Abstract
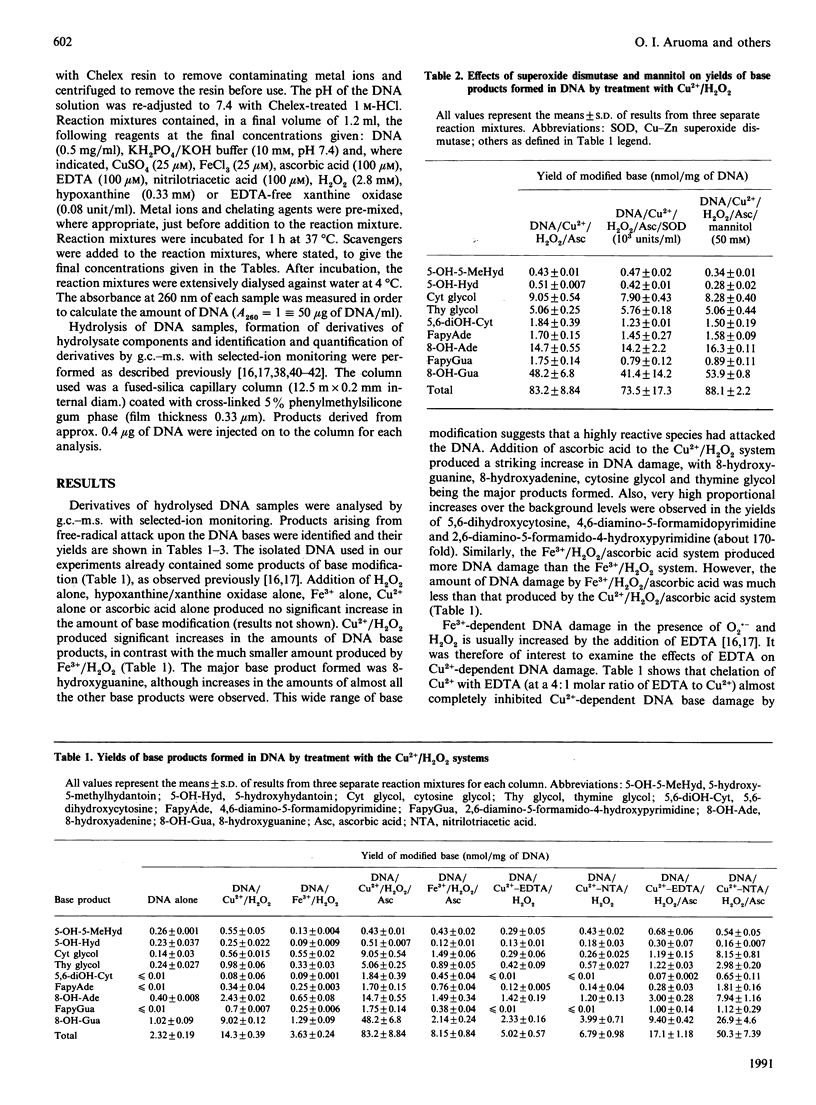
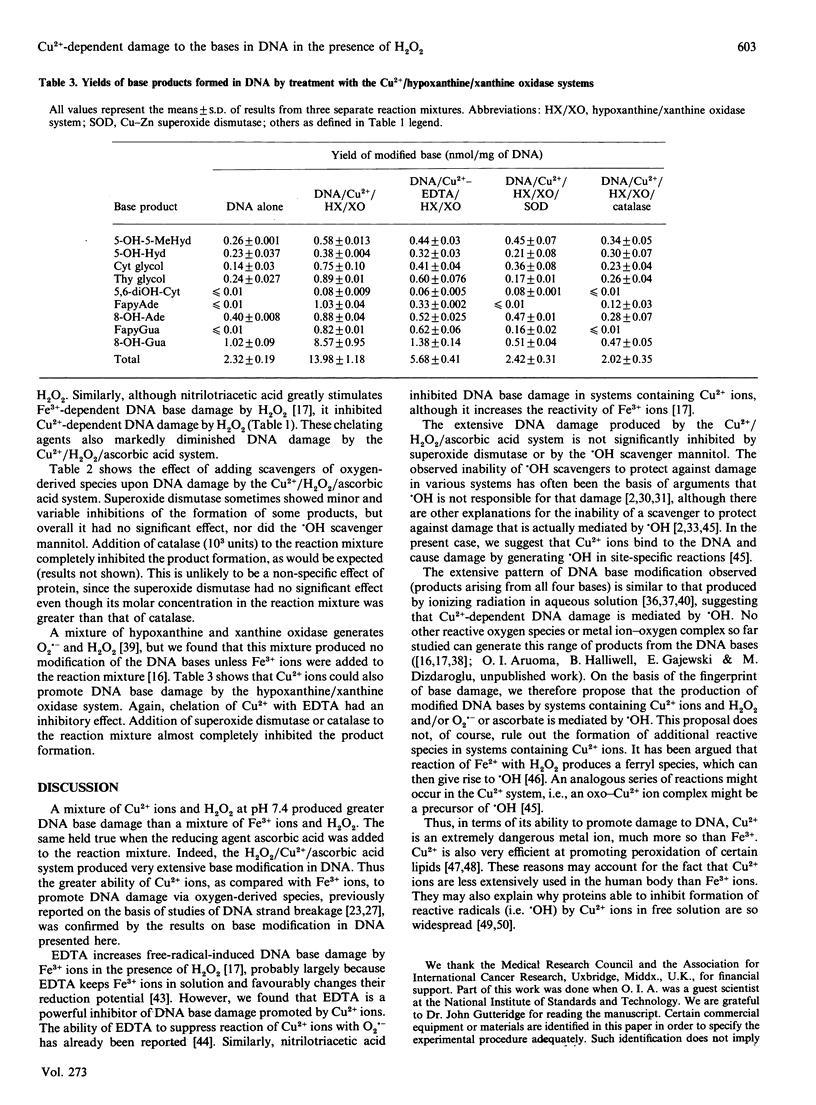
Mixtures of Cu2+ and H2O2 at pH 7.4 caused damage to the bases in DNA greater than that caused by mixtures of Fe3+ and H2O2. Addition of ascorbic acid to the Cu2+/H2O2 system caused a very large increase in base damage, much greater than that produced by the Fe3+/H2O2/ascorbic acid system. The products of base damage in the presence of Cu2+ were typical products that have been shown to result from attack of hydroxyl radicals upon the DNA bases. Cytosine glycol, thymine glycol, 8-hydroxyadenine and especially 8-hydroxyguanine were the major products in both the Cu2+/H2O2 and the Cu2+/H2O2/ascorbic acid systems. Base damage in DNA by these systems was inhibited by the chelating agents EDTA and nitrilotriacetic acid and by catalase, but not by superoxide dismutase, nor by the hydroxyl-radical scavenger mannitol. It is proposed that Cu2+ ions bound to the DNA react with H2O2 and ascorbic acid to generate hydroxyl radicals, which then immediately attack the DNA bases in a site-specific manner. A hypoxanthine/xanthine oxidase system also caused damage to the DNA bases in the presence of Cu2+ ions. This was inhibited by superoxide dismutase and catalase. The high activity of Cu2+ ions, when compared with Fe3- ions, in causing hydroxyl-radical-dependent damage to DNA and to other biomolecules, means that the availability of Cu2+ ions in vivo must be carefully controlled.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruoma O. I., Halliwell B., Dizdaroglu M. Iron ion-dependent modification of bases in DNA by the superoxide radical-generating system hypoxanthine/xanthine oxidase. J Biol Chem. 1989 Aug 5;264(22):13024–13028. [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Gajewski E., Dizdaroglu M. Damage to the bases in DNA induced by hydrogen peroxide and ferric ion chelates. J Biol Chem. 1989 Dec 5;264(34):20509–20512. [PubMed] [Google Scholar]
- Brawn K., Fridovich I. DNA strand scission by enzymically generated oxygen radicals. Arch Biochem Biophys. 1981 Feb;206(2):414–419. doi: 10.1016/0003-9861(81)90108-9. [DOI] [PubMed] [Google Scholar]
- Brawn M. K., Fridovich I. Increased superoxide radical production evokes inducible DNA repair in Escherichia coli. J Biol Chem. 1985 Jan 25;260(2):922–925. [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Chiou S. H. DNA- and protein-scission activities of ascorbate in the presence of copper ion and a copper-peptide complex. J Biochem. 1983 Oct;94(4):1259–1267. doi: 10.1093/oxfordjournals.jbchem.a134471. [DOI] [PubMed] [Google Scholar]
- Czapski G., Goldstein S., Meyerstein D. What is unique about superoxide toxicity as compared to other biological reductants? A hypothesis. Free Radic Res Commun. 1988;4(4):231–236. doi: 10.3109/10715768809055147. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M. Application of capillary gas chromatography-mass spectrometry to chemical characterization of radiation-induced base damage of DNA: implications for assessing DNA repair processes. Anal Biochem. 1985 Feb 1;144(2):593–603. doi: 10.1016/0003-2697(85)90158-7. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M., Aruoma O. I., Halliwell B. Modification of bases in DNA by copper ion-1,10-phenanthroline complexes. Biochemistry. 1990 Sep 11;29(36):8447–8451. doi: 10.1021/bi00488a035. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M., Gajewski E. Selected-ion mass spectrometry: assays of oxidative DNA damage. Methods Enzymol. 1990;186:530–544. doi: 10.1016/0076-6879(90)86147-n. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Striegl G., Puhl H., Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun. 1989;6(1):67–75. doi: 10.3109/10715768909073429. [DOI] [PubMed] [Google Scholar]
- Feldberg R. S., Carew J. A., Paradise R. Probing Cu(II)/H2O2 damage in DNA with a damage-specific DNA binding protein. J Free Radic Biol Med. 1985;1(5-6):459–466. doi: 10.1016/0748-5514(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986 May 15;247(1):1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Fuciarelli A. F., Wegher B. J., Gajewski E., Dizdaroglu M., Blakely W. F. Quantitative measurement of radiation-induced base products in DNA using gas chromatography-mass spectrometry. Radiat Res. 1989 Aug;119(2):219–231. [PubMed] [Google Scholar]
- Fujimoto S., Adachi Y., Ishimitsu S., Ohara A. Release of bases from deoxyribonucleic acid by ascorbic acid in the presence of Cu2+. Chem Pharm Bull (Tokyo) 1986 Nov;34(11):4848–4851. doi: 10.1248/cpb.34.4848. [DOI] [PubMed] [Google Scholar]
- Grootveld M., Halliwell B. An aromatic hydroxylation assay for hydroxyl radicals utilizing high-performance liquid chromatography (HPLC). Use to investigate the effect of EDTA on the Fenton reaction. Free Radic Res Commun. 1986;1(4):243–250. doi: 10.3109/10715768609051634. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Age pigments: role of iron and copper salts in the formation of fluorescent lipid complexes. Mech Ageing Dev. 1984 Apr-May;25(1-2):205–214. doi: 10.1016/0047-6374(84)90141-6. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Wilkins S. Copper salt-dependent hydroxyl radical formation. Damage to proteins acting as antioxidants. Biochim Biophys Acta. 1983 Aug 23;759(1-2):38–41. doi: 10.1016/0304-4165(83)90186-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Albumin--an important extracellular antioxidant? Biochem Pharmacol. 1988 Feb 15;37(4):569–571. doi: 10.1016/0006-2952(88)90126-8. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Grootveld M., Gutteridge J. M. Methods for the measurement of hydroxyl radicals in biomedical systems: deoxyribose degradation and aromatic hydroxylation. Methods Biochem Anal. 1988;33:59–90. doi: 10.1002/9780470110546.ch2. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987 Nov;1(5):358–364. [PubMed] [Google Scholar]
- Halliwell B. The superoxide dismutase activity of iron complexes. FEBS Lett. 1975 Aug 1;56(1):34–38. doi: 10.1016/0014-5793(75)80105-0. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Kyle M. E., Nakae D., Sakaida I., Miccadei S., Farber J. L. Endocytosis of superoxide dismutase is required in order for the enzyme to protect hepatocytes from the cytotoxicity of hydrogen peroxide. J Biol Chem. 1988 Mar 15;263(8):3784–3789. [PubMed] [Google Scholar]
- Lesko S. A., Lorentzen R. J., Ts'o P. O. Role of superoxide in deoxyribonucleic acid strand scission. Biochemistry. 1980 Jun 24;19(13):3023–3028. doi: 10.1021/bi00554a029. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Nicotera P., Wyllie A. H., Orrenius S. Stimulation of endogenous endonuclease activity in hepatocytes exposed to oxidative stress. Toxicol Lett. 1988 Aug;42(2):123–130. doi: 10.1016/0378-4274(88)90069-0. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Mello Filho A. C., Hoffmann M. E., Meneghini R. Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. Biochem J. 1984 Feb 15;218(1):273–275. doi: 10.1042/bj2180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody C. S., Hassan H. M. Mutagenicity of oxygen free radicals. Proc Natl Acad Sci U S A. 1982 May;79(9):2855–2859. doi: 10.1073/pnas.79.9.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Kaneko M., Kodama M., Nagata C. Cigarette smoke induces DNA single-strand breaks in human cells. Nature. 1985 Apr 4;314(6010):462–464. doi: 10.1038/314462a0. [DOI] [PubMed] [Google Scholar]
- Nassi-Calò L., Mello-Filho C., Meneghini R. o-phenanthroline protects mammalian cells from hydrogen peroxide-induced gene mutation and morphological transformation. Carcinogenesis. 1989 Jun;10(6):1055–1057. doi: 10.1093/carcin/10.6.1055. [DOI] [PubMed] [Google Scholar]
- Reed C. J., Douglas K. T. Single-strand cleavage of DNA by Cu(II) and thiols: a powerful chemical DNA-cleaving system. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1111–1117. doi: 10.1016/0006-291x(89)90788-2. [DOI] [PubMed] [Google Scholar]
- Rowley D. A., Halliwell B. DNA damage by superoxide-generating systems in relation to the mechanism of action of the anti-tumour antibiotic adriamycin. Biochim Biophys Acta. 1983 Nov 22;761(1):86–93. doi: 10.1016/0304-4165(83)90365-3. [DOI] [PubMed] [Google Scholar]
- Rowley D. A., Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals in the presence of copper salts: a physiologically significant reaction? Arch Biochem Biophys. 1983 Aug;225(1):279–284. doi: 10.1016/0003-9861(83)90031-0. [DOI] [PubMed] [Google Scholar]
- Sagripanti J. L., Kraemer K. H. Site-specific oxidative DNA damage at polyguanosines produced by copper plus hydrogen peroxide. J Biol Chem. 1989 Jan 25;264(3):1729–1734. [PubMed] [Google Scholar]
- Schraufstätter I., Hyslop P. A., Jackson J. H., Cochrane C. G. Oxidant-induced DNA damage of target cells. J Clin Invest. 1988 Sep;82(3):1040–1050. doi: 10.1172/JCI113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoewe R., Prütz W. A. Copper-catalyzed DNA damage by ascorbate and hydrogen peroxide: kinetics and yield. Free Radic Biol Med. 1987;3(2):97–105. doi: 10.1016/s0891-5849(87)80003-5. [DOI] [PubMed] [Google Scholar]
- Sutton H. C., Winterbourn C. C. On the participation of higher oxidation states of iron and copper in Fenton reactions. Free Radic Biol Med. 1989;6(1):53–60. doi: 10.1016/0891-5849(89)90160-3. [DOI] [PubMed] [Google Scholar]
- Tachon P. Ferric and cupric ions requirement for DNA single-strand breakage by H2O2. Free Radic Res Commun. 1989;7(1):1–10. doi: 10.3109/10715768909088155. [DOI] [PubMed] [Google Scholar]
- Teoule R., Cadet J. Radiation-induced degradation of the base component in DNA and related substances--final products. Mol Biol Biochem Biophys. 1978;27:171–203. doi: 10.1007/978-3-642-81196-8_9. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Weitberg A. B., Clark E. P., Stossel T. P. Phagocytes as carcinogens: malignant transformation produced by human neutrophils. Science. 1985 Mar 8;227(4691):1231–1233. doi: 10.1126/science.3975611. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Kawanishi S. Hydroxyl free radical is not the main active species in site-specific DNA damage induced by copper (II) ion and hydrogen peroxide. J Biol Chem. 1989 Sep 15;264(26):15435–15440. [PubMed] [Google Scholar]


