Abstract
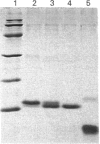
Leucocyte elastase in catalytic amounts was observed to rapidly cleave the Val-10-Gly-11 bond of the human cysteine-proteinase inhibitor cystatin C at neutral pH. The resulting modified inhibitor had size and amino acid composition consistent with a cystatin C molecule devoid of the N-terminal Ser-1-Val-10 decapeptide. Leucocyte-elastase-modified cystatin C had more than 240-fold lower affinity than native cystatin C for papain. Removal of the N-terminal decapeptide of human cystatin C also decreased inhibition of human cathepsins B and L by three orders of magnitude, but decreased inhibition of cathepsin H by only 5-fold. A tripeptidyldiazomethane analogue of of the N-terminal portion of cystatin C was a good inhibitor of cathepsins B and L but a poor inhibitor of cathepsin H. It therefore appears that amino acid side chains of the N-terminal segment of cystatin C bind in the substrate-binding pockets of cathepsins B and L but not in those of cathepsin H. It is argued that the N-terminal cystatin C interaction with cathepsin B is physiologically important and hence that leucocyte elastase could have a function as a regulator of extracellular cysteine-proteinase inhibitory activity at sites of inflammation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson M., Barrett A. J., Salvesen G., Grubb A. Isolation of six cysteine proteinase inhibitors from human urine. Their physicochemical and enzyme kinetic properties and concentrations in biological fluids. J Biol Chem. 1986 Aug 25;261(24):11282–11289. [PubMed] [Google Scholar]
- Abrahamson M., Dalbøge H., Olafsson I., Carlsen S., Grubb A. Efficient production of native, biologically active human cystatin C by Escherichia coli. FEBS Lett. 1988 Aug 15;236(1):14–18. doi: 10.1016/0014-5793(88)80276-x. [DOI] [PubMed] [Google Scholar]
- Abrahamson M., Grubb A., Olafsson I., Lundwall A. Molecular cloning and sequence analysis of cDNA coding for the precursor of the human cysteine proteinase inhibitor cystatin C. FEBS Lett. 1987 Jun 1;216(2):229–233. doi: 10.1016/0014-5793(87)80695-6. [DOI] [PubMed] [Google Scholar]
- Abrahamson M., Olafsson I., Palsdottir A., Ulvsbäck M., Lundwall A., Jensson O., Grubb A. Structure and expression of the human cystatin C gene. Biochem J. 1990 Jun 1;268(2):287–294. doi: 10.1042/bj2680287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson M., Ritonja A., Brown M. A., Grubb A., Machleidt W., Barrett A. J. Identification of the probable inhibitory reactive sites of the cysteine proteinase inhibitors human cystatin C and chicken cystatin. J Biol Chem. 1987 Jul 15;262(20):9688–9694. [PubMed] [Google Scholar]
- Assfalg-Machleidt I., Jochum M., Klaubert W., Inthorn D., Machleidt W. Enzymatically active cathepsin B dissociating from its inhibitor complexes is elevated in blood plasma of patients with septic shock and some malignant tumors. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):263–269. [PubMed] [Google Scholar]
- Baici A., Gyger-Marazzi M. The slow, tight-binding inhibition of cathepsin B by leupeptin. A hysteretic effect. Eur J Biochem. 1982 Dec;129(1):33–41. doi: 10.1111/j.1432-1033.1982.tb07017.x. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Davies M. E., Grubb A. The place of human gamma-trace (cystatin C) amongst the cysteine proteinase inhibitors. Biochem Biophys Res Commun. 1984 Apr 30;120(2):631–636. doi: 10.1016/0006-291x(84)91302-0. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Fritz H., Grubb A., Isemura S., Järvinen M., Katunuma N., Machleidt W., Müller-Esterl W., Sasaki M., Turk V. Nomenclature and classification of the proteins homologous with the cysteine-proteinase inhibitor chicken cystatin. Biochem J. 1986 May 15;236(1):312–312. doi: 10.1042/bj2360312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
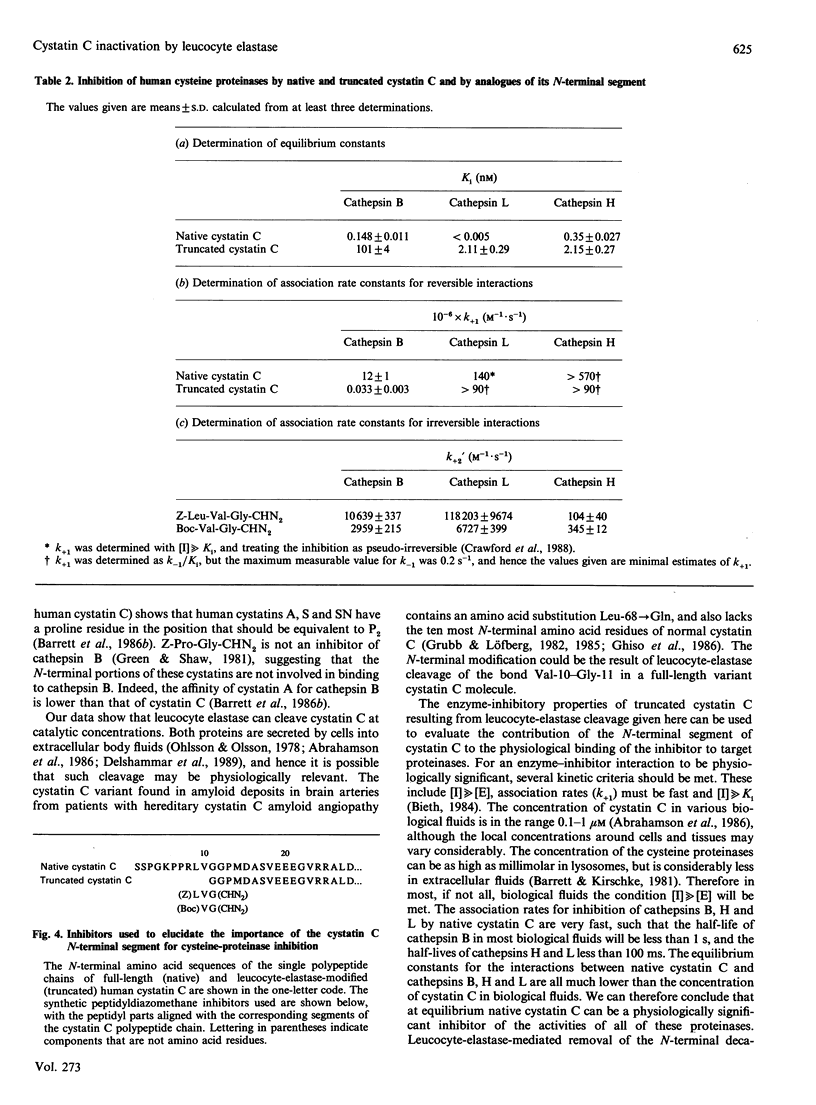
- Bieth J. G. In vivo significance of kinetic constants of protein proteinase inhibitors. Biochem Med. 1984 Dec;32(3):387–397. doi: 10.1016/0006-2944(84)90046-2. [DOI] [PubMed] [Google Scholar]
- Björck L., Akesson P., Bohus M., Trojnar J., Abrahamson M., Olafsson I., Grubb A. Bacterial growth blocked by a synthetic peptide based on the structure of a human proteinase inhibitor. Nature. 1989 Jan 26;337(6205):385–386. doi: 10.1038/337385a0. [DOI] [PubMed] [Google Scholar]
- Blumberg S., Schechter I., Berger A. The purification of papain by affinity chromatography. Eur J Biochem. 1970 Jul;15(1):97–102. doi: 10.1111/j.1432-1033.1970.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Bode W., Engh R., Musil D., Thiele U., Huber R., Karshikov A., Brzin J., Kos J., Turk V. The 2.0 A X-ray crystal structure of chicken egg white cystatin and its possible mode of interaction with cysteine proteinases. EMBO J. 1988 Aug;7(8):2593–2599. doi: 10.1002/j.1460-2075.1988.tb03109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttle D. J., Barrett A. J. Chymopapain. Chromatographic purification and immunological characterization. Biochem J. 1984 Oct 1;223(1):81–88. doi: 10.1042/bj2230081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttle D. J., Burnett D., Abrahamson M. Levels of neutrophil elastase and cathepsin B activities, and cystatins in human sputum: relationship to inflammation. Scand J Clin Lab Invest. 1990 Sep;50(5):509–516. doi: 10.1080/00365519009089165. [DOI] [PubMed] [Google Scholar]
- Buttle D. J., Ritonja A., Dando P. M., Abrahamson M., Shaw E. N., Wikstrom P., Turk V., Barrett A. J. Interactions of papaya proteinase IV with inhibitors. FEBS Lett. 1990 Mar 12;262(1):58–60. doi: 10.1016/0014-5793(90)80153-a. [DOI] [PubMed] [Google Scholar]
- Cejka J., Fleischmann L. E. Post- -globulin: isolation and physicochemical characterization. Arch Biochem Biophys. 1973 Jul;157(1):168–176. doi: 10.1016/0003-9861(73)90402-5. [DOI] [PubMed] [Google Scholar]
- Crawford C., Mason R. W., Wikstrom P., Shaw E. The design of peptidyldiazomethane inhibitors to distinguish between the cysteine proteinases calpain II, cathepsin L and cathepsin B. Biochem J. 1988 Aug 1;253(3):751–758. doi: 10.1042/bj2530751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaissé J. M., Eeckhout Y., Vaes G. In vivo and in vitro evidence for the involvement of cysteine proteinases in bone resorption. Biochem Biophys Res Commun. 1984 Dec 14;125(2):441–447. doi: 10.1016/0006-291x(84)90560-6. [DOI] [PubMed] [Google Scholar]
- Delshammar M., Lasson A., Ohlsson K. Proteases and protease inhibitor balance in peritonitis with different causes. Surgery. 1989 Sep;106(3):555–562. [PubMed] [Google Scholar]
- Gauthier F., Fryksmark U., Ohlsson K., Bieth J. G. Kinetics of the inhibition of leukocyte elastase by the bronchial inhibitor. Biochim Biophys Acta. 1982 Jan 18;700(2):178–183. doi: 10.1016/0167-4838(82)90095-4. [DOI] [PubMed] [Google Scholar]
- Ghiso J., Jensson O., Frangione B. Amyloid fibrils in hereditary cerebral hemorrhage with amyloidosis of Icelandic type is a variant of gamma-trace basic protein (cystatin C). Proc Natl Acad Sci U S A. 1986 May;83(9):2974–2978. doi: 10.1073/pnas.83.9.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. D., Shaw E. Peptidyl diazomethyl ketones are specific inactivators of thiol proteinases. J Biol Chem. 1981 Feb 25;256(4):1923–1928. [PubMed] [Google Scholar]
- Grubb A., Abrahamson M., Olafsson I., Trojnar J., Kasprzykowska R., Kasprzykowski F., Grzonka Z. Synthesis of cysteine proteinase inhibitors structurally based on the proteinase interacting N-terminal region of human cystatin C. Biol Chem Hoppe Seyler. 1990 May;371 (Suppl):137–144. [PubMed] [Google Scholar]
- Grubb A., Löfberg H. Human gamma-trace, a basic microprotein: amino acid sequence and presence in the adenohypophysis. Proc Natl Acad Sci U S A. 1982 May;79(9):3024–3027. doi: 10.1073/pnas.79.9.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppsson J. O., Laurell C. B., Franzén B. Agarose gel electrophoresis. Clin Chem. 1979 Apr;25(4):629–638. [PubMed] [Google Scholar]
- Johnson D. A., Barrett A. J., Mason R. W. Cathepsin L inactivates alpha 1-proteinase inhibitor by cleavage in the reactive site region. J Biol Chem. 1986 Nov 5;261(31):14748–14751. [PubMed] [Google Scholar]
- Johnson D., Travis J. Inactivation of human alpha 1-proteinase inhibitor by thiol proteinases. Biochem J. 1977 Jun 1;163(3):639–641. doi: 10.1042/bj1630639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppel P., Baici A., Keist R., Matzku S., Keller R. Cathepsin B-like proteinase as a marker for metastatic tumor cell variants. Exp Cell Biol. 1984;52(5):293–299. doi: 10.1159/000163273. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Machleidt W., Thiele U., Laber B., Assfalg-Machleidt I., Esterl A., Wiegand G., Kos J., Turk V., Bode W. Mechanism of inhibition of papain by chicken egg white cystatin. Inhibition constants of N-terminally truncated forms and cyanogen bromide fragments of the inhibitor. FEBS Lett. 1989 Jan 30;243(2):234–238. doi: 10.1016/0014-5793(89)80135-8. [DOI] [PubMed] [Google Scholar]
- Mason R. W., Green G. D., Barrett A. J. Human liver cathepsin L. Biochem J. 1985 Feb 15;226(1):233–241. doi: 10.1042/bj2260233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W. Interaction of lysosomal cysteine proteinases with alpha 2-macroglobulin: conclusive evidence for the endopeptidase activities of cathepsins B and H. Arch Biochem Biophys. 1989 Sep;273(2):367–374. doi: 10.1016/0003-9861(89)90495-5. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Recklies A. D., Poole A. R. Extracellular presence of the lysosomal proteinase cathepsin B in rheumatoid synovium and its activity at neutral pH. Arthritis Rheum. 1984 May;27(5):509–515. doi: 10.1002/art.1780270505. [DOI] [PubMed] [Google Scholar]
- Nicklin M. J., Barrett A. J. Inhibition of cysteine proteinases and dipeptidyl peptidase I by egg-white cystatin. Biochem J. 1984 Oct 1;223(1):245–253. doi: 10.1042/bj2230245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson K., Olsson A. S. Immunoreactive granulocyte elastase in human serum. Hoppe Seylers Z Physiol Chem. 1978 Nov;359(11):1531–1539. doi: 10.1515/bchm2.1978.359.2.1531. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Tegner H. Granulocyte collagenase, elastase and plasma protease inhibitors in purulent sputum. Eur J Clin Invest. 1975 Jun 12;5(3):221–227. doi: 10.1111/j.1365-2362.1975.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Pietras R. J., Szego C. M., Mangan C. E., Seeler B. J., Burtnett M. M., Orevi M. Elevated serum cathepsin B1 and vaginal pathology after prenatal DES exposure. Obstet Gynecol. 1978 Sep;52(3):321–327. [PubMed] [Google Scholar]
- Rich D. H., Brown M. A., Barrett A. J. Purification of cathepsin B by a new form of affinity chromatography. Biochem J. 1986 May 1;235(3):731–734. doi: 10.1042/bj2350731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz W. N., Barrett A. J. Human cathepsin H. Biochem J. 1980 Nov 1;191(2):487–497. doi: 10.1042/bj1910487. [DOI] [PMC free article] [PubMed] [Google Scholar]