Abstract
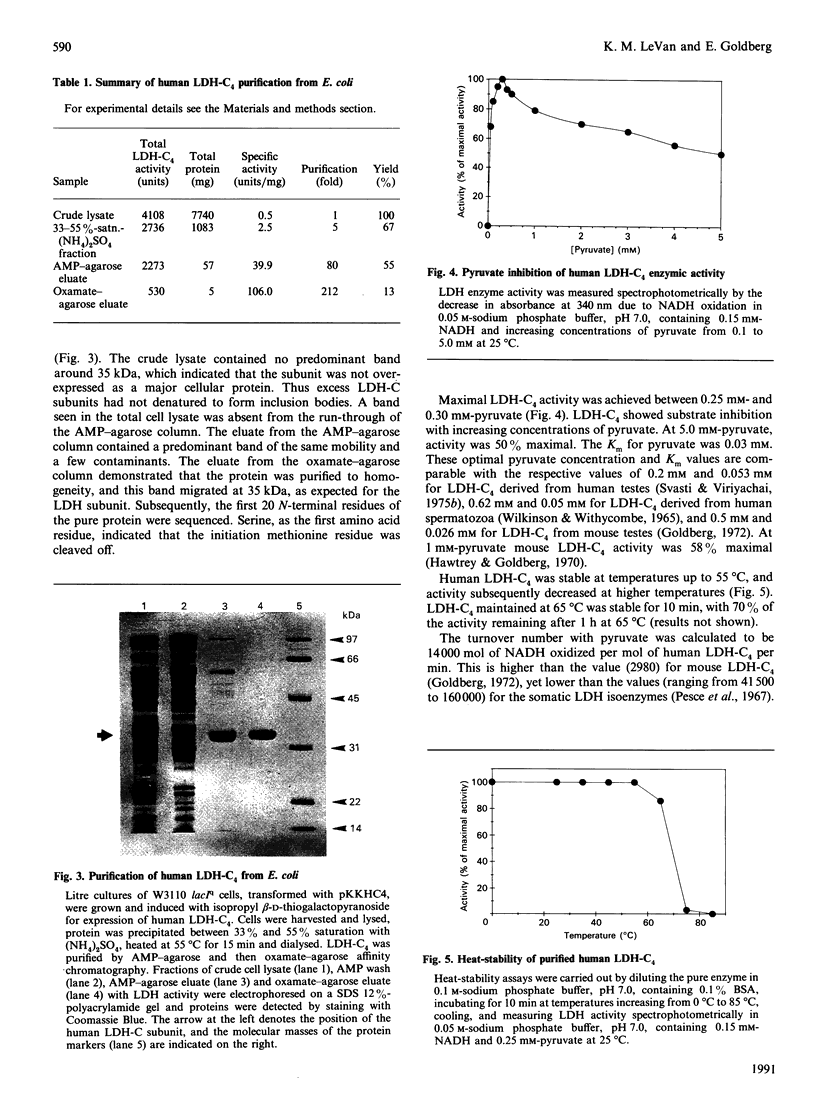
The cDNA encoding the C4 isoenzyme of lactate dehydrogenase (LDH-C4) was engineered for expression in Escherichia coli. The Ldh-c open reading frame was constructed as a cassette for production of the native protein. The modified Ldh-c cDNA was subcloned into the prokaryotic expression vector pKK223-3. Transformed E. coli cells were grown to mid-exponential phase, and induced with isopropyl beta-D-thiogalactopyranoside for positive regulation of the tac promoter. Induced cells expressed the 35 kDa subunit, which spontaneously formed the enzymically active 140 kDa tetramer. Human LDH-C4 was purified over 200-fold from litre cultures of cells by AMP and oxamate affinity chromatography to a specific activity of 106 units/mg. The enzyme was inhibited by pyruvate concentrations above 0.3 mM, had a Km for pyruvate of 0.03 mM, a turnover number (nmol of NADH oxidized/mol of LDH-C4 per min at 25 degrees C) of 14,000 and was heat-stable.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez J. G., Storey B. T. Assessment of cell damage caused by spontaneous lipid peroxidation in rabbit spermatozoa. Biol Reprod. 1984 Mar;30(2):323–331. doi: 10.1095/biolreprod30.2.323. [DOI] [PubMed] [Google Scholar]
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Blanco A., Zinkham W. H. Lactate Dehydrogenases in Human Testes. Science. 1963 Feb 15;139(3555):601–602. doi: 10.1126/science.139.3555.601. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Brosius J., Ullrich A., Raker M. A., Gray A., Dull T. J., Gutell R. R., Noller H. F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981 Jul;6(1):112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Erickson R. P., Friend D. S., Tennenbaum D. Localization of lactate dehydrogenase-X on the surfaces of mouse spermatozoa. Exp Cell Res. 1975 Mar 1;91(1):1–5. doi: 10.1016/0014-4827(75)90134-2. [DOI] [PubMed] [Google Scholar]
- Everse J., Kaplan N. O. Lactate dehydrogenases: structure and function. Adv Enzymol Relat Areas Mol Biol. 1973;37:61–133. doi: 10.1002/9780470122822.ch2. [DOI] [PubMed] [Google Scholar]
- GOLDBERG E. LACTATE DEHYDROGENASES AND MALATE DEHYDROGENASES IN SPERM: STUDIED BY POLYACRYLAMIDE GEL ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:560–570. doi: 10.1111/j.1749-6632.1964.tb14226.x. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Goldberg E. Amino acid composition and properties of crystalline lactate dehydrogenase X from mouse testes. J Biol Chem. 1972 Apr 10;247(7):2044–2048. [PubMed] [Google Scholar]
- Goldberg E. Antigenic sites of lactate dehydrogenase-C4. Isozymes Curr Top Biol Med Res. 1987;14:103–122. [PubMed] [Google Scholar]
- Goldberg E., Hawtrey C. The ontogeny of sperm specific lactate dehydrogenase in mice. J Exp Zool. 1967 Apr;164(3):309–316. doi: 10.1002/jez.1401640302. [DOI] [PubMed] [Google Scholar]
- Goldberg E. Immunochemical specificity of lactate dehydrogenase-X. Proc Natl Acad Sci U S A. 1971 Feb;68(2):349–352. doi: 10.1073/pnas.68.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg E. Lactic and Malic Dehydrogenases in Human Spermatozoa. Science. 1963 Feb 15;139(3555):602–603. doi: 10.1126/science.139.3555.602. [DOI] [PubMed] [Google Scholar]
- Hawtrey C. O., Goldberg E. Some kinetic aspects of sperm specific lactate dehydrogenase in mice. J Exp Zool. 1970 Aug;174(4):451–461. doi: 10.1002/jez.1401740408. [DOI] [PubMed] [Google Scholar]
- Hogrefe H. H., Griffith J. P., Rossmann M. G., Goldberg E. Characterization of the antigenic sites on the refined 3-A resolution structure of mouse testicular lactate dehydrogenase C4. J Biol Chem. 1987 Sep 25;262(27):13155–13162. [PubMed] [Google Scholar]
- Hogrefe H. H., Kaumaya P. T., Goldberg E. Immunogenicity of synthetic peptides corresponding to flexible and antibody-accessible segments of mouse lactate dehydrogenase (LDH)-C4. J Biol Chem. 1989 Jun 25;264(18):10513–10519. [PubMed] [Google Scholar]
- Kolk A. H., van Kuyk L., Boettcher B. Isolation of human lactate dehydrogenase isoenzyme X by affinity chromatography. Biochem J. 1978 Sep 1;173(3):767–771. doi: 10.1042/bj1730767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latner A. L., Siddiqui S. A., Skillen A. W. Pyruvate inhibition of lactate dehydrogenase activity in human tissue extracts. Science. 1966 Oct 28;154(3748):527–529. [PubMed] [Google Scholar]
- Lee C. Y., Yuan J. H., Goldberg E. Lactate dehydrogenase isozymes from mouse. Methods Enzymol. 1982;89(Pt 500):351–358. doi: 10.1016/s0076-6879(82)89063-0. [DOI] [PubMed] [Google Scholar]
- Li S. S., Feldmann R. J., Okabe M., Pan Y. C. Molecular features and immunological properties of lactate dehydrogenase C4 isozymes from mouse and rat testes. J Biol Chem. 1983 Jun 10;258(11):7017–7028. [PubMed] [Google Scholar]
- Liang Z. G., Shelton J. A., Goldberg E. Non-cross-reactivity of antibodies to murine LDH-C4 with LDH-A4 and LDH-B4. J Exp Zool. 1986 Dec;240(3):377–384. doi: 10.1002/jez.1402400312. [DOI] [PubMed] [Google Scholar]
- McKee R. W., Longstaff E., Latner A. L. Lactate dehydrogenase in human tests. Clin Chim Acta. 1972 Jun;39(1):221–227. doi: 10.1016/0009-8981(72)90319-1. [DOI] [PubMed] [Google Scholar]
- Miki T., Yasukochi T., Nagatani H., Furuno M., Orita T., Yamada H., Imoto T., Horiuchi T. Construction of a plasmid vector for the regulatable high level expression of eukaryotic genes in Escherichia coli: an application to overproduction of chicken lysozyme. Protein Eng. 1987 Aug-Sep;1(4):327–332. doi: 10.1093/protein/1.4.327. [DOI] [PubMed] [Google Scholar]
- Millan J. L., Driscoll C. E., LeVan K. M., Goldberg E. Epitopes of human testis-specific lactate dehydrogenase deduced from a cDNA sequence. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5311–5315. doi: 10.1073/pnas.84.15.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musick W. D., Rossmann M. G. The structure of mouse testicular lactate dehydrogenase isoenzyme C4 at 2.9 A resolution. J Biol Chem. 1979 Aug 25;254(16):7611–7620. doi: 10.2210/pdb1ldx/pdb. [DOI] [PubMed] [Google Scholar]
- O'Carra P., Barry S. Affinity chromatography of lactate dehydrogenase Model studies demonstrating the potential of the technique in the mechanistic investigation as well as in the purification of multi-substrate enzymes. FEBS Lett. 1972 Apr 1;21(3):281–285. doi: 10.1016/0014-5793(72)80183-2. [DOI] [PubMed] [Google Scholar]
- O'Carra P., Barry S., Corcoran E. Affinity chromatographic differentiation of lactate dehydrogenase isoenzymes on the basis of differential abortive complex formation. FEBS Lett. 1974 Jul 15;43(2):163–168. doi: 10.1016/0014-5793(74)80992-0. [DOI] [PubMed] [Google Scholar]
- Pallas D. C., Schley C., Mahoney M., Harlow E., Schaffhausen B. S., Roberts T. M. Polyomavirus small t antigen: overproduction in bacteria, purification, and utilization for monoclonal and polyclonal antibody production. J Virol. 1986 Dec;60(3):1075–1084. doi: 10.1128/jvi.60.3.1075-1084.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. C., Sharief F. S., Okabe M., Huang S., Li S. S. Amino acid sequence studies on lactate dehydrogenase C4 isozymes from mouse and rat testes. J Biol Chem. 1983 Jun 10;258(11):7005–7016. [PubMed] [Google Scholar]
- Pesce A., Fondy T. P., Stolzenbach F., Castillo F., Kaplan N. O. The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of vertebrates. J Biol Chem. 1967 May 10;242(9):2151–2167. [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Spielmann H., Erickson R. P., Epstein C. J. The separation of lactate dehydrogenase X from other lactate dehydrogenase isozymes of mouse testes by affinity chromatography. FEBS Lett. 1973 Sep 1;35(1):19–23. doi: 10.1016/0014-5793(73)80568-x. [DOI] [PubMed] [Google Scholar]
- Storey B. T., Kayne F. J. Energy metabolism of spermatozoa. VI. Direct intramitochondrial lactate oxidation by rabbit sperm mitochondria. Biol Reprod. 1977 May;16(4):549–556. [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Chemical characterization of D-lactate dehydrogenase from Escherichia coli B. J Biol Chem. 1968 May 25;243(10):2579–2586. [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Kinetics of Escherichia coli B D-lactate dehydrogenase and evidence for pyruvate-controlled change in conformation. J Biol Chem. 1968 May 25;243(10):2587–2596. [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wheat T. E., Goldberg E. Antigenic domains of the sperm-specific lactate dehydrogenase C4 isozyme. Mol Immunol. 1985 Jun;22(6):643–649. doi: 10.1016/0161-5890(85)90093-8. [DOI] [PubMed] [Google Scholar]
- Wheat T. E., Shelton J. A., Gonzales-Prevatt V., Goldberg E. The antigenicity of synthetic peptide fragments of lactate dehydrogenase C4. Mol Immunol. 1985 Oct;22(10):1195–1199. doi: 10.1016/0161-5890(85)90008-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. H., Withycombe W. A. Organ specificity and lactate-dehydrogenase activity. Some properties of human spermatozoal lactate dehydrogenase. Biochem J. 1965 Dec;97(3):663–668. doi: 10.1042/bj0970663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]