Abstract
The insulin and insulin-like growth factor-1 (IGF-1) are considered to play important roles in brain development; and their cognate receptors -InsR and IGF-1R- localized within distinct brain regions including cerebellum. Using Real-Time PCR and western blot analysis, we compared the expression of InsR and IGF-1R in male and female developing rat cerebellum at P0, P7, and P14. At all time points studied, the cerebellar expression of IGF-1R, both at mRNA and protein levels was higher than that of InsR. The lowest InsR and IGF-1R mRNA and protein levels were measured in the neonate cerebellum, independent of gender. In males, the highest InsR and IGF-1R mRNA and protein expression were found at P7. InsR and IGF-1R expression increased significantly between P0 and P7, followed by a marked downregulation at P14. In contrast, in females, mRNA and protein levels of InsR and IGF-1R remain unchanged between P0 and P7, and are upregulated at P14. Therefore, peaked InsR and IGF-1R expression in female cerebelli occurred at P14. Interestingly, changes in mRNA expression and in protein levels followed the same developmental pattern, indicating that InsR and IGF-1R transcription is not subject to modulatory effects during the first 2 weeks of development. These findings indicate that there are prominent sexual differences in InsR and IGF-1R expression in the developing rat cerebellum, suggesting a probable mechanism for the control of gender differences in development and function of the cerebellum.
Keywords: Insulin-like growth factor 1 receptor, Insulin receptor, Cerebellum, Rat newborn
Introduction
Insulin and Insulin-like growth factor-1 (IGF-1) are highly homologous polypeptides and widely expressed in central nervous system (CNS), particularly during development. Multiple lines of evidence indicate that these factors are essential for normal brain growth and development (Anlar et al. 1999; Baron-Van Evercooren et al. 1991; Baskin et al. 1988; Chiu and Cline 2010; D’Ercole et al. 1996; de Pablo and de la Rosa 1995; Gammeltoft et al. 1985; Kar et al. 1993; Popken et al. 2004).
During the past decade, the joint efforts of several laboratories have firmly established biological functions for insulin and IGF-1 in the CNS development. In vitro and in vivo studies indicated that IGF-1 stimulates the proliferation of neuronal progenitors, inhibits neuronal apoptosis, induces the differentiation of oligodendrocytes, and increases the survival of neurons and oligodendrocytes (Anderson et al. 2002; Anlar et al. 1999; Bains et al. 2009; Baron-Van Evercooren et al. 1991; Baskin et al. 1988; Beck et al. 1995; Bondy 1991; Carson et al. 1993; Cheng et al. 2001; Chrysis et al. 2001; D’Ercole et al. 1996, 2002; Gammeltoft et al. 1985; Hodge et al. 2004; Popken et al. 2004; Russo et al. 2005). Insulin also has pleiotropic effects on neurons, including the regulation of neuronal proliferation, apoptosis, role in synaptic transmission, direct and indirect effects over various neurotransmitter systems, neuronal degeneration, synaptic plasticity, cognition, memory formation, and learning (Agrawal et al. 2011; Chiu and Cline 2010; Dou et al. 2005; Nelson et al. 2008; Plum et al. 2005; Reagan 2007; Zhao et al. 1999, 2004; Zhao and Alkon 2001).
Insulin and IGF-1 exert their pleiotropic functions by binding to and activating their membrane-bound tyrosine-kinase receptors: Insulin receptor (InsR) and IGF-1 receptor (IGF-1R). According to previous studies, both receptors show considerable similarities with regard to structure, sequence homology, and function. Both receptors have heterotetrameric structures consisting of two extracellular α-subunits and two transmembrane β-subunits with a tyrosine-kinase domain in the cytoplasmic portion (Bondy and Cheng 2004; Nakae et al. 2001; Navarro et al. 1999; Zhao and Alkon 2001; Zhao et al. 2004).
Using a variety of techniques including in situ hybridization and ligand binding autoradiography, animal studies have reported the localization of InsR and IGF-1R in the hippocampus, cerebral cortex, cerebellum, and in other rat brain regions where it is most abundant in large projection neurons (Bach et al. 1991; Bartlett et al. 1991; Beck et al. 1988; Marks et al. 1991, 1990). In rats, previous reports demonstrated that IGF-1R mRNA is expressed in the brain as early as embryonic day 13 (E13) and InsR mRNA is expressed abundantly at embryonic day 20 (E20) and at the day of birth (Hill et al. 1986; Kar et al. 1993; Marks et al. 1991; 1990; Werther et al. 1990).
The cerebellum has been known to be a critical brain structure for the coordination and control of voluntary movement (Brooks 1981; Eccles 1981; Ghez and Fahn 1985). However, recent evidence indicates that the cerebellum also plays a role in cognitive, behavioral, and emotional functions (Allen et al. 1997; Beaton and Marien 2010; Bugalho et al. 2006; Diamond 2000; Dolan 1998; Hayter et al. 2007; Middlenton and Strick 1994; Schmahmann and Caplan 2006; Schmahmann et al. 1998; Schmahmann and Sherman 1998; Schutter and van Honk 2006; Tavano et al. 2007; Turner et al. 2007). It is generally believed that in both humans and rodents there are some sex differences in the cerebellum (Abel et al. 2011; Nguon et al. 2005). The male human cerebellum is larger than that of the female, and the female rat cerebellum has more extensive dendritic branching (Nguon et al. 2005).
In support of a biological basis of sex difference, a study done in infants indicated that, while males and females are very similar in their motor development, females develop fine motor skills slightly sooner and males develop gross motor skills sooner (Touwen 1976). It has also been shown in infants that females have greater synchrony between the joints of their arms than males (Piek et al. 2002). In a study of Portuguese elementary school children, boys were shown to have significantly better motor speed and coordination than girls (Martins et al. 2005).
In addition, several developmental neuropsychiatry disorders such as autism, attention deficit hyperactivity disorder (ADHD), dyslexia, and schizophrenia involve the cerebellar structures and affect a significantly larger proportion of males. For instances, boys are more at risk for autism and ADHD than girls are, and schizophrenia manifests at an earlier age in men. In the other hand, mood disorders, Alzheimer’s disease, depression, and anxiety are more prevalent in women (Andreasen et al. 1996; Hoppenbrouwers et al. 2008; Kern 2002; Konarski et al. 2005; Nguon et al. 2005; Parker and Brotchie 2010; Rucklidge 2010; Tavano et al. 2007). Cerebellar involvement in some sexually dimorphic disorders suggests that sex differences in the cerebellum may have implications for human motor coordination, as well as cognitive difficulties (Hoppenbrouwers et al. 2008; Kern 2002; Konarski et al. 2005; Nguon et al. 2005; Rucklidge 2010; Tavano et al. 2007).
The previous work in our lab showed a prominent gender and laterality differences in InsR and IGF-1R expression in the developing rat hippocampus, as a probable mechanism for the control of gender and laterality differences in development and function of the hippocampus (Hami et al. 2012).
The objective of the present study was to compare the cerebellar expression of Insulin and IGF-1 receptors of male and female rats at first two postnatal weeks using Quantitative Real-Time PCR, and western blot techniques. The study reports for the first time the mRNA and protein levels of InsR and IGF-1R in the cerebellum and compares these markers between the male and female rat neonates at P0, P7, and P14. The data presented here are important to the understanding of the probable mechanisms involved in the sexual dimorphism on the developing cerebellum.
Methods
Animals
All procedures involving animals were in accordance with the Guide for the Care and Use of Laboratory Animals of the Mashhad University of Medical Sciences, Iran.
Timed-pregnant Wistar rats (200–250 g bodyweight, 6–8 weeks old) were shipped from Mashhad University of Medical Sciences Experimental Animal House (Mashhad, Iran) at gestational day (G)2 (G1 defined as the first day after males and females are co-housed and on which the female has either a sperm plug or a sperm-positive vaginal smear). Dams were housed individually under standard conditions (12-h light cycle, lights on at 6 AM and off at 6 PM, at 21–24 °C). Standard laboratory rat chow and water were available ad libitum.
At the end of pregnancy, the animals were allowed to deliver naturally, the day of birth was defined as postnatal day 0 (P0). A total of 42 newborns from 6 different litters were used in the present study. Pups were sexed and randomly assigned to three age groups, P0 (n = 7), P7 (n = 7), and P14 (n = 7). These time points coincide with the peak expression of InsR and IGF-1RmRNA in the rat brain (Bartlett et al. 1991; Kar et al. 1993; Marks et al. 1990; Werther et al. 1990). Efforts were made to retain equal numbers of males and females in each group.
Pups were anaesthetized with chloroform and killed by cervical dislocation at P0, P7, and P14. Cerebelli were rapidly dissected and the meninges were removed carefully.
RNA Isolation and cDNA Synthesis
Newborn’s cerebelli were collected in 1 ml of RNA stabilization reagent (RNAlater, Qiagen, Germany) and stored at −80 °C until further analysis. Total RNA was isolated from cerebellum tissue using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Final RNA pellets were dissolved in diethylpyrocarbonate (DPEC)-treated water, and the yield of RNA was quantified by measuring optical density at 260 nm. The integrity of the RNA was checked by visualization of 18S and 28S ribosomal bands on 1 % agarose gel with ethidium bromide.
We used 1 μg of total RNA for reverse transcription to synthesize the first strand cDNA using oligo(dT)18 primers following the instructions of the RevertAid First Strand cDNA synthesis kit (Fermentas Life Science, Vilnius, Lithuania). cDNA samples were stored at −20 °C.
Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Real-time PCR was performed to analyze cerebellar expression of IGF-1R and InsR genes. The primer sets were designed based on sequences from the NCBI database and checked for specificity using the NCBI BLAST tool (www.ncbi.nlm.nih.gov/BLAST); primers with no significant similarity to other loci were selected. The following primers were used: 5′- GCCGTGCTGTG- CCTGTCCTAAAAC -3′ (forward) and 5′- GCTACCGTGGTGTTCCTGCTTCG -3′ (reverse) for IGF-1R, 5′- GGCCCGATGCTGAGAACA -3′ (forward) and 5′- CGTCATTCCAAAGTCTCCGA -3′ (reverse) for InsR, and 5′- AACTCCCATT CTTCCACCTTTG -3′ (forward) and 5′- CTGTAGCCATATTCATTGTCATACCAG -3′ (reverse) for GAPDH. Each 25 μL real-time PCR reaction containing 2 μL cDNA was performed with SYBR Premix Ex. TaqTM Kit (TaKaRa, Biotechnology Co., LTD, Dalian, China) and PCR parameters were 95 °C for 30 s, 40 cycles of 95 °C for 5 s, 60 °C for 15 s, and 72 °C for 15 s.
The real-time detection of emission intensity of SYBR Green bound to double-stranded DNAs was performed using Corbett Research Rotor-Gene 6000 real-time DNA analysis system (Corbett Research, Sydney, Australia). At the end of the runs, melting curves were obtained to make sure that there were no primer–dimmer artifacts.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control to measure the relative quantitation of the expression of the target genes. Relative differences in the expression of target genes were calculated using the Relative Expression Software Tool (REST-384). Efficiency of primers was also calculated and used for REST analysis.
Western Blot Analysis
Western blotting was used to detect changes in the protein levels of IGF-1R and InsR in newborn’s cerebelli. Cerebellar protein was extracted as previously described (Klugmann et al. 1997), and stored at −80 °C until use.
Aliquots of 50 μg protein were separated on 7.5 % sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, USA). Proteins of interest were detected using their specific antibodies: rabbit anti-IGF-1R (antibody) (1:500, Signalway Antibody SAB, Pearland, USA); rabbit anti-InsR (antibody) (1:500, Abnova, Taipei, Taiwan), and anti-B-Actin (antibody) (1:1000, SIGMA-ALDRICH, USA).
Following four washes in TBST, membranes were incubated with HRP conjugated secondary antibody (1:5000, SAB, Signalway Antibody SAB, Pearland, USA). The IGF-1R protein immunoreactive bands were detected using the enhanced chemiluminescence (ECL) detection system from Amersham Corporation (Bioimaging, System, Syngene, UK) and quantified using ImageJ software.
Statistical Analysis
Statistical analyses were performed using the SPSS statistical package, version 15.0 (SPSS). Differences between groups were determined using an independent sample T test and a one-way analysis of variance (ANOVA) followed by Tukey’s tests. Results are expressed as mean ± S.E. and were regarded as being significant at P ≤ 0.05.
Results
Real-Time Quantitative PCR
We used quantitative real-time PCR to evaluate the mRNA expression of InsR and IGF-1R gene in cerebelli of rat newborns at P0, P7, and P14.
InsR Expression
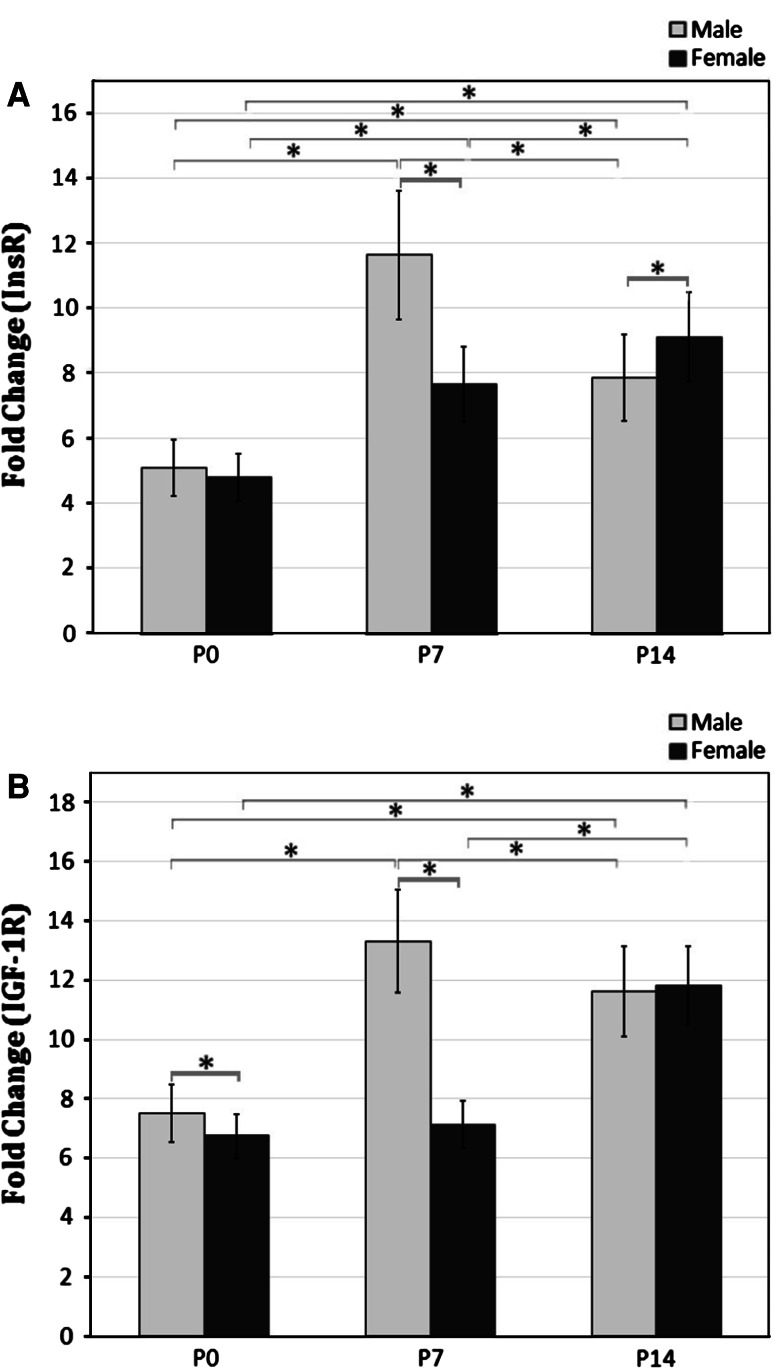
The detailed differential expression of InsR mRNA in cerebelli of male/female rat neonates is presented in Fig. 1a. As noted, InsR mRNA expression was the lowest at P0 in both male and female cerebelli.
Fig. 1.
The graphs represent the relative transcription levels of InsR (a) and IGF-1R (b) mRNA expression in the male and female rat cerebellum at P0, P7, and P14. Values represent the mean ± SE (n = 7). The transcription levels are quantified in a relative way to the standard curve. *Significant differences (P ≤ 0.05)
At P7, InsR mRNA expression was significantly upregulated in cerebelli independent of gender (P < 0.01) when compared to P0 animals. In addition, our results indicate that expression was significantly higher in the male pups than in female animals (P < 0.001).
In males, InsR expression in the cerebellum was markedly downregulated at P14 (P < 0.01); whereas we found a significant upregulation in expression of InsR in the females cerebellum at the same time (P < 0.05).
Interestingly, the results indicated that maximum expression of InsR in the male cerebellum occurred at P7. In contrast, in the female cerebellum peak expression was detected at P14.
IGF-1R Expression
Figure 1b, demonstrates the differential expression of the IGF-1R gene in male and female newborns’ cerebelli. Comparison of Fig. 1a, b reveals that the expression of the IGF-1R in cerebellum exhibits the same pattern as that of InsR. However, IGF-1R expression in neonate’s cerebelli was higher at all studied time points, in comparison to expression of InsR.
The lowest IGF-1R mRNA expression was measured at P0 in both male and female cerebellumi. In male cerebellum, IGF-1R mRNA levels were significantly higher at P7 than those measured P0 (P < 0.001). Furthermore, the highest IGF-1R expression in males occurred at P7. There was no significant difference in IGF-1R expression in female cerebellum between P0 and P7 (P > 0.05).
At P14, IGF-1R mRNA expression was significantly increased in the female cerebellum (P < 0.001), thus reaching the highest value of all examined time points. We found no differences in male cerebellum in expression of IGF-1R mRNA between P14 with P7 (P > 0.05).
Western Blot Analysis
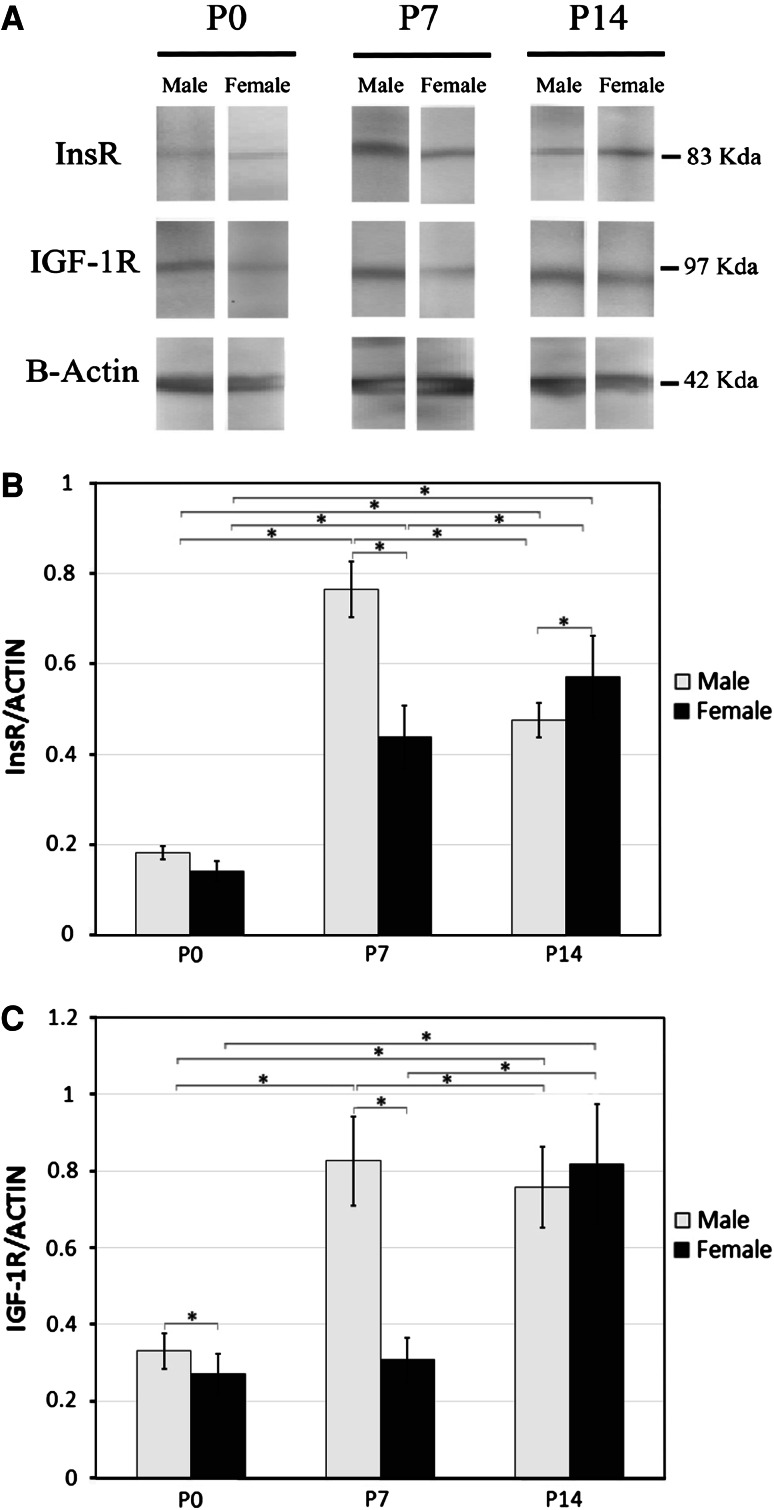
To determine the amount of IGF-1R and InsR protein in male and female neonate cerebelli, we performed Western blot analysis. The analysis for InsR showed a single immunoreactive band at ~83 kDa, which corresponds to the known molecular mass of the β-subunit of InsR, and a single band for IGF-1R at 97 kDa. Then, we analyzed our data densitometrically using Image J software. The results of this analysis are presented in Fig. 2.
Fig. 2.
Western immunoblots analysis of the abundance of the InsR and IGF1R in the developing cerebellum of early postnatal rats (a). Protein levels of InsR (b) and IGF-1R (c) in P0, P7, and P14 cerebellum were quantified using imageJ analysis software and normalized to β-actin expression. Data are presented as mean ± S.E. *Significant differences (P ≤ 0.05)
Interestingly, in both male and female cerebelli, the changes we found in InsR and IGF-1R for protein levels followed the same time course as those described for mRNA expression. That is, InsR protein level was the lowest at P0 and markedly increased at P7 (Fig. 2b). This was true for both the male and female comparisons. InsR protein level increased further at P14 in female cerebelli, but it decreased in males (Fig. 2b). In the male cerebellum we found the highest InsR protein level at P7, whereas in females cerebellar InsR protein levels peaked at P14. The differences in cerebellar InsR protein levels at P7 and P14 between male and female rat neonates was statistically significant (P7 P < 0.001, P14, P < 0.05) (Fig. 2b).
Concerning the IGF-1R, we also found the same developmental pattern at the protein level as that described for mRNA expression. Protein level was higher at P7 than at P0 in male cerebellum, but without any change in females (Fig. 2c). We also found a significant difference between IGF-1R protein concentration in cerebellum between male and female newborn rat (P < 0.001, Fig. 2c). Furthermore, whereas IGF-1R protein level increased drastically between P7 and P14 in the cerebellum of female rats, there was no difference between the IGF-1R protein levels in male rats at the same time point (Fig. 2c).
Discussion
The present study utilized Real-time quantitative PCR and western blot techniques to investigate the expression of InsR and IGF-1R at the mRNA and protein level in order to determine the differential expression of these genes between male and female newborn cerebellum. The cerebellum has been traditionally associated with motor control, physical coordination, and balance. Recent data also suggest that this brain structure has a significant role in behavior, cognition, and learning (Allen et al. 1997; Beaton and Marien 2010; Bugalho et al. 2006; Diamond 2000; Dolan 1998; Hayter et al. 2007; Middlenton and Strick 1994; Schmahmann and Caplan 2006; Schmahmann et al. 1998; Schmahmann and Sherman 1998; Schutter and van Honk 2006; Tavano et al. 2007; Turner et al. 2007).
Insulin and IGF-1 have been shown to affect CNS development/growth, including that of the cerebellum, since they promote neural cell proliferation, survival, differentiation, and maturation as well as synaptogenesis and myelination (Anderson et al. 2002; Anlar et al. 1999; Bains et al. 2009; Baron-Van Evercooren et al. 1991; Baskin et al. 1988; Beck et al. 1995; Bondy 1991; Carson et al. 1993; Cheng et al. 2001; Chrysis et al. 2001; D’Ercole et al. 1996; D’Ercole et al. 2002; Gammeltoft et al. 1985; Hodge et al. 2004; Popken et al. 2004; Russo et al. 2005). Additionally, the developmental regulation of insulin and IGF-1 receptor transcripts is partially coordinated with the appearance of their ligand transcripts (Baron-Van Evercooren et al. 1991).
In this study, we focused on the first two postnatal weeks, an important period for neurogenesis and synaptogenesis in rat cerebellum; because the cerebellum in rodents develops relatively late, and its development occurs largely after birth (Swinny et al. 2005). According to previous reports, the Purkinje cells in rats, which are responsible for all cerebellar cortex output, are born between embryonic days 14–16 (E14–16). On E14-E17, these cells mature and migrate to their final positions as a single cellular layer. By the day of birth (P0), dendrites from settled Purkinje cells begin to sprout from all aspects of the soma. From P5 to 10 the Purkinje cells are arranged in a single monolayer. However, it is not until the second postnatal week that the greatest synapse formation occurs between Purkinje cells and parallel fibers, the excitatory main input to Purkinje cells. The complex branching of the Purkinje cell dendritic tree reaches its adult width by P13. By P15, synaptic contact with multiple climbing fibers has been reduced to contact with a single climbing fiber per Purkinje cell. Starting on E16 the external granule layer (EGL), eventual birth place of the granule cells, spreads across the surface of the developing cerebellum. Granule cells migrate to the internal granule cell layer (IGL) during the first 2 weeks of life. The Golgi cells are born starting on E19 and continuing on to postnatal day 3 (P3). Basket cells are born on postnatal day 6–7 (P6–7) and begin to take on mature morphology by the middle of the second week of life; Stellate cells are also born next on P8–P11 (Altman and Bayer 1996; Goldowitz and Hamre 1998; Smeyne et al. 1995; Wang and Zoghibi 2001; Wechsler-Reya and Scott 1999).
The structure of the cerebellum has been considered to be a fairly monomorphic structure with few reliable sex differences (Ghez and Fahn 1985; Swinny et al. 2005). Nevertheless, several studies have found basic differences between the sexes in terms of cerebellum anatomy and function (Chung et al. 2005; Cosgrove et al. 2007; de Vries et al. 1984; Escalona et al. 1991; Filipek et al. 1994; Hu et al. 2008; Luft et al. 1998; Raz et al. 1998, 2001). By neuroimaging and MR techniques, many studies have shown that men had larger gross cerebellar hemispheres, cerebellar vermis, as compared with women (Chung et al. 2005; de Vries et al. 1984; Escalona et al. 1991; Raz et al. 1998, 2001). In children and adolescents, MRI reveals an 8 % difference in cerebellum volume, with the cerebelli of males being larger (Giedd et al. 1996). In juvenile rats, regional increases in the cerebellar volume of males versus females has also been found (Kornack et al. 1991).
In a postmortem morphologic study, the researchers reported that the total number of Purkinje cells in the male was 6–8 % higher than in the female (Hall et al. 1975). In addition, some differences were reported in glial structure and development in cerebellum between men with women. In a study by Kornack et al. (1991), mRNA level of nerve growth factor (NGF), an important neurotrophic factor, was higher in the developing cerebellum of female rats as compared to males (Kornack et al. 1991).
Recent data suggest the existence of sexual differences in behaviors such as cognition, mood, and motor skills (Andreano and Cahill 2009; Cohen-Bendahan et al. 2005; Dalla and Shors 2009; Jazin and Cahill 2010). Moreover, it is well known that the cerebellum is involved in several cognitive and developmental neuropsychiatry disorders such as schizophrenia, autism, attention deficit hyperactivity disorder (ADHD), and dyslexia (Andreasen et al. 1996; Dean and McCarthy 2008; Gowen and Miall 2007; Hoppenbrouwers et al. 2008; Keller et al. 2003; Kern 2002; Kibby et al. 2008; Konarski et al. 2005; Rucklidge 2010; Szeszko et al. 2003; Tavano et al. 2007). The expression of these disorders is also sexually dimorphic with autism, ADHD, and schizophrenia being more prevalent in males and mood disorders being more prevalent in females (Hoppenbrouwers et al. 2008; Nguon et al. 2005; Parker and Brotchie 2010; Rucklidge 2010). Therefore, sexual dimorphism in cerebellar functions under normal and pathological conditions indicates that any sex differences present in the cerebellum can have major implications for motor coordination as well as higher cognitive functions and neurological disorders.
In this work, we hypothesized that the sexually dimorphic differences in volume or cellular numbers in cerebellum reported in earlier reports; on the other hand, sex-differences existing in the prevalence of the neurodevelopmental and neurocognitive impairments that involve the cerebellum may be mediated, at least in part, via alterations in IGF-1R and InsR mRNA and/or protein levels.
Taken together, the current study results showed that InsR and IGF-1R in the rat cerebellum have a relatively uniform male and female expressional and transcriptional pattern (Fig. 1a, b). Although, the cerebellar expression of IGF-1R both in mRNA and protein levels were higher than those of InsR (Fig. 1a, b). We also found that in male cerebellum, InsR expression was significantly upregulated at P7, followed by a significant downregulation around P14 (Fig. 1a). Our results indicated that maximum expression of InsR in the male cerebellum occurred at P7 (Fig. 1a). In comparison, in the female cerebellum, we could observe a different pattern: InsR expression was upregulated at P7 and the peak expression occurred at P14 (Fig. 1a). In the case of IGF-1R expression, our data showed that there is a different pattern between male and female developing cerebelli. In male cerebellum, the expression of IGF-1R is markedly upregulated at P7 and the expression remains high until P14 (Fig. 1b). While, from P0 to P7, the expression of IGF-1R remains unchanged in the female cerebellum and then upregulated until P14 (Fig. 1b). In addition, in the male cerebelli, the expression of IGF-1R reaches its highest level at P7, whereas in the female one it occurs at P14 (Fig. 1b). The expression of IGF-1R is higher in male cerebelli compared to females; regardless of which time points were analyzed. This could be due to a higher neurogenesis and probably higher volume, or the higher number of Purkinje and glial cells in male cerebelli comparing to female (Hall et al. 1975; Giedd et al. 1996; Nguon et al. 2005). Moreover, data obtained from Real-time PCR and western blot analyses followed the same developmental pattern; i.e., changes found for protein levels followed the same time course as those described for mRNA expression (Figs. 1, 2). Thus, we can conclude that transcription of IGF-1R and of InsR in the rat cerebellum is not subject to any kind of modulatory effects during the first 2 weeks of development.
In conclusion, our present study results indicate the existence of a differential expression of InsR and IGF-1R in male–female developing rat cerebellum. Together with other mechanisms such as environmental conditions (Nguon et al. 2005) and endogenous steroid levels (Abel et al. 2011; Cohen-Bendahan et al. 2005; Dean and McCarthy 2008; Nguon et al. 2005), may underlie sexual dimorphism in volume, and number of Purkinje and glial cells, as well as, function of the cerebellum. Moreover, male–female differences in cerebellum developmental strategy may lead to differences in susceptibility to early developmental injury. Understanding the underlying neural mechanisms regulating sex differences can offer important insights into neurobehavioral diseases with strong sex biases. The present report may provide insight into the sex bias in neurodevelopmental diseases associated with cerebellar pathology, such as autism, ADHD, and schizophrenia.
Acknowledgments
This work was supported by a Mashhad University of Medical Sciences (MUMS) grant (No. 88631).
References
- Abel JM, Witt DM, Rissman EF (2011) Sex differences in the cerebellum and frontal cortex: roles of estrogen receptor alpha and sex chromosome genes. Neuroendocrinol 93:230–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal R, Tyagi E, Shukla R, Nath C (2011) Insulin receptor signaling in rat hippocampus: a study in STZ (ICV) induced memory deficit model. Eur Neuropsychopharmacol 21:261–273 [DOI] [PubMed] [Google Scholar]
- Allen G, Buxton RB, Wong EC, Courchesne E (1997) Attentional activation of the cerebellum independent of motor involvement. Science 275:1940–1943 [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA (1996) Development of the cerebellar system. CRC Press, Oxford [Google Scholar]
- Anderson MF, Aberg MA, Nilsson M, Eriksson PS (2002) Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res Dev Brain Res 134(1–2):115–122 [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L (2009) Sex influences on the neurobiology of learning and memory. Learn Mem 16:248–266 [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto L (1996) Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional pre-frontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA 93:9985–9990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anlar B, Sullivan KA, Feldman EL (1999) Insulin-like growth factor-I and central nervous system development. Horm Metab Res 31(2–3):120–125 [DOI] [PubMed] [Google Scholar]
- Bach MA, Shen-Orr Z, Lowe WL Jr, Roberts CT Jr, LeRoith D (1991) Insulin-like growth factor I mRNA levels are developmentally regulated in specific regions of the rat brain. Brain Res Mol Brain Res 10(1):43–48 [DOI] [PubMed] [Google Scholar]
- Bains M, Florez-McClure ML, Heidenreich KA (2009) Insulin-like growth factor-I prevents the accumulation of autophagic vesicles and cell death in purkinje neurons by increasing the rate of autophagosome-to-lysosome fusion and degradation. J Biol Chem 284:20398–20407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Van Evercooren A, Olichon-Berthe C, Kowalski A, Visciano G, Van Obberghen E (1991) Expression of IGF-I and insulin receptor genes in the rat central nervous system: a developmental, regional, and cellular analysis. J Neurosci Res 28(2):244–253 [DOI] [PubMed] [Google Scholar]
- Bartlett WP, Li XS, Williams M, Benkovic S (1991) Localization of insulin-like growth factor-1 mRNA in murine central nervous system during postnatal development. Dev Biol 147(1):239–250 [DOI] [PubMed] [Google Scholar]
- Baskin DG, Wilcox BJ, Figlewicz DP, Dorsa DM (1988) Insulin and insulin-like growth factors in the CNS. Trends Neurosci 11(3):107–111 [DOI] [PubMed] [Google Scholar]
- Beaton A, Marien P (2010) Language, cognition and the cerebellum: grappling with an enigma. Cortex 46:811–820 [DOI] [PubMed] [Google Scholar]
- Beck F, Samani NJ, Byrne S, Morgan K, Gebhard R, Brammar WJ (1988) Histochemical localization of IGF-I and IGF-II mRNA in the rat between birth and adulthood. Development 104(1):29–39 [DOI] [PubMed] [Google Scholar]
- Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F (1995) Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron 14(4):717–730 [DOI] [PubMed] [Google Scholar]
- Bondy CA (1991) Transient IGF-I gene expression during the maturation of functionally related central projection neurons. J Neurosci 11(11):3442–3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy CA, Cheng CM (2004) Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol 490(1–3):25–31 [DOI] [PubMed] [Google Scholar]
- Brooks VB (1981) Comment: on functions of the “cerebellar circuit” in movement control. Can J Physiol Pharmacol 59:776–778 [DOI] [PubMed] [Google Scholar]
- Bugalho P, Correa B, Viana-Baptista M (2006) Role of cerebellum in cognitive and behavioral control: scientific basis and investigation models. Acta Med Port 19:257–268 [PubMed] [Google Scholar]
- Carson MJ, Behringer RR, Brinster RL, McMorris FA (1993) Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron 10(4):729–740 [DOI] [PubMed] [Google Scholar]
- Cheng CM, Cohen M, Tseng V, Bondy CA (2001) Endogenous IGF1 enhances cell survival in the postnatal dentate gyrus. J Neurosci Res 64:341–347 [DOI] [PubMed] [Google Scholar]
- Chiu SL, Cline HT (2010) Insulin receptor signaling in the development of neuronal structure and function. Neural Dev 5:7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysis D, Calikoglu AS, Ye P, D’Ercole AJ (2001) Insulin-like growth factor-I overexpression attenuates cerebellar apoptosis by altering the expression of Bcl family proteins in a developmentally specific manner. J Neurosci 21:1481–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SC, Lee BY, Tack GRL, S.Y, Eom JS, Sohn JH (2005) Effects of age, gender, and weight on the cerebellar volume of Korean people. Brain Res 1042(2):233–235 [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CC, van de Beek C, Berenbaum SA (2005) Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci Biobehav Rev 29:353–384 [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK (2007) Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 62:847–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Shors TJ (2009) Sex differences in learning processes of classical and operant conditioning. Physiol Behav 97:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pablo F, de la Rosa EJ (1995) The developing CNS: a scenario for the action of proinsulin, insulin and insulin-like growth factors. Trends Neurosci 18(3):143–150 [DOI] [PubMed] [Google Scholar]
- de Vries GJ, de Bruin JPC, Uylings HMM, Corner MA (1984) Sex differences in the brain: the relation between structure and function. Prog Brain Res 61:VII–VIII [Google Scholar]
- Dean SL, McCarthy MM (2008) Steroids, sex and the cerebellar cortex: implications for human disease. Cerebellum 7:38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ercole AJ, Ye P, Calikoglu AS, Gutierrez-Ospina G (1996) The role of the insulin-like growth factors in the central nervous system. Mol Neurobiol 13(3):227–255 [DOI] [PubMed] [Google Scholar]
- D’Ercole AJ, Ye P, O’Kusky JR (2002) Mutant mouse models of insulin-like growth factor actions in the central nervous system. Neuropeptides 36(2–3):209–220 [DOI] [PubMed] [Google Scholar]
- Diamond A (2000) Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev 71:44–56 [DOI] [PubMed] [Google Scholar]
- Dolan RJ (1998) A cognitive affective role for the cerebellum. Brain 121:545–546 [DOI] [PubMed] [Google Scholar]
- Dou JT, Chen M, Dufour F, Alkon DL, Zhao WQ (2005) Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn Mem 12(6):646–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC (1981) Physiology of motor control in man. Appl Neurophysiol 44:5–15 [DOI] [PubMed] [Google Scholar]
- Escalona PR, McDonald WM, Doraiswamy PM, Boyko OB, Husain MM, Figiel GS, Laskowitz D, Ellinwood EH Jr, Krishnan KR (1991) In vivo stereological assessment of human cerebellar volume: effects of gender and age. Am J Neuroradiol 12:927–929 [PMC free article] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS Jr (1994) The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex 4:344–360 [DOI] [PubMed] [Google Scholar]
- Gammeltoft S, Fehlmann M, Van Obberghen E (1985) Insulin receptors in the mammalian central nervous system: binding characteristics and subunit structure. Biochimie 67:1147–1153 [DOI] [PubMed] [Google Scholar]
- Ghez C, Fahn S (1985) The cerebellum, in, principles of neural science. Elsevier, New York, pp 502–522 [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D et al (1996) Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex 6:551–560 [DOI] [PubMed] [Google Scholar]
- Goldowitz D, Hamre K (1998) The cells and molecules that make a cerebellum. Trends Neurosci 21:375–382 [DOI] [PubMed] [Google Scholar]
- Gowen E, Miall RC (2007) The cerebellum and motor dysfunction in neuropsychiatric disorders. Cerebellum 6:268–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TC, Miller AKH, Corsellis JAN (1975) Variations in the human Purkinje cell population according to sex and age. Neuropathol Appl Neurobiol 1:267–292 [Google Scholar]
- Hami J, Sadr-Nabavi A, Sankian M, Haghir H (2012) Sex differences and left-right asymmetries in expression of insulin and insulin-like growth factor-I receptors in developing rat hippocampus. Brain Struct Funct 217(2):293–302 [DOI] [PubMed] [Google Scholar]
- Hayter AL, Langdon DW, Ramnani N (2007) Cerebellar contributions to working memory. Neuroimage 36:943–954 [DOI] [PubMed] [Google Scholar]
- Hill JM, Lesniak MA, Pert CB, Roth J (1986) Autoradiographic localization of insulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience 17(4):1127–1138 [DOI] [PubMed] [Google Scholar]
- Hodge RD, D’Ercole AJ, O’Kusky JR (2004) Insulin-like growth factor-I accelerates the cell cycle by decreasing G1 phase length and increases cell cycle reentry in the embryonic cerebral cortex. J Neurosci 24:10201–10210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppenbrouwers SS, Schutter DJ, Fitzgerald PB, Chen R, Daskalakis ZJ (2008) The role of the cerebellum in the pathophysiology and treatment of neuropsychiatric disorders: a review. Brain Res Rev 59(1):185–200 [DOI] [PubMed] [Google Scholar]
- Hu D, Shen H, Zhou Z (2008) Functional asymmetry in the cerebellum: a brief review. Cerebellum 7:304–313 [DOI] [PubMed] [Google Scholar]
- Jazin E, Cahill L (2010) Sex differences in molecular neuroscience: from fruit flies to humans. Nat Rev Neurosci 11:9–17 [DOI] [PubMed] [Google Scholar]
- Kar S, Chabot JG, Quirion R (1993) Quantitative autoradiographic localization of [125I]insulin-like growth factor I, [125I]insulin-like growth factor II, and [125I]insulin receptor binding sites in developing and adult rat brain. J Comp Neurol 333(3):375–397 [DOI] [PubMed] [Google Scholar]
- Keller A, Castellanos FX, Vaituzis AC, Jeffries NO, Giedd JN, Rapoport JL (2003) Progressive loss of cerebellar volume in childhood-onset schizophrenia. Am J Psychiatr 160:128–133 [DOI] [PubMed] [Google Scholar]
- Kern JK (2002) The Possible role of the cerebellum in autism/PDD: disruption of a multisensory feedback loop. Med Hypoth 59:255–260 [DOI] [PubMed] [Google Scholar]
- Kibby MY, Fancher JB, Markanen R, Hynd GW (2008) A quantitative magnetic resonance imaging analysis of the cerebellar deficit hypothesis of dyslexia. J Child Neurol 23:368–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugmann M, Schwab MH, Pühlhofer A, Schneider A, Zimmermann F, Griffiths IR, Nave KA (1997) Assembly of CNS myelin in the absence of proteolipid protein. Neuron 18:59–70 [DOI] [PubMed] [Google Scholar]
- Konarski JK, McIntyre RS, Grupp LA, Kennedy SH (2005) Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J Psychiatry Neurosci 30:178–186 [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Lu B, Black IB (1991) Sexually dimorphic expression of the NGF receptor gene in the developing rat brain. Brain Res 542:171–174 [DOI] [PubMed] [Google Scholar]
- Luft AR, Skalej M, Welte D, Kolb R, Burk K, Schulz JB, Klockgether T, Voigt K (1998) A new semiautomated, three-dimensional technique allowing precise quantification of total and regional cerebellar volume using MRI. Diabetes Care Magn Reson 40:143–151 [DOI] [PubMed] [Google Scholar]
- Marks JL, Porte D Jr, Stahl WL, Baskin DG (1990) Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinol 127(6):3234–3236 [DOI] [PubMed] [Google Scholar]
- Marks JL, Porte D Jr, Baskin DG (1991) Localization of type I insulin-like growth factor receptor messenger RNA in the adult rat brain by in situ hybridization. Mol Endocrinol 5(8):1158–1168 [DOI] [PubMed] [Google Scholar]
- Martins IP, Castro-Caldas A, Townes BD, Ferreira G, Rodrigues P, Marques S, Derouen T (2005) Age and sex differences in neurobehavioral performance: a study of portuguese elementary school children. Int J Neurosci 115:1687–1709 [DOI] [PubMed] [Google Scholar]
- Middlenton FA, Strick PL (1994) Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science 266:458–461 [DOI] [PubMed] [Google Scholar]
- Nakae J, Kido Y, Accili D (2001) Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev 22(6):818–835 [DOI] [PubMed] [Google Scholar]
- Navarro I, Leibush B, Moon TW, Plisetskaya EM, Banos N, Mendez E, Planas JV, Gutierrez J (1999) Insulin, insulin-like growth factor-I (IGF-I) and glucagon: the evolution of their receptors. Comp Biochem Physiol B 122(2):137–153 [DOI] [PubMed] [Google Scholar]
- Nelson TJ, Sun MK, Hongpaisan J, Alkon DL (2008) Insulin, PKC signaling pathways and synaptic remodeling during memory storage and neuronal repair. Eur J Pharmacol 585(1):76–87 [DOI] [PubMed] [Google Scholar]
- Nguon K, Ladd B, Baxter MG, Sajdel-Sulkowska EM (2005) Sexual dimorphism in cerebellar structure, function, and response to environmental perturbations. Prog Brain Res 148:341–351 [DOI] [PubMed] [Google Scholar]
- Parker G, Brotchie H (2010) Gender differences in depression. Int Rev Psychiatr (Abingdon, England) 22(5):429–436 [DOI] [PubMed] [Google Scholar]
- Piek JP, Gasson N, Barrett N, Case I (2002) Limb and gender differences in the development of coordination in early infancy. Hum Mov Sci 21(5–6):621–639 [DOI] [PubMed] [Google Scholar]
- Plum L, Schubert M, Bruning JC (2005) The role of insulin receptor signaling in the brain. Trends Endocrinol Metab 16(2):59–65 [DOI] [PubMed] [Google Scholar]
- Popken GJ, Hodge RD, Ye P, Zhang J, Ng W, O’Kusky JR, D’Ercole AJ (2004) In vivo effects of insulin-like growth factor-I (IGF-I) on prenatal and early postnatal development of the central nervous system. Eur J Neurosci 19(8):2056–2068 [DOI] [PubMed] [Google Scholar]
- Raz N, Dupuis JH, Briggs SD, McGavran C, Acker JD (1998) Differential effects of age and sex on the cerebellar hemispheres and the vermis: a prospective MR study. Am J Neuroradiol 19:65–71 [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD (2001) Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. Am J Neuroradiol 22:1161–1167 [PMC free article] [PubMed] [Google Scholar]
- Reagan LP (2007) Insulin signaling effects on memory and mood. Curr Opin Pharmacol 7(6):633–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucklidge JJ (2010) The Gender differences in attention-deficit/hyperactivity disorder. Psychiatr Clin North Am 33(2):357–373 [DOI] [PubMed] [Google Scholar]
- Russo VC, Gluckman PD, Feldman EL, Werther GA (2005) The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev 26(7):916–943 [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Caplan D (2006) Cognition, emotion and the cerebellum. Brain Res 129:290–292 [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC (1998) The cerebellar cognitive affective syndrome. Brain Res 121:561–579 [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Loeber RT, Marjani J, Hurwitz AS (1998) Topographic organization of cognitive function in the human cerebellum. A meta-analysis of functional imaging studies. NeuroImage 7:S721 [Google Scholar]
- Schutter DJ, van Honk J (2006) An electrophysiological link between the cerebellum, cognition and emotion: frontal theta EEG activity to single-pulse cerebellar TMS. Neuroimage 33:1227–1231 [DOI] [PubMed] [Google Scholar]
- Smeyne RJ, Chu T, Lewin A, Bian F, S-Crisman S, Kunsch C, Lira SA, Oberdick J (1995) Local control of granule cell generation by cerebellar Purkinje cells. Mol Cell Neurosci 6:230–251 [DOI] [PubMed] [Google Scholar]
- Swinny JD, van der Want JJL, Gramsbergen A (2005) Cerebellar development and plasticity: perspectives for motor coordination strategies, for motor skills, and for therapy. Neural Plasticity 12(2–3):153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Gunning-Dixon F, Ashtari M, Snyder PJ, Lieberman JA, Bilder RM (2003) Reversed cerebellar asymmetry in men with first-episode schizophrenia. Biol Psychiatr 53:450–459 [DOI] [PubMed] [Google Scholar]
- Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F (2007) Disorders of cognitive and affective development in cerebellar malformations. Brain 130:2646–2660 [DOI] [PubMed] [Google Scholar]
- Touwen B (1976) Neurological development in infancy. Spastics International Medical Publications, London [Google Scholar]
- Turner BM, Paradiso S, Marvel CL, Pierson R, Boles Ponto LL, Hichwa RD (2007) The cerebellum and emotional experience. Neuropsychol 45:1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YW, Zoghibi HY (2001) Genetic regulation of cerebellar development. Nat Rev Neurosci 2:484–491 [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP (1999) Control of neuronal precursor proliferation in the cerebellum by Sonic hedgehog. Neuron 22:103–114 [DOI] [PubMed] [Google Scholar]
- Werther GA, Abate M, Hogg A, Cheesman H, Oldfield B, Hards D, Hudson P, Power B, Freed K, Herington AC (1990) Localization of insulin-like growth factor-I mRNA in rat brain by in situ hybridization–relationship to IGF-I receptors. Mol Endocrinol 4:773–778 [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Alkon DL (2001) Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol 177(1–2):125–134 [DOI] [PubMed] [Google Scholar]
- Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, Alkon DL (1999) Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem 274(49):34893–34902 [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Chen H, Quon MJ, Alkon DL (2004) Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol 490(1–3):71–81 [DOI] [PubMed] [Google Scholar]