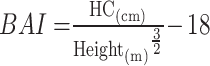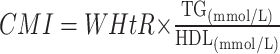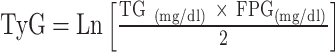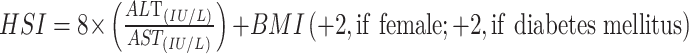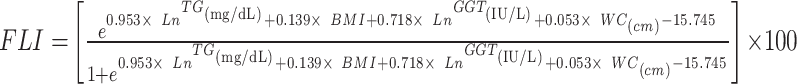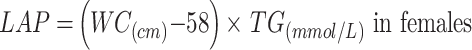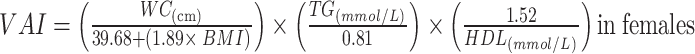Abstract
Purpose
The aim of this study was to comprehensively compare the predictive effect of 10 body fat indexes on MAFLD in different sex, age and BMI subgroups.
Patients and Methods
A total of 5403 physical examination data were included and divided into the MAFLD group (N=2632) and non-MAFLD group (N=2771). The differences and correlation of 10 promising indicators between the two groups were compared, including fatty liver index (FLI), hepatic steatosis index (HSI), lipid accumulation product (LAP), visceral fat index (VAI), cardiometabolic index (CMI), body adiposity index (BAI), and triglyceride-glucose index (TyG), waist circumference index (WC), body mass index (BMI), waist to height ratio (WHtR). Logistic regression was used to analyze the risk of MAFLD under different adjustment conditions. The operating characteristic curve of different genders, BMI levels and age subgroups was plotted.
Results
Male gender, smoking, alcohol drinking, and higher age are risk factors for MAFLD. In addition to BAI, the other 9 indicators had a high correlation with MAFLD, the area under the curve (AUC) value was >0.7, and the prediction effect was better in females, BMI<24 kg/m2, age <35 years subgroup, among which FLI (AUC: 0.912, 95% CI: 0.905–0.920), LAP (AUC: 0.894, 95% CI: 0.8866–0.903), and HSI (AUC: 0.881, 95% CI: 0.872–0.890) have better prediction effects.
Conclusion
Our study confirmed the accuracy of body fat-related indexes in predicting MAFLD in people of different sexes, ages, and BMI levels. Among them, FLI, LAP and HSI have high predictive value and can be utilized as simple and cost-effective tools for screening MAFLD in clinical settings.
Keywords: metabolic dysfunction-associated fatty liver disease, body fat index, metabolic index, cross-sectional study
Introduction
In 2022, an international panel of experts redefined non-alcoholic fatty liver disease (NAFLD) as metabolic dysfunction-associated fatty liver disease (MAFLD).1 The prevalence of MAFLD is approximately 25% globally, affecting an estimated 173 to 310 million individuals in China. According to the latest data on the global burden of liver disease in 2019, the prevalence of MAFLD 25% in Europe and Japan. In China, the prevalence increased from 17% in 2003 to 22.4% in 2012.2,3 In the United States, the prevalence of MAFLD has increased from 34.4% to 38.1% over the past decade.4 Recently, the prevalence of MAFLD has increased annually and is trending younger. This condition can progress to hepatitis, liver cirrhosis, and liver cancer, posing a serious threat to health,5 It has become a leading cause of liver disease burden in many countries and regions worldwide, representing a significant global public health challenge in the 21st century.6,7 Therefore, the early and timely detection and treatment of MAFLD is crucial for delaying the progression of the disease.
Currently, liver tissue biopsy is considered the gold standard for diagnosing MAFLD. However, its clinical use is limited due to its invasive nature.8 Magnetic resonance imaging (MRI) and computed tomography (CT) are expensive non-invasive diagnostic methods, while the MAFLD Fibrosis Score and the Fibrosis 4 Index have limitations in predictive performance and risk stratification,9 and the accuracy of diagnosis was affected by age and transaminase levels.10 Although the commonly used ultrasound technique is safe, it is not highly effective for identifying subjects with a fat infiltration degree of less than 30%. Therefore, the rapid identification and diagnosis of MAFLD in a convenient and efficient manner hold significant health-economic importance for preventing and delaying the progression of MAFLD, as well as the occurrence of related complications.
With the increasing trend of obesity and type 2 diabetes, More and more studies have confirmed that the occurrence of MAFLD is related to fat infiltration and metabolic processes in liver tissue, as well as insulin resistance (IR). The combination of lipid accumulation and metabolites, as well as the extent of lipid accumulation, is clinically significant for the diagnosis and evaluation of MAFLD.11–13 Other studies have shown an association between muscular steatosis, as measured by visual muscle mass maps in computed tomography, and NAFLD severity,14,15 and circulating lipid metabolites may also play a role in exacerbating muscle metabolic disorders.16,17 Fatty Liver Index (FLI), Hepatic Steatosis Index (HSI), Lipid Accumulation Product (LAP), Visceral Fat Index (VAI), Cardiometabolic Index (CMI), Body Adiposity Index (BAI), and Triglyceride-Glucose (TyG) Index are novel body fat indexes that more accurately reflect the distribution of body fat, the extent of lipid accumulation, and associated metabolic abnormalities.18–20 Studies have demonstrated that these indexes are closely related to lipid metabolism disorders, IR and various cardiovascular risk factors, thereby verifying their utility in screening for metabolic diseases.21–23 However, due to regional differences in the epidemiology of MAFLD, the predictive effectiveness of various indexes across different populations varies. Additionally, there are few studies examining the comprehensive use of these indexes for predicting MAFLD in China. Therefore, further comprehensive evaluations and comparisons of their predictive value for MAFLD in the general population are necessary.
Therefore, the aim of this study is to analyze the correlation between LAP, VAI, CMI, TyG, BAI, HSI, FLI, BMI, WC, WHtR, and MAFLD. Additionally, it seeks to evaluate and compare their predictive effects on the risk of MAFLD across different subgroups, providing new insights into the prevention and diagnosis of MAFLD.
Materials and Methods
Study Design and Participants
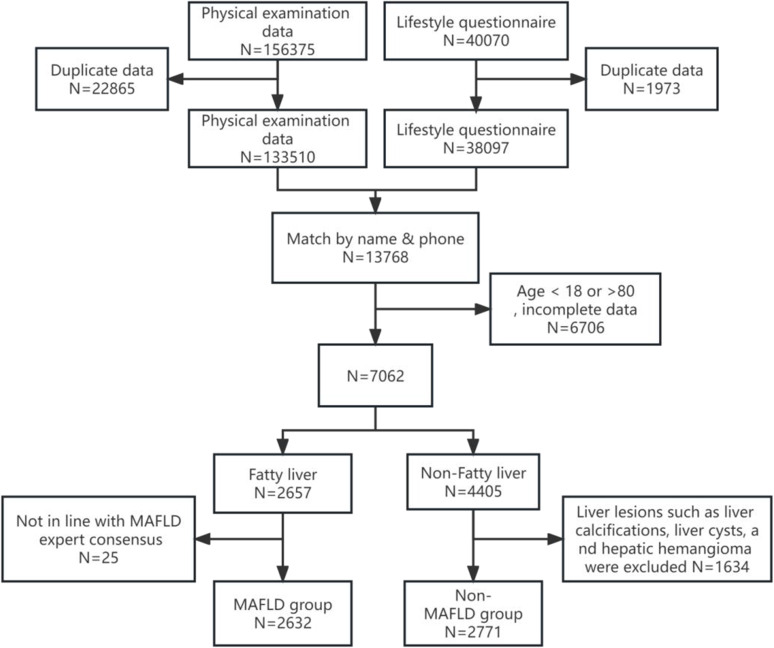
This is a retrospective cross-sectional study conducted from January 2021 to June 2023 at the First Affiliated Hospital of the Army Medical University in China. The study extracted a total of 156,375 health records from the physical examination database. The inclusion criteria included an age range of 18 to 80 years, complete basic physical examination information, lifestyle questionnaires, imaging data, laboratory examination indicators, and imaging results indicated the presence of hepatic steatosis. Exclusion criteria include pregnant or lactating women, the physical examination revealed hepatic calcification, as well as severe hepatic and renal insufficiency (Figure 1). According to the diagnostic criteria for MAFLD, participants were classified into the MAFLD group and the non-MAFLD group. The differences and correlations among LAP, VAI, CMI, TyG, BAI, HSI, FLI, BMI, WC, WHtR, and other biochemical indices were compared between the two groups.
Figure 1.
Flow chart of the study subjects.
Sample Size Calculation
The parameters for the sample size calculation were established as follows: a confidence coefficient (Z) of 1.96, based on previous research findings; an expected incidence (P) of 0.25; an allowable error (d) of 0.05; and a confidence interval of 95%. The calculated usable sample size is 288 individuals. After accounting for a 10% data loss, the minimum required sample size is 317 individuals.
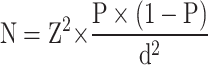 |
Data Collection and Definition
The physical examinations were conducted in the morning while the subjects were fasting. All assessments were carried out by professionally trained medical staff. The participants were instructed not to wear shoes or heavy clothing, and their height, weight, and body mass index (BMI) were measured using the Natural Station Stereoscopic Examination Instrument (Model: Intelligent Health Examination Integrated Machine HW-900A, Henan Lejia Electronic Technology Co., Ltd, China). Medical staff used a soft ruler to manually measure waist circumference (WC), waist-to-height ratio (WHtR), and hip circumference (HC). At rest, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using an electronic sphygmomanometer (Instrument name and model: Medical Automatic Electronic Blood Pressure Monitor HBP-9021, Omron Electronics LLC, Japan). Blood samples were collected from the median cubital vein. The chemical analysis was performed using the BECKMAN COULTER Chemistry Analyzer AU5800 (Beckman Coulter, Inc., USA). The following parameters were analyzed: alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), total cholesterol (TC), fasting plasma glucose (FPG), total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), total bile acids (TBA), uric acid (UA), albumin (Alb), total protein (TP), and globulin (G). The auto hematology analyzer was utilized to measure hemoglobin (HGB), the number of white blood cells (WBC), platelet count (PLT), and other relevant indicators (Instrument name and model: Auto Hematology Analyzer BC-6800, Shenzhen Mindray Bio-Medical Electronics Co., Ltd, China). Basic demographic information, including gender, age, smoking habits, and alcohol drinking history was gathered through lifestyle questionnaires.
Calculation of Body Fat-Related Indexes
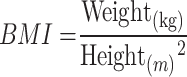 |
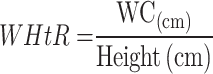 |
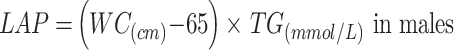 |
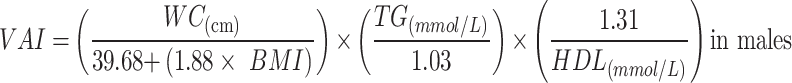 |
To avoid 0 or negative LAP values, the waist circumference ≤ 58 cm is adjusted to 59 cm for women and 66 cm for men ≤ 65 cm. The unit conversions are as follows: FPG: 1 mmol/L=18 mg/dl, TG: 1 mmol/L=88.5mg/dl.
MAFLD Definition
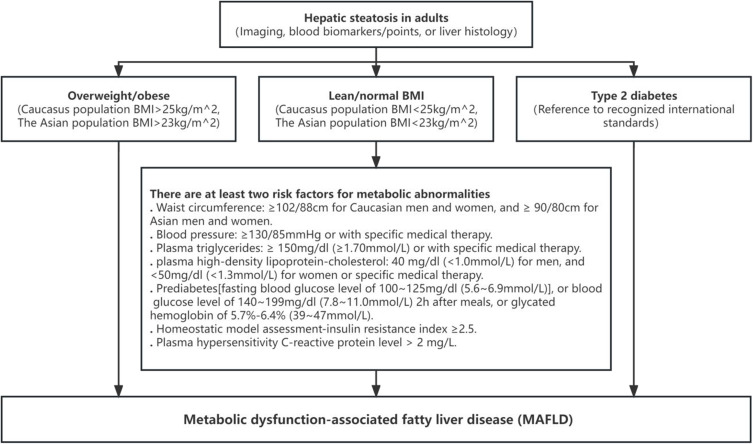
Refer to the International Expert Consensus on the New Definition of Metabolic Dysfunction-Associated Fatty Liver Disease, the diagnostic criteria for MAFLD in this study are based on imaging and the presence of at least one of the following three conditions: overweight/obesity, type 2 diabetes, or metabolic dysfunction1 (Figure 2).
Figure 2.
MAFLD definition.
Statistical Analysis
Continuous variables were described using the mean ± standard deviation (X ± S) or median (M) with interquartile range (P25–P75), depending on their distribution. The t-test or Mann–Whitney U-test was employed for group comparisons. Categorical variables were described using frequency and percentage, and the chi-square (χ²) test was employed for between-group comparisons. According to the quartile levels of LAP, VAI, CMI, TyG, BAI, HSI, FLI, BMI, WC, and WHtR, Cramer’s V coefficient was employed to evaluate the strength of the correlation. The correlation intensity was categorized as follows: low intensity correlation (0.1 < Cramer’s V < 0.3), moderate intensity correlation (0.3 ≤ Cramer’s V < 0.5), and high intensity correlation (Cramer’s V ≥ 0.5). The correlation analysis of each indicator was conducted using the Spearman test. Logistic regression was employed to analyze the odds ratio (OR) and the 95% confidence interval (CI) for the risk of MAFLD under various adjustment conditions. The specific model designs were as follows: Model 1 did not adjust for any confounders, using the first group of each body fat index as the reference to measure the OR for the risk of MAFLD at different levels. Model 2 adjusted for gender (female assignment: 0, male assignment: 1), age, alcohol drinking, and smoking status. Model 3 adjusted for SBP, DBP, ALT, AST, GGT, ALP, TG, HDL, LDL, TC, FPG, UA, ALb, TP, HGB, WBC, PLT and other factors were further corrected. The receiver operating characteristic (ROC) curve was plotted to predict the occurrence of MAFLD across different genders, BMI levels, and age groups. The area under the ROC curve (AUC), sensitivity, specificity, Youden index, and corresponding cut-off values were calculated.
All data were statistically analyzed using SPSS version 27.0. A difference was considered statistically significant at P < 0.05.
Results
Subjects Characteristics
A total of 5403 participants were included in the study, comprising 3029 males (56.1%) with an average age of 40.86 ± 11.02 years, and 2374 females (43.9%) with an average age of 39.09 ± 11.83 years. Among these participants, 2632 were classified in the MAFLD group, with an average age of 43.02 ± 11.03 years. The non-MAFLD group comprised 2771 participants with a mean age of 37.09 ± 11 years. Significant differences were observed in gender, alcohol drinking history, and smoking history. Compared to the non-MAFLD group, the MAFLD group exhibited higher levels of age, SBP, DBP, GGT, ALP, TG, LDL-C, TC, FPG, TBIL, IBIL, UA, ALb, TP, HGB, WBC, PLT, ALT, AST, TBA, BMI, WC, WHtR, HSI, TyG, BAI, LAP, VAI, CMI, FLI, etc, and the HDL-C level was statistically significant (p < 0.05). There was no significant difference in DBIL and G levels between the two groups (Table 1).
Table 1.
Basic Characteristics of Participants According to the MAFLD and Non-MAFLD Group
| Characteristics | Total | Non-MAFLD Group | MAFLD Group | t/z/ χ2 | P-value |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 2374 (43.9%) | 1716 (61.9%) | 658 (25.0%) | 747.253 | <0.001*** |
| Male | 3029 (56.1%) | 1055 (38.1%) | 1974 (75.0%) | ||
| Alcohol drinking | |||||
| No | 2966 (54.9%) | 1824 (65.8%) | 1142 (43.4%) | 274.362 | <0.001*** |
| Yes | 2437 (45.1%) | 947 (34.2%) | 1490 (56.6%) | ||
| Smoking | |||||
| No | 3860 (71.4%) | 2254 (81.3%) | 1606 (61.0%) | 273.245 | <0.001*** |
| Yes | 1543 (28.6%) | 517 (18.7%) | 1026 (39.0%) | ||
| Age | 39.98±11.41 | 37.09±11.00 | 43.02±11.03 | −19.762 | <0.001*** |
| SBP (mmHg) | 123.33±16.54 | 117.36±14.34 | 129.61±16.37 | −29.189 | <0.001*** |
| DBP (mmHg) | 77.38±11.97 | 72.94±10.29 | 82.05±11.84 | −30.132 | <0.001*** |
| GGT (IU/L) | 38.28±50.38 | 24.22±37.30 | 53.09±57.62 | −21.735 | <0.001*** |
| ALP (IU/L) | 74.23±22.40 | 68.37±21.81 | 80.39±21.35 | −20.452 | <0.001*** |
| TG (mmol/L) | 1.79±1.57 | 1.18±0.91 | 2.44±1.83 | −31.841 | <0.001*** |
| LDL-C (mmol/L) | 3.14±0.71 | 2.93±0.67 | 3.35±0.70 | −22.474 | <0.001*** |
| HDL-C (mmol/L) | 1.29±0.30 | 1.41±0.30 | 1.17±0.25 | 32.056 | <0.001*** |
| TC (mmol/L) | 5.04±0.99 | 4.82±0.91 | 5.28±1.01 | −17.901 | <0.001*** |
| FPG (mmol/L) | 5.78±1.37 | 5.41±0.73 | 6.16±1.74 | −20.369 | <0.001*** |
| TBIL (umol/L) | 14.52±5.59 | 14.23±5.83 | 14.79±5.34 | −3.157 | 0.002 |
| DBIL (umol/L) | 2.64±1.10 | 2.62±1.16 | 2.66±1.03 | −1.161 | 0.246 |
| IBIL (umol/L) | 11.88±4.75 | 11.61±4.90 | 12.13±4.59 | −3.441 | 0.001*** |
| UA (umol/L) | 372.91±101.68 | 330.45±84.65 | 417.62±98.93 | −34.711 | <0.001*** |
| Alb (g/L) | 44.78±2.42 | 44.65±2.37 | 44.92±2.47 | −3.998 | <0.001*** |
| TP (g/L) | 74.64±3.87 | 74.53±3.86 | 74.76±3.88 | −2.140 | 0.032 |
| G (g/L) | 29.86±3.21 | 29.88±3.21 | 29.84±3.21 | 0.447 | 0.655 |
| HGB (g/L) | 148.16±16.29 | 142.4±15.93 | 154.23±14.33 | −28.724 | <0.001*** |
| WBC (10^9/L) | 5.99±1.58 | 5.66±1.51 | 6.34±1.58 | −16.193 | <0.001*** |
| PLT(10^9/L) | 222.32±55.49 | 220.32±54.72 | 224.42±56.22 | −2.718 | 0.007 |
| ALT (IU/L) | 22.80 (14.90~36.10) | 19.10 (13.40~28.70) | 33.00 (24.20~50.60) | −39.953 | <0.001*** |
| AST (IU/L) | 22.40 (18.80 ~28.20) | 21.70 (18.5~26.60) | 25.70 (21.4~32.70) | −27.916 | <0.001*** |
| TBA (mmol/L) | 2.90 (1.90~4.50) | 2.70 (1.80~4.40) | 3.10 (2.10~4.50) | −3.677 | <0.001*** |
| BMI | 24.82±3.72 | 22.6±2.88 | 27.15±3.01 | −56.799 | <0.001*** |
| WC (cm) | 83.58±10.94 | 76.79±8.80 | 90.72±8.06 | −60.750 | <0.001*** |
| WHtR | 0.51±0.06 | 0.47±0.05 | 0.55±0.05 | −57.116 | <0.001*** |
| HSI | 34.52±5.89 | 30.9±4.30 | 38.34±4.81 | −59.863 | <0.001*** |
| TyG | 8.77±0.71 | 8.38±0.54 | 9.19±0.64 | −50.361 | <0.001*** |
| BAI | 28.12±3.56 | 27.35±3.39 | 28.94±3.56 | −16.846 | <0.001*** |
| LAP | 29.97 (13.64~56.1) | 17.28 (9.36~31.32) | 55.89 (36.48~90.24) | −50.175 | <0.001*** |
| VAI | 1.60 (0.98~2.65) | 1.14 (0.79~1.82) | 2.54 (1.71~3.92) | −42.194 | <0.001*** |
| CMI | 0.56 (0.30~1.01) | 0.38 (0.24~0.60) | 1.01 (0.67~1.62) | −46.909 | <0.001*** |
| FLI | 30.66 (8.28~61.99) | 13.74 (5.19~33.93) | 64.05 (43.13~80.63) | −52.452 | <0.001*** |
Notes: ***P<0.001. Categorical variables are described in N(%), Continuous variables are described by X±S or M (P25~P75) according to whether they are normally distributed.
Abbreviations: SBP, Systolic blood pressure; DBP, diastolic blood pressure; GGT, gamma glutamyl transferase; ALP, alkaline phosphatase; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol; FPG, fasting plasma glucose; TBIL, total bilirubin; DBIL, direct bilirubin; IBIL, indirect bilirubin; UA, uric acid; Alb, albumin; TP, total protein; G, globulin; HGB, hemoglobin; WBC, white blood cells; PLT, platelet count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBA, total bile acids; BMI, body mass index; WC, waist circumference index; WHtR, waist to height ratio; HSI, hepatic steatosis index; TyG, triglyceride-glucose index; BAI, body adiposity index; LAP, accumulation product; VAI, visceral fat index; CMI, lipid cardiometabolic index; FLI, fatty liver index.
Correlation Between Different Body Fat Index Levels and MAFLD
Subjects were categorized into four groups based on the quartiles of LAP, VAI, CMI, TyG, BAI, HSI, FLI, BMI, WC, and WHtR. The prevalence of MAFLD increases with the subgroup level of each index. All indices, except for BAI (Cramer’s V: BAI = 0.201), had Cramer’s V coefficients greater than 0.5 for MAFLD (Cramer’s V: FLI = 0.702; LAP = 0.675; HSI = 0.652), indicating a strong correlation (Table S1).
Univariate and Multivariate Logistic Regression Analysis
Univariate logistic regression analysis indicated that male gender, alcohol drinking, smoking, older age, and various body fat indices (including LAP, VAI, CMI, TyG, BAI, HSI, FLI, BMI, WC, and WHtR), SBP, DBP, GGT, and ALP were all identified as risk factors for MAFLD. HDL-C was found to be a protective factor. The adjustment results of the multi-factor regression model were comparable to those of the single-factor model. As the model was gradually adjusted, the effect values decreased; however, the correlation strength between FLI (OR: 118.52, 95% CI: 57.36–244.93), LAP (OR: 36.53, 95% CI: 21.00–63.57), HSI (OR: 40.81, 95% CI: 25.39–65.60), and MAFLD remained significant. The positive predictive value, negative predictive value, sensitivity, and consistency rate for each index in the model exceeded 80% (Tables 2 and 3, Tables S2 and S3).
Table 2.
Univariate Logistic Regression Analysis of the Risk of Developing MAFLD
| Characteristics | B | SE | Wald χ2 | OR (95% CI) | P-value |
|---|---|---|---|---|---|
| Gender | 1.585 | 0.060 | 706.34 | 4.88 (4.34–5.49) | <0.001*** |
| Age | 0.048 | 0.003 | 344.24 | 1.05 (1.04–1.06) | <0.001*** |
| Alcohol drinking | 0.921 | 0.056 | 269.48 | 2.51 (2.25–2.81) | <0.001*** |
| Smoking | 1.024 | 0.063 | 263.95 | 2.79 (2.46–3.15) | <0.001*** |
| LAP | 0.061 | 0.002 | 1132.88 | 1.06 (1.06–1.07) | <0.001*** |
| VAI | 0.888 | 0.032 | 767.15 | 2.43 (2.28–2.59) | <0.001*** |
| CMI | 2.720 | 0.090 | 921.43 | 15.18 (12.74–18.09) | <0.001*** |
| TyG | 2.487 | 0.070 | 1245.73 | 12.03 (10.48–13.81) | <0.001*** |
| BAI | 0.133 | 0.008 | 255.25 | 1.14 (1.12–1.16) | <0.001*** |
| HSI | 0.368 | 0.010 | 1381.54 | 1.45 (1.42–1.47) | <0.001*** |
| FLI | 0.075 | 0.002 | 1598.49 | 1.08 (1.07–1.08) | <0.001*** |
| BMI | 0.553 | 0.015 | 1332.50 | 1.74 (1.69–1.79) | <0.001*** |
| WC (cm) | 0.193 | 0.005 | 1386.43 | 1.21 (1.20–1.23) | <0.001*** |
| WHtR | 1.478 | 0.038 | 1490.75 | 4.39 (4.07–4.73) | <0.001*** |
| SBP (mmHg) | 0.055 | 0.002 | 637.45 | 1.06 (1.05–1.06) | <0.001*** |
| DBP (mmHg) | 0.077 | 0.003 | 672.06 | 1.08 (1.07–1.09) | <0.001*** |
| ALT (IU/L) | 0.064 | 0.002 | 727.43 | 1.07 (1.06–1.07) | <0.001*** |
| AST (IU/L) | 0.072 | 0.004 | 355.06 | 1.07 (1.07–1.08) | <0.001*** |
| GGT (IU/L) | 0.035 | 0.002 | 513.22 | 1.04 (1.03–1.04) | <0.001*** |
| ALP (IU/L) | 0.028 | 0.001 | 362.64 | 1.03 (1.03–1.03) | <0.001*** |
| TG (mmol/L) | 1.344 | 0.046 | 853.24 | 3.84 (3.51–4.20) | <0.001*** |
| LDL (mmol/L) | 0.911 | 0.044 | 421.74 | 2.49 (2.28–2.71) | <0.001*** |
| HDL (mmol/L) | −3.313 | 0.122 | 736.36 | 0.04 (0.03–0.05) | <0.001*** |
| TC (mmol/L) | 0.521 | 0.031 | 285.78 | 1.68 (1.59–1.79) | <0.001*** |
| FPG (mmol/L) | 0.984 | 0.050 | 392.02 | 2.67 (2.43–2.95) | <0.001*** |
| TBIL (umol/L) | 0.018 | 0.006 | 9.88 | 1.02 (1.01–1.03) | 0.002** |
| DBIL (umol/L) | 0.034 | 0.030 | 1.33 | 1.04 (0.98–1.10) | 0.249 |
| IBIL (umol/L) | 0.023 | 0.007 | 11.72 | 1.02 (1.01–1.04) | 0.001** |
| TBA (mmol/L) | 0.021 | 0.014 | 2.20 | 1.02 (0.99–1.05) | 0.138 |
| UA (umol/L) | 0.011 | 0 | 829.27 | 1.01 (1.01–1.01) | <0.001*** |
| Alb (g/L) | 0.045 | 0.011 | 15.90 | 1.05 (1.02–1.07) | <0.001*** |
| TP (g/L) | 0.015 | 0.007 | 4.58 | 1.02 (1.00–1.03) | 0.032* |
| G (g/L) | −0.004 | 0.008 | 0.20 | 1.00 (0.98–1.01) | 0.655 |
| HGB (g/L) | 0.052 | 0.002 | 638.02 | 1.05 (1.05–1.06) | <0.001*** |
| WBC (10^9/L) | 0.303 | 0.020 | 240.91 | 1.35 (1.30–1.41) | <0.001*** |
| PLT (10^9/L) | 0.001 | 0 | 7.36 | 1.00 (1.00–1.00) | 0.007** |
Notes: Assignment of categorical variables, Gender (Female: 0, Male: 1); Drinking (No: 0, Yes: 1); Smoking (No: 0, Yes: 1). WHtR was used as a continuity variable according to the grouping (WHtR1-WHtR4). *P<0.05, **P<0.01,***P<0.001.
Abbreviations: SBP, Systolic blood pressure; DBP, diastolic blood pressure; GGT, gamma glutamyl transferase; ALP, alkaline phosphatase; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol; FPG, fasting plasma glucose; TBIL, total bilirubin; DBIL, direct bilirubin; IBIL, indirect bilirubin; UA, uric acid; Alb, albumin; TP, total protein; G, globulin; HGB, hemoglobin; WBC, white blood cells; PLT, platelet count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBA, total bile acids; BMI, body mass index; WC, waist circumference index; WHtR, waist to height ratio; HSI, hepatic steatosis index; TyG, triglyceride-glucose index; BAI, body adiposity index; LAP, accumulation product; VAI, visceral fat index; CMI, lipid cardiometabolic index; FLI, fatty liver index.
Table 3.
Logistic Regression Analysis of MAFLD Risk for Each Index in Model 3
| Groups | OR (95% CI) | P-value | Specificity | Sensitivity | Consistency Rate | PPV | NPV |
|---|---|---|---|---|---|---|---|
| LAP(1) | 80.1% | 85.8% | 83.0% | 82.3% | 83.9% | ||
| LAP(2) | 6.18 (3.99–9.58) | <0.001*** | |||||
| LAP(3) | 17.85 (11.28–28.27) | <0.001*** | |||||
| LAP(4) | 36.53 (21.00–63.57) | <0.001*** | |||||
| VAI(1) | 79.9% | 83.9% | 82.0% | 81.9% | 82.2% | ||
| VAI(2) | 1.93 (1.42–2.63) | <0.001*** | |||||
| VAI(3) | 3.69 (2.57–5.30) | <0.001*** | |||||
| VAI(4) | 4.92 (2.95–8.22) | <0.001*** | |||||
| CMI(1) | 81.0% | 86.7% | 83.9% | 83.1% | 84.9% | ||
| CMI(2) | 3.87 (2.63–5.72) | <0.001*** | |||||
| CMI(3) | 9.76 (6.22–15.33) | <0.001*** | |||||
| CMI(4) | 14.96 (8.30–26.94) | <0.001*** | |||||
| TyG(1) | 78.8% | 85.4% | 82.2% | 81.3% | 83.3% | ||
| TyG(2) | 1.83 (1.33–2.50) | <0.001*** | |||||
| TyG(3) | 3.09 (2.16–4.42) | <0.001*** | |||||
| TyG(4) | 2.86 (1.70–4.82) | <0.001*** | |||||
| BAI(1) | 79.8% | 86.9% | 83.5% | 82.3% | 84.9% | ||
| BAI(2) | 1.91 (1.47–2.47) | <0.001*** | |||||
| BAI(3) | 2.11 (1.61–2.76) | <0.001*** | |||||
| BAI(4) | 4.56 (3.37–6.16) | <0.001*** | |||||
| HSI(1) | 80.2% | 85.9% | 83.1% | 82.4% | 84.0% | ||
| HSI(2) | 5.13 (3.51–7.49) | <0.001*** | |||||
| HSI(3) | 16.85 (11.38–24.94) | <0.001*** | |||||
| HSI(4) | 40.81 (25.39–65.60) | <0.001*** | |||||
| FLI(1) | 79.9% | 83.9% | 82.0% | 81.9% | 82.2% | ||
| FLI(2) | 13.06 (6.93–24.61) | <0.001*** | |||||
| FLI(3) | 50.77 (26.34–97.85) | <0.001*** | |||||
| FLI(4) | 118.52 (57.36–244.93) | <0.001*** | |||||
| BMI(1) | 81.0% | 86.7% | 83.9% | 83.1% | 84.9% | ||
| BMI(2) | 3.43 (2.36–4.99) | <0.001*** | |||||
| BMI(3) | 8.12 (5.58–11.81) | <0.001*** | |||||
| BMI(4) | 18.87 (12.60–28.28) | <0.001*** | |||||
| WC(1) | 78.8% | 85.4% | 82.2% | 81.3% | 83.3% | ||
| WC(2) | 4.89 (3.13–7.65) | <0.001*** | |||||
| WC(3) | 11.27 (7.10–17.87) | <0.001*** | |||||
| WC(4) | 24.42 (15.06–39.61) | <0.001*** | |||||
| WHtR(1) | 79.8% | 86.9% | 83.5% | 82.3% | 84.9% | ||
| WHtR(2) | 4.59 (3.08–6.83) | <0.001*** | |||||
| WHtR(3) | 10.26 (6.86–15.33) | <0.001*** | |||||
| WHtR(4) | 19.61 (12.86–29.91) | <0.001*** |
Notes: ***P<0.001. Corrected for gender (female assignment: 0, male assignment: 1), age, Alcohol drinking, smoking, SBP, DBP, ALT, AST, GGT, ALP, TG, HDL, LDL, TC, FPG, UA, ALb, TP, HGB, WBC, PLT factors.
Abbreviations: LAP, Lipid accumulation product; VAI, visceral fat index; CMI, cardiometabolic index; TyG, triglyceride-glucose index; BAI, body adiposity index; HSI, hepatic steatosis index; FLI, fatty liver index; BMI, body mass index; WC, waist circumference.
Effect of Body Fat Index on the Risk of MAFLD Stratified by Subgroups
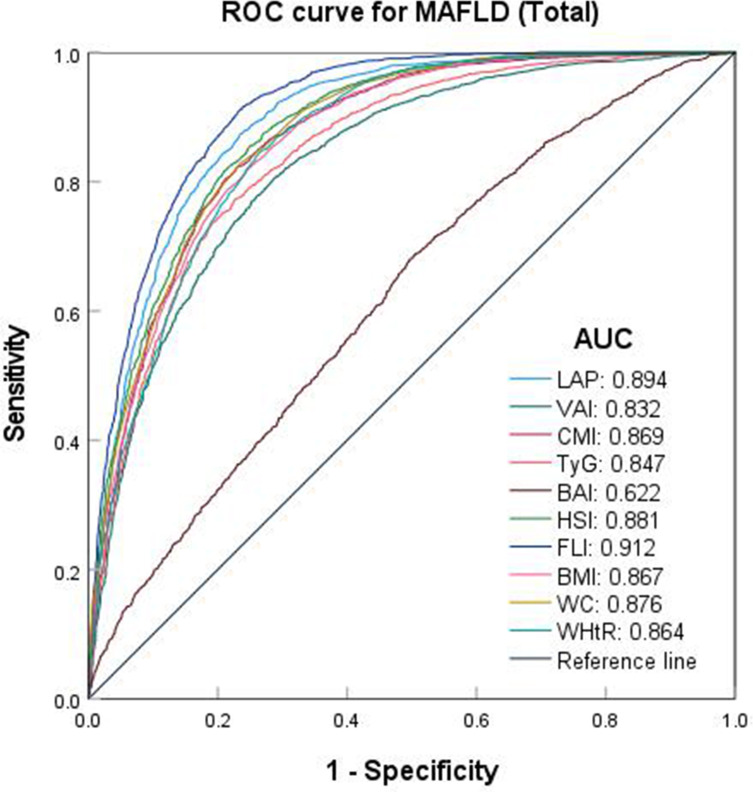
In the overall population, body fat indexes other than BAI (AUC: 0.622, 95% CI: 0.608–0.637) exhibited the highest predictive capability (AUC>0.8) for MAFLD, and FLI (AUC: 0.912, 95% CI: 0.905–0.920) had the highest predictive effect, with a cut-off values of 22.968 and a sensitivity of 0.916. This was followed by LAP (AUC: 0.894, 95% CI: 0.886–0.903) and HSI (AUC: 0.881, 95% CI: 0.872–0.890) (Table 4 and Figure 3).
Table 4.
Curve Analysis of Body Fat Indexes Predicting the Risk of MAFLD
| Index | AUC (95% CI) | P | Cut-off value | Sensitivity | Specificity | Youden Index |
|---|---|---|---|---|---|---|
| LAP | 0.894 (0.886–0.903) | <0.001*** | 26.070 | 0.879 | 0.759 | 0.638 |
| VAI | 0.832 (0.821–0.842) | <0.001*** | 1.475 | 0.804 | 0.715 | 0.519 |
| CMI | 0.869 (0.859–0.878) | <0.001*** | 0.543 | 0.822 | 0.771 | 0.593 |
| TyG | 0.847 (0.837–0.858) | <0.001*** | 8.717 | 0.773 | 0.773 | 0.546 |
| BAI | 0.622 (0.608–0.637) | <0.001*** | 26.967 | 0.689 | 0.494 | 0.183 |
| HSI | 0.881 (0.872–0.890) | <0.001*** | 34.060 | 0.822 | 0.785 | 0.607 |
| FLI | 0.912 (0.905–0.920) | <0.001*** | 22.968 | 0.916 | 0.762 | 0.678 |
| BMI | 0.867 (0.858–0.877) | <0.001*** | 24.550 | 0.818 | 0.755 | 0.573 |
| WC | 0.876 (0.867–0.885) | <0.001*** | 83.100 | 0.829 | 0.764 | 0.593 |
| WHtR | 0.864 (0.855–0.874) | <0.001*** | 0.503 | 0.848 | 0.732 | 0.580 |
Note: ***P<0.001.
Abbreviations: LAP, Lipid accumulation product; VAI, visceral fat index; CMI, cardiometabolic index; TyG, triglyceride-glucose index; BAI, body adiposity index; HSI, hepatic steatosis index; FLI, fatty liver index; BMI, body mass index; WC, waist circumference.
Figure 3.
ROC curve of each body fat index predicting the risk of MAFLD (Total).
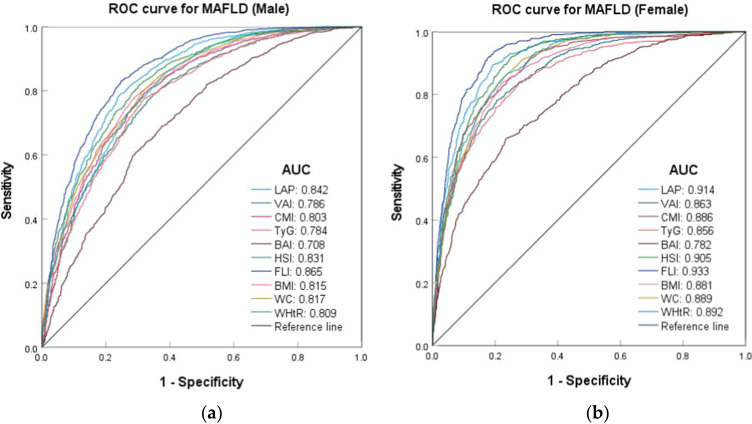
In various gender subgroups, all body fat indices demonstrated good predictive value for MAFLD (AUC > 0.7). The predictive effect was more pronounced in females than in males, and this difference was statistically significant (p < 0.001). Except for the BAI, the area under the curve (AUC) values for various body fat indexes in females were greater than 0.85. Notably, the AUC values of LAP, HSI, and Fatty Liver Index (FLI) exceeded 0.9, with sensitivities approaching 0.9. FLI demonstrated the highest predictive effect in both the male group (AUC: 0.865, 95% CI: 0.851–0.879) and the female group (AUC: 0.933, 95% CI: 0.924–0.943), with cut-off values of 13.317 and 39.818, respectively. While the BAI was predicted in the male group (AUC: 0.708, 95% CI: 0.689–0.728), the female group (AUC: 0.782, 95% CI: 0.762–0.802) exhibited the lowest predictive effect, with cut-off values of 30.684 and 26.289, respectively. The cut-off values for VAI and BAI in males were lower than those in females, whereas the cut-off values for LAP, CMI, TyG, HSI, FLI, BMI, WC, and WHtR were higher (Table S4 and Figure 4).
Figure 4.
ROC curves for the risk of MAFLD by body fat indexes of different genders. (a) ROC curves for MAFLD (Male); (b) ROC curves for MAFLD (Female).
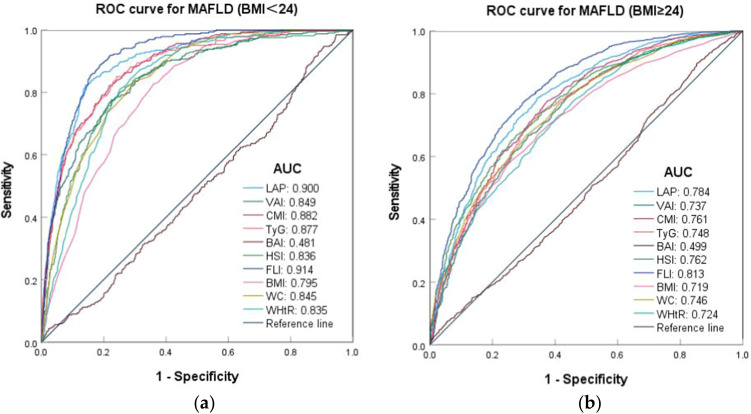
In various subgroups of BMI levels, body fat index other than BAI demonstrated a strong predictive value for MAFLD (AUC > 0.7), and the prediction effect in the BMI < 24 kg/m2 group was better than that in the BMI ≥ 24 kg/m2 group, the cut-off values was lower, and the difference was statistically significant (p < 0.05). Among the various indexes, the AUC values for LAP (AUC: 0.900, 95% CI: 0.883–0.916) and FLI (AUC: 0.914, 95% CI: 0.901–0.928) exceeded 0.9 in the BMI < 24 kg/m² group. Similarly, FLI demonstrated the highest predictive capability across different BMI categories, with cut-off values of 13.282 for the BMI < 24 kg/m² group and 42.154 for the BMI ≥ 24 kg/m² group. In contrast, BAI did not exhibit any predictive value in either group (Table S5 and Figure 5).
Figure 5.
ROC curves of body fat indexes predicting the risk of MAFLD at different BMI levels. (a) ROC curves for MAFLD (BMI<24); (b) ROC curves for MAFLD (BMI≥24).
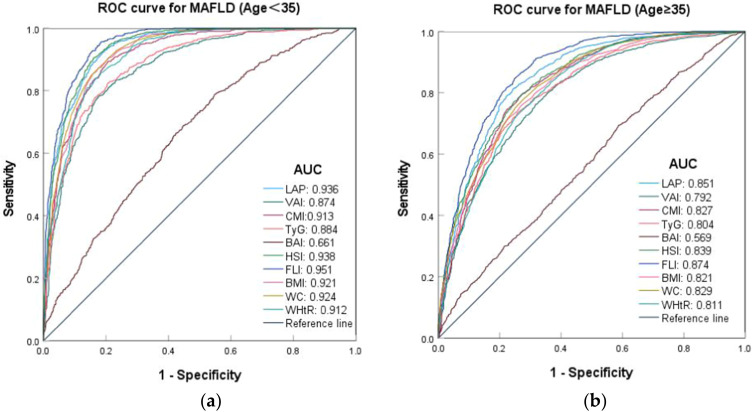
In various different age subgroups, body fat index other than BAI demonstrated a strong predictive value for MAFLD (AUC>0.7), and predictive effectiveness was particularly pronounced in the group aged under 35. Except for the VAI, the cut-off values of the other indexes were lower in the age group under 35 years, and the difference was statistically significant (p < 0.05). In the age group under 35, the AUC values and sensitivities of the other indexes were greater than 0.9, with the exception of VAI (AUC: 0.874, 95% CI: 0.859–0.89), TyG (AUC: 0.884, 95% CI: 0.869–0.899), and BAI (AUC: 0.661, 95% CI: 0.637–0.685). Similarly, the FLI demonstrated the highest predictive effect across the various age groups, with cut-off values of 20.653 and 29.197, respectively. In contrast, the BAI exhibited no significant predictive value in either group (Table S6 and Figure 6).
Figure 6.
ROC curves of body fat indexes predicting the risk of MAFLD at different age groups. (a) ROC curves for MAFLD (Age<35); (b) ROC curves for MAFLD (Age≥35).
Discussion
Many studies have examined various indexes related to body fat and metabolism to predict the risk of MAFLD. However, there are few studies that comprehensively compare body fat indexes and examine the differences among various subgroups and regions in China. In this cross-sectional study, we included 10 body fat indexes for a thorough comparison and analyzed their predictive effects across different genders, ages, and BMI levels. This research can provide certain reference values for the early prediction of MAFLD using body fat indexes.
This study found that, compared to the non-MAFLD group, the MAFLD group exhibited higher levels of BMI, WC, WHtR, SBP, DBP, ALP, TG, LDL-C, TC, and other biochemical indicators, while showing lower levels of HDL-C. These metabolic indicators were independently associated with an increased risk of MAFLD. Numerous cross-sectional and prospective studies have confirmed that the clinical indicators and outcomes for MAFLD patients are worse than those for non-MAFLD patients, highlighting significant clinical differences between the two groups.31
In the basic data and univariate regression analysis, it was found that alcohol drinking and smoking were the risk factors for MAFLD, and the probability of MAFLD was 2.25 times and 2.78 times that of non-drinking and non-smoking. Males are also an important risk factor for MAFLD, with their incidence being 4.88 times higher than that of females. Studies have confirmed that the prevalence of MAFLD in males is significantly higher than in females under 50 years of age (males: 22.4%; females: 7.1%, p<0.001),32 which is consistent with the findings that males are more prone to MAFLD than females.
According to the proportion of MAFLD in each group after quartile grouping of each index, and in conjunction with Cramer’s V coefficient, there is a strong correlation between all indexes except for BAI and MAFLD (Cramer’s V > 0.5). All indicators, whether considered continuous variables or ordered categorical variables, exhibit a dose-response relationship with the risk of MAFLD. The risk of MAFLD increases as the index level rises, which aligns with previous research findings.33,34 At the same time, a multivariate logistic regression analysis was conducted in Model 3, controlling for gender, age, smoking, alcohol consumption, and biochemical markers associated with MAFLD. The consistency rate, sensitivity, specificity, positive predictive value, and negative predictive value of all indexes except BAI in the diagnostic test of Model 3 exceeded 75%. This further suggests that the aforementioned body fat index is closely related to the occurrence and progression of MAFLD.33,35,36
In Model 3 of this study, a higher Body Mass Index (BMI) is associated with an increased risk of MAFLD, with individuals in the 4th quantile facing an 18-fold greater risk compared to those in the 1st quantile. A meta-analysis indicated that obese individuals have a 3.5 times higher risk of developing NAFLD than those with a normal BMI, and the severity of NAFLD tends to escalate in individuals with elevated BMI levels.37,38 Related studies indicate that individuals with a higher BMI tend to have increased visceral adipose tissue (VAT) and elevated levels of inflammatory cytokines, such as C-reactive protein, tumor necrosis factor-α, and interleukin-639. These factors are more likely to contribute to macrophage infiltration and chronic inflammation.40 At the same time, the adipokine chemokines secreted by adipocytes, along with the excess free fatty acids (FFA) released during adipocyte catabolism, can lead to metabolic disorders and other complications, further leading to the occurrence and development of fibrosis, inflammation and liver injury, and thus promoting the occurrence of MAFLD.39–41
Although BMI is the most commonly used clinical indicator of systemic obesity, WC is more appropriate for assessing central obesity. Both measures are widely utilized in the evaluation of obesity. However, they have a limited ability to differentiate between abdominal fat mass, as well as visceral and subcutaneous fat deposits.33,42,43 In another meta-analysis, approximately 40% of patients with metabolic fatty liver disease are not obese.44 In the univariate regression analysis of this study, although WHtR, BMI, and WC were independent risk factors for MAFLD, WHtR (OR: 4.385, 95% CI: 4.068–4.727) had significantly higher OR values than BMI (OR: 1.739, 95% CI: 1.688–1.792) and WC (OR: 1.213, 95% CI: 1.200–1.225). In a survey study in the western Chinese male population, WHtR (AUC 0.859, 95% CI: 0.843–0.874) also showed outstanding diagnostic value for MAFLD compared with BMI (AUC: 0.863, 95% CI: 0.848–0.879),45 similar to the results of this study. The explanation provided by several studies is that WHtR is an adjustment for WC and height, so it is a better indicator of abdominal obesity than WC and BMI.34,46,47
Notably, this study found that these 10 body fat indices were more effective at identifying MAFLD in women, young individuals, and non-obese individuals. The reasons may include the fact that, on one hand, the frequency, quantity, and proportion of smoking and alcohol drinking in males are significantly higher than those among females. These habits are important risk factors for the development of MAFLD.48,49 On the other hand, the majority of body fat in females (80–90%) is stored subcutaneously, which aids in maintaining glucose-insulin homeostasis and helps prevent damage from hypertriglyceridemia. However, in males, body fat is often stored in internal organs, which is strongly associated with central obesity.50,51 In addition, numerous studies have demonstrated that estrogen exerts a protective effect against fat accumulation. Compared to premenopausal women, men face a higher risk of developing more severe liver fibrosis.48,52,53 Therefore, related risk factors, specific estrogens, and regional fat distribution may be the primary reasons for the gender differences observed in the recognition of body fat index in MAFLD.33,48,52 As for the differences in predicted effects across various BMI subgroups, there is increasing evidence that metabolic health plays a crucial role beyond what is captured in the definition of obesity. Metabolically unhealthy lean individuals may experience greater ectopic fat accumulation, primarily manifested as visceral fat distribution. Non-obese patients with metabolically unhealthy MAFLD face a higher risk of liver damage and cardiovascular disease compared to their metabolically healthy counterparts.1 Therefore, the health level of fat metabolism is more effective in evaluating and predicting the occurrence of MAFLD in non-obese individuals than fat storage indices, such as obesity. In addition, changes in metabolism, body composition, and coexisting diseases occur with age. Young people tend to accumulate excess body fat due to irregular diets and insufficient exercise.54,55 Therefore, it is reasonable to conclude that relevant body fat indices predict MAFLD more accurately in younger populations.42,55
From the ROC curves predicting the risk of MAFLD in the overall population and various subgroups, FLI had the largest AUC value in the ROC analysis of each group, followed by LAP and HSI, while the prediction effect of BAI was not ideal. Observational studies utilizing individual health examination data have demonstrated that FLI and HSI exhibit strong discriminative ability in the general population, as well as among individuals at metabolic risk within Asian populations.28,44 Studies conducted across various races and environments have confirmed that the FLI is an effective predictor of fatty liver disease. This conclusion is also supported by the findings of this study.56 Cai et al showed that LAP exhibited the highest diagnostic value in MAFLD compared to other anthropometric measures (AUC 0.868, 95% CI, 0.853 to 0.883) with cut-off value of 24.49.45 In this study, although the AUC value of LAP is not the highest, its predictive performance is second only to that of FLI. It is noteworthy that LAP requires fewer parameters and computational structures than other indices, suggesting that LAP may be a more valuable and convenient predictor of MAFLD.34,36
Studies have also shown that VAI can more accurately diagnose MAFLD in healthy people. When the VAI > 2.33, the patient is likely to have MAFLD.18 Unlike the Yi et al study, in this study, the threshold value of VAI in the overall case is 1.475, and its AUC value is 0.832, which does not show a better prediction effect than other indexes. Studies have given an explanation that, in terms of ethnicity, VAI is more diagnostic value in European populations than in Asian populations, suggesting that VAI can more accurately diagnose MAFLD in European populations.18 Thus, this means that MAFLD is a heterogeneous disease whose epidemiology is not only related to gender, age, ethnicity, genetic variation, alcohol consumption, obesity, metabolism, lifestyle, and education level.57 Due to uneven economic development, varying regional cultures, and diverse lifestyles, the epidemiology also exhibits significant regional differences.36 This suggests that metabolic markers such as FLI, LAP, HSI, and VAI can serve as significant risk indicators for the diagnosis of MAFLD.18 However, when selecting indicators to assess and predict the risk of MAFLD, it is important to consider the variations that may arise from the aforementioned factors.
Some limitations of this study should be noted. First, this research is a single-center study that utilized a non-probability sampling method rather than a random sampling approach. Consequently, the results may not be representative of the entire population of China, as they may vary by region. However, we addressed this limitation by including data from as many participants as possible compared to previous studies. Second, the diagnosis of fatty liver is not confirmed histologically by biopsy but was determined using ultrasound technique.
Conclusion
In summary, this study found that male gender, smoking, alcohol drinking, and advanced age as risk factors for MAFLD. Compared to the non-MAFLD group, there were certain differences in the levels of biochemical indicators such as SBP, DBP, ALP, TG, and so on. Except for BAI, there was a strong correlation with MAFLD, and these indicators can more accurately predict the occurrence of MAFLD in women, non-obese individuals, and those under 35 years of age. Among these, the best prediction effect, followed by LAP and HSI, This study will aid in stratifying metabolic risk in patients with hepatic steatosis. Although these indices exhibited strong predictive value across various subgroups, it is essential to recognize that the effectiveness of each index in predicting the occurrence of MAFLD may differ among population groups in different regions.
Acknowledgments
The authors would like to thank all subjects who participated in this study.
Funding Statement
This study was funded by Army Medical University Humanities and Social Science foundation project (Grant no. 2022XRW15).
Data Sharing Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
Ethics Approval
This study was conducted in accordance with the Declaration of Helsinki. The Ethics Committee of the First Affiliated Hospital of Army Medical University approved the study protocol (Grant No. [A]KY202227). An informed consent was obtained from each participant. Data were treated with strict confidentiality and used only for scientific purposes.
Author Contributions
Zhen Cheng and Chunyu Hu are first authors. All authors made a significant contribution to the work reported, whether that in the conception, study design, acquisition of data, analysis, and interpretation, or in all these areas in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The author(s) report no conflicts of interest in this work.
References
- 1.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 2.Xiao J, Wang F, Wong NK, et al. Global liver disease burdens and research trends: analysis from a Chinese perspective. J Hepatol. 2019;71:212–221. doi: 10.1016/j.jhep.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 3.Fan J, Luo S, Ye Y, et al. Prevalence and risk factors of metabolic associated fatty liver disease in the contemporary South China population. Nutr Metab. 2021;18:82. doi: 10.1186/s12986-021-00611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong RJ, Cheung R. Trends in the prevalence of metabolic dysfunction-associated fatty liver disease in the United States, 2011-2018. Clin Gastroenterol Hepatol. 2022;20:e610–e613. doi: 10.1016/j.cgh.2021.01.030 [DOI] [PubMed] [Google Scholar]
- 5.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370 [DOI] [PubMed] [Google Scholar]
- 6.Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–2224. doi: 10.1016/S0140-6736(20)32511-3 [DOI] [PubMed] [Google Scholar]
- 7.Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0 [DOI] [PubMed] [Google Scholar]
- 8.Davison BA, Harrison SA, Cotter G, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. 2020;73:1322–1332. doi: 10.1016/j.jhep.2020.06.025 [DOI] [PubMed] [Google Scholar]
- 9.Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1264–1281. doi: 10.1053/j.gastro.2018.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van kleef LA, Sonneveld MJ, de Man RA, et al. Poor performance of fib-4 in elderly individuals at risk for chronic liver disease - implications for the clinical utility of the easl nit guideline. J Hepatol. 2022;76:245–246. doi: 10.1016/j.jhep.2021.08.017 [DOI] [PubMed] [Google Scholar]
- 11.Cicero A, Gitto S, Fogacci F, et al. Fatty liver index is associated to pulse wave velocity in healthy subjects: data from the brisighella heart study. Eur J Intern Med. 2018;53:29–33. doi: 10.1016/j.ejim.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 12.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65(8):1038–1048. doi: 10.1016/j.metabol.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 13.Okada A, Yamada G, Kimura T, et al. Diagnostic ability using fatty liver and metabolic markers for metabolic-associated fatty liver disease stratified by metabolic/glycemic abnormalities. J Diabetes Investig. 2023;14(3):463–478. doi: 10.1111/jdi.13966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarantino G, Sinatti G, Citro V, et al. Sarcopenia, a condition shared by various diseases: can we alleviate or delay the progression? Intern Emerg Med. 2023;18:1887–1895. doi: 10.1007/s11739-023-03339-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tandon P, Ney M, Irwin I, et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18(10):1209–1216. doi: 10.1002/lt.23495 [DOI] [PubMed] [Google Scholar]
- 16.Bucci L, Yani SL, Fabbri C, et al. Circulating levels of adipokines and igf-1 are associated with skeletal muscle strength of young and old healthy subjects. Biogerontology. 2013;14(3):261–272. doi: 10.1007/s10522-013-9428-5 [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Cheng KY, Tong X, et al. The role of obesity in sarcopenia and the optimal body composition to prevent against sarcopenia and obesity. Front Endocrinol. 2023;14:1077255. doi: 10.3389/fendo.2023.1077255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi X, Zhu S, Zhu L. Diagnostic accuracy of the visceral adiposity index in patients with metabolic-associated fatty liver disease: a meta-analysis. Lipids Health Dis. 2022;21(1):28. doi: 10.1186/s12944-022-01636-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu C, Ma Z, Wang Y, et al. Visceral adiposity index as a predictor of NAFLD: a prospective study with 4-year follow-up. Liver Int. 2018;38:2294–2300. doi: 10.1111/liv.13941 [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Cong HL, Zhang JX, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19:80. doi: 10.1186/s12933-020-01054-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Wu Q, Yan G, et al. Cardiometabolic index: a new tool for screening the metabolically obese normal weight phenotype. J Endocrinol Invest. 2021;44(6):1253–1261. doi: 10.1007/s40618-020-01417-z [DOI] [PubMed] [Google Scholar]
- 23.Tutunchi H, Naeini F, Mobasseri M, et al. Triglyceride glucose (tyg) index and the progression of liver fibrosis: a cross-sectional study. Clin Nutr Espen. 2021;44:483–487. doi: 10.1016/j.clnesp.2021.04.025 [DOI] [PubMed] [Google Scholar]
- 24.Bergman RN. A better index of body adiposity. Obesity. 2012;20(6):1135. doi: 10.1038/oby.2012.99 [DOI] [PubMed] [Google Scholar]
- 25.Wakabayashi I, Daimon T. The “cardiometabolic index” as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clin Chim Acta. 2015;438:274–278. doi: 10.1016/j.cca.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 26.Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–3351. doi: 10.1210/jc.2010-0288 [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503–508. doi: 10.1016/j.dld.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 28.Meffert PJ, Baumeister SE, Lerch MM, et al. Development, external validation, and comparative assessment of a new diagnostic score for hepatic steatosis. Am J Gastroenterol. 2014;109:1404–1414. doi: 10.1038/ajg.2014.155 [DOI] [PubMed] [Google Scholar]
- 29.Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. Bmc Cardiovasc Disord. 2005;5:26. doi: 10.1186/1471-2261-5-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amato MC, Giordano C, Galia M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920–922. doi: 10.2337/dc09-1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim D, Konyn P, Sandhu KK, et al. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75:1284–1291. doi: 10.1016/j.jhep.2021.07.035 [DOI] [PubMed] [Google Scholar]
- 32.Zhou YJ, Li YY, Nie YQ, et al. Prevalence of fatty liver disease and its risk factors in the population of South China. World J Gastroenterol. 2007;13:6419–6424. doi: 10.3748/wjg.v13.i47.6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Zhang Y, Luo H, et al. The lipid accumulation product is a powerful tool to diagnose metabolic dysfunction-associated fatty liver disease in the United States adults. Front Endocrinol. 2022;13:977625. doi: 10.3389/fendo.2022.977625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng H, Pan L, Ran S, et al. Prediction of mafld and NAFLD using different screening indexes: a cross-sectional study in U.S. adults. Front Endocrinol. 2023;14:1083032. doi: 10.3389/fendo.2023.1083032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai H, Wang W, Chen R, et al. Lipid accumulation product is a powerful tool to predict non-alcoholic fatty liver disease in Chinese adults. Nutr Metab. 2017;14(1):49. doi: 10.1186/s12986-017-0206-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Zhang Y, Liu Y, et al. Comparison between traditional and new obesity measurement index for screening metabolic associated fatty liver disease. Front Endocrinol. 2023;14:1163682. doi: 10.3389/fendo.2023.1163682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Liu DW, Yan HY, et al. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev. 2016;17:510–519. doi: 10.1111/obr.12407 [DOI] [PubMed] [Google Scholar]
- 38.Lim G, Tang A, Ng CH, et al. An observational data meta-analysis on the differences in prevalence and risk factors between mafld vs NAFLD. Clin Gastroenterol Hepatol. 2023;21:619–629. doi: 10.1016/j.cgh.2021.11.038 [DOI] [PubMed] [Google Scholar]
- 39.Fontana L, Eagon JC, Trujillo ME, et al. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656 [DOI] [PubMed] [Google Scholar]
- 40.Antraco VJ, Hirata B, de Jesus SJ, et al. Omega-3 polyunsaturated fatty acids prevent nonalcoholic steatohepatitis (Nash) and stimulate adipogenesis. Nutrients. 2021;14:13. doi: 10.3390/nu14010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stefan N, Kantartzis K, Haring HU. Causes and metabolic consequences of fatty liver. Endocr Rev. 2008;29:939–960. [DOI] [PubMed] [Google Scholar]
- 42.Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8:616–627. doi: 10.1016/S2213-8587(20)30110-8 [DOI] [PubMed] [Google Scholar]
- 43.Schulze MB, Stefan N. Metabolically healthy obesity: from epidemiology and mechanisms to clinical implications. Nat Rev Endocrinol. 2024;20:633–646. doi: 10.1038/s41574-024-01008-5 [DOI] [PubMed] [Google Scholar]
- 44.Eslam M, Fan JG, Mendez-Sanchez N. Non-alcoholic fatty liver disease in non-obese individuals: the impact of metabolic health. Lancet Gastroenterol Hepatol. 2020;5:713–715. doi: 10.1016/S2468-1253(20)30090-X [DOI] [PubMed] [Google Scholar]
- 45.Cai J, Lin C, Lai S, et al. Waist-to-height ratio, an optimal anthropometric indicator for metabolic dysfunction associated fatty liver disease in the western Chinese male population. Lipids Health Dis. 2021;20:145. doi: 10.1186/s12944-021-01568-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stefan N, Schiborn C, Machann J, et al. Impact of higher bmi on cardiometabolic risk: does height matter? Lancet Diabetes Endocrinol. 2024;12:514–515. doi: 10.1016/S2213-8587(24)00164-5 [DOI] [PubMed] [Google Scholar]
- 47.Sweatt K, Garvey WT, Martins C. Strengths and limitations of bmi in the diagnosis of obesity: what is the path forward? Curr Obes Rep. 2024;13:584–595. doi: 10.1007/s13679-024-00580-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang S, Ungvari GS, Forester BP, et al. Gender differences in general mental health, smoking, drinking and chronic diseases in older adults in Jilin province, China. Psychiatry Res. 2017;251:58–62. doi: 10.1016/j.psychres.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Jiang Y, Zhang M, et al. Drinking behaviour among men and women in China: the 2007 china chronic disease and risk factor surveillance. Addiction. 2011;106:1946–1956. doi: 10.1111/j.1360-0443.2011.03514.x [DOI] [PubMed] [Google Scholar]
- 50.Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11:90–100. [DOI] [PubMed] [Google Scholar]
- 51.Amati F, Pennant M, Azuma K, et al. Lower thigh subcutaneous and higher visceral abdominal adipose tissue content both contribute to insulin resistance. Obesity. 2012;20:1115–1117. doi: 10.1038/oby.2011.401 [DOI] [PubMed] [Google Scholar]
- 52.Yang JD, Abdelmalek MF, Pang H, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:1406–1414. doi: 10.1002/hep.26761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tramunt B, Smati S, Grandgeorge N, et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.JafariNasabian P, Inglis JE, Reilly W, et al. Aging human body: changes in bone, muscle and body fat with consequent changes in nutrient intake. J Endocrinol. 2017;234(1):R37–R51. doi: 10.1530/JOE-16-0603 [DOI] [PubMed] [Google Scholar]
- 55.Schneider BC, Dumith SC, Orlandi SP, et al. Diet and body fat in adolescence and early adulthood: a systematic review of longitudinal studies. Cien Saude Colet. 2017;22(5):1539–1552. doi: 10.1590/1413-81232017225.13972015 [DOI] [PubMed] [Google Scholar]
- 56.Han AL. Validation of fatty liver index as a marker for metabolic dysfunction-associated fatty liver disease. Diabetol Metab Syndr. 2022;14:44. doi: 10.1186/s13098-022-00811-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang TD, Behary J, Zekry A. Non-alcoholic fatty liver disease: a review of epidemiology, risk factors, diagnosis and management. Intern Med J. 2020;50:1038–1047. doi: 10.1111/imj.14709 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.