Abstract
Somatic cells do not have telomerase activity but immortalized cell lines and more than 85 % of the cancer cells show telomerase activation to prevent the telomere from progressive shortening. The activation of this enzyme has been found in a variety of human tumors and tumor-derived cell lines, but only few studies on telomerase activity in human brain tumors have been reported. Here, we evaluated telomerase activity in different grades of human astrocytoma and meningioma brain tumors. In this study, assay for telomerase activity performed on 50 eligible cases consisted of 26 meningioma, 24 astrocytoma according to the standard protocols. In the brain tissues, telomerase activity was positive in 39 (65 %) of 50 patients. One sample t test showed that the telomerase activity in meningioma and astrocytoma tumors was significantly positive entirely (P < 0.001). Also, grade I of meningioma and low grades of astrocytoma (grades I and II) significantly showed telomerase activity. According to our results, we suggest that activation of telomerase is an event that starts mostly at low grades of brain including meningioma and astrocytoma tumors.
Keywords: Telomerase activity, Brain tumor, Astrocytoma, Meningioma
Introduction
Somatic cells do not have telomerase activity but immortalized cell lines and more than 85 % of the cancer cells show telomerase activation to prevent the telomere from progressive shortening (Harley 1991). When telomere length reaches a critical point, a length-sensing feedback mechanism would lead to this activation (Harley 1991).
Telomerase or telomere terminal transferase is a ribonucleoprotein that makes de novo synthesis and elongation of the telomeric repeats onto the chromosome ends using the RNA template complementary to the (TTAGGG)n repeats, and prevents the replication-dependent loss of telomere and cellular senescence in highly proliferative cells of the germline and in the majority of cancers (Blackburn 1991; Feng et al. 1995; Blasco 2005). However, human somatic cells do not normally have this telomerase activation (Kim et al. 1994).
The lack of telomerase activity in normal human somatic cells can be considered as a tumor suppressor mechanism which prevents limitless clonal expanding. This idea is supported by the finding that more than 90 % of human tumors are telomerase positive (Falchetti et al. 2002). Cells with activity of this enzyme appear to escape from progressive telomeric shortening and acquire an indefinite growth potential (Counter et al. 1992; Kim et al. 1994). The activation of this enzyme has been found in a variety of human tumors and tumor-derived cell lines (Counter et al. 1994; Kim et al. 1994; Hiyama et al. 1995a; Hiyama et al. 1995b; Garcia-Aranda et al. 2006) but only few studies on telomerase activity in human brain tumors have been reported (Langford et al. 1995; Nakatani et al. 1997). Telomerase activity can be detected in a very small amount of tissues such as collected cells in fine-needle aspiration cytology or cells in the body fluid of cancer patients (Hiyama and Hiyama 2002).
The conventional primer-extension-based assay for detecting telomerase activity requires large numbers of cells or sample amounts of tissue and only allows detection of telomerase with limited sensitivity using radioactive label. Furthermore, unspecific amplification products have been observed. These disadvantages have been overcome by the Telomeric Repeat Amplification Protocol (TRAP), in which the telomerase-reaction product is amplified by PCR (Kim et al. 1994).
Here, we evaluated telomerase activity in human brain tumors in different grades of astrocytoma and meningioma tumors.
Materials and Methods
Patients and Samples
Eligible cases in this study consisted of 50 patients were enrolled in study between April 2008 and March 2009. Patients underwent surgical resection for brain tumor (meningioma in 26 and astrocytoma in 24) in Shariati hospital of Tehran province. The tumor tissue was immediately placed in liquid nitrogen in operation room and then stored frozen at −80 °C.
These samples were graded on the basis of the classification of the World Health Organization (WHO) (Kleihues et al. 1993; Tatter et al. 1995). The study was performed in Tehran University of Medical Sciences, according to the instructions of the local Ethics Committee with the informed patient’s consent.
Assay for Telomerase Activity
Assays performed using Telo TAGGG Telomerase PCR ELISAPLUS Kit (Roche Diagnostics GmbH, Germany) according to the manufacturer’s protocol. Each frozen sample was suspended into a sterile reaction tube containing 200 μl ice-cold lysis reagent and homogenized on ice until uniform consistency. After incubation on ice for 30 min, centrifuged the lysate at 16,000×g for 20 min at 4 °C, 175 μl of supernatant was removed and transferred to another tube.
Assays were carried out in two steps. In the first step, for each tumor sample and the control template, 25 μl of reaction mixture and 5 μl of the internal standard (IS) were transferred into a suitable tube for PCR amplification. Telomerase activity was measured twice through an independent experiment using 1 μl cell extract per sample tube corresponding to 200–300 ng of total protein. The amount of 1 μl of the corresponding heat-treated cell extract was added (which corresponds to 200–300 ng of total protein) per each negative control tube. We transferred 1 μl of control template and 1 μl of Lysis reagent into two separate tubes. Total volume of each tube adjusted to 50 μl with sterile water. The thermal cycling parameters were a primer elongation step of 30 min at 25 °C by PCR using Taq polymerase, the P1-TS, P2 primers and nucleotides, followed by a telomerase inactivation step of 5 min at 94 °C and then 33 cycles at 94 °C for 30 s for denaturation, Finally, 50 °C for 30 s for annealing and 72 °C for 90 s for polymerization on a DNA thermocycler (Corbett CG1-96). In the second step, using the ELISA method, after immobilization the amplified products onto streptavidin-coated microtiter plates (MP) via biotin–streptavidin interaction, detection could be possible by anti-digoxigenin (DIG) antibody conjugated to peroxidase. Then the peroxidase substrate (3,3′,5,5′-tetramethylbenzidine) was added and the amount of TRAP products determined by measurement of their absorbance at 450 nm with a reference wavelength of 690 nm. The results were considered as negative or positive when the OD (optic density) values were ≤0.2 or >0.2, respectively. Moreover, to confirm the ELISA results, amplified products were systemically run on 12 % non-denaturing polyacrylamide gel.
Quantification of Telomerase Activity and Statistical Analysis
The level of telomerase activity in a given sample is determined by comparing the signal from the sample to the signal obtained using a known amount of a control template which are provided with the TeloTAGGG Telomerase PCR ELISAPLUS and are ready to use solutions. All statistical tests were two sided, and P ≤ 0.05 was considered significant. Statistical analysis was performed with SPSS software package (Version 16).
Results
Clinical–Pathologic Variables
A case series study included 50 primary brain tumors and the clinical–pathologic variables of patients are listed in Table 1.
Table 1.
Clinicopathologic characteristics of 50 patients affected with brain tumor
| Character | Meningioma (non-neuroepithelial) | Astrocytoma (neuroepithelial) |
|---|---|---|
| Mean of age ± SD | 55.65 ± 14.5 | 36.95 ± 15.4 |
| Sex | ||
| Male | 5 | 13 |
| Female | 21 | 11 |
| Grade | ||
| Grade I | 20 (76.9 %) | 2 (8.3 %) |
| Grade II | 6 (23.1 %) | 5 (20.0 %) |
| Grade III | 5 (20.0 %) | |
| Grade IV | 12 (50.0 %) | |
| Mean tumors size (cm) ± SD | 4.5 ± 2.3 | 4.1 ± 1.82 |
| Total | 26 | 24 |
Our samples included 26 meningioma and 24 astrocytoma tumors. According to the WHO grading system and surgical-pathologic tumor grading, 20 (76.92 %) of 26 meningioma tumor samples had grade I, and 6 tumors (23.07 %) were classified as grade II. The grading of astrocytoma tumor samples exhibited 2 (8.3 %) as grade I, 5 (20.0 %) as grade II, 5 (20.0 %) as grade III, and 12 (50.0 %) as grade IV. The mean size of meningioma and astrocytoma tumors was 4.5 and 4.1 cm, respectively.
Analysis of Telomerase Activity in Brain Tumors
In brain tumors, telomerase activity was positive in 39 (65 %) of 50 patients (Figs. 1, 2). Whereas, in meningioma tumors, 22 (84.61 %) of 26 patients and 17 (70.83 %) of 24 astrocytoma tumors revealed to have positive telomerase activity.
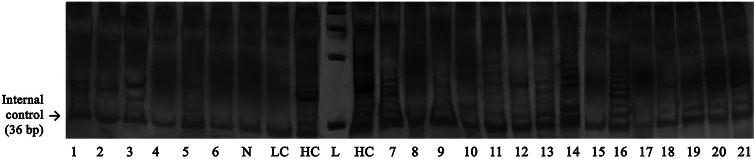
Fig. 1.
TRAP assay for telomerase activity in surgical specimens of brain tumors. TS oligonucleotides were amplified by PCR, with the downstream primer CX [5′-(CCCTTA)3 CCCTAA-3′] and the upstream primer TS, in the presence of internal TRAP assay standard. Reaction products were resolved on a 12 % polyacrylamide gel. The numbers of lines 1–21 are according to numbers of gray part in Table 2. Lines 1–3, 5–10, and 12–13 are M (meningioma) and lines 4, 11, and 14–21 are A (astrocytoma). N Negative control; LC Low-control template; HC High-control template; L molecular weight marker (50 bp) (Color figure online)
Fig. 2.
Comparison of telomerase activity between high- and low-grade brain tumors. a meningioma; b astrocytoma. TA Telomerase Activity, M–I, and M–II grade I and grade II of meningiomas, A–H High grades (III and IV) of astrocytomas, A–L Low grades (I and II) of astrocytomas, Neg Negative and Pos Positive, respectively
In grade I of meningioma tumors, 16 (80 %) of 20 samples exhibited telomerase activity and in 4 (20 %) samples no activity could be found. In addition, 5 out of 6 grade II meningioma tumors had telomerase activity, except one sample.
In grade I of astrocytoma tumors, 1 of 2 samples exhibited telomerase activity. Three (60 %) of 5 samples in grade II of astrocytoma tumors had telomerase activity and others did not show telomerase activity. In grade III of astrocytomas, telomerase activity was found positive in 4 (80 %) of 5 patients with brain tumor. In grade IV of astrocytoma tumors, 9 (80 %) of 12 samples exhibited telomerase activity, but in 3 (20 %) samples no activity could be find. On the whole, 4 (57.1 %) of 7 low-grade astrocytoma (grades I and II) had telomerase activity, whereas 13 (76.5 %) of 17 high-grade astrocytoma (grades III and IV) showed function of telomerase.
One sample t test showed that the overall telomerase activity was significantly positive (P < 0.001) in meningioma and astrocytoma tumors. However, Telomerase activity in grade I of meningioma tumors was significantly positive (P < 0.001). Low grades of astrocytoma (grades I and II) significantly showed telomerase activity (P = 0.03). Telomerase activity in high grade of meningioma tumors was significantly positive (P = 0.004). High grades of astrocytoma tumors exhibited a high positive telomerase activity (P < 0.001) (Fig. 2).
We had previously measured the telomere length of these samples (Kheirollahi et al. 2011), but there was not any correlation between telomere length and activity of telomere. In addition, no relation between tumor size and telomerase activity was observed. We did not find any significant correlation between activity of telomeres and age or sex (Table 2).
Table 2.
Telomerase activity of 50 brain tumors and age of patients
| No. | Ref. | Telomerase activity | Pathology | Age | No. | Ref. | Telomerase activity | Pathology | Age |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | ++ | M-II | 70 | 26 | 27 | + | M-I | 82 |
| 2 | 64 | + | M-I | 37 | 27 | 28 | − | M-I | 56 |
| 3 | 65 | + | M-I | 66 | 28 | 30 | + | M-I | 68 |
| 4 | 76 | − | A-IV | 35 | 29 | 37 | + | M-I | 53 |
| 5 | 78 | ++ | M-I | 69 | 30 | 53 | + | M-I | 47 |
| 6 | 80 | + | M-I | 43 | 31 | 55 | ++ | M-I | 80 |
| 7 | 50 | ++ | M-I | 65 | 32 | 66 | + | M-I | 56 |
| 8 | 39 | − | M-I | 55 | 33 | 77 | + | M-I | 50 |
| 9 | 34 | + | M-I | 53 | 34 | 45 | − | M-II | 34 |
| 10 | 29 | + | M-II | 77 | 35 | 74 | + | M-II | 58 |
| 11 | 26 | ++ | A-IV | 7 | 36 | 31 | − | A-I | 28 |
| 12 | 20 | ++ | M-I | 62 | 37 | 22 | − | A-II | 30 |
| 13 | 13 | ++ | M-II | 36 | 38 | 70 | + | A-II | 32 |
| 14 | 11 | ++ | A-II | 41 | 39 | 16 | + | A-III | 45 |
| 15 | 82 | − | A-II | 20 | 40 | 40 | + | A-III | 23 |
| 16 | 75 | ++ | A-II | 46 | 41 | 84 | − | A-III | 42 |
| 17 | 67 | − | A-IV | 22 | 42 | 17 | + | A-IV | 42 |
| 18 | 14 | ++ | A-III | 43 | 43 | 33 | + | A-IV | 50 |
| 19 | 60 | ++ | A-IV | 50 | 44 | 35 | ++ | A-IV | 50 |
| 20 | 56 | ++ | A-III | 34 | 45 | 41 | + | A-IV | 66 |
| 21 | 71 | ++ | A-I | 8 | 46 | 54 | + | A-IV | 31 |
| 22 | 7 | + | M-I | 65 | 47 | 47 | + | M-II | 37 |
| 23 | 8 | + | M-I | 52 | 48 | 57 | + | A-IV | 25 |
| 24 | 12 | − | M-I | 37 | 49 | 72 | ++ | A-IV | 49 |
| 25 | 23 | ++ | M-I | 57 | 50 | 89 | − | A-IV | 68 |
+ Positive, ++ Highly positive, − Negative, M–I, and M–II grade I and grade II of meningiomas, A–H High grades (III and IV) of astrocytomas, A–L Low grades (I and II) of astrocytomas
Comparison of telomerase activity between astrocytoma and meningioma tumors showed that lower grades of astrocytomas (including grades I and II) have a wide range of telomerase activity than higher grades (grades III and IV), while this situation can be seen in higher grade (grade II) of meningioma (Fig. 3).
Fig. 3.
The average of telomerase activity (±SD) by ELISA analysis for each group of tumors and grades of tumors. The values obtained with 1 μl of the control template, low, and 1 μl of the control template, high, should be in the range of 0.2–0.5 and 2.0–4.0, respectively, after 10 min substrate reaction. M–I, and M–II grade I and grade II of meningiomas, A–H High grades (III and IV) of astrocytomas, A–L Low grades (I and II) of astrocytomas, respectively
Discussion
The growth and development of brain tumors vary, but low- and high-grade brain tumors are capable to invade normal brain tissue. It means that surgery is rarely able to heal them, as it is generally not possible for the surgeon to remove all of the cancerous cells without removing an unacceptably large number of normal brain cells as well (Lichtenstein et al. 2009). Unlike the other tissues, brain cells have a limit number of cell division, and therefore, studies of telomerase activity and telomere length in brain tumors are more attractive. The results may be reflecting more clear insight into cancerous cells in future and considered as biomarker(s).
The enzymatic activity of telomerase correlates with tumor malignancy (Langford et al. 1995; DeMasters et al. 1997; Sallinen et al. 1997; Hiraga et al. 1998; Kleinschmidt-Demasters et al. 1998; Falchetti et al. 1999; Weil et al. 1999). Telomerase activity was observed in malignant tumors of non-neuroepithelial tumors, such as germ cell tumors, lymphomas, metastatic adenocarcinomas, hemangiopericytomas, and an anaplastic meningioma. In contrast, telomerase activity was not found in benign tumors, including meningiomas, pituitary adenomas, hemangioblastomas, and schwannomas, except for one hemangioblastoma that displayed malignant features at the fourth recurrence (Hiraga et al. 1998). Finally, they concluded that telomerase activity can be an indicator of malignant potential or malignancy itself in non-neuroepithelial brain tumors (Hiraga et al. 1998). In another study, high telomerase activity and hTERT mRNA expression were detected solely in glioblastoma and medulloblastoma tumors with a high proliferation rate (Cabuy and Ridder 2001). In our study, we observed telomerase activity in 65 % of patients. Whereas, in meningioma tumors, 22 (84.61 %) of the patients revealed to have positive telomerase activity. In grade I of meningioma tumors, 16 (80 %) of the samples exhibited telomerase activity and in 4 (20 %) of the samples did not find this activity. Telomerase activity was negative in only one sample with grade II of meningioma tumor, and in other 5, it was positive. By comparing these results with those found in grade I of meningioma, it may be suggested that beginning of telomerase activity in meningioma tumors is related to low-grade tumors.
Furthermore, it was demonstrated that telomerase activity pattern in anaplastic astrocytomas (WHO grade III) and glioblastomas (WHO grade IV), with a high percentage of glioblastomas (ranging from 80 to 100 %) revealed to show different values, which was opposed to anaplastic astrocytomas in those only a minor percentage of samples (ranging from 20 to 30 %) expressed telomerase activity (Langford et al. 1995; Falchetti et al. 1999). This is a challenging result, because secondary glioblastomas are thought to be originated, through progression, from pre-existing astrocytomas. It appears that the transformation from anaplastic astrocytoma to glioblastoma, implies the activation of telomerase, and that secondary glioblastomas express telomerase in a higher percentage of cases than do primary glioblastomas (Falchetti et al. 2002). But according to our results, high frequency (4 (57.1 %) of 7) of low-grade (grades I and II) astrocytoma tumors showed telomerase activity.
Furthermore in our study, 1 of 2 diffuse fibrilary samples (grade II) exhibited telomerase activity. We also had one oligodendroglioma tumor with a positive telomerase activity. Therefore, we suggest that the initiation of telomerase activity in astrocytoma might be occurred in low-grade tumors as well.
Shortening of telomere length and tumor size are correlated, probably due to increased duration of proliferation in the tumor, and tumor aggression. A lower rate of cell division and the presence of telomerase activity could be reasons for recurrent case (Matsuo et al. 2007). In general, telomerase activity and histological grade are too closely correlated (Langford et al. 1995; Simon et al. 2000). Available data suggests that telomerase is an important prognostic indicator of survival in patients with brain tumors (Kim et al. 2006). But it seems that there is a correlation between telomerase activity and prognosis in the patients who had positive telomerase activity, leading to a poor prognosis.
The result of study on the 41 brain tumor samples has indicated that telomerase activity may be an important malignancy marker in brain tumor. Furthermore, the change from negative activity to positive activity in the recurrent tumors appeared to be a useful prognosticator for malignant astrocytic tumor (Nakatani et al. 1997). We suggest that activation of telomerase is an event that may be initiated at low-grade brain tumors including meningioma and astrocytoma. In addition, our previous experience shows that the shortening process of telomere could be an early event in human brain tumors (Kheirollahi et al. 2011). However, we did not find any correlation between telomerase activity and telomere length. Apparently, the regulation of telomerase activity and telomere length is a complex and dynamic event that is tightly related to cell cycle regulation in human stem cells (Hiyama and Hiyama 2007) and elongation of telomere is not only by telomerase and studies have confirmed a role for recombination in telomere maintenance by “Alternative lengthening of telomeres” (ALT); however, the exact mechanism of this pathway is yet to be determined (Dunham et al. 2000).
Finally, by relying on more complementary research (Sampl et al. 2012), then telomerase activity might be considered as a constructive biomarker and combined with our information obtained from the telomere length, may be alteration of telomere length and telomerase activity altogether are appropriate markers to precise diagnosis of brain tumor cells.
Acknowledgments
Conflict of interest
The authors report no conflict of interest.
References
- Blackburn EH (1991) Structure and function of telomeres. Nature (Lond) 350:569–573 [DOI] [PubMed] [Google Scholar]
- Blasco MA (2005) Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6:611–622 [DOI] [PubMed] [Google Scholar]
- Cabuy E, Ridder Ld (2001) Telomerase activity and expression of telomerase reverse transcriptase correlated with cell proliferation in meningiomas and malignant brain tumors in vivo. Virchows Arch 439:176–184 [DOI] [PubMed] [Google Scholar]
- Counter CM, Avillion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB (1992) Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J 11:1921–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter CM, Hirte HW, Bacchetti S, Harely CB (1994) Telomerase activity in human ovarian carcinoma. Proc Natl Acad Sci USA 91:2900–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMasters BK, Markham N, Lillehei KO, Shroyer KR (1997) Differential telomerase expression in human primary intracranial tumors. Am J Clin Pathol 107:548–554 [DOI] [PubMed] [Google Scholar]
- Dunham MA, Neumann AA, Fasching CL, RR RRR, (2000) Telomere maintenance by recombination in human cells. Nat Genet 26(4):447–450 [DOI] [PubMed] [Google Scholar]
- Falchetti ML, Pallini R, Larocca LM, Verna R, D’Ambrosio E (1999) Telomerase expression in intracranial tumors: prognostic potential for malignant gliomas and meningiomas. J Clin Pathol 52:234–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchetti ML, Larocca LM, Pallini R (2002) Telomerase in brain tumors. Childs Nerv Syst 18:112–117 [DOI] [PubMed] [Google Scholar]
- Feng J, Funk WD, Wang S, Weinrich SL, Avilion AA, Chiu C, Adams R, Chang E, Allsopp RC (1995) The RNA component of human telomerase. Science 269:1236–1241 [DOI] [PubMed] [Google Scholar]
- Garcia-Aranda C, Cd Juan, Diaz-Lopez A, Sanchez-Pernaute A, Torres A-J, Diaz-Rubio E, Balibrea J-L, Benito M, Iniesta P (2006) Correlations of telomere length, telomerase activity, and telomeric-repeat binding factor 1 expression in colorectal carcinoma. Cancer 106:541–551 [DOI] [PubMed] [Google Scholar]
- Harley CB (1991) Telomere loss: mitotic clock or genetic time bomb? Mutat Res 256:271–282 [DOI] [PubMed] [Google Scholar]
- Hiraga S, Ohnishi T, Izumoto S, Miyahara E, Kanemura Y, Matsumura H, Arita N (1998) Telomerase activity and alterations in telomere length in human brain tumors. Cancer Res 58:2117–2125 [PubMed] [Google Scholar]
- Hiyama E, Hiyama K (2002) Clinical utility of telomerase in cancer. Oncogene 21:643–649 [DOI] [PubMed] [Google Scholar]
- Hiyama E, Hiyama K (2007) Telomere and telomerase in stem cells. Br J Cancer 96:1020–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama E, Yokoyama T, Tatsumoto N, Hiyama K, Imamura Y, Murakami Y (1995a) Telomerase activity in gastric cancer. Cancer Res 55:3258–3262 [PubMed] [Google Scholar]
- Hiyama K, Hiyama E, Ishioka S, Yamakido M, Inai K, Gazdar AF (1995b) Telomerase activity in small-cell and non-small cell lung cancers. J Natl Cancer Inst 1:249–255 [DOI] [PubMed] [Google Scholar]
- Kheirollahi M, Mehrazin M, Kamalian N, Mehdipour P (2011) Alterations of telomere length in human brain tumors. Med Oncol 28(3):864–870 [DOI] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PLC (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011–2015 [DOI] [PubMed] [Google Scholar]
- Kim CH, Cheong JH, Bak KH, Kim JM, Oh SJ (2006) Prognostic implication of telomerase activity in patients with brain tumors. J Korean Med Sci 21:126–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleihues P, Burger PC, Scheithauer BW (1993) The new WHO classification of brain tumours. Brain Pathol 3(3):255–268 [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-Demasters BK, Hashizumi TL, Sze CI, Lillehei KO, Shroyer AL, Shroyer KR (1998) Telomerase expression shows differences across multiple regions of oligodendroglioma versus high grade astrocytomas but shows correlation with MIB/1 labelling. J Clin Pathol 51:284–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford LA, Piatyszek MA, Schold SCJ, Shay JW (1995) Telomerase activity in human brain tumours. Lancet 346:1267–1268 [DOI] [PubMed] [Google Scholar]
- Lichtenstein A, Lichtenstein M, Lichtenstein D, Lichtenstein E (2009) http://voicesagainstbraincancer.org/Initiatives/RaiseYourVoiceProgram/2WhatisCancer/tabid/85/Default.aspx. Accessed 12 Nov 2011
- Matsuo T, Hiyama E, Sugita T, Shimose S, Kubo T, Mochizuki Y, Adachi N, Ochi M (2007) Telomere length and telomerase activity in extra-abdominal desmoid tumors. Anticancer Res 27(1A):411–415 [PubMed] [Google Scholar]
- Nakatani K, Yoshimi N, Mori H, Yoshimura S-i, Sakai H, Shinoda J, Sakai N (1997) The significant role of telomerase activity in human brain tumors. Cancer 80:471–476 [DOI] [PubMed] [Google Scholar]
- Sallinen P, Miettinen H, Sallinen SL, Haapasalo H, Helin H, Kononen J (1997) Increased expression of telomerase RNA component is associated with increased cell proliferation in human astrocytomas. Am J Pathol 150:1159–1164 [PMC free article] [PubMed] [Google Scholar]
- Sampl S, Pramhas S, Stern C, Preusser M, Marosi C, Holzmann K (2012) Expression of telomeres in astrocytoma WHO Grade 2 to 4: TERRA level correlates with telomere length, telomerase activity, and advanced clinical grade. Transl Oncol 5(1):56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M, Park TW, Leuenroth S, Hans VH, Loning T, Schramm J (2000) Telomerase activity and expression of the telomerase catalytic subunit, hTERT, in meningioma progression. J Neurosurg 92:832–840 [DOI] [PubMed] [Google Scholar]
- Tatter SB, Wilson CB, Harsh GRIV (1995) Neuroepithelial tumors of the adult brain. W.B. Saunders Co, Philadelphia, pp 2612–2684 [Google Scholar]
- Weil RJ, Wu YY, Wortmeyer AO, Moon YW, Delgado RM, Fuller BG, Lonser RR, Remaley AT, Zhuang Z (1999) Telomerase activity in microdissected human gliomas. Mod Pathol 12:41–46 [PubMed] [Google Scholar]