Abstract
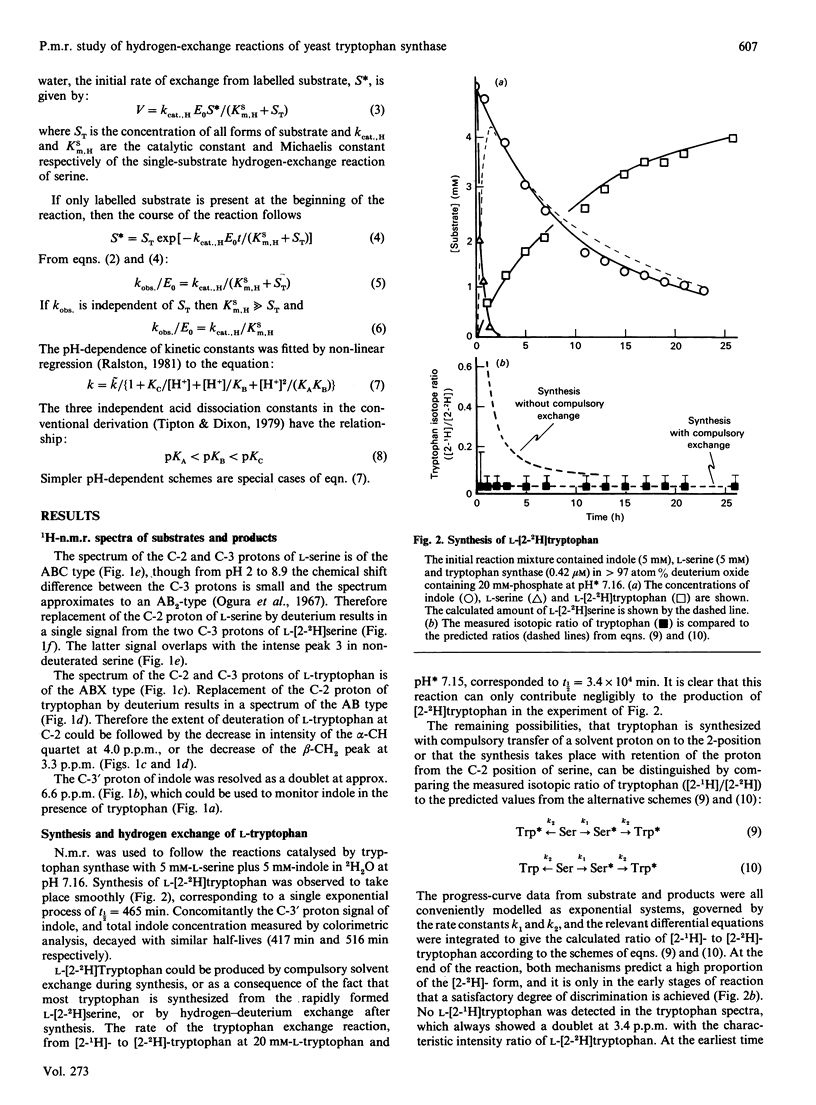
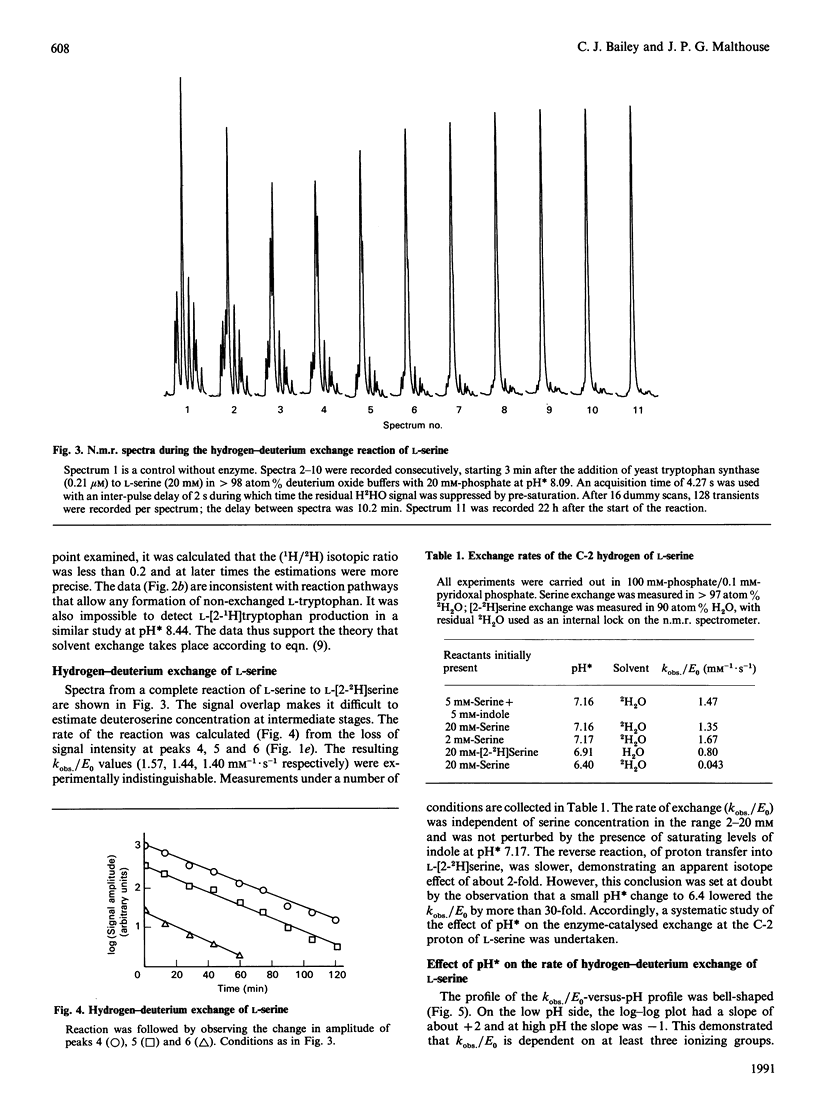
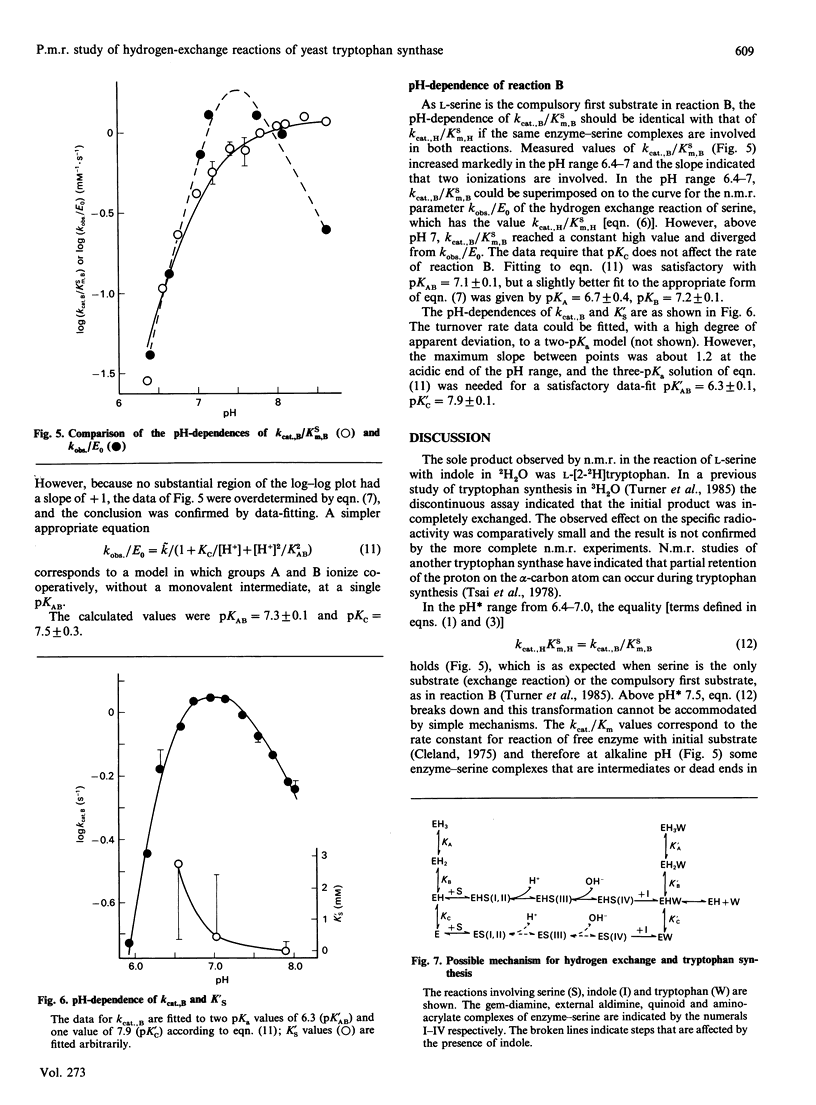
1H n.m.r. was used to observe tryptophan formation from indole and L-serine, proton exchange at C-2 of L-tryptophan, and proton exchange at C-2 of L-serine, catalysed by yeast tryptophan synthase in the presence of 2H2O. Tryptophan synthesis took place with compulsory replacement of C-2 hydrogen by solvent hydrogen. The exponential decay rate (kobs) of the serine exchange reaction was insensitive to serine concentration in the range 2-20mM and was used to calculate kcat./Km values. However, kobs. was very sensitive to pH* values in the range 6.5-8.5 and the data require that the free enzyme is active in the base form resulting from two inseparable ionizations of pKa 7.3, and inactive after a third ionization controlled by a pKa of 7.5. Initial rates measured by u.v. absorbance and colorimetric procedures were used to calculate kinetic parameters of the tryptophan synthesis reaction. From pH 6.5 to 7, kcat./Km values for L-serine in the tryptophan synthesis and hydrogen exchange reactions were indistinguishable and increased rapidly under the control of two acid-base groups of pKa 6.7 and 7.2. Above pH 7, this equivalence breaks down because the exchange reaction alone is responsive to the third pKa value of the free enzyme. The pH dependence of the catalytic constant for tryptophan synthesis was qualitatively similar to that of the kobs. for serine exchange. A mechanism to explain the results is contrasted with recent proposals for the Escherichia coli system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey C. J., Turner P. D. Purification and properties of tryptophan synthase from baker's yeast (Saccharomyces cerevisiae). Biochem J. 1983 Jan 1;209(1):151–157. doi: 10.1042/bj2090151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland W. W. Partition analysis and the concept of net rate constants as tools in enzyme kinetics. Biochemistry. 1975 Jul 15;14(14):3220–3224. doi: 10.1021/bi00685a029. [DOI] [PubMed] [Google Scholar]
- Drewe W. F., Jr, Dunn M. F. Detection and identification of intermediates in the reaction of L-serine with Escherichia coli tryptophan synthase via rapid-scanning ultraviolet-visible spectroscopy. Biochemistry. 1985 Jul 16;24(15):3977–3987. doi: 10.1021/bi00336a027. [DOI] [PubMed] [Google Scholar]
- Faeder E. J., Hammes G. G. Kinetic studies of tryptophan synthetase. Interaction of substrates with the B subunit. Biochemistry. 1970 Oct 13;9(21):4043–4049. doi: 10.1021/bi00823a003. [DOI] [PubMed] [Google Scholar]
- Goldberg M. E., York S., Stryer L. Fluorescence studies of substrate and subunit interactions of the beta-2 protein of Escherichia coli tryptophan synthetase. Biochemistry. 1968 Oct;7(10):3662–3667. doi: 10.1021/bi00850a045. [DOI] [PubMed] [Google Scholar]
- Hyde C. C., Ahmed S. A., Padlan E. A., Miles E. W., Davies D. R. Three-dimensional structure of the tryptophan synthase alpha 2 beta 2 multienzyme complex from Salmonella typhimurium. J Biol Chem. 1988 Nov 25;263(33):17857–17871. [PubMed] [Google Scholar]
- Kirschner K., Bisswanger H. Multifunctional proteins. Annu Rev Biochem. 1976;45:143–166. doi: 10.1146/annurev.bi.45.070176.001043. [DOI] [PubMed] [Google Scholar]
- Lane A. N., Kirschner K. The catalytic mechanism of tryptophan synthase from Escherichia coli. Kinetics of the reaction of indole with the enzyme--L-serine complexes. Eur J Biochem. 1983 Jan 1;129(3):571–582. doi: 10.1111/j.1432-1033.1983.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Lane A. N., Kirschner K. The mechanism of binding of L-serine to tryptophan synthase from Escherichia coli. Eur J Biochem. 1983 Jan 1;129(3):561–570. doi: 10.1111/j.1432-1033.1983.tb07086.x. [DOI] [PubMed] [Google Scholar]
- Lane A. N., Kirschner K. The mechanism of tryptophan binding to tryptophan synthase from Escherichia coli. Eur J Biochem. 1981 Nov;120(2):379–387. doi: 10.1111/j.1432-1033.1981.tb05715.x. [DOI] [PubMed] [Google Scholar]
- Miles E. W., Kawasaki H., Ahmed S. A., Morita H., Morita H., Nagata S. The beta subunit of tryptophan synthase. Clarification of the roles of histidine 86, lysine 87, arginine 148, cysteine 170, and cysteine 230. J Biol Chem. 1989 Apr 15;264(11):6280–6287. [PubMed] [Google Scholar]
- Miles E. W., Kumagai H. Modification of essential histidyl residues of the beta 2 subunit of tryptophan synthetase by photo-oxidation in the presence of pyridoxal 5'-phosphate and L-serine and by diethylpyrocarbonate. J Biol Chem. 1974 May 10;249(9):2843–2851. [PubMed] [Google Scholar]
- Miles E. W. Tryptophan synthase: structure, function, and subunit interaction. Adv Enzymol Relat Areas Mol Biol. 1979;49:127–186. doi: 10.1002/9780470122945.ch4. [DOI] [PubMed] [Google Scholar]
- Mozzarelli A., Peracchi A., Rossi G. L., Ahmed S. A., Miles E. W. Microspectrophotometric studies on single crystals of the tryptophan synthase alpha 2 beta 2 complex demonstrate formation of enzyme-substrate intermediates. J Biol Chem. 1989 Sep 25;264(27):15774–15780. [PubMed] [Google Scholar]
- Srere P. A. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- Tipton K. F., Dixon H. B. Effects of pH on enzymes. Methods Enzymol. 1979;63:183–234. doi: 10.1016/0076-6879(79)63011-2. [DOI] [PubMed] [Google Scholar]
- Tsai M. D., Schleicher E., Potts R., Skye G. E., Floss H. G. Stereochemistry and mechanism of reactions catalyzed by tryptophan synthetase and its beta2 subunit. J Biol Chem. 1978 Aug 10;253(15):5344–5349. [PubMed] [Google Scholar]
- Turner P. D., Loughrey H. C., Bailey C. J. Hydrogen exchange kinetics and the mechanism of reaction B of yeast tryptophan synthase. Biochim Biophys Acta. 1985 Dec 20;832(3):280–287. doi: 10.1016/0167-4838(85)90261-4. [DOI] [PubMed] [Google Scholar]
- Zalkin H., Yanofsky C. Yeast gene TRP5: structure, function, regulation. J Biol Chem. 1982 Feb 10;257(3):1491–1500. [PubMed] [Google Scholar]


