the erythrocyte glutathione, glutathione peroxidase, superoxide dismutase and serum lipid peroxidation in epileptic children. Pharmacol Res 41(4):423–425 [DOI] [PubMed] [Google Scholar]
- Chang TK, Abbott FS (2006) Oxidative stress as a mechanism of valproic acid-associated hepatotoxicity. Drug Metab Rev 38(4):627–639 [DOI] [PubMed] [Google Scholar]
- Daoud AS, Batieha A, Abu-Ekteish F, Gharaibeh N, Ajlouni S, Hijazi S (2002) Iron status: a possible risk factor for the first febrile seizure. Epilepsia 43(7):740–743 [DOI] [PubMed] [Google Scholar]
- Delgado-Escueta AV, Horan MP (1980) Phenytoin: biochemical membrane studies. Adv Neurol 27:377–398 [PubMed] [Google Scholar]
- Espino J, Pariente JA, Rodríguez AB (2012) Oxidative stress and immunosenescence: therapeutic effects of melatonin. Oxid Med Cell Longev 2012:670294. doi:10.1155/2012/670294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekmekcioğlu C, Zodl B, Humpeler S, Zeiner M, Gundacker C, Steffan I. (2008) Ascorbic Acid Mediated Iron Toxicity in Caco-2 Cells: Effects of Different Iron Species Cell Membr, Free Radic Res 2(2): 92–97 [Google Scholar]
- Ganesh R, Janakiraman L, Meenakshi B (2011) Serum zinc levels are low in children with simple febrile seizures compared with those in children with epileptic seizures and controls. Ann Trop Paediatr 31(4):345–349 [DOI] [PubMed] [Google Scholar]
- González A, Granados MP, Pariente JA, Salido GM (2006) H2O2 mobilizes Ca2+ from agonist- and thapsigargin-sensitive and insensitive intracellular stores and stimulates glutamate secretion in rat hippocampal astrocytes. Neurochem Res 31:741–750 [DOI] [PubMed] [Google Scholar]
- González A, Pariente JA, Salido GM (2007) Ethanol stimulates ROS generation by mitochondria through Ca2+ mobilization and increases GFAP content in rat hippocampal astrocytes. Brain Res 1178:28–37 [DOI] [PubMed] [Google Scholar]
- Gupta RC, Milatovic D, Dettbarn WD (2001) Depletion of energy metabolites following acetylcholinesterase inhibitor-induced status epilepticus: protection by antioxidants. Neurotoxicology 22(2):271–282 [DOI] [PubMed] [Google Scholar]
- Gupta YK, Veerendra Kumar MH, Srivastava AK (2003) Effect of Centella asiatica on pentylenetetrazole-induced kindling, cognition and oxidative stress in rats. Pharmacol Biochem Behav 74(3):579–585 [DOI] [PubMed] [Google Scholar]
- Hamed SA, Abdellah MM, El-Melegy N (2004) Blood levels of trace elements, electrolytes, and oxidative stress/antioxidant systems in epileptic patients. J Pharmacol Sci 96(4):465–473 [DOI] [PubMed] [Google Scholar]
- Hayashi M (2009) Oxidative stress in developmental brain disorders. Neuropathology 29(1):1–8 [DOI] [PubMed] [Google Scholar]
- Hellmich HL, Eidson KA, Capra BA, Garcia JM, Boone DR, Hawkins BE, Uchida T, Dewitt DS, Prough DS (2007) Injured Fluoro-Jade-positive hippocampal neurons contain high levels of zinc after traumatic brain injury. Brain Res 1127(1):119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Drislane FW, Schomer DL, Pennell PB, Bromfield EB, Dworetzky BA, Farina EL, Frye CA (2005) Differential effects of antiepileptic drugs on sexual function and hormones in men with epilepsy. Neurology 65(7):1016–1020 [DOI] [PubMed] [Google Scholar]
- Hirsch LJ, Arif H, Nahm EA, Buchsbaum R, Resor SR Jr, Bazil CW (2008) Cross-sensitivity of skin rashes with antiepileptic drug use. Neurology 71(19):1527–1534 [DOI] [PubMed] [Google Scholar]
- Holland KD, Bouley MG, Covey DF, Ferrendelli JA (1993) Alkyl-substituted gamma-butyrolactones act at a distinct site allosterically linked to the TBPS/picrotoxinin site on the GABAA receptor complex. Brain Res 615(1):170–174 [DOI] [PubMed] [Google Scholar]
- Jesberger JA, Richardson JS (1991) Oxygen free radicals and brain dysfunction. Int J Neurosci 57(1–2):1–17 [DOI] [PubMed] [Google Scholar]
- Kubera M, Budziszewska B, Jaworska-Feil L, Basta-Kaim A, Leskiewicz M, Tetich M, Maes M, Kenis G, Marciniak A, Czuczwar SJ, Jagla G, Nowak W, Lason W (2004) Effect of topiramate on the kainate-induced status epilepticus, lipid peroxidation and immunoreactivity of rats. Pol J Pharmacol 56(5):553–561 [PubMed] [Google Scholar]
- Kurekci AE, Alpay F, Tanindi S, Gokcay E, Ozcan O, Akin R, Isimer A, Sayal A (1995) Plasma trace element, plasma glutathione peroxidase, and superoxide dismutase levels in epileptic children receiving antiepileptic drug therapy. Epilepsia 36(6):600–604 [DOI] [PubMed] [Google Scholar]
- Liang LP, Patel M (2004) Mitochondrial oxidative stress and increased seizure susceptibility in Sod2(−/+) mice. Free Radic Biol Med 36(5):542–554 [DOI] [PubMed] [Google Scholar]
- Liu CS, Wu HM, Kao SH, Wei YH (1997) Phenytoin-mediated oxidative stress in serum of female epileptics: a possible pathogenesis in the fetal hydantoin syndrome. Hum Exp Toxicol 16(3):177–181 [DOI] [PubMed] [Google Scholar]
- Lukyanetz EA, Shkryl VM, Kostyuk PG (2002) Selective blockade of N-type calcium channels by levetiracetam. Epilepsia 43(1):9–18 [DOI] [PubMed] [Google Scholar]
- Mahyar A, Ayazi P, Fallahi M, Javadi A (2010) Correlation between serum selenium level and febrile seizures. Pediatr Neurol 43(5):331–334 [DOI] [PubMed] [Google Scholar]
- Martinez-Ballesteros C, Pita-Calandre E, Sanchez-Gonzalez Y, Rodriguez-Lopez CM, Agil A (2004) Lipid peroxidation in adult epileptic patients treated with valproic acid. Rev Neurol 38(2):101–106 [PubMed] [Google Scholar]
- Michoulas A, Tong V, Teng XW, Chang TK, Abbott FS, Farrell K (2006) Oxidative stress in children receiving valproic acid. J Pediatr 149(5):692–696 [DOI] [PubMed] [Google Scholar]
- Miller AD, Krauss GL, Hamzeh FM (2004) Improved CNS tolerability following conversion from immediate- to extended-release carbamazepine. Acta Neurol Scand 109(6):374–377 [DOI] [PubMed] [Google Scholar]
- Murata M (2004) Novel therapeutic effects of the anti-convulsant, zonisamide, on Parkinson’s disease. Curr Pharm Des 10(6):687–693 [DOI] [PubMed] [Google Scholar]
- Muriach M, Lopez-Pedrajas R, Barcia JM, Sanchez-Villarejo MV, Almansa I, Romero FJ (2010) Cocaine causes memory and learning impairments in rats: involvement of nuclear factor kappa B and oxidative stress, and prevention by topiramate. J Neurochem 114(3):675–684 [DOI] [PubMed] [Google Scholar]
- Nazıroglu M, Karaoğlu A, Aksoy AO. (2004) Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 195(2–3):221–230 [DOI] [PubMed] [Google Scholar]
- Nazıroglu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32(11):1990–2001 [DOI] [PubMed] [Google Scholar]
- Nazıroglu M (2011) TRPM2 cation channels, oxidative stress and neurological diseases: where are we now? Neurochem Res 36(3):355–366 [DOI] [PubMed] [Google Scholar]
- Nazıroğlu M. (2012) Molecular role of catalase on oxidative stress-induced Ca(2+) signaling and TRP cation channel activation in nervous system. J Recept Signal Transduct Res. 32(3):134–141 [DOI] [PubMed] [Google Scholar]
- Nazıroğlu M, Dikici DM, Dursun S. (2012) Role of oxidative stress and Ca2 signaling on molecular pathways of neuropathic pain in diabetes: focus on TRP channels. Neurochem Res. 37(10):2065–2075 [DOI] [PubMed] [Google Scholar]
- Nazıroglu M, Kutluhan S, Yilmaz M (2008) Selenium and topiramate modulates brain microsomal oxidative stress values, Ca2+-ATPase activity, and EEG records in pentylentetrazol-induced seizures in rats. J Membr Biol 225(1–3):39–49 [DOI] [PubMed] [Google Scholar]
- Nazıroğlu M, Kutluhan S, Uguz AC, Celik O, Bal R, Butterworth PJ (2009) Topiramate and vitamin E modulate the electroencephalographic records, brain microsomal and blood antioxidant redox system in pentylentetrazol-induced seizure of rats. J Membr Biol 229(3):131–140 [DOI] [PubMed] [Google Scholar]
- Oki G, Wada T, Iba K, Aiki H, Sasaki K, Imai S, Sohma H, Matsumoto K, Yamaguchi M, Fujimiya M, Yamashita T, Kokai Y (2012) Metallothionein deficiency in the injured peripheral nerves of complex regional pain syndrome as revealed by proteomics. Pain 153(3):532–539 [DOI] [PubMed] [Google Scholar]
- Oliveira AA, Almeida JP, Freitas RM, Nascimento VS, Aguiar LM, Junior HV, Fonseca FN, Viana GS, Sousa FC, Fonteles MM (2007) Effects of levetiracetam in lipid peroxidation level, nitrite–nitrate formation and antioxidant enzymatic activity in mice brain after pilocarpine-induced seizures. Cell Mol Neurobiol 27(3):395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozmen I, Naziroglu M, Alici HA, Sahin F, Cengiz M, Eren I (2007) Spinal morphine administration reduces the fatty acid contents in spinal cord and brain by increasing oxidative stress. Neurochem Res 32(1):19–25 [DOI] [PubMed] [Google Scholar]
- Ozolins TR, Siksay DL, Wells PG (1996) Modulation of embryonic glutathione peroxidase activity and phenytoin teratogenicity by dietary deprivation of selenium in CD-1 mice. J Pharmacol Exp Ther 277(2):945–953 [PubMed] [Google Scholar]
- Oztas B, Akgul S, Seker FB (2007) Gender difference in the influence of antioxidants on the blood–brain barrier permeability during pentylenetetrazol-induced seizures in hyperthermic rat pups. Biol Trace Elem Res 118(1):77–83 [DOI] [PubMed] [Google Scholar]
- Pariente JA, Camello C, Camello PJ, Salido GM (2001) Release of calcium from mitochondrial and nonmitochondrial intracellular stores in mouse pancreatic acinar cells by hydrogen peroxide. J Membr Biol 179:27–35 [DOI] [PubMed] [Google Scholar]
- Patel MN (2002) Oxidative stress, mitochondrial dysfunction, and epilepsy. Free Radic Res 36(11):1139–1146 [DOI] [PubMed] [Google Scholar]
- Patel M (2004) Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Radic Biol Med 37(12):1951–1962 [DOI] [PubMed] [Google Scholar]
- Poulain P, Margineanu DG (2002) Levetiracetam opposes the action of GABAA antagonists in hypothalamic neurones. Neuropharmacology 42(3):346–352 [DOI] [PubMed] [Google Scholar]
- Ramaekers VT, Calomme M, Vanden Berghe D, Makropoulos W (1994) Selenium deficiency triggering intractable seizures. Neuropediatrics 25(4):217–223 [DOI] [PubMed] [Google Scholar]
- Rayman MP (2000) The importance of selenium to human health. Lancet 356(9225):233–241 [DOI] [PubMed] [Google Scholar]
- Reeta KH, Mehla J, Gupta YK (2009) Curcumin is protective against phenytoin-induced cognitive impairment and oxidative stress in rats. Brain Res 1301:52–60 [DOI] [PubMed] [Google Scholar]
- Rowley S, Patel M (2013) Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic Biol Med. doi:10.1016/j.freeradbiomed.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaskan NE, Brauer AU, Kuhbacher M, Eyupoglu IY, Kyriakopoulos A, Ninnemann O, Behne D, Nitsch R (2003) Selenium deficiency increases susceptibility to glutamate-induced excitotoxicity. FASEB J 17(1):112–114 [DOI] [PubMed] [Google Scholar]
- Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA (1992) Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med 175(5):1181–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchmann S, Buchheim K, Meierkord H, Heinemann U (1999) A relative energy failure is associated with low-Mg2+ but not with 4-aminopyridine induced seizure-like events in entorhinal cortex. J Neurophysiol 81(1):399–403 [DOI] [PubMed] [Google Scholar]
- Schweizer U, Schomburg L, Savaskan NE (2004) The neurobiology of selenium: lessons from transgenic mice. J Nutr 134:707–710 [DOI] [PubMed] [Google Scholar]
- Seven M, Basaran SY, Cengiz M, Unal S, Yuksel A (2012) Deficiency of selenium and zinc as a causative factor for idiopathic intractable epilepsy. Epilepsy Res 104:35–39 [DOI] [PubMed] [Google Scholar]
- Shin EJ, Jeong JH, Chung YH, Kim WK, Ko KH, Bach JH, Hong JS, Yoneda Y, Kim HC (2011) Role of oxidative stress in epileptic seizures. Neurochem Int 59(2):122–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowiej E, Sobaniec W (2003) The effect of antiepileptic drug therapy on antioxidant enzyme activity and serum lipid peroxidation in young patients with epilepsy. Neurol Neurochir Pol 37(5):991–1003 [PubMed] [Google Scholar]
- Stanton PK, Moskal JR (1991) Diphenylhydantoin protects against hypoxia-induced impairment of hippocampal synaptic transmission. Brain Res 546(2):351–354 [DOI] [PubMed] [Google Scholar]
- Stettner M, Dehmel T, Mausberg AK, Kohne A, Rose CR, Kieseier BC (2011) Levetiracetam exhibits protective properties on rat Schwann cells in vitro. J Peripher Nerv Syst 16(3):250–260 [DOI] [PubMed] [Google Scholar]
- Thaakur SR, Pushpakumari B (2007) Influence of spirulina on the phenytoin induced haematological changes. Anc Sci Life 26(3):9–15 [PMC free article] [PubMed] [Google Scholar]
- Tufan K, Oztanir N, Ofluoglu E, Ozogul C, Uzum N, Dursun A, Pasaoglu H, Pasaoglu A (2008) Ultrastructure protection and attenuation of lipid peroxidation after blockade of presynaptic release of glutamate by lamotrigine in experimental spinal cord injury. Neurosurg Focus 25(5):E6 [DOI] [PubMed] [Google Scholar]
- Tütüncüoğlu S, Kütükçüler N, Kepe L, Coker C, Berdeli A, Tekgül H. (2001) Proinflammatory cytokines, prostaglandins and zinc in febrile convulsions. Pediatr Int. 43(3):235–239 [DOI] [PubMed] [Google Scholar]
- Verrotti A, Basciani F, Trotta D, Pomilio MP, Morgese G, Chiarelli F (2002) Serum copper, zinc, selenium, glutathione peroxidase and superoxide dismutase levels in epileptic children before and after 1 year of sodium valproate and carbamazepine therapy. Epilepsy Res 48(1–2):71–75 [DOI] [PubMed] [Google Scholar]
- Weber GF, Maertens P, Meng XZ, Pippenger CE (1991) Glutathione peroxidase deficiency and childhood seizures. Lancet 337(8755):1443–1444 [DOI] [PubMed] [Google Scholar]
- White HS (2005) Molecular pharmacology of topiramate: managing seizures and preventing migraine. Headache 45(Suppl 1):S48–S56 [DOI] [PubMed] [Google Scholar]
- Wirth EK, Conrad M, Winterer J, Wozny C, Carlson BA, Roth S, Schmitz D, Bornkamm GW, Coppola V, Tessarollo L, Schomburg L, Kohrle J, Hatfield DL, Schweizer U (2010) Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. FASEB J 24(3):844–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciak RW, Mojs E, Stanislawska-Kubiak M, Samborski W (2013) The serum zinc, copper, iron, and chromium concentrations in epileptic children. Epilepsy Res 104(1–2):40–44 [DOI] [PubMed] [Google Scholar]
- Yaari Y, Selzer ME, Pincus JH (1986) Phenytoin: mechanisms of its anticonvulsant action. Ann Neurol 20(2):171–184 [DOI] [PubMed] [Google Scholar]
- Yis U, Seckin E, Kurul SH, Kuralay F, Dirik E (2009) Effects of epilepsy and valproic acid on oxidant status in children with idiopathic epilepsy. Epilepsy Res 84(2–3):232–237 [DOI] [PubMed] [Google Scholar]
- Yuksel A, Cengiz M, Seven M, Ulutin T (2001) Changes in the antioxidant system in epileptic children receiving antiepileptic drugs: two-year prospective studies. J Child Neurol 16(8):603–606 [DOI] [PubMed] [Google Scholar]
- Yürekli VA, Nazıroğlu M (2013) Selenium and topiramate attenuates blood oxidative toxicity in patients with Epilepsy: A Clinical Pilot Study. Biol Trace Elem Res. doi:10.1007/s12011-013-9616-9 [DOI] [PubMed] [Google Scholar]
- Yurekli VA, Gurler S, Naziroglu M, Uguz AC, Koyuncuoglu HR (2012) Zonisamide attenuates MPP(+)-induced oxidative toxicity through modulation of Ca(2+) signaling and caspase-3 activity in neuronal PC12 cells. Cell Mol Neurobiol 33(2):205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]