Laryngeal motor control is crucial in a variety of fundamental behaviors, including swallowing and breathing, and in humans, spoken language and vocalization. Proximate control of the laryngeal muscles during speech production is known to be supported by ventral laryngeal motor cortex (vLMC) [1]. More controversially, a human-unique and recently evolved dorsal laryngeal motor cortex area (dLMC) with direct (mono-synaptic) control of laryngeal muscles through the nucleus retroambiguus has been proposed [2,3]. This dorsal laryngeal motor control area is the focus of the current investigation.
Patient AJ presented at the age of 27 with a tumor in the right frontal lobe undercutting the superior and middle frontal gyri (Fig. 1a). AJ had no discernible cognitive, sensory or motor impairments prior to surgery (Supplemental Online Materials). Because of the proximity of the lesion to motor cortex, and because pre-operative fMRI suggested involvement of right frontal regions in speech production, the surgery for removal of the tumor was carried out using an asleep-awake-asleep procedure for language and motor mapping [4]. The rare clinical opportunity to explore direct electrical stimulation mapping of dLMC in the right hemisphere of a left-language-dominant individual allowed us to assess the specific role of dLMC in speech production.
Fig. 1. Stimulation of dorsal Laryngeal Motor Cortex disrupts vocalization during speech production.
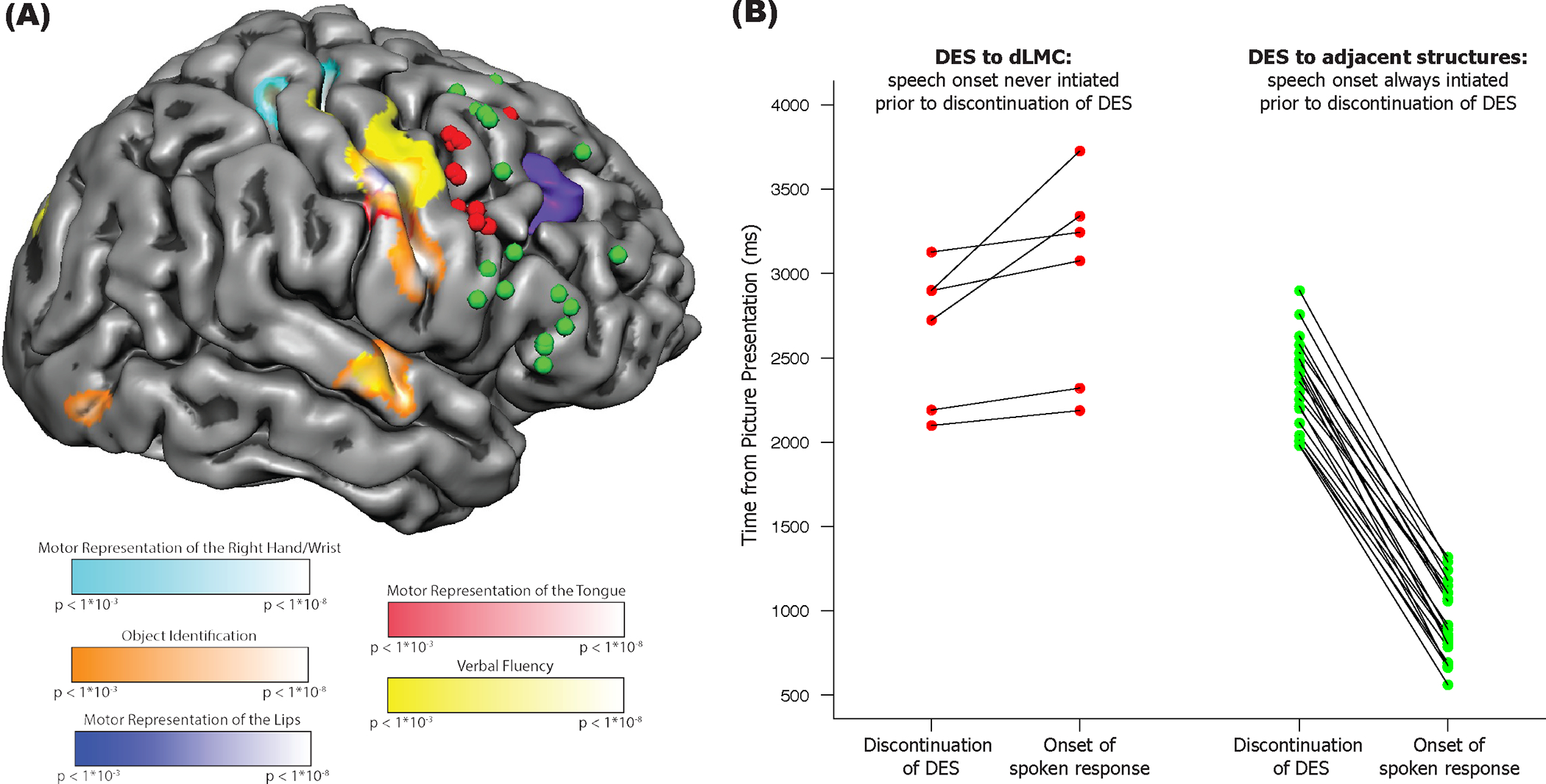
(A) Overview of intra-operative direct electrical stimulation mapping and pre-operative functional MRI. Reconstruction of the right hemisphere of Patient AJ’s brain, with locations of intraoperative stimulation associated with errors (red circles) and correct trials (green circles). The tumor in the right frontal lobe is represented in purple (visible in the mesh where it came to the cortical surface). Also plotted on the cortical surface are the results of pre-operative functional MRI for wrist, lip, and tongue movements, object identification, and verbal fluency. (B) Speech production is disrupted by stimulation to dLMC but not to adjacent structures. All data points are temporally aligned (y-axis) with respect to the onset of the picture stimulus on the corresponding trial. The left panel plots data for stimulation to dLMC (red circles) while the right panel plots data for stimulation to structures adjacent to (but not including) dLMC. The left column of data points in each panel corresponds to the time-point, after picture presentation, when direct electrical stimulation to the cortical surface was discontinued; the right column in each panel plots the time point (post picture onset) at which speech initiated (the two measures for each trial connected by line). The key observation is that for trials involving stimulation to dLMC (left panel), it was always the case that onset of a correct response occurred after electrical stimulation was discontinued. By contrast, when stimulation was applied to structures adjacent to dLMC (right panel), the patient was always able to initiate a correct response prior to discontinuation of stimulation. This pattern was substantiated with formal analysis: For those trials in which the patient eventually produced an accurate response after stimulation to dLMC, the average response time (from picture stimulus onset to initiation of a correct response) was 2983ms (SD=605ms). That response time was longer than the average response time for the 21 accurate trials associated with stimulation to structures adjacent to, but not overlapping with, dLMC (Response Time=939ms, SD=229ms; Welch Two Sample t-test, t=8.11, p<.001). Importantly, the bipolar stimulator was not in contact with the brain for a longer period of time on trials of transient speech arrest involving stimulation to dLMC (mean = 2277ms, range = 1800:2740ms) compared to trials with stimulation to surrounding structures (mean = 2103ms, range = 1530:3200ms; t =1.02, p=.33).
During the awake portion of his surgery, AJ completed 70 trials of a picture naming task in which he read a short preamble (‘This is a…) and then named a target picture (e.g., …CAT.”). 7 of the 70 trials were excluded from analyses because of interruptions unrelated to stimulation (e.g. patient talking to clinicians). Of the remaining 63 trials, 33 were paired with DES to the cortical surface and 30 were without stimulation. Of the 33 trials paired with stimulation, 12 were characterized by disruptions to the patient’s speech production. Of the 30 trials not paired with stimulation, three trials were marked by observable errors; there was a significant effect of stimulation on the likelihood of error (12/33 vs 3/30; χ2 = 4.7; p < .05; see Figure 1A for all locations of DES, plotted on a 3D reconstruction of AJ’s brain).
Eleven of the 33 stimulation trials were localized to a ~2cm region (i.e. 9mm radius) centered on the anterior aspect of right dorsal BA 6, anterior to speech motor cortex. This is the region previously identified as dorsal laryngeal motor cortex (dLMC) [3,5,6]. All (i.e., 11/11) trials with stimulation to dLMC resulted in an observable disruption to speech. There were several types of errors caused by stimulation of dLMC. First, AJ was unable to initiate coherent speech for the duration that the stimulator was in contact with cortex (see below, and Figure 1B, for additional analyses). Second, he made involuntary guttural vocalizations and non-linguistic voiced intrusions. And third, when AJ did initiate recognizable speech on a trial involving stimulation to dLMC, the resulting utterance was dysfluent and slurred (see Supporting Video, https://youtu.be/AotZG22KvEs). By contrast, of the 22 trials paired with stimulation to structures surrounding dLMC, the patient produced an error on one trial, and fluent, accurate responses on 21 trials. The higher incidence of errors in association with stimulation to dLMC compared to stimulation to surrounding structures was significant (11/11 vs. 1/22; χ2=24.90; p< .001). Furthermore, there was no difference in response time for accurate trials without stimulation (n=27; 1059ms, SD=403ms) compared to trials with stimulation to structures adjacent to (but not overlapping) dLMC (Welch Two Sample t-test, t=1.31, p=.199). This lack of a difference in response times indicates that stimulation of structures adjacent to dLMC did not, even subtly, disrupt the initiation of a correct linguistic response. This pattern is also consistent with the assumption, based on pre-operative functional MRI (Figure 1A) that the right hemisphere is the non-dominant hemisphere in this right-handed individual.
Of the 11 picture-naming trials in which stimulation was applied to dLMC, AJ eventually produced a correct response on 6 of those trials and never produced a correct response on the remaining 5 trials. A salient aspect of the patient’s behavior on the 6 trials in which he eventually produced the correct response was what we refer to as ‘transient speech arrest’: the patient was unable to initiate a correct response until after the stimulator discontinued contact with the surface of the brain. Furthermore, when the patient did initiate speech after stimulation to dLMC, he was able to do so within 200ms of the stimulator discontinuing contact with the brain (Fig. 1b). That pattern of exceedingly rapid recovery to speech onset indicates that the entire utterance was retrieved and planned, and thus that stimulation of the dLMC disrupted speech at a very peripheral stage of processing (namely, vocalization).
Our observations provide causal evidence in support of the inference that dLMC has direct feedforward control of laryngeal muscles [3,5,6]. In considering the evolution of vocal control in primates, the traditional perspective emphasizes human-unique morphological modifications of vocal anatomy (for review see [7]). However, much recent work suggests that key innovations underlying precise laryngeal control occurred at the neural level [6–8; see also [9]], and that the neural systems controlling the larynx developed in concert with the dorsal speech-processing stream [10]. In this regard, the dLMC may be an example of a neural substrate unique to humans and which supported the human-unique capacity for speech [2,3,6].
Supplementary Material
Acknowledgements:
This work was supported by NIH Grants R01NS089069 and R01EY028535 to BZM, NSF Grant BCS-1349042 to BZM and EN, and a core grant to the Center for Vision Sciences at the University of Rochester (P30 EY001319). Preparation of the manuscript was supported, in part, by training grant T32GM081760 from the National Institute of General Medical Sciences (to JRB), NSF training grant (NSF DGE-1449828) support for BLC, and a University of Rochester Center for Visual Science pre-doctoral training fellowship (NIH training Grant 5T32EY007125–24) to FEG. The authors are grateful to Sarah Gannon for assistance with intra-operative testing and neurophysiological monitoring, and Keith Parkins for his work on the development of StrongView. The Program for Translational Brain Mapping at the University of Rochester was established, in part, with support from Norman and Arlene Leenhouts, and with a grant from the Wilmot Cancer Institute to Drs. Kevin Walter and Bradford Z. Mahon. Information about the Program for Translational Brain Mapping at the University of Rochester Medical Center can be found at: www.tbm.urmc.edu.
Footnotes
Declaration of Competing Interest:
Authors BZM and MS have intellectual property (patent pending, PCT/US2019/064015) associated with the software and hardware systems (StrongView) used to collect data for this report, and are co-founders of MindTrace Technologies, Inc., which licenses said intellectual property from Carnegie Mellon University.
References:
- 1.Loucks TM, Poletto CJ, Simonyan K, Reynolds CL, & Ludlow CL (2007). Human brain activation during phonation and exhalation: Common volitional control for two upper airway functions. Neuroimage, 36(1), 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonyan K (2014). The laryngeal motor cortex: its organization and connectivity. Current opinion in neurobiology, 28, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belyk M, & Brown S (2017). The origins of the vocal brain in humans. Neuroscience & Biobehavioral Reviews, 77, 177–193. [DOI] [PubMed] [Google Scholar]
- 4.Manninen PH, Balki M, Lukitto K, & Bernstein M (2006). Patient satisfaction with awake craniotomy for tumor surgery: a comparison of remifentanil and fentanyl in conjunction with propofol. Anesthesia & Analgesia, 102(1), 237–242. [DOI] [PubMed] [Google Scholar]
- 5.Breshears JD, Molinaro AM, & Chang EF (2015). A probabilistic map of the human ventral sensorimotor cortex using electrical stimulation. Journal of neurosurgery, 123(2), 340–349 [DOI] [PubMed] [Google Scholar]
- 6.Dichter BK, Breshears JD, Leonard MK, & Chang EF (2018). The control of vocal pitch in human laryngeal motor cortex. Cell, 174(1), 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitch WT (2000). The evolution of speech: a comparative review. Trends in cognitive sciences, 4(7), 258–267. [DOI] [PubMed] [Google Scholar]
- 8.Fitch WT, De Boer B, Mathur N, & Ghazanfar AA (2016). Monkey vocal tracts are speech-ready. Science advances, 2(12), e1600723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieberman P, Fecteau S, Théoret H, Garcia RR, Aboitiz F, MacLarnon A, … & Lieberman P. (2007). The evolution of human speech: Its anatomical and neural bases. Current anthropology, 48(1), 39–66. [Google Scholar]
- 10.Hickok G (2017). A cortical circuit for voluntary laryngeal control: Implications for the evolution language. Psychonomic bulletin & review, 24(1), 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


