Abstract
Neurons rely on glutathione (GSH) and its degradation product cysteinylglycine released by astrocytes to maintain their antioxidant defences. This is particularly important under conditions of inflammation and oxidative stress, as observed in many neurodegenerative diseases including Alzheimer’s disease (AD). The effects of inflammatory activation on intracellular GSH content and the extracellular thiol profile (including cysteinylglycine and homocysteine) of astrocytes were investigated. U373 astroglial cells exposed to IL-1β and TNF-α for up to 96 h showed a dose-dependent increase in IL-6 release, indicative of increasing pro-inflammatory cellular activation. With increasing concentrations of IL-1β and TNF-α (0.01–1 ng/ml), an increase in both intracellular and extracellular GSH levels was observed, followed by a return to control levels in response to higher concentrations of IL-1β and TNF-α. Extracellular levels of cysteinylglycine decreased in response to all concentrations of IL-1β and TNF-α. In contrast, levels of the neurotoxic thiol homocysteine increased in a dose-dependent manner to IL-1β and TNF-α-induced activation. Our results suggest that chronically activated astrocytes in the brain might fail to adequately maintain GSH substrate delivery to neurons, thus promoting neuronal vulnerability. They might also explain the elevated levels of homocysteine found in the brains and serum of patients with AD.
Keywords: Astrocyte, Glutathione, Cysteinylglycine, Homocysteine, Inflammation, Alzheimer’s disease
Introduction
Oxidative stress refers to an imbalance between the production of reactive oxygen species (ROS) and their detoxification. Inadequate detoxification of ROS can lead to detrimental oxidation of DNA, lipids and proteins, which can cause cellular dysfunction and cell death. The brain is particularly vulnerable to oxidative stress due to a number of reasons such as a high oxygen usage compared to the rest of the body (~20 % of total), a high iron content (which can increase the generation of ROS) and relatively low levels of enzymes that detoxify ROS, including superoxide dismutase, catalase and glutathione peroxidise (Dringen 2000).
Oxidative stress is thought to play a significant role in the neuronal degeneration evident in Parkinson’s disease (PD) and Alzheimer’s disease (AD). Another characteristic of these diseases is the presence of chronic inflammation in the brain. Due to the widespread and early appearance of both inflammation and oxidative stress in PD and AD, it has been proposed that inflammation induced oxidative stress, or vice versa, might be central to the pathogenesis of such diseases.
Glutathione (GSH) is the key regulator of the intracellular redox state and is required for the detoxification of ROS, peroxides, electrophilic xenobiotic compounds and the regeneration of other endogenous and exogenous antioxidants. GSH is a tripeptide consisting of glutamate, cysteine and glycine and is synthesised by the consecutive actions of the enzymes γ-glutamyl cysteine ligase (γGCL) and glutathione synthetase (Chen et al. 2005). GSH can non-enzymatically detoxify ROS, such as superoxide and hydroxyl radicals, as well as act as an electron donor for the reduction of peroxides, catalysed by glutathione peroxidase (Brannan et al. 1980). In both reactions, two GSH molecules become oxidised and join together via a disulfide bond between the cysteine residues to form glutathione disulfide (GSSG) (Meister 1988). GSH can be regenerated from the oxidised disulfide form back to the reduced form by the action of glutathione reductase or it can be synthesised de novo.
Extracellular cysteine, the rate-limiting substrate for GSH synthesis, is readily oxidised to form cystine leading to a plasma cystine/cysteine ratio >4 (Jones et al. 2000). Hence, cystine transport mechanisms are essential to provide brain cells with sufficient cysteine (Yoshiba-Suzuki et al. 2011). Cystine uptake is the Xc-system, a Na+-independent cystine/glutamate exchange transporter that is expressed at lower levels in neurons compared to astrocytes (Bannai 1984; Sagara et al. 1993; Shanker and Aschner 2001). As a result, cystine is taken up much more efficiently by astrocytes than neurons (Kranich et al. 1996).
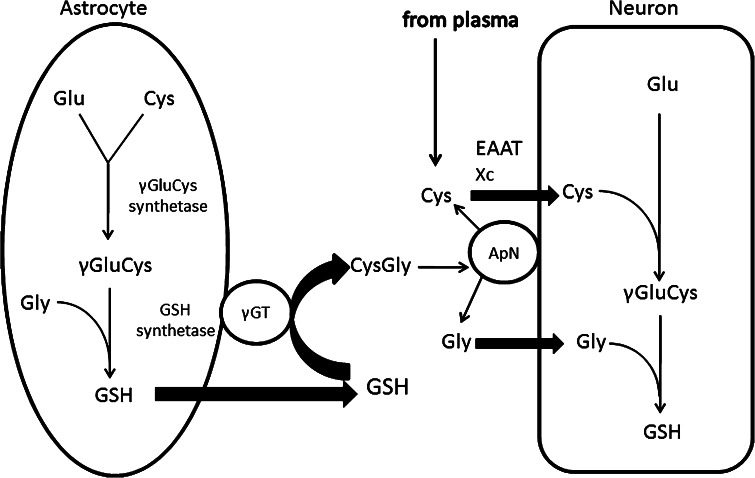
Cysteine uptake of neurons is mostly (80–90 %) mediated by the sodium-dependent excitatory amino acid transporter (EAAT) (Chen and Swanson 2003), with the remaining transport capacity provided by the Na+-independent cystine/glutamate exchange transporter (Shanker et al. 2001). One additional pool of cysteine in the extracellular space is provided through the release of GSH from astrocytes. Astrocytes store high levels of GSH (up to 8 mM), and release ~10 % of their intracellular GSH content per hour via the multidrug resistance protein 1 transporter (Dringen and Hamprecht 1997; Minich et al. 2006). Extracellular GSH released from astrocytes is then metabolised by γ-glutamyltranspeptidase (γ-GT) to form the dipeptide, cysteinylglycine, which is then processed by the neuronal ectopeptidase, aminopeptidase N, allowing neurons to immediately take up the resultant cysteine and glycine (Fig. 1). Therefore, neurons partly rely on the release and subsequent cleavage of GSH by astrocytes in order to maintain optimal intracellular GSH levels (Dringen and Hamprecht 1999).
Fig. 1.
GSH synthesis and metabolism in astrocytes and neurons. In astrocytes, γGluCys synthetase uses glutamate and cysteine to generate the dipeptide γGluCys, which is combined with glycine by glutathione synthetase to generate GSH. GSH is exported from the astrocyte whereupon extracellular GSH serves as a substrate for the astroglial ectoenzyme γ-glutamyl transpeptidase (γGT), producing the dipeptide CysGly, an important exogenous precursor of neuronal GSH. CysGly is broken down into Cys and Gly by aminopeptidase N. Cys transport into neurons is mediated by two cysteine transport systems, EAAT and Xc. In neurons, Cys, Gly and Glu are then resynthesised into GSH by the consecutive actions of neuronal γGluCys synthetase and GSH synthetase
In neurodegenerative diseases such as AD and PD, neurons need an optimal GSH supply to defend themselves against free radicals, such as superoxide and nitric oxide, released from activated microglia and astrocytes (Aschner 2000; Steele and Robinson 2011). The effect of chronic inflammation on the ability of astrocytes to provide neurons with substrates for GSH synthesis is of particular interest as reductions in neuronal GSH levels may contribute to neuronal cell death in a pro-oxidative, pro-inflammatory environment.
A second thiol of particular interest in neurodegenerative diseases is homocysteine. Homocysteine is a sulphydryl-containing amino acid that is metabolically linked to GSH via the transsulfuration pathway (Bolin et al. 2005). An elevated plasma level of homocysteine (hyperhomocysteinemia) is recognised as an independent risk factor for a variety of chronic diseases, including AD (Dwyer et al. 2004; Li et al. 2003; Pietrzik 2006; Seshadri et al. 2002).
For these reasons, it is important to understand the effect of acute and chronic pro-inflammatory activation on astrocytic production and release of GSH precursors and homocysteine.
Methods
Cell Culture and Experimental Protocols
The U373-MG human astrocytoma cell line was kindly provided by Dr. Peter Lock (The Royal Melbourne Hospital, Australia). U373 cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing 25 mM glucose, supplemented with 200 U/ml penicillin, 200 μg/ml streptomycin, 2.6 μg/ml fungizone, 200 mM glutamine and 5 % foetal bovine serum (FBS). Cells were grown in 175 cm2 tissue culture flasks and incubated at 37 °C in 5 % CO2. Cells were harvested with a solution containing 0.05 % trypsin and 0.02 % EDTA in phosphate buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) and seeded into 96-well, flat-bottom, tissue culture plates at a density of 9 × 103 cells/well. FBS concentration was reduced to 3 % to minimise proliferation and the total volume of media in each well was 100 μl. 24 h after plating the media was replaced with fresh media containing 1 % FBS and 0, 0.01, 0.1, 1 or 10 ng/ml IL-1β + TNF-α for up to 96 h. All cell culture materials were from Invitrogen (Mulgrave, Australia).
Measurement of IL-6 in Conditioned Media
Media was collected from U373 cells treated with 0–10 ng/ml IL-1β + TNF-α for 12, 24, 36, 48, 60, 72, 84 or 96 h, placed in fresh 96-well plate and stored at −20 °C until analysis. On thawing, supernatants were diluted with distilled water by a factor of 130 and samples and standards assayed for IL-6 concentration by ELISA (PeproTech) as per manufacturer’s instructions. Absorbance was measured at 355 nm in a POLARstar Omega microplate reader.
Analysis of Cell Viability
Cell viability was assessed in terms of the metabolic capability of cells to convert the fluorogenic redox indicator, resazurin, into its highly fluorescent product, resorufin. A modified version of the resazurin-reduction assay was used (Buranrat et al. 2008). Resazurin was dissolved in PBS to give a concentration of 0.001 % (w/v). This solution was sterile filtered (0.22 μm), protected from light with aluminium foil and stored at 4 °C for up to 6 months. To determine cell viability, incubation media was removed from wells and replaced with 100 μl of resazurin solution. Plates were incubated at 37 °C, with 5 % CO2 for 45 min and then fluorescence was measured with excitation at 530 nm and emission at 590 nm in a POLARstar Omega microplate reader (BMG Labtech). For every plate, background fluorescence determined in cell-free wells was subtracted from all wells, and values were expressed as a percentage of untreated control cells.
Determination of Intracellular GSH by the Tietze Assay
Following co-treatment of U373 cells with equal concentrations of IL-1β + TNF-α (0.01–10 ng/ml) for 24, 48, 72 or 96 h and removal of conditioned media, cells were lysed by subjection to three freeze–thaw (−80 and 25 °C) cycles. Cell lysates were dissolved in sterile distilled water (100 μl/well) and analysed for total GSH concentration by the Tietze assay (1969) and total protein by the Bradford assay (1976). The Tietze assay is a kinetic assay based on the reaction of GSH with 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) to form GSSG and 5-thio-2-nitrobenzoic acid (TNB). GSSG is converted back to GSH by glutathione reductase allowing further production of TNB. The rate of TNB formation, which is measurable at an absorbance of 412 nm, is proportional to the concentration of total GSH (GSH + GSSG) in the sample and is expressed as nmol of GSH per mg of protein. Cell lysate samples and GSH standards (20 μl) were added in triplicate to wells of a 96-well plate. Equal volumes of freshly prepared DTNB and glutathione reductase solutions were mixed together and 120 μl was added to each well. Following this, 60 μl of 0.8 mM NADPH was added to each well and absorbance was measured every 30 s for 3 min using a Bio-Rad Model 680 microplate reader. Total GSH in the samples was determined by use of standard concentrations between 0.8 and 50 μM. To determine total protein in cell lysates, 10 μl samples were added to a 96-well plate, followed by 40 μl of Bradford reagent and 150 μl of distilled water. Absorbance was measured at 595 nm using a Bio-Rad Model 680 microplate reader. A standard curve consisting of 0–0.125 mg/ml bovine serum albumin (BSA) was used to determine total protein concentration in samples. GSH concentration was standardised to total protein for each sample and expressed as nmol per mg of protein.
Determination of Extracellular GSH and Related Thiols by High Performance Liquid Chromatography and Fluorescence Detection
A Dionex HPLC system consisting of an ASI-100 automated sample injector, a P680 solvent pump, a TCC-100 thermostatted column compartment and an RF-2000 fluorescence detector was used for all chromatographic analyses. The system was equipped with a Luna C18(2) column (150 × 4.6 mm id, 3 μm) protected by a SecurityGuardC18 Cartridge (4.0 × 3.0 mm) in a SecurityGuardCartridge Holder supplied by Phenomenex. The Chromeleon 6.8 Chromatography Data System from Dionex was used to control instruments, acquire data and quantify peak areas.
Media taken from U373 cells treated with 0–10 ng/ml IL-1β + TNF-α for 24, 48, 72 or 96 h was centrifuged at 200×g for 5 min at 4 °C to pellet cellular debris. Following centrifugation, the supernatant was mixed with an equal volume of 1 % 5-sulfosalicylic acid containing 1 mM EDTA, centrifuged at 14,000×g for 10 min at 4 °C to precipitate protein, and the resulting supernatant placed in fresh tubes and stored at −80 °C until analysis. Upon thawing of samples and standard solutions, fresh microcentrifuge vials were placed in a heating block at 35 °C and 50 μl of sample or standard added. To reduce all disulfide bonds, 30 μl of a 1 mM solution of the reducing agent tris(2-carboxyethyl)phosphine hydrochloride was added. For the derivatisation reaction, vials were incubated for 5 min at 35 °C before the addition of 100 μl of borate buffer (0.1 M, pH 9.3, with 1 mM EDTA) and 30 μl of the derivatising agent 4-fluoro-7-aminosulfonylbenzofurazan (ABD-F; Novachem) (1 mg/ml in 0.1 M borate buffer). Samples were incubated at 35 °C for 10 min, before the reaction was stopped by addition of 50 μl of 2 M hydrochloric acid. Vials were then centrifuged at 14,000×g for 5 min at 4 °C in order to pellet any particulates that could potentially damage the HPLC system. Supernatants were placed into fresh vials and loaded into an autosampler. The autosampler maintained sample temperatures at 8 °C to prevent evaporation and injected 10 μl aliquots for analysis. The mobile phase used for separation of ABD-F-derivatised thiols was 0.1 M acetate buffer (pH 4)–methanol [86:14]. An isocratic program with a flow rate of 1 ml/min was used and column temperature was maintained at 35 °C. The fluorescence detector was set to an excitation and emission wavelengths of 390 and 510 nm, respectively, with high level sensitivity.
Statistics
Data presented are the mean of three independent experiments and error bars denote standard error of the mean (SEM). For Figs. 6, 7, 8, 9, the mean values from three independent experiments were used to calculate percent of the mean control from all three experiments. Significant differences caused by varying cytokine concentration were assessed by one-way ANOVA with Dunnett’s post-hoc tests and shown as *p < 0.05, **p < 0.01 and ***p < 0.001.
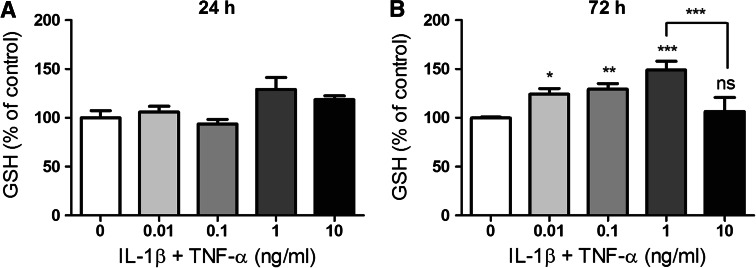
Fig. 6.
Concentration of total GSH in media collected from U373 cells after 24- and 72-h incubation. U373 cells were treated with IL-1β and TNF-α (0.01–10 ng/ml) for either 24 (a) or 72 h (b). Levels of total GSH in the media were then determined by HPLC with fluorescence detection. Mean ± SEM are plotted from three independent experiments and *p < 0.05, **p < 0.01 or ***p < 0.001 designates a significant difference from the non-activated control (0 ng/ml IL-1β and TNF-α). Significant differences were assessed by one-way ANOVA with Dunnett’s post hoc tests
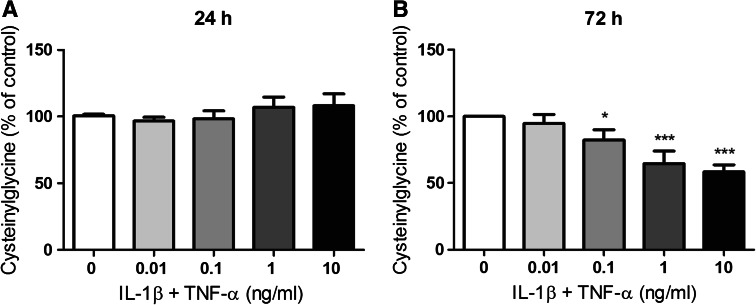
Fig. 7.
Concentration of total cysteinylglycine in media collected from U373 cells after 24- and 72-h incubation. U373 cells were treated with IL-1β and TNF-α (0.01–10 ng/ml) for either 24 (a) or 72 h (b). Levels of total cysteinylglycine in the media were then determined by HPLC with fluorescence detection. Mean ± SEM are plotted from three independent experiments and *p < 0.05 or ***p < 0.001 designates significant difference from the non-activated control (0 ng/ml IL-1β and TNF-α). Significant differences were assessed by one-way ANOVA with Dunnett’s post-hoc tests
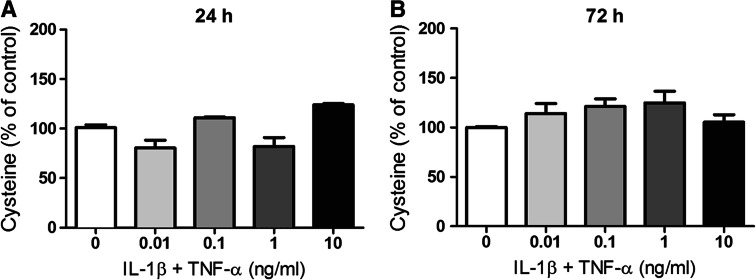
Fig. 8.
Concentration of total cysteine in media collected from U373 cells after 24- and 72-h incubation. U373 cells were treated with IL-1β and TNF-α (0.01–10 ng/ml) for either 24 (a) or 72 h (b). No significantly changes in the levels of total cysteine in the media were observed. Mean ± SEM are from three independent experiments are shown
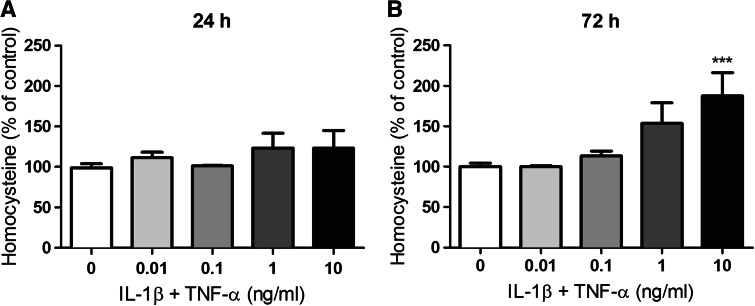
Fig. 9.
Concentration of total homocysteine in media collected from U373 cells after 24- and 72-h incubation. U373 cells were treated with IL-1β and TNF-α (0.01–10 ng/ml) for either 24 (a) or 72 h (b). Levels of total homocysteine in the media were then determined by HPLC with fluorescence detection. Mean ± SEM are plotted from three independent experiments and ***p < 0.001 designates significant difference from the non-activated control (0 ng/ml IL-1β and TNF-α). Significant differences were assessed by one-way ANOVA with Dunnett’s post-hoc tests
Results
IL-1β + TNF-α Induced Activation of U373 Cells
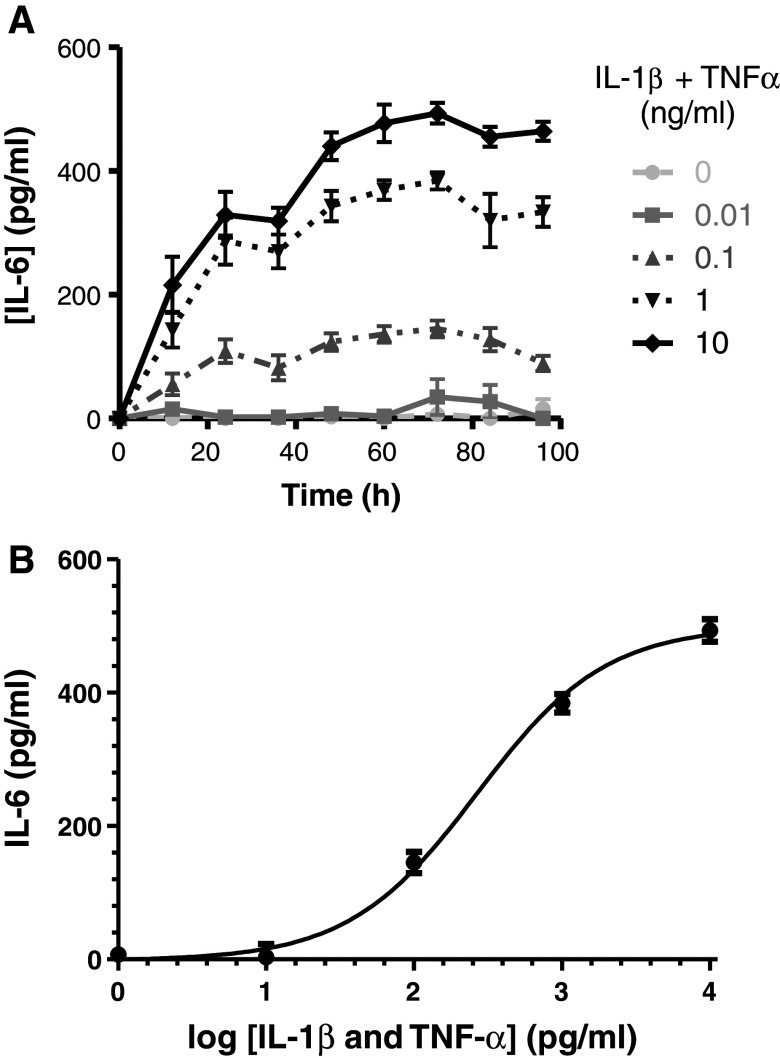
In order to study the effect of inflammation on levels of GSH and its derivatives, U373 human astroglial cells were treated with 0.01, 0.1, 1 or 10 ng/ml of the pro-inflammatory activators IL-1β + TNF-α for 24, 48, 72 or 96 h. As a measure of pro-inflammatory activation, levels of IL-6 were determined in cell culture media (Fig. 2). Activation of cells with 0.1–10 ng/ml of IL-1β + TNF-α led to a time- and dose-dependent increase in the IL-6 concentration in the media. At all concentrations of pro-inflammatory activators, maximal IL-6 concentration in the media was measured after 72-h incubation. Half-maximal IL-6 release (for 0.1, 1 and 10 ng/ml of IL-1β + TNF-α) was calculated as 19.7 ± 7.9, 17.2 ± 3. 7 and 19.8 ± 4.5 h, respectively (data points between 0 and 72 h used).
Fig. 2.
IL-6 production by U373 cells treated with IL-1β + TNF-α. U373 astroglia cells were incubated with 0.01–10 ng/ml IL-1β + TNF-α for up to 96 h. Data presented are the mean ± SEM of three independent experiments (a). Using the log (agonist) versus response (3 parameter) model, the EC50 was calculated as 0.266 ± 0.008 ng/ml IL-1β + TNF-α and the maximal possible IL-6 production as 500 ± 15.2 pg/ml (b)
At 72 h, an IL-6 concentration of 34 ± 20 pg/ml was detected in media collected from cells treated with 0.01 ng/ml IL-1β + TNF-α. Higher IL-6 production was identified in cells treated with 0.1, 1 and 10 ng/ml IL-1β + TNF-α, with the concentration of IL-6 in the media reaching 145 ± 16, 384 ± 14 and 493 ± 17 pg/ml, respectively (Fig. 2a). Using the log (agonist) versus response (3 parameter) model, the EC50 was calculated as 0.266 ± 0.008 ng/ml IL-1β + TNF-α and the maximal possible IL-6 production as 500 ± 15.2 pg/ml (Fig. 2b).
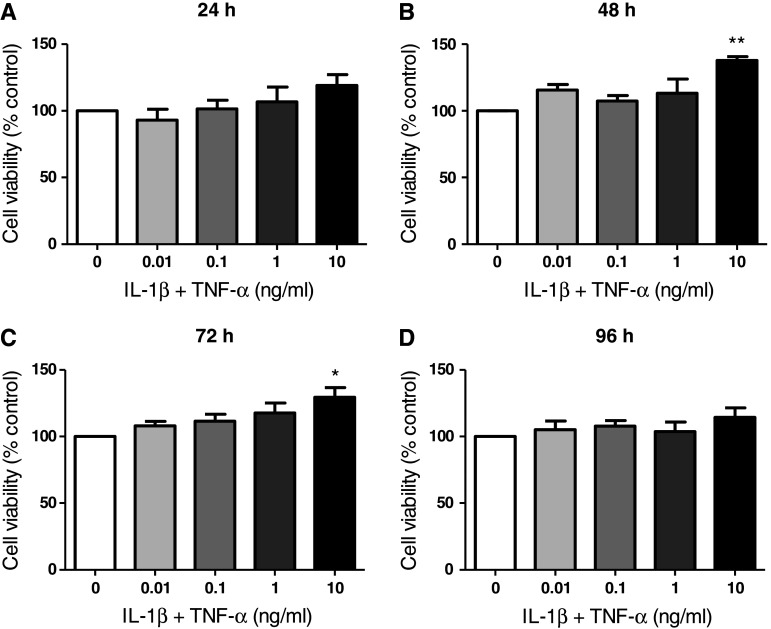
To exclude the possibility that a high concentration of pro-inflammatory activators might lead to cell death, the effect of IL-1β + TNF-α on cell viability was determined by the resazurin (Alamar Blue) assay. No significant reduction in cell viability was observed for concentrations of IL-1β + TNF-α up to 10 ng/ml at all time points up to 96 h (Fig. 3a–d). Importantly, this indicates that the selected concentrations of IL-1β + TNF-α were non-toxic to cells for the duration of the experiment. Interestingly, slight increases in cell viability at increasing concentrations of pro-inflammatory activators were identified. For example, cell viabilities of 138 ± 3 and 130 ± 7 % of control cells were observed following 48 and 72 h treatment with 10 ng/ml IL-1β + TNF-α, respectively (Fig. 3b, c).
Fig. 3.
Cell viability results for U373 cells treated with IL-1β + TNF-α. U373 cells were treated with 0.01, 0.1, 1, 5 or 10 ng/ml IL-1β and TNF-α for 24, 48, 72 or 96 hours. Cell viability was measured by the resazurin reduction assay and data are expressed as percentage of non-activated control cells ± SEM of 3 independent experiments. *p < 0.05 and **p < 0.01 designate a significant difference to the non-activated control (0 ng/ml IL-1β + TNF-α). Significant differences were assessed by one-way ANOVA with Dunnett’s post hoc tests
Effect of IL-1β + TNF-α Treatment of U373 Cells on Intracellular GSH Content
Inflammatory conditions are associated with increased levels of reactive oxygen and nitrogen species (Dedon and Tannenbaum 2004; Winrow et al. 1993). It can therefore be assumed that cells exposed to chronic inflammation would require more GSH in order to defend themselves against oxidant-induced damage.
To investigate the effect of pro-inflammatory activation of U373 cells by IL-1β + TNF-α on their GSH content, intracellular GSH was determined by the Tietze assay after 24, 48, 72 and 96 h treatment (Fig. 4). Although cells contain both GSH and GSSG, it has been calculated previously that GSSG makes up <1 % of total GSH (GSH + GSSG) in both control and cytokine-treated cells (Gavillet et al. 2008). Therefore, total GSH measured here represents predominantly reduced GSH.
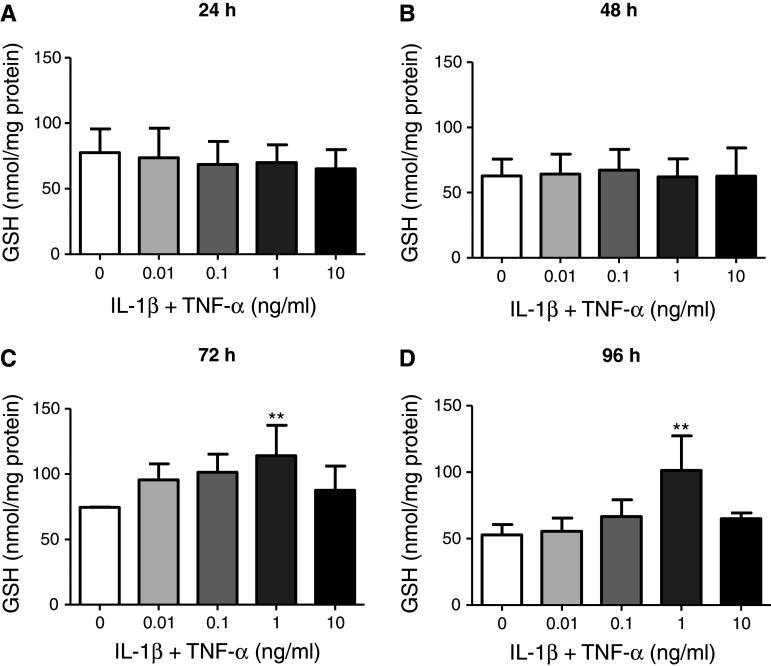
Fig. 4.
Total intracellular GSH in U373 cells after 24-, 48-, 72- and 96-h incubation. U373 cells were treated with 0.01–10 ng/ml IL-1β + TNF-α for 24 (a), 48 (b), 72 (c) or 96 (d) h. Levels of total intracellular GSH were determined by the Tietze assay and data presented are the mean ± SEM of three independent experiments. **p < 0.01 designates a significant difference from the non-activated control (0 ng/ml IL-1β + TNF-α). Significant differences were assessed by one-way ANOVA with Dunnett’s post-hoc tests
No significant changes in intracellular GSH content were observed after 24 or 48 h of treatment with 0.01–10 ng/ml IL-1β + TNF-α (Fig. 4a, b). Interestingly, after 72- and 96-h treatment, GSH content in U373 cells increased dose dependently up to 1 ng/ml IL-1β + TNF-α (to 153 ± 31 and 192 ± 49 % of the non-activated controls, respectively) but showed similar levels of GSH as non-activated cells (113 ± 14 and 121 ± 6 %, respectively) at 10 ng/ml IL-1β + TNF-α (Fig. 4c, d).
Effect of IL-1β + TNF-α Treatment of U373 Cells on Levels of Extracellular GSH and Related Compounds
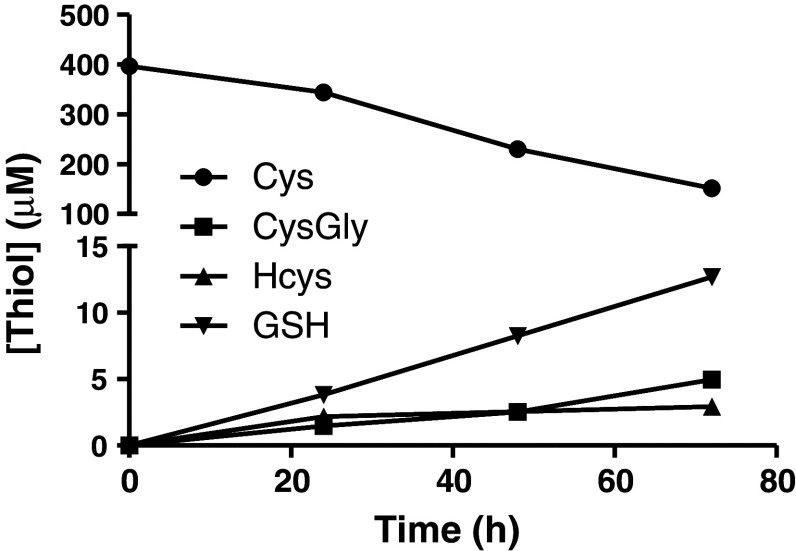
In order to follow cysteine consumption and the release of GSH and related thiols under baseline conditions (from non-activated cells), media was collected from cultured U373 astroglial cells after 24-, 48- and 72-h incubation. The levels of cysteine (added as cystine to DMEM by the manufacturer), cysteinylglycine, homocysteine and GSH changed over time between 0 and 72 h. Fresh media was found to contain 396.6 ± 9.5 μM cysteine, consistent with the manufacturer’s specification of 400 μM. Cysteine concentration decreased to 151.7 ± 10.4 μM after 72-h incubation with U373 cells. As expected, cysteinylglycine, homocysteine or GSH were not detected in fresh media. Cysteinylglycine, homocysteine and GSH accumulated in the media over time, reaching concentrations of 5.0 ± 0.3, 2.9 ± 0.1 and 12.7 ± 1.1 μM, respectively, after 72-h incubation (Fig. 5). It should be noted that the concentration of total cysteine levels in the media after 72 h was still above the physiological plasma levels in humans, which is ~100 μM (Park et al. 2010) suggesting that any responses at this time point are not simply caused by non-physiologically low cysteine levels.
Fig. 5.
Concentrations of total Cys (closed circle), CysGly (closed square), Hcys (closed triangle) and GSH (closed inverted triangle) measured in fresh media (0 h) and media collected from non-activated U373 cells after 24, 48 or 72 h. Fresh media was found to contain 396.6 ± 9.5 μM Cys. Cys concentration decreased to 151.7 ± 10.4 μM after 72-h incubation with U373 cells. CysGly, Hcys and GSH accumulated in the media over time, reaching concentrations of 5.0 ± 0.3, 2.9 ± 0.1 and 12.7 ± 1.1 μM, respectively, after 72 h. Data represent means ± SEM of three independent experiments
In order to investigate how pro-inflammatory activation influences the amount of astrocytic released GSH, and the conversion of GSH into its downstream metabolite cysteinylglycine, the effect of short-term (24 h) and long-term (72 h) activation with 0.01–10 ng/ml IL-1β + TNF-α on levels of GSH and cysteinylglycine in the media were measured. As astrocytes release ~10 % of their intracellular GSH content per hour via the multidrug resistance protein 1 transporter (Dringen and Hamprecht 1997; Minich et al. 2006), it was hypothesized that the inflammation-induced changes in extracellular GSH would mirror the changes in intracellular GSH.
IL-1β + TNF-α treatment had no significant effect on GSH concentration in the media after 24-h incubation (3.8 ± 0.3 μM) (Fig. 6a). However, after 72-h treatment, GSH concentration in media increased dose dependently from 12.1 ± 0.4 μM GSH in non-activated cells to 18 ± 0.6 μM GSH when cells were activated with 1 ng/ml IL-1β + TNF-α, then returned to 12.9 ± 1.6 GSH when activated with 10 ng/ml IL-1β + TNF-α (Fig. 6b).
Neurons rely mainly on extracellular cysteine for GSH synthesis because they lack a means of direct GSH uptake. To provide cysteine to neurons, astrocytes convert GSH to cysteinylglycine via the extracellularly located enzyme γ-GT (Dringen et al. 1999), before cysteinylglycine is degraded to cysteine and glycine on the extracellular surface of the neurons. It is thus likely that extracellular cysteinylglycine levels are more relevant than extracellular GSH levels in terms of cysteine supply to neurons. Therefore, total cysteinylglycine levels were also measured in U373 media after 24- and 72-h treatment with IL-1β + TNF-α. No significant changes in cysteinylglycine release were measured after 24-h incubation (1.5 ± 0.1 μM) (Fig. 7a). However, after 72 h a dose-dependent decrease in extracellular cysteinylglycine was measured. A significant decrease in cysteinylglycine levels was observed with concentrations as low as 0.1 ng/ml IL-1β + TNF-α, leading to maximal reduction (42 ± 2 %) in extracellular cysteinylglycine (2.1 ± 0.1 μM) compared to control cells (3.6 ± 0.2 μM) at 10 ng/ml IL-1β + TNF α (Fig. 7b).
Total cysteine consumption was also monitored by measuring cysteine + cystine in the media (Fig. 8) to ensure that changes in GSH and related thiols (particularly the decreases above 0.1 ng/ml IL 1β + TNF-α) were not caused by inflammation-induced cysteine depletion. While there were some small fluctuations in cysteine levels in response to different concentrations of IL-1β + TNF-α, the changes were not significantly different from non-activated control cells after 24-h (347.0 ± 4.8 μM) or 72-h (153.0 ± 6.1 μM) activation. This observation indicates that the observed changes in levels of GSH and cysteinylglycine were not simply due to depletion of cysteine and cystine in the media.
Effect of IL-1β + TNF-α Treatment of U373 Cells on Extracellular Levels of Homocysteine
Homocysteine, which is released by astrocytes, can be toxic to neurons in vitro at concentrations as low as 0.5 μM (Kruman et al. 2000). Therefore, in addition to investigating changes in the levels of GSH and GSH precursors in the media of activated U373 cells, we were also interested in any activation-induced changes in homocysteine levels. While no significant changes were observed after 24-h incubation with IL-1β + TNF-α (2.2 ± 0.1 μM), after 72-h incubation, a cytokine concentration-dependent increase in homocysteine was observed (Fig. 9). The concentration of homocysteine in the media of cells treated with 10 ng/ml IL-1β + TNF-α for 72 h was significantly elevated to 189 % (6.6 ± 0.9 μM) of the non-activated control (3.5 ± 0.3 μM) (Fig. 9). Therefore, extended activation of U373 cells resulted in an extracellular homocysteine concentration 13 times higher than concentrations previously shown to be toxic to neurons (Kruman et al. 2000).
Discussion
Effect of Pro-inflammatory Activation on Intracellular and Extracellular GSH Levels
In order to effectively protect themselves against oxidative stress, neurons rely on astroglial provision of GSH and its degradation product, cysteinylglycine, which can be further processed extracellularly by neurons into cysteine and glycine (Fig. 1). This study investigated whether pro-inflammatory activation affects this neurosupportive role of astrocytes. Human U373 astroglial cells were activated with IL-1β + TNF-α and both intracellular and extracellular levels of GSH, as well as extracellular levels of the downstream GSH metabolite cysteinylglycine, and the neurotoxic thiol homocysteine were measured.
Whereas there was no change at shorter time points, after 72 h both intracellular and extracellular levels of GSH increased dose dependently up to 1 ng/ml IL-1β + TNF-α, however, were similar to control levels at 10 ng/ml IL-1β + TNF-α. Importantly, the dose response curve of extracellular GSH levels closely mirrors that of intracellular GSH levels. These findings are consistent with the published observation that astrocytes release ~10 % of their intracellular GSH content per hour via the multidrug resistance protein 1 transporter (Dringen and Hamprecht 1997; Minich et al. 2006). This adaptive response, i.e. the increase in GSH in response to higher inflammatory activation, might be explained by oxidative stress-mediated induction of GSH synthesis or the cystine transport system.
The rate of GSH synthesis is controlled largely by the activity of γGCL, the first enzyme required for GSH synthesis, and by the availability of cysteine/cystine. Expression of the catalytic (γGCL-C) and modulatory (γGCL-M) subunits of γGCL and of the Xc-system, which facilitates cystine uptake, are regulated by the redox-sensitive transcription factor, nuclear factor erythroid-2-related factor 2 (Nrf2) (Correa et al. 2011). Nrf2 also regulates the expression of glutathione synthetase (the second enzyme required for synthesis of GSH), glutathione reductase (recycles GSSG to GSH) and glutathione peroxidases (detoxify peroxides using GSH as the reducing agent) (Chan and Kwong 2000; Chanas et al. 2002; Copple et al. 2008; Harvey et al. 2009; Wild et al. 1999). A recent study in human monocytes found that TNF-α induces sustained expression and activation of Nrf2, leading to the upregulation of γGCL-M, amongst other cytoprotective genes (Rushworth et al. 2011). Furthermore, inflammatory activation of cells has been shown to also increase cystine import into cells. This increases the availability of cysteine, the rate-limiting substrate for GSH synthesis. In macrophages, the transport activity for cystine can be potently induced by bacterial lipopolysaccharide (LPS), and enhanced by TNF-α (Sato et al. 1995). Astrocytes were also shown to increase cystine uptake following IL-1β exposure, mediated by increased expression of the Xc-(cystine/glutamate exchange) transporter (Jackman et al. 2010). It has previously been shown that TNF-α increases glutamate release by astrocytes (Bezzi et al. 2001) and that elevated extracellular levels of glutamate inhibit cystine uptake via the Xc-system (Bannai and Kitamura 1980). Therefore, one possible explanation for the lack of increased GSH levels observed in response to the highest concentration of cytokines might be a decreased availability of intracellular cysteine.
It can be theorised that neurons in a highly pro-inflammatory environment (e.g. in the presence of 10 ng/ml IL-1β and TNF-α) would require more GSH substrates released from astrocytes than those in low or non-inflammatory environments (0–1 ng/ml IL-1β and TNF-α). However, our results show that with increasing inflammatory activation astrocytic GSH levels increase and then subside, though the reason for the lack of increase of GSH levels at high activation is unclear.
Effect of Pro-inflammatory Activation on Extracellular Cysteinylglycine Levels
In contrast to GSH, cysteinylglycine levels in the media did not increase at low to medium levels of inflammation, but rather decreased in response to activation with 0.1 ng/ml IL-1β + TNF-α. The fact that enzymatic activity of γ-GT determines extracellular levels of cysteinylglycine, coupled with our observation that increasing cytokine concentrations up to 1 ng/ml had opposite effects on extracellular GSH and cysteinylglycine levels suggests there is a dose-dependent inhibition or reduction in expression of γ-GT in pro-inflammatory conditions. This explanation is supported by the findings of Malaplate-Armand and colleagues (2000) that IL-1β induces a dose-dependent reduction in γ-GT activity in U373 cells treated for 48 or 72 h. Therefore, our results suggest that even low level inflammation may be detrimental to neuronal GSH levels as the adaptive increase in astrocytic GSH might be useless if cysteinylglycine, the key substrate required for neuronal GSH synthesis, decreases.
Effect of Pro-inflammatory Activation on Extracellular Homocysteine Levels
When levels of homocysteine were analysed in media collected from cultures of U373 cells treated with IL-1β + TNF-α for 72 h a dose-dependent increase was observed. Homocysteine is a non-essential amino acid that is derived from the breakdown of dietary-obtained methionine, by a multi-step process involving removal of a methyl group. The three primary fates of homocysteine are reversible methylation back to methionine, transsulfuration to form cystathionine and then cysteine, the rate-limiting substrate for GSH synthesis (Vitvitsky et al. 2006) or export from the cell via neutral amino acid transporters (Tsitsiou et al. 2009). Astrocytes are deficient in cystathionine β-synthase, the enzyme that catalyses the conversion of homocysteine to cystathionine (Kohl and Quay 1979; Robert et al. 2003). Furthermore, it has been shown that exogenous homocysteine does not stimulate total GSH synthesis in rat astrocytes (Jin and Brennan 2008) and that activation of catechol-O-methyltransferase (an enzyme involved in methionine–homocysteine conversion) in astrocytes stimulates homocysteine synthesis and export (Huang et al. 2005). Together, these findings suggest that astrocytes have a reduced capacity for transsulfuration and therefore must export homocysteine and take up cysteine and cystine for maintenance of GSH levels. Most likely, the link between inflammation and homocysteine synthesis and export involves nitric oxide. Activated astrocytes produce large volumes of nitric oxide (Park and Murphy 1994) and nitric oxide inhibits methionine synthetase, the enzyme that converts homocysteine to methionine (Nicolaou et al. 1996). In two human fibroblast cell lines exposed to nitric oxide, the resulting inhibition of homocysteine remethylation increased homocysteine efflux, leading to elevated extracellular levels of homocysteine (Nicolaou et al. 1996). Therefore, nitric oxide-mediated inhibition of methionine synthetase could explain the elevated levels of extracellular homocysteine in cytokine-treated U373 cells in our experiments.
Homocysteine is regarded as a neurotoxin and has been shown to activate neuronal NMDA-type glutamatergic receptors, causing excessive Ca2+ influx, ROS generation, tau phosphorylation and apoptosis at concentrations ranging from 10 to 250 μM (Althausen and Paschen 2000; Ho et al. 2002; Lipton et al. 1997). In another study, extended treatment of primary rat neurons with exogenous homocysteine at concentrations as low as 0.5 μM induced apoptosis (Kruman et al. 2000). Thus, it is possible that inflammation-induced elevated efflux of homocysteine by astrocytes in vivo might contribute to the neurodegeneration observed in chronic, inflammatory and neurodegenerative diseases.
Relevance to AD
Interestingly, the altered extracellular thiol profile observed for chronically activated U373 cells in this study closely reflects the reported changes in thiol levels in AD patients. For example, a recent study by Mandal and colleagues (2012), in which magnetic resonance spectroscopy was used to non-invasively measure brain GSH content, found a significant decrease in GSH level in the brains of AD patients compared to both young and old healthy controls. In another study, AD patients were found to have reduced plasma levels of cysteinylglycine compared to controls (Hernanz et al. 2007). Furthermore, not only have elevated levels of homocysteine been measured in the plasma (McCaddon et al. 2003) and CSF (Popp et al. 2009) of AD patients, hyperhomocysteinemia was suggested as an independent risk factor for AD (Morris 2003; Seshadri 2006).
Overall, our data suggests that chronic exposure to pro-inflammatory cytokines induces changes in astrocytic thiol metabolism that might be potentially harmful to neurons. Although our results cannot be directly extrapolated to an in vivo situation, it is tempting to consider the possibility that chronically activated astrocytes, might show similar changes to those observed here. In particular, decreased astrocytic provision of the neuronal GSH substrate, cysteinylglycine and increased astrocytic release of the neurotoxic compound, homocysteine, could contribute to neuronal toxicity. If indeed inflammation-induced impaired thiol metabolism is a key contributor to the neurodegeneration seen in diseases such as AD, it might be possible to affect disease outcomes by modulation of GSH levels. For example, the use of astroglial-targeted GSH boosters (e.g. Nrf2 activators), direct supplementation with neuronal GSH substrates (e.g. cysteinylglycine and γ-glutamylcysteine) or administration of exogenous antioxidants or anti-inflammatory agents might be explored (Steele et al. 2007; Steele and Robinson 2011).
Acknowledgments
We thank UWS for support through the College Research Grant scheme and Alzheimer’s Australia for their support through the Dementia Research grants program. Megan L. Steele was supported by the Hunter Postgraduate Research Scholarship from Alzheimer’s Australia.
Abbreviations
- ABD-F
4-Flouro-7-aminosulfonylbenzofurazan
- AD
Alzheimer’s disease
- ANOVA
Analysis of variance
- ARE
Antioxidant response element
- CSF
Cerebral spinal fluid
- DMEM
Dulbecco’s modified eagle medium
- DTNB
5,5′-Dithio-bis(2-nitrobenzoic acid)
- EDTA
Ethylenediaminetetraacetic acid
- ELISA
Enzyme-linked immunosorbent sandwich assay
- FBS
Foetal bovine serum
- GCL
Glutamate cysteine ligase
- GSH
Glutathione
- GSSG
Glutathione disulfide
- HPLC
High performance liquid chromatography
- IL-1β
Interleukin-1 beta
- IL-6
Interleukin-6
- Keap1
Kelch like-ECH-associated protein 1
- MRP1
Multidrug resistance protein 1
- NADPH
Reduced nicotinamide adenine dinucleotide phosphate
- NMDA
N-Methyl-D-aspartate
- Nrf2
Nuclear factor erythroid-2-related factor 2
- PD
Parkinson’s disease
- ROS
Reactive oxygen species
- TNB
5-Thio-2-nitrobenzoic acid
- TNF-α
Tumour necrosis factor-alpha
- γ-GT
γ-Glutamyltranspeptidase
- γ-GCL-C
Catalytic glutamate cysteine ligase subunit
- γGCL-M
Modulatory glutamate cysteine ligase subunit
References
- Althausen S, Paschen W (2000) Homocysteine-induced changes in mRNA levels of genes coding for cytoplasmic- and endoplasmic reticulum-resident stress proteins in neuronal cell cultures. Brain Res Mol Brain Res 84:32–40 [DOI] [PubMed] [Google Scholar]
- Aschner M (2000) Neuron-astrocyte interactions: implications for cellular energetics and antioxidant levels. Neurotoxicology 21:1101–1107 [PubMed] [Google Scholar]
- Bannai S (1984) Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta 779:289–306 [DOI] [PubMed] [Google Scholar]
- Bannai S, Kitamura E (1980) Transport interaction of l-cystine and l-glutamate in human diploid fibroblasts in culture. J Biol Chem 255:2372–2376 [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A (2001) CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci 4:702–710 [DOI] [PubMed] [Google Scholar]
- Bolin LM, Zhaung A, Strychkarska-Orczyk I, Nelson E, Huang I, Malit M, Nguyen Q (2005) Differential inflammatory activation of IL-6 (−/−) astrocytes. Cytokine 30:47–55 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Brannan TS, Maker HS, Weiss C, Cohen G (1980) Regional distribution of glutathione peroxidase in the adult rat brain. J Neurochem 35:1013–1014 [DOI] [PubMed] [Google Scholar]
- Buranrat B, Prawan A, Kukongviriyapan V (2008) Optimization of resazurin-based assay for cytotoxicity test in cholangiocarcinoma cells. KKU Res J 8:73–80 [Google Scholar]
- Chan JY, Kwong M (2000) Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim Biophys Acta 1517:19–26 [DOI] [PubMed] [Google Scholar]
- Chanas SA, Jiang Q, McMahon M, McWalter GK, McLellan LI, Elcombe CR, Henderson CJ, Wolf CR, Moffat GJ, Itoh K, Yamamoto M, Hayes JD (2002) Loss of the Nrf2 transcription factor causes a marked reduction in constitutive and inducible expression of the glutathione S-transferase Gsta1, Gsta2, Gstm1, Gstm2, Gstm3 and Gstm4 genes in the livers of male and female mice. Biochem J 365:405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Swanson RA (2003) The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J Neurochem 84:1332–1339 [DOI] [PubMed] [Google Scholar]
- Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP (2005) Glutamate cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J Biol Chem 280:33766–33774 [DOI] [PubMed] [Google Scholar]
- Copple IM, Goldring CE, Kitteringham NR, Park BK (2008) The Nrf2-Keap1 defence pathway: role in protection against drug-induced toxicity. Toxicology 246:24–33 [DOI] [PubMed] [Google Scholar]
- Correa F, Ljunggren E, Mallard C, Nilsson M, Weber SG, Sandberg M (2011) The Nrf2-inducible antioxidant defense in astrocytes can be both up- and down-regulated by activated microglia: involvement of p38 MAPK. Glia 59:785–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedon PC, Tannenbaum SR (2004) Reactive nitrogen species in the chemical biology of inflammation. Arch Biochem Biophys 423:12–22 [DOI] [PubMed] [Google Scholar]
- Dringen R (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62:649–671 [DOI] [PubMed] [Google Scholar]
- Dringen R, Hamprecht B (1997) Involvement of glutathione peroxidase and catalase in the disposal of exogenous hydrogen peroxide by cultured astroglial cells. Brain Res 759:67–75 [DOI] [PubMed] [Google Scholar]
- Dringen R, Hamprecht B (1999) N-Acetylcysteine, but not methionine or 2-oxothiazolidine-4-carboxylate, serves as cysteine donor for the synthesis of glutathione in cultured neurons derived from embryonal rat brain. Neurosci Lett 259:79–82 [DOI] [PubMed] [Google Scholar]
- Dringen R, Pfeiffer B, Hamprecht B (1999) Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci 19:562–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer BE, Raina AK, Perry G, Smith MA (2004) Homocysteine and Alzheimer’s disease: a modifiable risk? Free Radic Biol Med 36:1471–1475 [DOI] [PubMed] [Google Scholar]
- Gavillet M, Allaman I, Magistretti P (2008) Modulation of astrocytic metabolic phenotype by proinflammatory cytokines. Glia 56:975–989 [DOI] [PubMed] [Google Scholar]
- Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, Fujii J, Myers A, Biswal S (2009) Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med 46:443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernanz A, De la Fuente M, Navarro M, Frank A (2007) Plasma aminothiol compounds, but not serum tumor necrosis factor receptor II and soluble receptor for advanced glycation end products, are related to the cognitive impairment in Alzheimer’s disease and mild cognitive impairment patients. NeuroImmunoModulation 14:163–167 [DOI] [PubMed] [Google Scholar]
- Ho PI, Ortiz D, Rogers E, Shea TB (2002) Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. J Neurosci Res 70:694–702 [DOI] [PubMed] [Google Scholar]
- Huang G, Dragan M, Freeman D, Wilson JX (2005) Activation of catechol-O-methyltransferase in astrocytes stimulates homocysteine synthesis and export to neurons. Glia 51:47–55 [DOI] [PubMed] [Google Scholar]
- Jackman NA, Uliasz TF, Hewett JA, Hewett SJ (2010) Regulation of system x(c)(−)activity and expression in astrocytes by interleukin-1beta: implications for hypoxic neuronal injury. Glia 58:1806–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Brennan L (2008) Effects of homocysteine on metabolic pathways in cultured astrocytes. Neurochem Int 52:1410–1415 [DOI] [PubMed] [Google Scholar]
- Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P (2000) Redox state of glutathione in human plasma. Free Radic Biol Med 28:625–635 [DOI] [PubMed] [Google Scholar]
- Kohl RL, Quay WB (1979) Cystathionine synthase in rat brain: regional and time-of-day differences and their metabolic implications. J Neurosci Res 4:189–196 [DOI] [PubMed] [Google Scholar]
- Kranich O, Hamprecht B, Dringen R (1996) Different preferences in the utilization of amino acids for glutathione synthesis in cultured neurons and astroglial cells derived from rat brain. Neurosci Lett 219:211–214 [DOI] [PubMed] [Google Scholar]
- Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, Mattson MP (2000) Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci 20:6920–6926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sun L, Zhang H, Liao Y, Wang D, Zhao B, Zhu Z, Zhao J, Ma A, Han Y, Wang Y, Shi Y, Ye J, Hui R (2003) Elevated plasma homocysteine was associated with hemorrhagic and ischemic stroke, but methylenetetrahydrofolate reductase gene C677T polymorphism was a risk factor for thrombotic stroke: a multicenter case-control study in China. Stroke 34:2085–2090 [DOI] [PubMed] [Google Scholar]
- Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudu PV, Arnelle DR, Stamler JS (1997) Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA 94:5923–5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaplate-Armand C, Gueguen Y, Bertrand P, Ferrari L, Batt AM (2000) U373-MG response to interleukin-1beta-induced oxidative stress. Cell Biol Toxicol 16:155–163 [DOI] [PubMed] [Google Scholar]
- Mandal PK, Tripathi M, Sugunan S (2012) Brain oxidative stress: detection and mapping of anti-oxidant marker ‘glutathione’ in different brain regions of healthy male/female, MCI and Alzheimer patients using non-invasive magnetic resonance spectroscopy. Biochem Biophys Res Commun 417:43–48 [DOI] [PubMed] [Google Scholar]
- McCaddon A, Hudson P, Hill D, Barber J, Lloyd A, Davies G, Regland B (2003) Alzheimer’s disease and total plasma aminothiols. Biol Psychiatry 53:254–260 [DOI] [PubMed] [Google Scholar]
- Meister A (1988) Glutathione metabolism and its selective modification. J Biol Chem 263:17205–17208 [PubMed] [Google Scholar]
- Minich T, Riemer J, Schulz JB, Wielinga P, Wijnholds J, Dringen R (2006) The multidrug resistance protein 1 (Mrp1), but not Mrp5, mediates export of glutathione and glutathione disulfide from brain astrocytes. J Neurochem 97:373–384 [DOI] [PubMed] [Google Scholar]
- Morris MS (2003) Homocysteine and Alzheimer’s disease. Lancet Neurol 2:425–428 [DOI] [PubMed] [Google Scholar]
- Nicolaou A, Kenyon SH, Gibbons JM, Ast T, Gibbons WA (1996) In vitro inactivation of mammalian methionine synthase by nitric oxide. Eur J Clin Invest 26:167–170 [DOI] [PubMed] [Google Scholar]
- Park SK, Murphy S (1994) Duration of expression of inducible nitric oxide synthase in glial cells. J Neurosci Res 39:405–411 [DOI] [PubMed] [Google Scholar]
- Park Y, Ziegler TR, Gletsu-Miller N, Liang Y, Yu T, Accardi CJ, Jones DP (2010) Postprandial cysteine/cystine redox potential in human plasma varies with meal content of sulfur amino acids. J Nutr 140:760–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzik K (2006) Homocysteine as a cardiovascular marker and risk factor. Clin Res Cardiol 95(Suppl 6):VI28–VI33 [DOI] [PubMed] [Google Scholar]
- Popp J, Lewczuk P, Linnebank M, Cvetanovska G, Smulders Y, Kolsch H, Frommann I, Kornhuber J, Maier W, Jessen F (2009) Homocysteine metabolism and cerebrospinal fluid markers for Alzheimer’s disease. J Alzheimers Dis 18:819–828 [DOI] [PubMed] [Google Scholar]
- Robert K, Vialard F, Thiery E, Toyama K, Sinet PM, Janel N, London J (2003) Expression of the cystathionine beta synthase (CBS) gene during mouse development and immunolocalization in adult brain. J Histochem Cytochem 51:363–371 [DOI] [PubMed] [Google Scholar]
- Rushworth SA, Shah S, MacEwan DJ (2011) TNF mediates the sustained activation of Nrf2 in human monocytes. J Immunol 187:702–707 [DOI] [PubMed] [Google Scholar]
- Sagara JI, Miura K, Bannai S (1993) Maintenance of neuronal glutathione by glial cells. J Neurochem 61:1672–1676 [DOI] [PubMed] [Google Scholar]
- Sato H, Fujiwara K, Sagara J, Bannai S (1995) Induction of cystine transport activity in mouse peritoneal macrophages by bacterial lipopolysaccharide. Biochem J 310(Pt 2):547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S (2006) Elevated plasma homocysteine levels: risk factor or risk marker for the development of dementia and Alzheimer’s disease? J Alzheimers Dis 9:393–398 [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA (2002) Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 346:476–483 [DOI] [PubMed] [Google Scholar]
- Shanker G, Aschner M (2001) Identification and characterization of uptake systems for cystine and cysteine in cultured astrocytes and neurons: evidence for methylmercury-targeted disruption of astrocyte transport. J Neurosci Res 66:998–1002 [DOI] [PubMed] [Google Scholar]
- Shanker G, Allen JW, Mutkus LA, Aschner M (2001) The uptake of cysteine in cultured primary astrocytes and neurons. Brain Res 902:156–163 [DOI] [PubMed] [Google Scholar]
- Steele ML, Robinson SR (2011) Reactive astrocytes give neurons less support: implications for Alzheimer’s disease. Neurobiol Aging 33: 423.e421–413 [DOI] [PubMed] [Google Scholar]
- Steele M, Stuchbury G, Munch G (2007) The molecular basis of the prevention of Alzheimer’s disease through healthy nutrition. Exp Gerontol 42:28–36 [DOI] [PubMed] [Google Scholar]
- Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522 [DOI] [PubMed] [Google Scholar]
- Tsitsiou E, Sibley CP, D’Souza SW, Catanescu O, Jacobsen DW, Glazier JD (2009) Homocysteine transport by systems L, A and y + L across the microvillous plasma membrane of human placenta. J Physiol 587:4001–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitvitsky V, Thomas M, Ghorpade A, Gendelman HE, Banerjee R (2006) A functional transsulfuration pathway in the brain links to glutathione homeostasis. J Biol Chem 281:35785–35793 [DOI] [PubMed] [Google Scholar]
- Wild AC, Moinova HR, Mulcahy RT (1999) Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem 274:33627–33636 [DOI] [PubMed] [Google Scholar]
- Winrow VR, Winyard PG, Morris CJ, Blake DR (1993) Free radicals in inflammation: second messengers and mediators of tissue destruction. Br Med Bull 49:506–522 [DOI] [PubMed] [Google Scholar]
- Yoshiba-Suzuki S, Sagara J, Bannai S, Makino N (2011) The dynamics of cysteine, glutathione and their disulphides in astrocyte culture medium. J Biochem 150:95–102 [DOI] [PubMed] [Google Scholar]